Ulvan as a Reducing Agent for the Green Synthesis of Silver Nanoparticles: A Novel Mouthwash
Abstract
1. Introduction
2. Materials and Methods
2.1. Ulvan Extraction from Ulva lactuca
2.2. Green Synthesis of Biogenic Silver Nanoparticles
2.3. Characterisation of AgNPs
2.4. Antioxidant Activity—DPPH Radical Assay
2.5. Cell Viability Assay
2.6. Preparation of Mouthrinse
2.7. Antimicrobial Activity of Ulvan-Mediated AgNP Mouthrinse against Oral Pathogens
3. Results
3.1. Characterisation of AgNPs
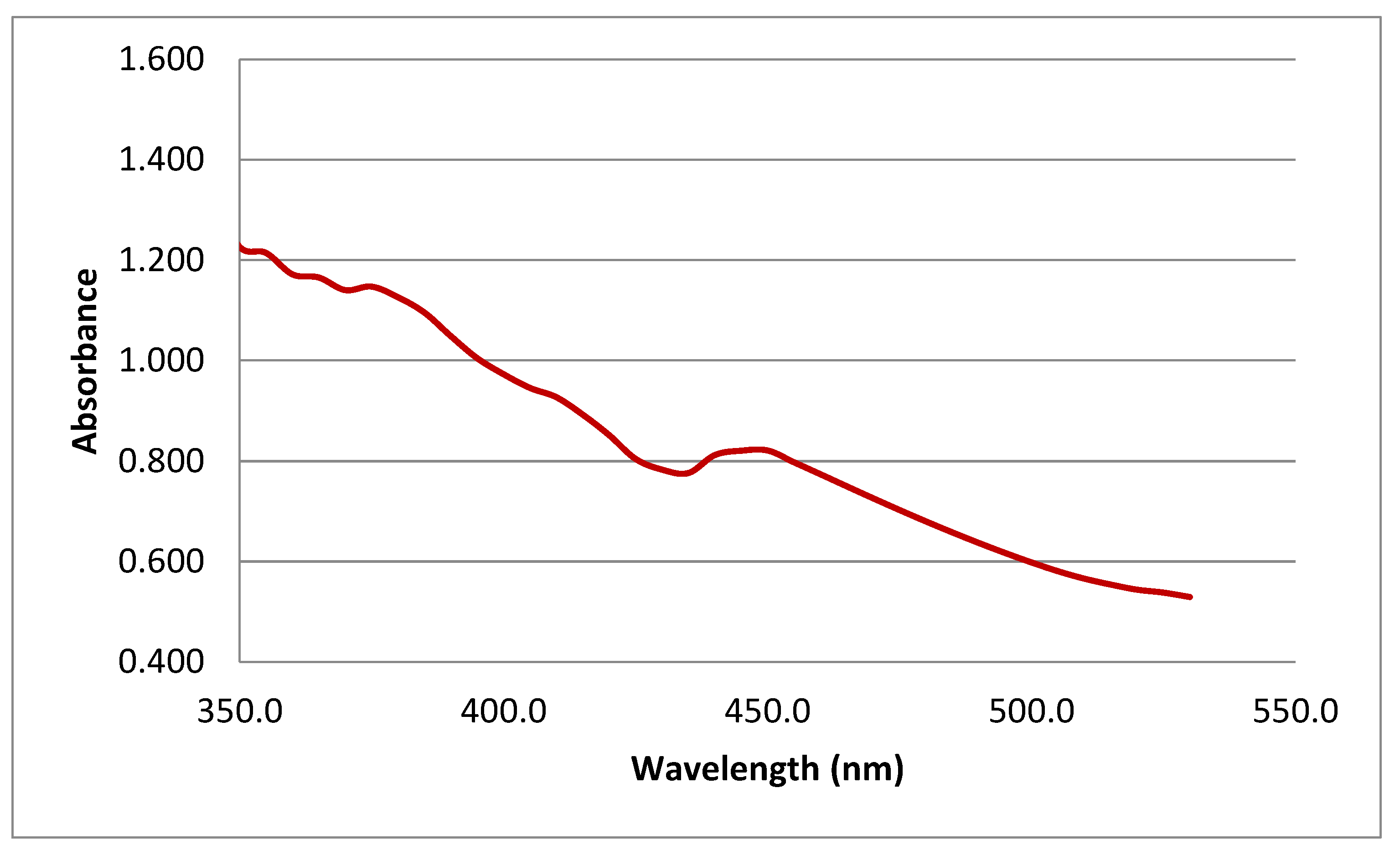
3.1.1. UV–Vis Spectra Analysis
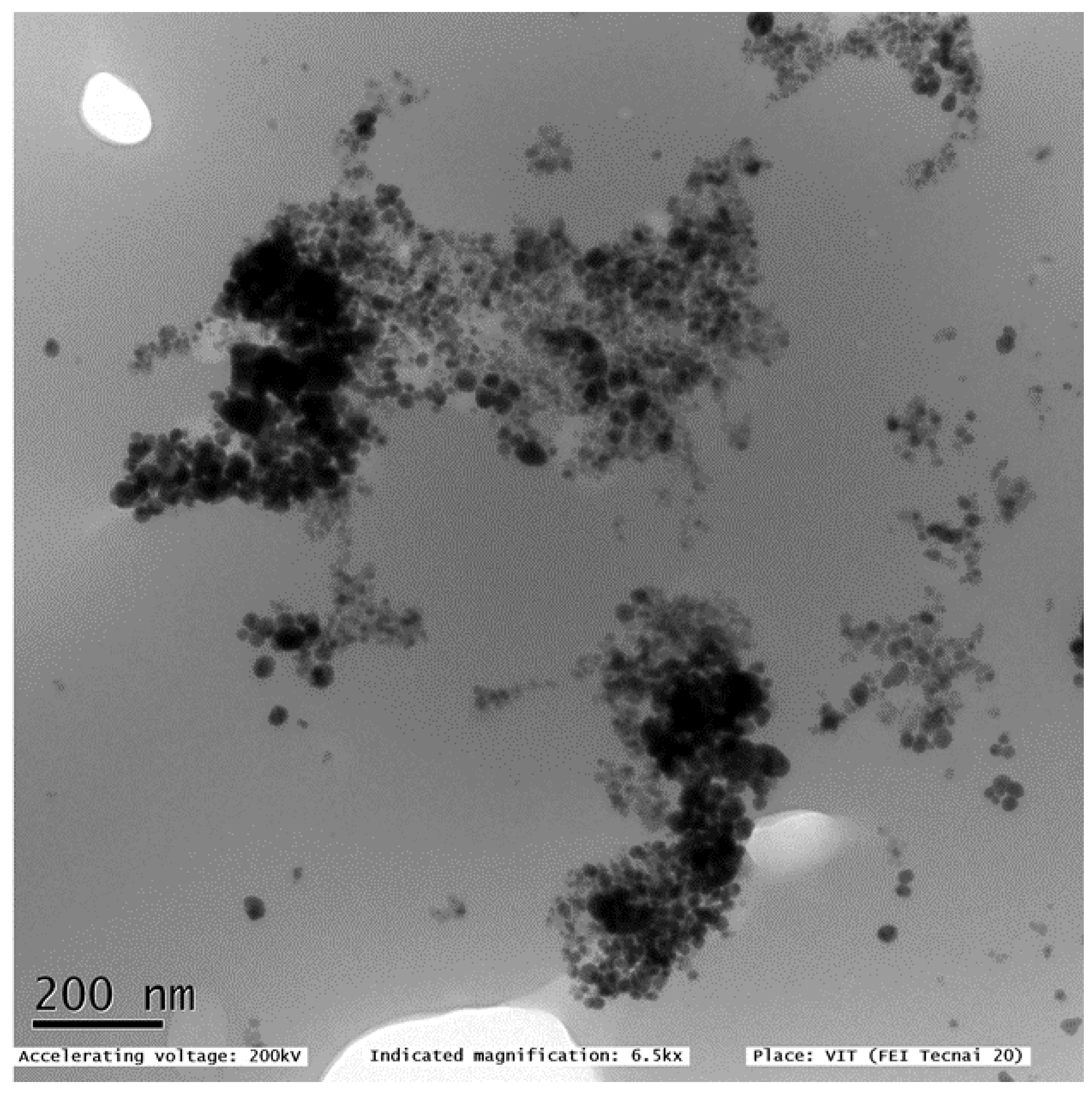
3.1.2. Transmission Electron Microscopy and X-ray Diffraction Analysis
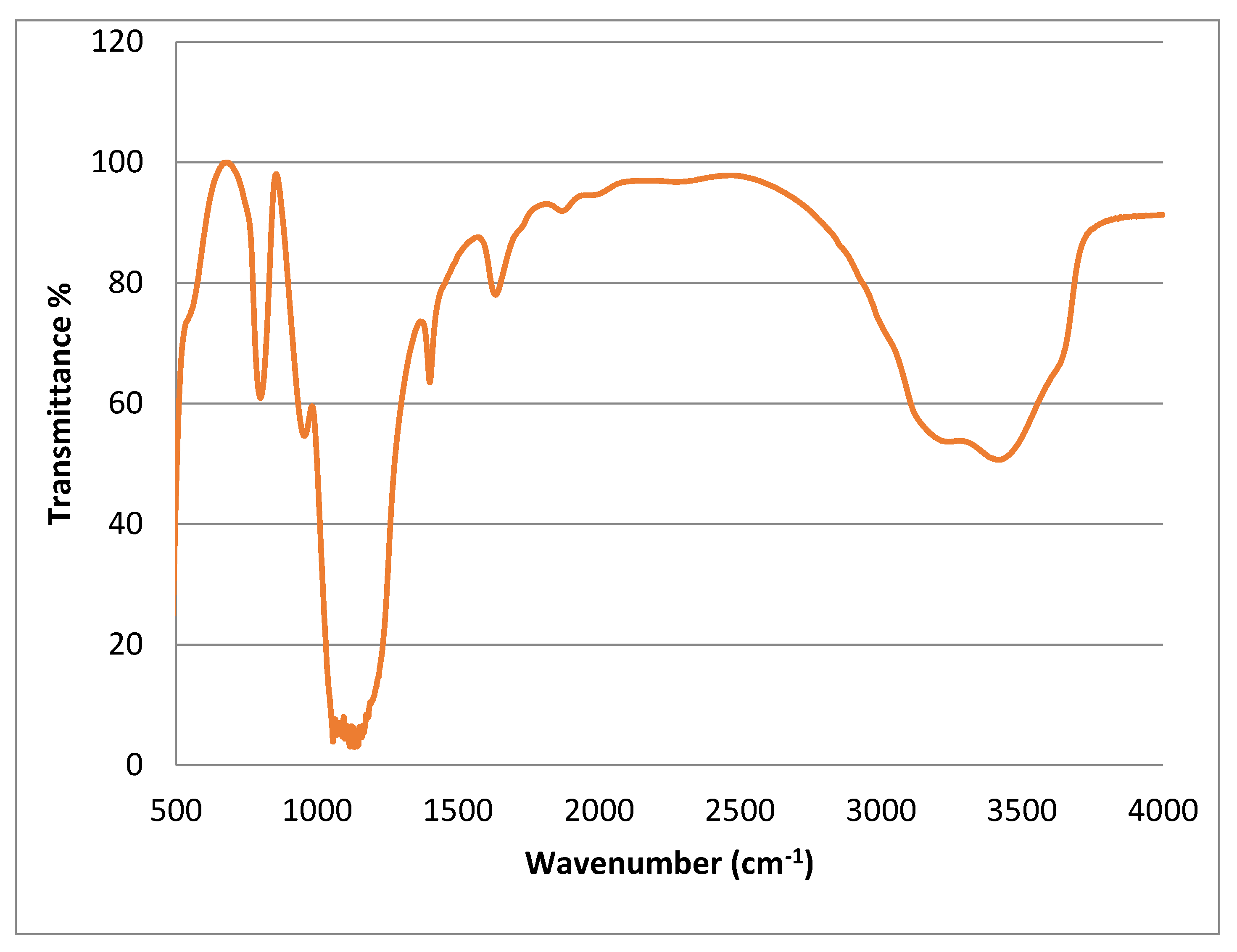
3.1.3. FT-IR Assessment
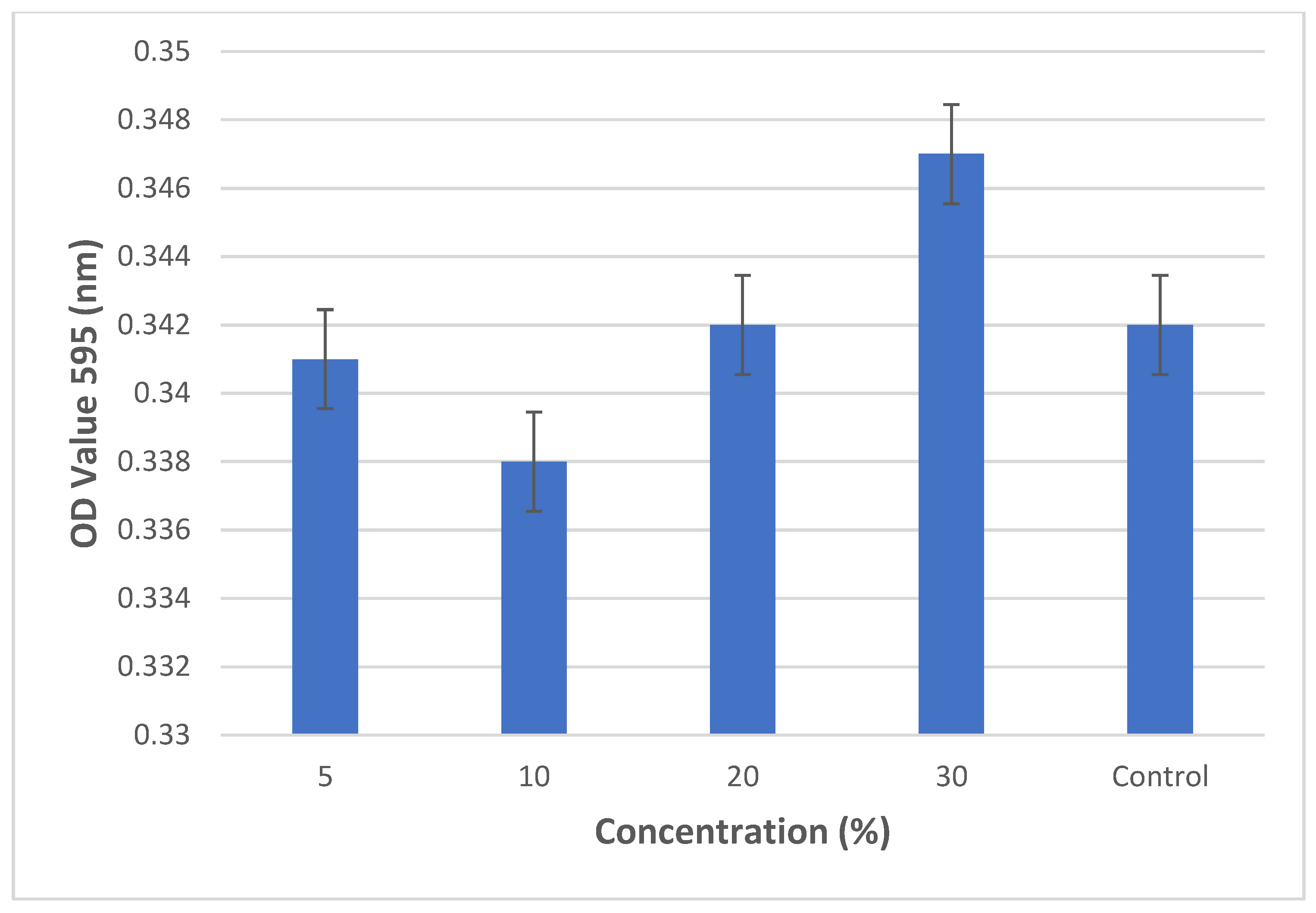
3.2. Cell Viability Assay
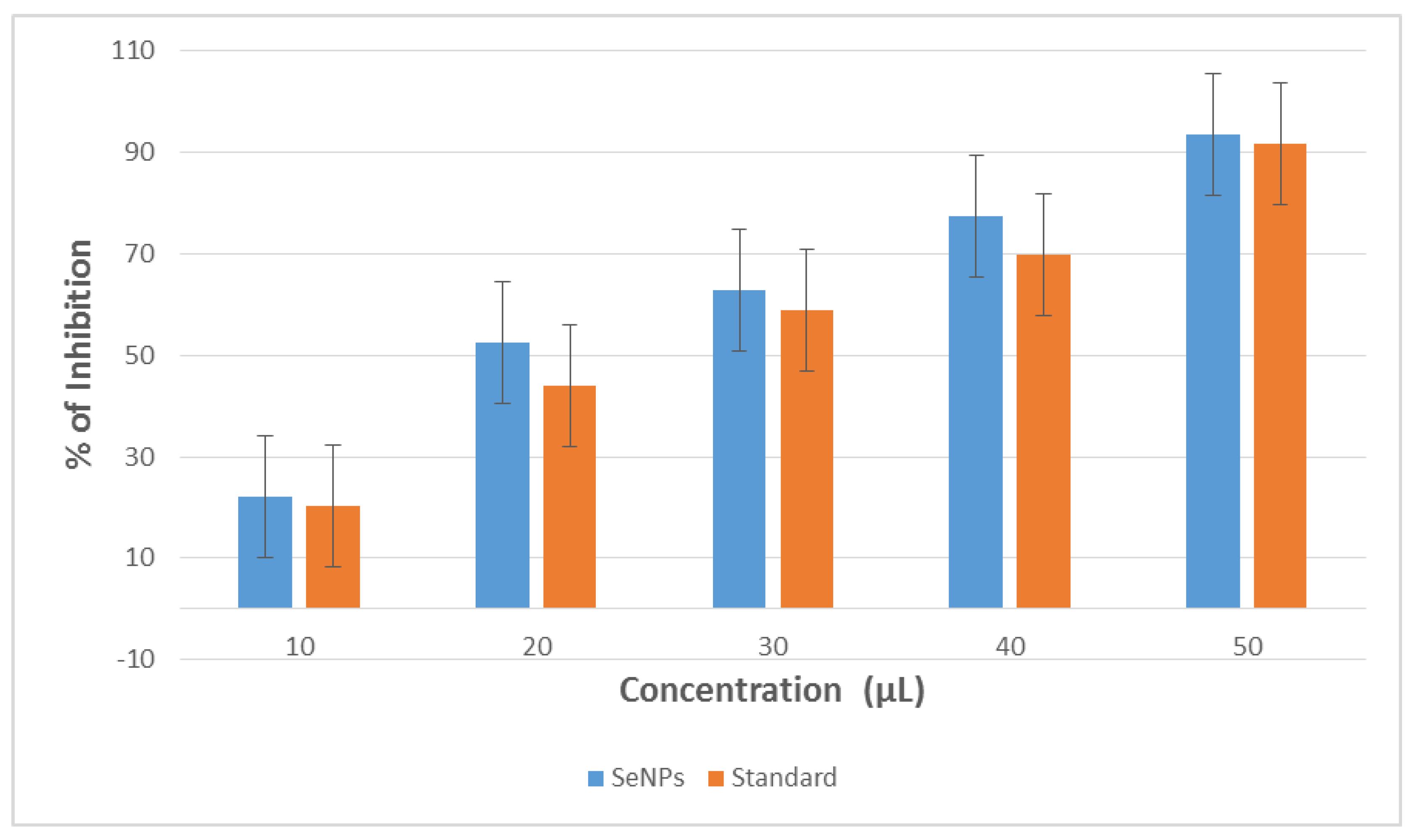
3.3. Antioxidant Activity
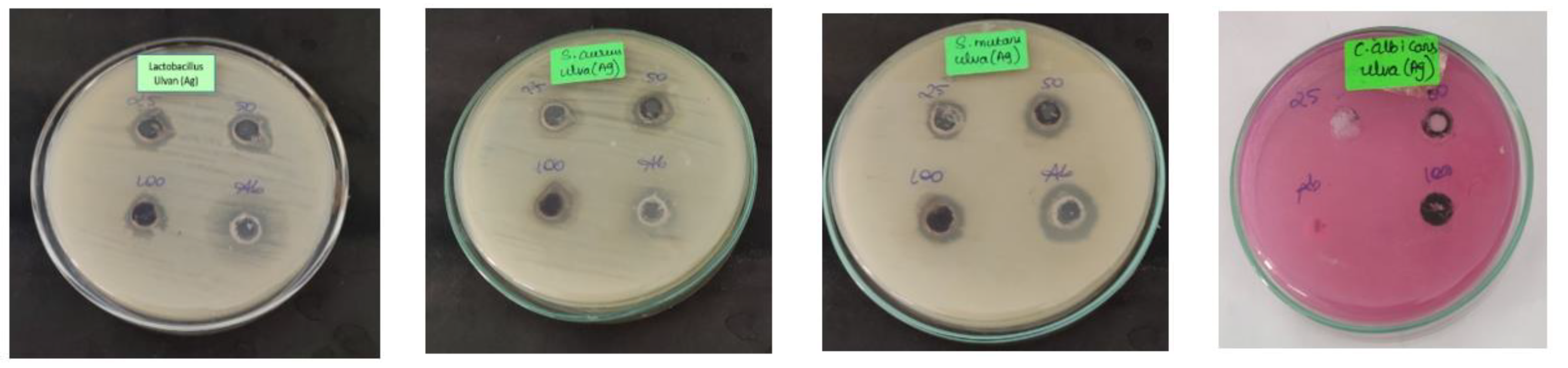
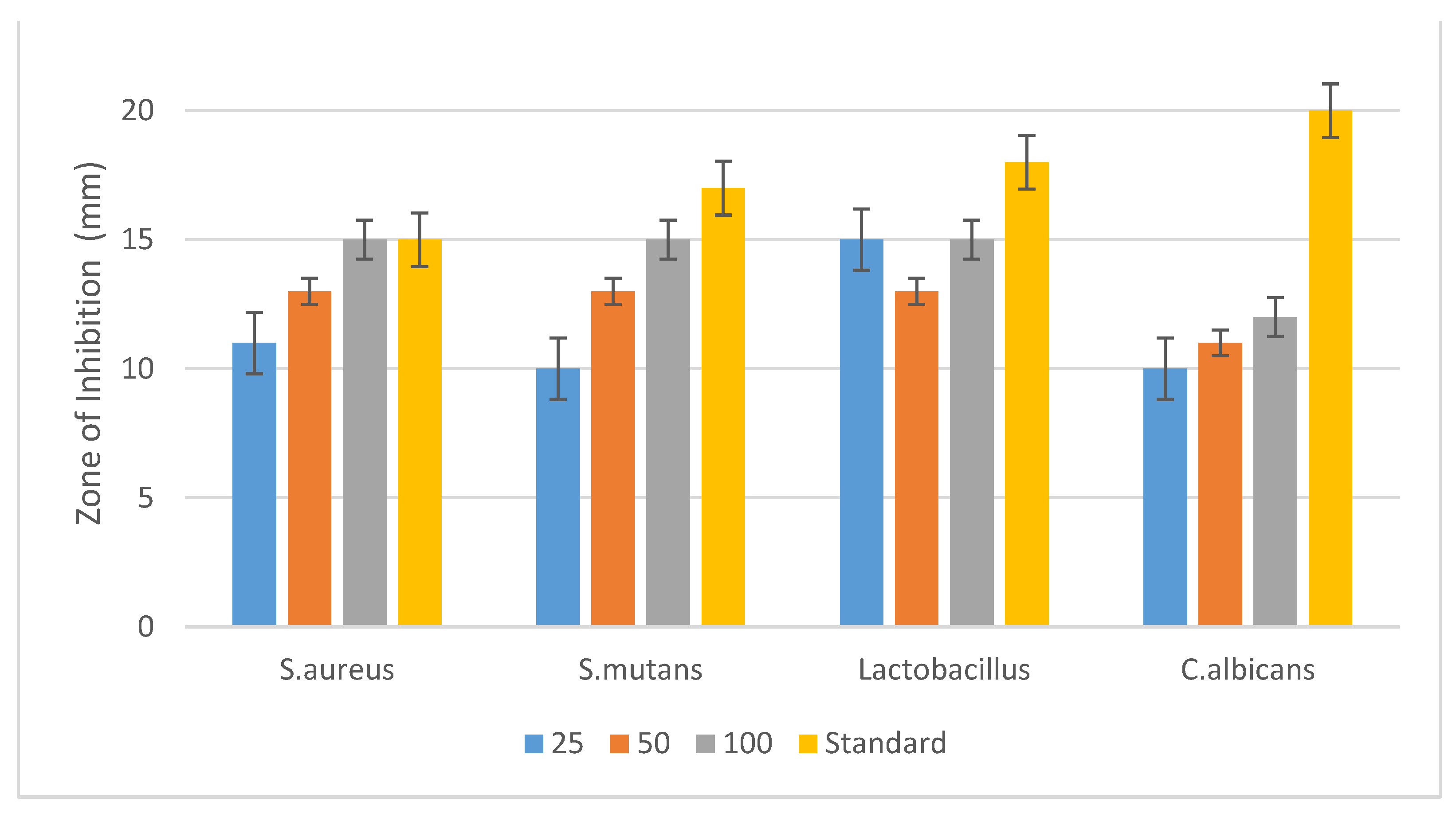
3.4. Antimicrobial Activity against Oral Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webster, T.J. Tran, Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011, 6, 1553. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Hu, D.; Ogawa, K.; Kajiyama, M.; Enomae, T. Characterization of self-assembled silver nanoparticle ink based on nanoemulsion method. R. Soc. Open Sci. 2020, 7, 200296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, H.; Bai, J.; Li, Y.; Yang, J.; Ma, Q.; Qu, Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 571, 9–16. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Sujatha, L.; Govardhan, L.; Rangaiah, G.S. Antibacterial Activity of Green Seaweeds on Oral Bacteria; Indian Journal of Natural Products and Resources: New Delhi, India, 2012. [Google Scholar]
- Tang, Y.-Q.; Mahmood, K.; Shehzadi, R.; Ashraf, M.F. Ulva Lactuca and Its Polysaccharides: Food and Biomedical Aspects. 2016. Available online: https://www.researchgate.net/publication/292156349 (accessed on 24 January 2020).
- Fernandes, G.L.; Delbem, A.C.B.; Amaral, J.G.D.; Gorup, L.F.; Fernandes, R.A.; Neto, F.N.D.S.; Souza, J.A.S.; Monteiro, D.R.; Hunt, A.M.A.; Camargo, E.R.; et al. Nanosynthesis of silver-calcium glycerophosphate: Promising association against oral pathogens. Antibiotics 2018, 7, 52. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of antimicrobial nanoparticles in dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef]
- Jeddy, N.; Ravi, S.; Radhika, T.; Lakshmi, L.J.S. Comparison of the efficacy of herbal mouth rinse with commercially available mouth rinses: A clinical trial. J. Oral Maxillofac. Pathol. 2018, 22, 332. [Google Scholar] [CrossRef]
- Silva Viana, R.L.; Pereira Fidelis, G.; Jane Campos Medeiros, M.; Antonio Morgano, M.; Gabriela Chagas Faustino Alves, M.; Domingues Passero, L.F.; Lima Pontes, D.; Cordeiro Theodoro, R.; Domingos Arantes, T.; Araujo Sabry, D.; et al. Green Synthesis of Antileishmanial and Antifungal Silver Nanoparticles Using Corn Cob Xylan as a Reducing and Stabilizing Agent. Biomolecules 2020, 10, 1235. [Google Scholar] [CrossRef]
- Hassabo, A.G.; Nada, A.A.; Ibrahim, H.M.; Abou-Zeid, N.Y. Impregnation of silver nanoparticles into polysaccharide substrates and their properties. Carbohydr. Polym. 2015, 122, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Vikneshan, M.; Saravanakumar, R.; Mangaiyarkarasi, R.; Rajeshkumar, S.; Samuel, S.R.; Suganya, M.; Baskar, G. Algal biomass as a source for novel oral nano-antimicrobial agent. Saudi J. Biol. Sci. 2020, 27, 3753–3758. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, Q.; Li, N.; Xu, Z.; Wang, Y.; Li, Z. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Qidwai, A.; Kumar, R.; Dikshit, A. Green synthesis of silver nanoparticles by seed of Phoenix sylvestris L. and their role in the management of cosmetics embarrassment. Green Chem. Lett. Rev. 2018, 11, 176–188. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; El-Mongy, M.A.; Eid, K.F. Antibacterial activity of silver nanoparticles using ulva fasciata extracts as reducing agent and sodium dodecyl sulfate as stabilizer. Int. J. Pharmacol. 2018, 14, 359–368. [Google Scholar] [CrossRef]
- Azizi, S.; Namvar, F.; Mahdavi, M.; Ahmad, M.B.; Mohamad, R. Biosynthesis of silver nanoparticles using brown marine macroalga, Sargassum muticum aqueous extract. Materials 2013, 6, 5942–5950. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, Y.; Zheng, W.; Fan, C.; Chen, T. Positive surface charge enhances selective cellular uptake and anticancer efficacy of selenium nanoparticles. Inorg. Chem. 2012, 51, 8956–8963. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Bao, Y.; Zhang, L. Nano red elemental selenium has no size effect in the induction of seleno-enzymes in both cultured cells and mice. Life Sci. 2004, 75, 237–244. [Google Scholar] [CrossRef]
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro evaluation of antioxidant and antibacterial potential of green synthesized silver nanoparticles using prosopis farcta fruit extract. Iran. J. Pharm. Res. 2019, 18, 430–445. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Boegli, L.; James, G.; Velasquillo, C.; Sánchez-Sánchez, R.; Martínez-Martínez, R.E.; Martínez-Castañón, G.A.; Martinez-Gutierrez, F. Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Mater. Sci. Eng. C 2015, 55, 360–366. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Martinez-Castanon, G.A.; Martínez-Martínez, R.E.; Loyola-Rodríguez, J.P.; Patiño-Marín, N.; Reyes-Macías, J.F.; Ruiz, F. Antimicrobial sensibility of Streptococcus mutans serotypes to silver nanoparticles. Mater. Sci. Eng. C 2012, 32, 896–901. [Google Scholar] [CrossRef]
- Panpaliya, N.P.; Dahake, P.T.; Kale, Y.J.; Dadpe, M.V.; Kendre, S.B.; Siddiqi, A.G.; Maggavi, U.R. In vitro evaluation of antimicrobial property of silver nanoparticles and chlorhexidine against five different oral pathogenic bacteria. Saudi Dent. J. 2019, 31, 76–83. [Google Scholar] [CrossRef]
- Hernández-Gómora, A.E.; Lara-Carrillo, E.; Robles-Navarro, J.B.; Scougall-Vilchis, R.J.; Hernández-López, S.; Medina-Solís, C.E.; Morales-Luckie, R.A. Biosynthesis of silver nanoparticles on orthodontic elastomeric modules: Evaluation of mechanical and antibacterial properties. Molecules 2017, 22, 1407. [Google Scholar] [CrossRef] [PubMed]
- Alnairat, N.; Abu Dalo, M.; Abu-Zurayk, R.; Abu Mallouh, S.; Odeh, F.; Al Bawab, A. Green Synthesis of Silver Nanoparticles as an Effective Antibiofouling Material for Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane. Polymers 2021, 13, 3683. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Al-Ahaidib, R.A.; Alharbi, R.I.; Al-Otaibi, R.M.; Albasher, G. Antimicrobial Potential of Biosynthesized Silver Nanoparticles by Aaronsohnia factorovskyi Extract. Molecules 2020, 26, 130. [Google Scholar] [CrossRef]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef]
- Jabir, M.S.; Saleh, Y.M.; Sulaiman, G.M.; Yaseen, N.Y.; Sahib, U.I.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Green Synthesis of Silver Nanoparticles Using Annona muricata Extract as an Inducer of Apoptosis in Cancer Cells and Inhibitor for NLRP3 Inflammasome via Enhanced Autophagy. Nanomaterials 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Hendiger, E.B.; Padzik, M.; Sifaoui, I.; Reyes-Batlle, M.; López-Arencibia, A.; Rizo-Liendo, A.; Bethencourt-Estrella, C.J.; Nicolás-Hernández, D.S.; Chiboub, O.; Rodríguez-Expósito, R.L.; et al. Silver Nanoparticles as a Novel Potential Preventive Agent against Acanthamoeba Keratitis. Pathogens 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohandoss, S.; Murugaboopathy, V.; Haricharan, P.B.; Hebbal, M.I.; Saadaldin, S.; Soliman, M.; Eldwakhly, E. Ulvan as a Reducing Agent for the Green Synthesis of Silver Nanoparticles: A Novel Mouthwash. Inorganics 2023, 11, 5. https://doi.org/10.3390/inorganics11010005
Mohandoss S, Murugaboopathy V, Haricharan PB, Hebbal MI, Saadaldin S, Soliman M, Eldwakhly E. Ulvan as a Reducing Agent for the Green Synthesis of Silver Nanoparticles: A Novel Mouthwash. Inorganics. 2023; 11(1):5. https://doi.org/10.3390/inorganics11010005
Chicago/Turabian StyleMohandoss, Suganya, Vikneshan Murugaboopathy, Praveen Bhoopathi Haricharan, Mamata Iranna Hebbal, Selma Saadaldin, Mai Soliman, and Elzahraa Eldwakhly. 2023. "Ulvan as a Reducing Agent for the Green Synthesis of Silver Nanoparticles: A Novel Mouthwash" Inorganics 11, no. 1: 5. https://doi.org/10.3390/inorganics11010005
APA StyleMohandoss, S., Murugaboopathy, V., Haricharan, P. B., Hebbal, M. I., Saadaldin, S., Soliman, M., & Eldwakhly, E. (2023). Ulvan as a Reducing Agent for the Green Synthesis of Silver Nanoparticles: A Novel Mouthwash. Inorganics, 11(1), 5. https://doi.org/10.3390/inorganics11010005






