Trimethylammonium Sn(IV) and Pb(IV) Chlorometalate Complexes with Incorporated Dichlorine
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of 1
2.2. Preparation of 2
2.3. X-Ray Diffractometry
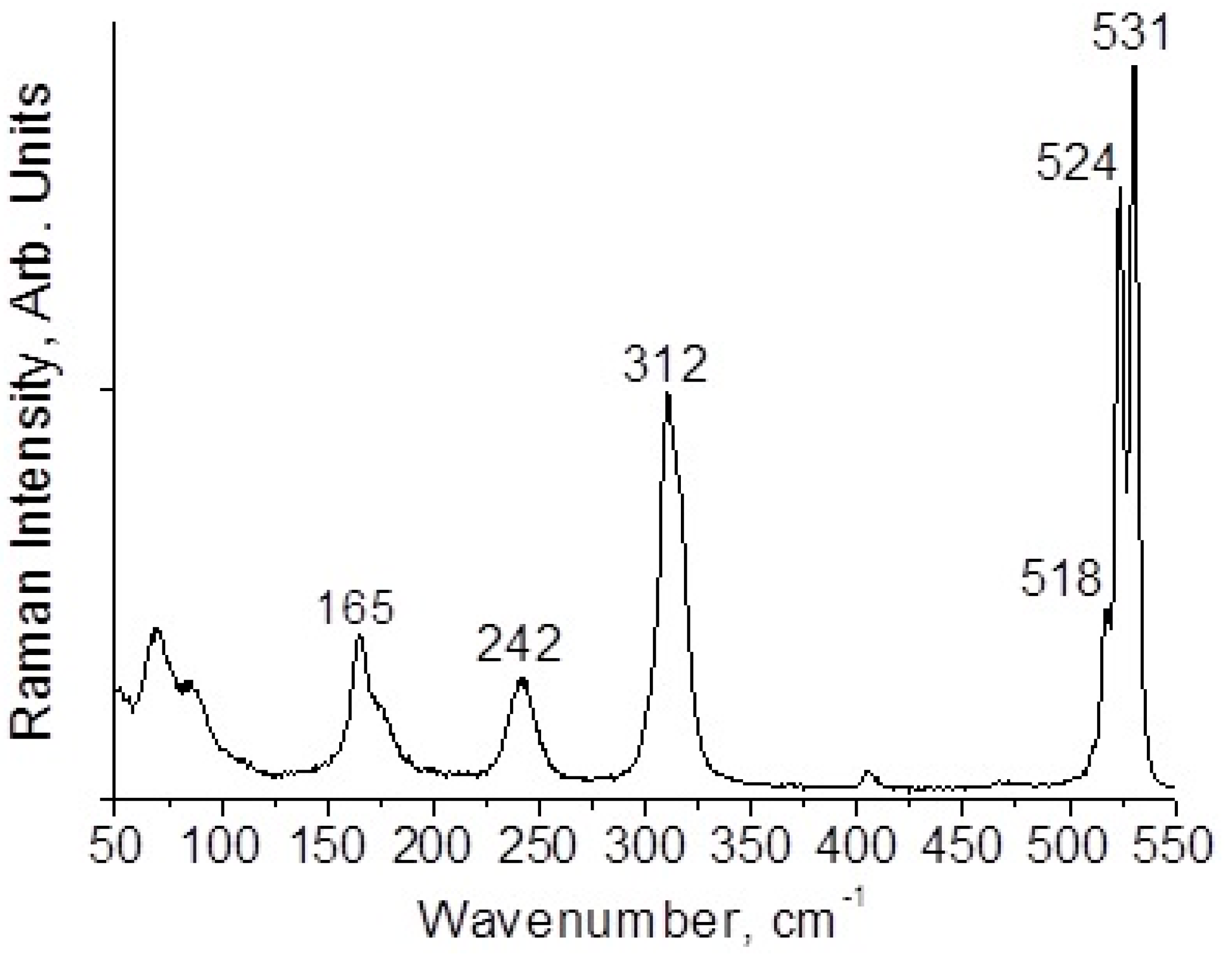
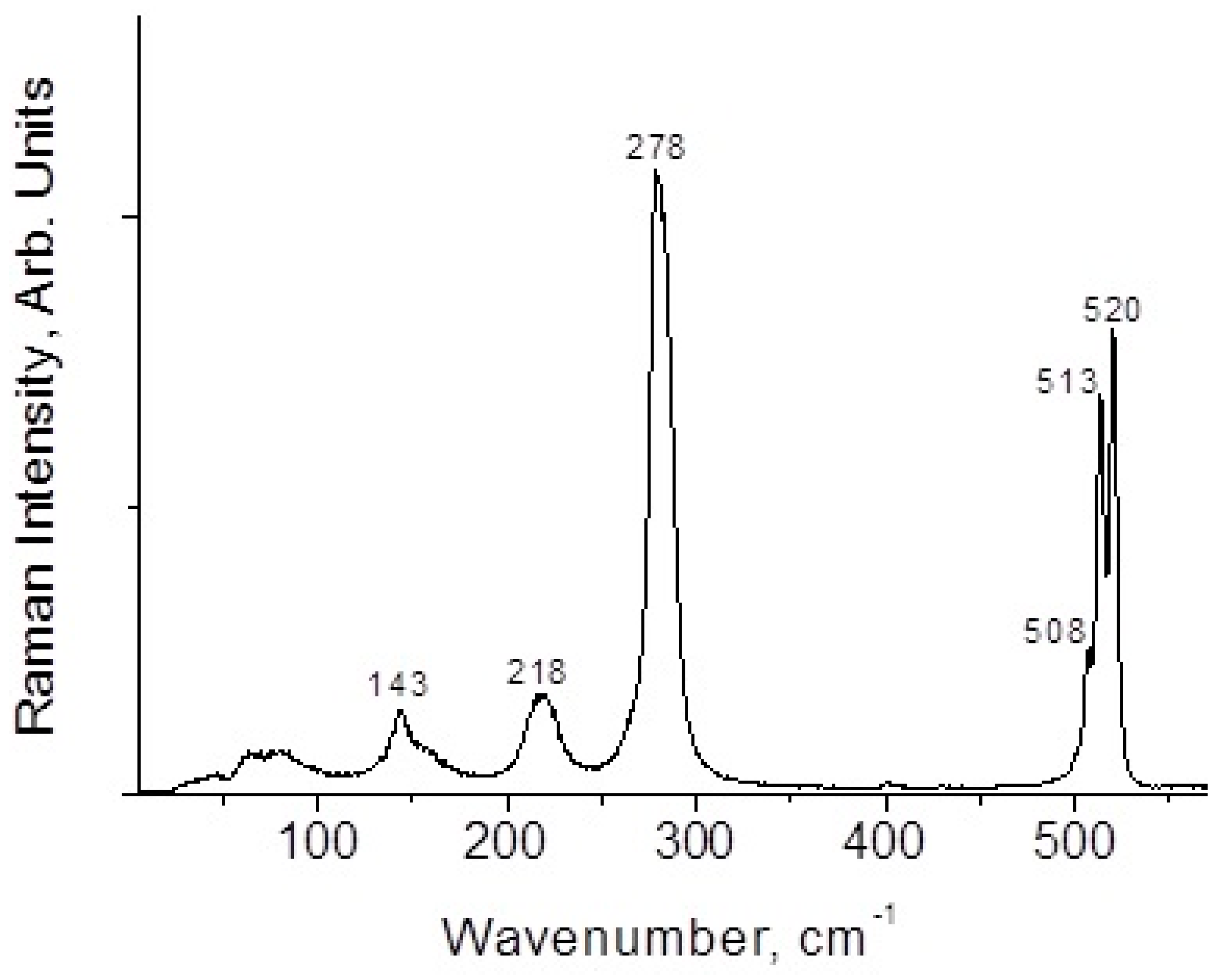
2.4. Raman Spectroscopy
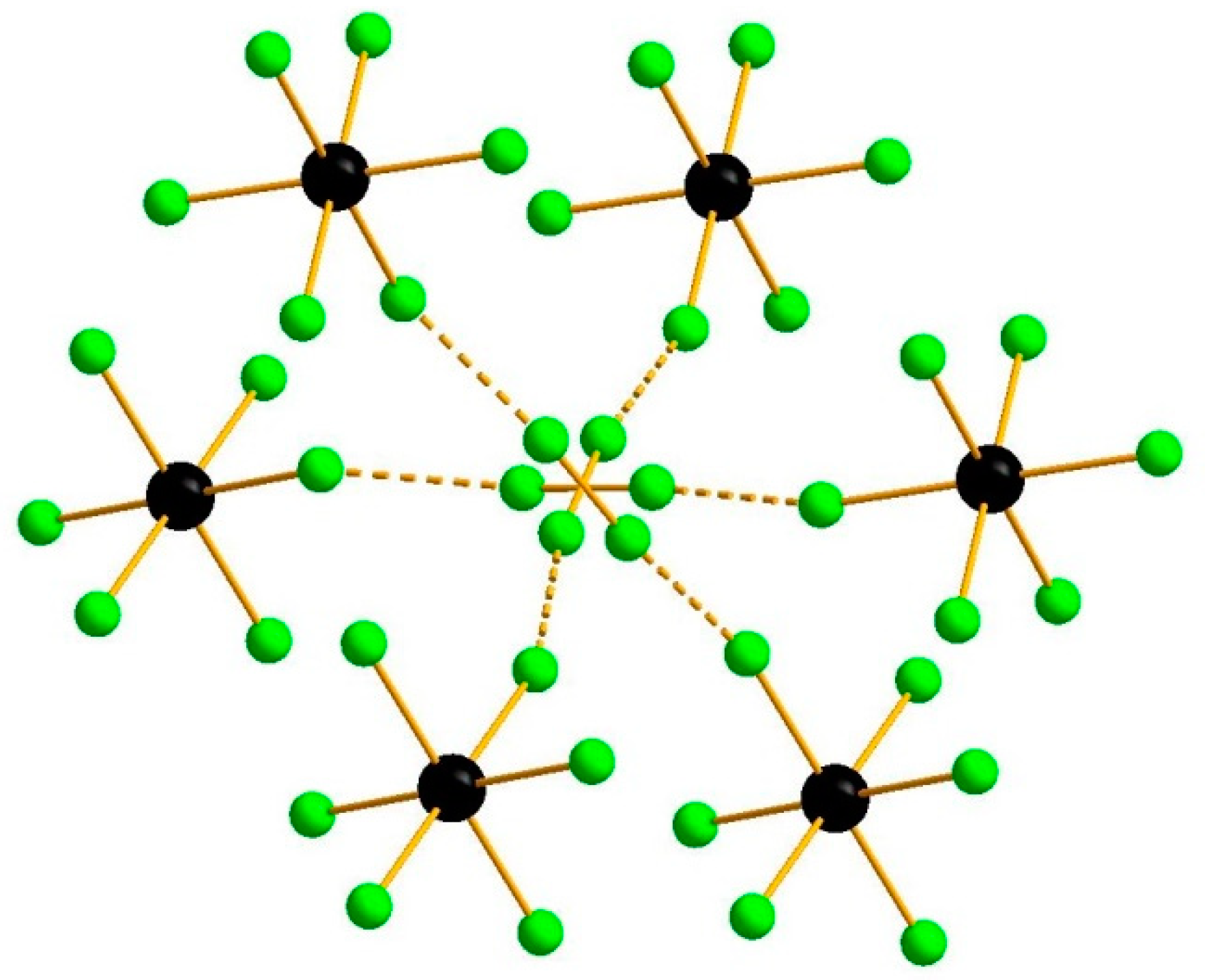
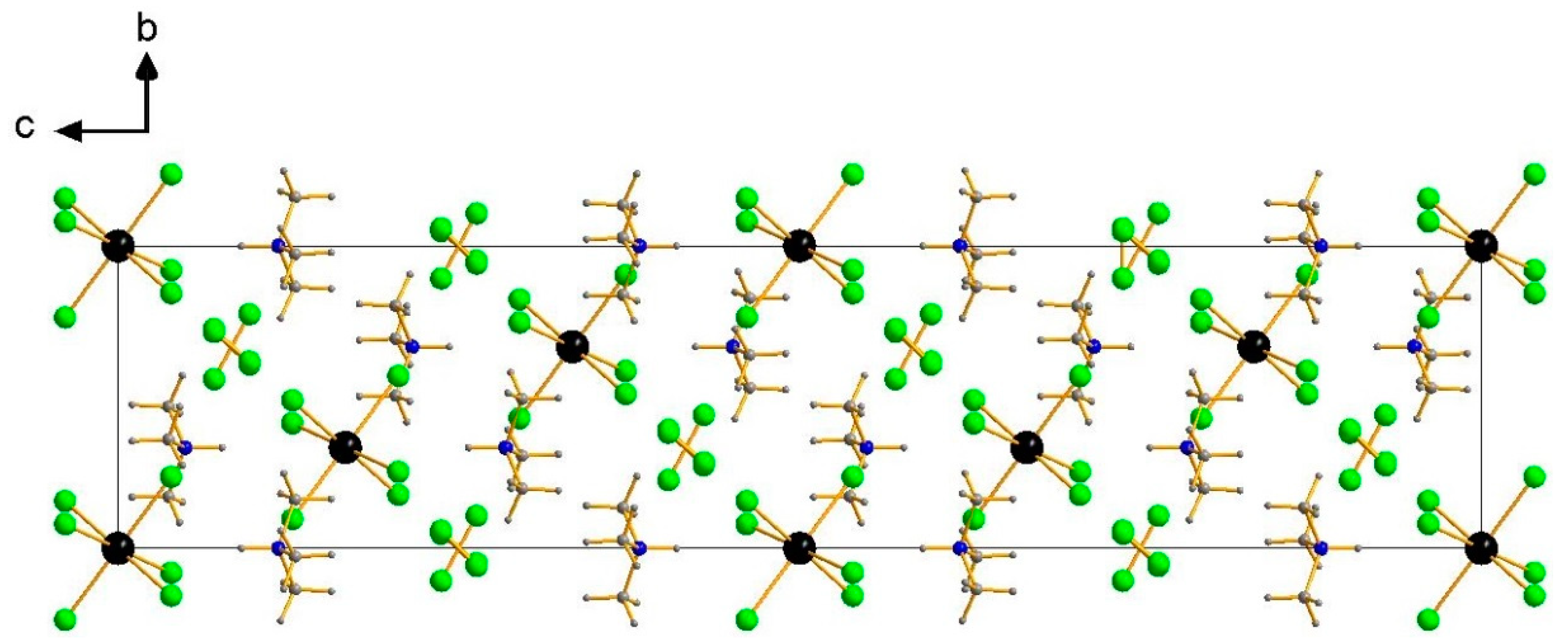
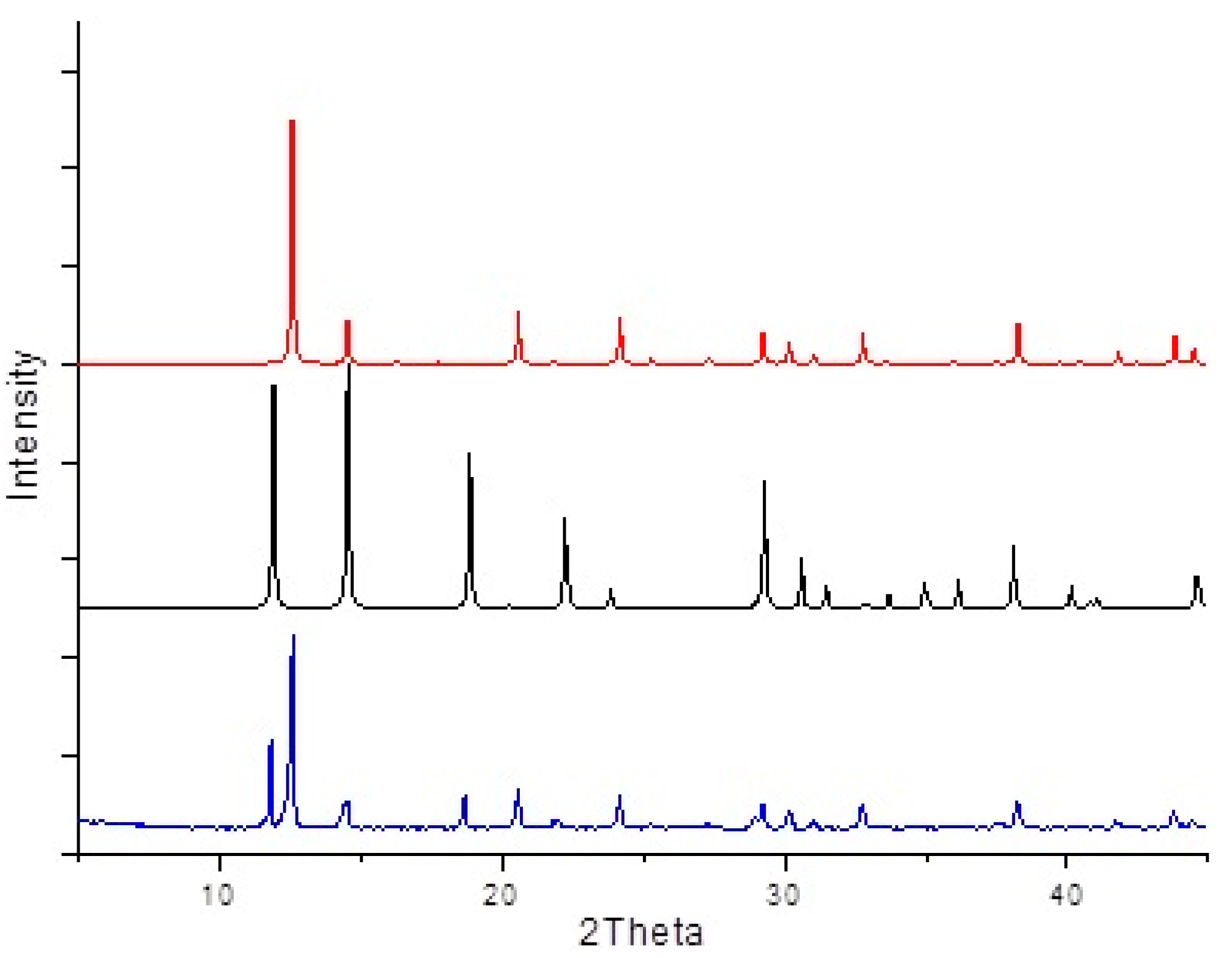
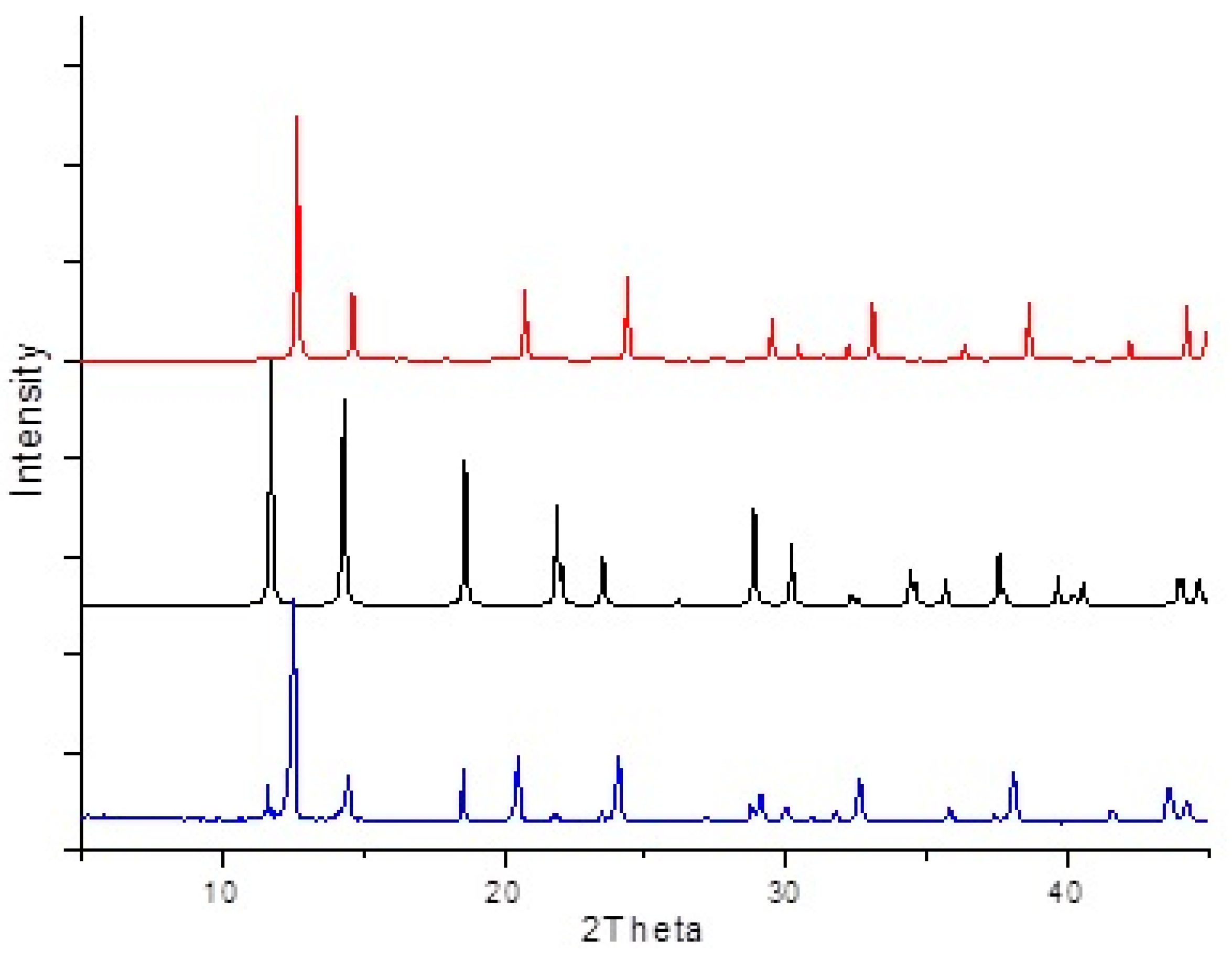
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szklarz, P.; Śmiałkowski, M.; Bator, G.; Jakubas, R.; Cichos, J.; Karbowiak, M.; Medycki, W.; Baran, J. Phase transitions and properties of 0D hybrid iodoantimonate(III) and iodobismuthate(III) semiconducting ferroics: [C(NH2)3]3Bi2I9 and [C(NH2)3]3Sb2I9. J. Mol. Struct. 2021, 1226, 129387. [Google Scholar] [CrossRef]
- Piecha, A.; Jakubas, R.; Pietraszko, A.; Baran, J. Structural characterization and spectroscopic properties of imidazolium chlorobismuthate(III): [C3H5N2]6[Bi4Cl18]. J. Mol. Struct. 2007, 844–845, 132–139. [Google Scholar] [CrossRef]
- Przesławski, J.; Piecha-Bisiorek, A.; Jakubas, R. Specific heat anomaly in ferroelectric: Bis(imidazolium) pentachloroantimonate(III) (C3N2H5)2[SbCl5]. J. Mol. Struct. 2016, 1110, 97–101. [Google Scholar] [CrossRef]
- Wojtaś, M.; Jakubas, R.; Ciunik, Z.; Medycki, W. Structure and phase transitions in [(CH3)4P]3[Sb2Br9] and [(CH3)4P]3[Bi2Br9]. J. Solid State Chem. 2004, 177, 1575–1584. [Google Scholar] [CrossRef]
- Ouasri, A.; Jeghnou, H.; Rhandour, A.; Roussel, P. Structures and phases transition in hexylenediammonium pentachlorobismuthate (III) [NH3(CH2)6NH3]BiCl5 crystal. J. Solid State Chem. 2013, 200, 22–29. [Google Scholar] [CrossRef]
- Hrizi, C.; Trigui, A.; Abid, Y.; Chniba-Boudjada, N.; Bordet, P.; Chaabouni, S. α- to β-[C6H4(NH3)2]2Bi2I10 reversible solid-state transition, thermochromic and optical studies in the p-phenylenediamine-based iodobismuthate(III) material. J. Solid State Chem. 2011, 184, 3336–3344. [Google Scholar] [CrossRef]
- Ahern, J.C.; Nicholas, A.D.; Kelly, A.W.; Chan, B.; Pike, R.D.; Patterson, H.H. A terbium chlorobismuthate(III) double salt: Synthesis, structure, and photophysical properties. Inorg. Chim. Acta 2018, 478, 71–76. [Google Scholar] [CrossRef]
- Kelly, A.W.; Nicholas, A.; Ahern, J.C.; Chan, B.; Patterson, H.H.; Pike, R.D. Alkali metal bismuth(III) chloride double salts. J. Alloys Compd. 2016, 670, 337–345. [Google Scholar] [CrossRef]
- Moyet, M.A.; Kanan, S.M.; Varney, H.M.; Abu-Farha, N.; Gold, D.B.; Lain, W.J.; Pike, R.D.; Patterson, H.H. Synthesis and characterization of (RPh3P)3 [Bi3I12] (R = Me, Ph) iodobismuthate complexes for photocatalytic degradation of organic pollutants. Res. Chem. Intermed. 2019, 45, 5919–5933. [Google Scholar] [CrossRef]
- Wu, L.-M.; Wu, X.-T.; Chen, L. Structural overview and structure–property relationships of iodoplumbate and iodobismuthate. Coord. Chem. Rev. 2009, 253, 2787–2804. [Google Scholar] [CrossRef]
- Buikin, P.A.; Ilyukhin, A.B.; Laurinavichyute, V.K.; Kotov, V.Y. Methylviologen Bromobismuthates. Russ. J. Inorg. Chem. 2021, 66, 133–138. [Google Scholar] [CrossRef]
- Buikin, P.A.; Rudenko, A.Y.; Ilyukhin, A.B.; Kotov, V.Y. Synthesis and Properties of Hybrid Halobismuthates of N-Acetonylpyridinium Derivatives. Russ. J. Inorg. Chem. 2021, 66, 482–489. [Google Scholar] [CrossRef]
- Buikin, P.A.; Rudenko, A.Y.; Ilyukhin, A.B.; Simonenko, N.P.; Yorov, K.E.; Kotov, V.Y. Bromobismuthates of 1,1′-(1,N-Alkanediyl)bis(picolines): Synthesis, Thermal Stability, Crystal Structures, and Optical Properties. Russ. J. Coord. Chem. 2020, 46, 111–118. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Sharutina, O.K.; Senchurin, V.S. Tetraphenylbismuth(V) Derivatives Ph4BiCl, [Ph4BiDMSO-O][PtBr3DMSO-S] and [Ph4Bi]2[PtCl6]: Synthesis and Structure. Russ. J. Inorg. Chem. 2020, 65, 1712–1717. [Google Scholar] [CrossRef]
- Zykova, A.R.; Sharutin, V.V.; Sharutina, O.K.; Senchurin, V.S. Synthesis and Structure of Organyltriphenylphosphonium and Stibonium Hexabromoplatinates. Russ. J. Gen. Chem. 2020, 90, 1483–1488. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Sharutina, O.K.; Lobanova, E.V. Hafnium Complexes [Ph3PR]2 [HfCl6], where R = Et, CH2C6H4CN−4, or CH2C6H4F−4: Synthesis and Structure. Russ. J. Inorg. Chem. 2020, 65, 870–873. [Google Scholar] [CrossRef]
- Yelovik, N.A.; Shestimerova, T.A.; Bykov, M.A.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Synthesis, structure, and properties of LnBiI6•13H2O (Ln = La, Nd). Russ. Chem. Bull. 2017, 66, 1196–1201. [Google Scholar] [CrossRef]
- Udalova, N.N.; Tutantsev, A.S.; Fateev, S.A.; Zharenova, E.A.; Belich, N.A.; Nemygina, E.M.; Ryabova, A.V.; Goodilin, E.A.; Tarasov, A.B. Crystallization Features of MAPbI3 Hybrid Perovskite during the Reaction of PbI2 with Reactive Polyiodide Melts. Russ. J. Inorg. Chem. 2021, 66, 153–162. [Google Scholar] [CrossRef]
- Belich, N.A.; Tychinina, A.S.; Kuznetsov, V.V.; Goodilin, E.A.; Grätzel, M.; Tarasov, A.B. Template synthesis of methylammonium lead iodide in the matrix of anodic titanium dioxide via the direct conversion of electrodeposited elemental lead. Mendeleev Commun. 2018, 28, 487–489. [Google Scholar] [CrossRef]
- Fateev, S.A.; Petrov, A.A.; Khrustalev, V.N.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Goodilin, E.A.; Tarasov, A.B. Solution Processing of Methylammonium Lead Iodide Perovskite from γ-Butyrolactone: Crystallization Mediated by Solvation Equilibrium. Chem. Mater. 2018, 30, 5237–5244. [Google Scholar] [CrossRef]
- Petrov, A.A.; Sokolova, I.P.; Belich, N.A.; Peters, G.S.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Khrustalev, V.N.; Petrov, A.V.; Grätzel, M.; Goodilin, E.A.; et al. Crystal Structure of DMF-Intermediate Phases Uncovers the Link between CH3NH3PbI3 Morphology and Precursor Stoichiometry. J. Phys. Chem. C 2017, 121, 20739–20743. [Google Scholar] [CrossRef]
- Frolova, L.A.; Anokhin, D.V.; Piryazev, A.A.; Luchkin, S.Y.; Dremova, N.N.; Stevenson, K.J.; Troshin, P.A. Highly Efficient All-Inorganic Planar Heterojunction Perovskite Solar Cells Produced by Thermal Coevaporation of CsI and PbI2. J. Phys. Chem. Lett. 2017, 8, 67–72. [Google Scholar] [CrossRef]
- Fateev, S.A.; Petrov, A.A.; Marchenko, E.I.; Zubavichus, Y.V.; Khrustalev, V.N.; Petrov, A.V.; Aksenov, S.M.; Goodilin, E.A.; Tarasov, A.B. FA2PbBr4: Synthesis, Structure, and Unusual Optical Properties of Two Polymorphs of Formamidinium-Based Layered (110) Hybrid Perovskite. Chem. Mater. 2021, 33, 1900–1907. [Google Scholar] [CrossRef]
- Tutantsev, A.S.; Udalova, N.N.; Fateev, S.A.; Petrov, A.A.; Petrov, A.A.; Chengyuan, W.; Maksimov, E.G.; Goodilin, E.A.; Goodilin, E.A.; Tarasov, A.B.; et al. New Pigeonholing Approach for Selection of Solvents Relevant to Lead Halide Perovskite Processing. J. Phys. Chem. C 2020, 124, 11117–11123. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Aliyarova, I.S.; Ivanov, D.M.; Soldatova, N.S.; Novikov, A.S.; Postnikov, P.S.; Yusubov, M.S.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Diaryliodonium Cations as Iodine(III)-Based Double-σ-Hole Donors. Cryst. Growth Des. 2021, 21, 1136–1147. [Google Scholar] [CrossRef]
- Mikherdov, A.S.; Novikov, A.S.; Boyarskiy, V.P.; Kukushkin, V.Y. The halogen bond with isocyano carbon reduces isocyanide odor. Nat. Commun. 2020, 11, 2921. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Postnikov, P.S.; Suslonov, V.V.; Kissler, T.Y.; Ivanov, D.M.; Yusubov, M.S.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. Diaryliodonium as a double s-hole donor: The dichotomy of thiocyanate halogen bonding provides divergent solid state arylation by diaryliodonium cations. Org. Chem. Front. 2020, 7, 2230–2242. [Google Scholar] [CrossRef]
- Bokach, N.A.; Suslonov, V.V.; Eliseeva, A.A.; Novikov, A.S.; Ivanov, D.M.; Dubovtsev, A.Y.; Kukushkin, V.Y.; Kukushkin, V.Y. Tetrachloroplatinate(ii) anion as a square-planar tecton for crystal engineering involving halogen bonding. CrystEngComm 2020, 22, 4180–4189. [Google Scholar]
- Lawton, S.L.; McAfee, E.R.; Benson, J.E.; Jacobson, R.A. Crystal structure of quinolinium hexabromoantimonate(V) tribromide, (C9H7NH)2SbVBr9. Inorg. Chem. 1973, 12, 2939–2944. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Jacobson, R.A. Molecular bromine bridging of SbIII2Br93− anions and the crystal structure of tetraethylammonium nonabromodiantimonate(III)-dibromine. Inorg. Chem. 1972, 11, 2247–2250. [Google Scholar] [CrossRef]
- Lawton, S.L.; Jacobson, R.A. Crystal structure of di-.alpha.-picolinium nonabromoantimonate(V). Inorg. Chem. 1968, 7, 2124–2134. [Google Scholar] [CrossRef]
- Siepmann, R.; von Schnering, H.G. Die Kristallstruktur von W6Br16. Eine Verbindung mit Polykationen [W6Br8]6+ und Polyanionen [Br4]2−. Z. Anorg. Allg. Chem. 1968, 357, 289–298. [Google Scholar] [CrossRef]
- Berkei, M.; Bickley, J.F.; Heaton, B.T.; Steiner, A. Polymeric anionic networks using dibromine as a crosslinker; the preparation and crystal structure of [(C4H9)4N]2[Pt2Br10]·(Br2)7 and [(C4H9)4N]2[PtBr4Cl2]·(Br2)6. Chem. Commun. 2002, 2180–2181. [Google Scholar] [CrossRef]
- Hausmann, D.; Feldmann, C. Bromine-rich Zinc Bromides: Zn6Br12(18-crown-6)2×(Br2)5, Zn4Br8(18-crown-6)2×(Br2)3, and Zn6Br12(18-crown-6)2×(Br2)2. Inorg. Chem. 2016, 55, 6141–6147. [Google Scholar] [CrossRef]
- Eich, A.; Köppe, R.; Roesky, P.W.; Feldmann, C. The Bromine-Rich Bromido Metallates [BMIm]2[SnBr6]·(Br2) and [MnBr(18-crown-6)]4[SnBr6]2·(Br2)4.5. Eur. J. Inorg. Chem. 2019, 2019, 1292–1298. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Samsonenko, D.G.; Plyusnin, P.E.; Sokolov, M.N.; Fedin, V.P. Bromine-rich complexes of bismuth: Experimental and theoretical studies. Dalt. Trans. 2018, 47, 2683–2689. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Golubev, N.A.; Bykov, M.A.; Mironov, A.V.; Fateev, S.A.; Tarasov, A.B.; Turkevych, I.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Molecular and supramolecular structures of triiodides and polyiodobismuthates of phenylenediammonium and its n,n-dimethyl derivative. Molecules 2021, 26, 5712. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Yelavik, N.A.; Mironov, A.V.; Kuznetsov, A.N.; Bykov, M.A.; Grigorieva, A.V.; Utochnikova, V.V.; Lepnev, L.S.; Shevelkov, A.V. From isolated anions to polymer structures through linking with I2: Synthesis, structure, and properties of two complex bismuth(III) iodine iodides. Inorg. Chem. 2018, 57, 4077–4087. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Mironov, A.V.; Bykov, M.A.; Grigorieva, A.V.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Assembling polyiodides and iodobismuthates using a template effect of a cyclic diammonium cation and formation of a low-gap hybrid iodobismuthate with high thermal stability. Molecules 2020, 25, 2765. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Golubev, N.A.; Yelavik, N.A.; Bykov, M.A.; Grigorieva, A.V.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Role of I2 Molecules and Weak Interactions in Supramolecular Assembling of Pseudo-Three-Dimensional Hybrid Bismuth Polyiodides: Synthesis, Structure, and Optical Properties of Phenylenediammonium Polyiodobismuthate(III). Cryst. Growth Des. 2018, 18, 2572–2578. [Google Scholar] [CrossRef]
- Storck, P.; Weiss, A. 35C1NQR and X-Ray Studies of Hexachloropalladates A2PdCl6 (A = Rb, Cs, NH4) and the Cl2—Clathrates Bis(tetramethylammonium)hexachloropalladate (Me4N)2PdCl6 • Cl2 and Bis(tetramethylammonium)hexachlorostannate (Me4N)2SnCl6 • Cl2. Zeitschrift fur Naturforsch.—Sect. B J. Chem. Sci. 1991, 46, 1214–1218. [Google Scholar] [CrossRef]
- Usoltsev, A.N.; Korobeynikov, N.A.; Kolesov, B.A.; Novikov, A.S.; Samsonenko, D.G.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. Rule, Not Exclusion: Formation of Dichlorine-Containing Supramolecular Complexes with Chlorometalates(IV). Inorg. Chem. 2021, 60, 4171–4177. [Google Scholar] [CrossRef]
- Usoltsev, A.N.; Adonin, S.A.; Kolesov, B.A.; Novikov, A.S.; Fedin, V.P.; Sokolov, M.N. Opening the Third Century of Polyhalide Chemistry: Thermally Stable Complex with “Trapped” Dichlorine. Chem.—A Eur. J. 2020, 26, 13776–13778. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Knop, O.; Cameron, T.S.; James, M.A.; Falk, M. Alkylammonium hexachlorostannates(IV), (RnNH4−n)2SnCl6: Crystal structure, infrared spectrum, and hydrogen bonding. Can. J. Chem. 1983, 61, 1620–1646. [Google Scholar] [CrossRef]
- Brückner, R.; Haller, H.; Ellwanger, M.; Riedel, S. Polychloride monoanions from [Cl3]− to [Cl9]−: A Raman spectroscopic and quantum chemical investigation. Chem.—A Eur. J. 2012, 18, 5741–5747. [Google Scholar] [CrossRef]
- Sonnenberg, K.; Pröhm, P.; Schwarze, N.; Müller, C.; Beckers, H.; Riedel, S. Investigation of Large Polychloride Anions: [Cl11]−, [Cl12]2−, and [Cl13]−. Angew. Chem. Int. Ed. 2018, 57, 9136–9140. [Google Scholar] [CrossRef]
- Brückner, R.; Pröhm, P.; Wiesner, A.; Steinhauer, S.; Müller, C.; Riedel, S. Structural Proof for the First Dianion of a Polychloride: Investigation of [Cl8]2−. Angew. Chem. Int. Ed. 2016, 55, 10904–10908. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.L.; Farrar, L.W.; Di Cecca, S.; Jeys, T.H. Raman spectra and cross sections of ammonia, chlorine, hydrogen sulfide, phosgene, and sulfur dioxide toxic gases in the fingerprint region 400–1400 cm−1. AIP Adv. 2016, 6, 025310. [Google Scholar] [CrossRef]
- Creighton, J.A.; Woodward, L.A. Raman spectrum of the hexachloroplumbate ion PbCl62− in solution. Trans. Faraday Soc. 1962, 58, 1077–1079. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, R.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Voßnacker, P.; Keilhack, T.; Schwarze, N.; Sonnenberg, K.; Seppelt, K.; Malischewski, M.; Riedel, S. From Missing Links to New Records: A Series of Novel Polychlorine Anions. Eur. J. Inorg. Chem. 2021, 2021, 1034–1040. [Google Scholar] [CrossRef]
| 1 | 2 | |
|---|---|---|
| Empirical formula | C6H20Cl8N2Sn | C6H20Cl8N2Pb |
| Formula weight | 522.53 | 611.03 |
| Temperature, K | 150(2) | 250(2) |
| Crystal system | Trigonal | trigonal |
| Space group | R–3c | R–3c |
| a, Å/α, ° | 9.4097(6)/90 | 9.5183(2)/90 |
| b, Å/β, ° | 9.4097(6)/90 | 9.5183(2)/90 |
| c, Å/γ, ° | 36.738(3)/120 | 37.3034(8)/120 |
| Volume, Å3 | 2817.1(4) | 2926.83(14) |
| Z | 6 | 6 |
| ρcalc, g/cm3 | 1.848 | 2.080 |
| μ, mm−1 | 2.482 | 9.726 |
| F(000) | 1536.0 | 1728.0 |
| Crystal size, mm3 | 0.13 × 0.08 × 0.05 | 0.15 × 0.15 × 0.15 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2θ range for data collection, ° | 5.47/62.95 | 6.55/63.03 |
| Index ranges | –13 ≤ h ≤ 13, –12 ≤ k ≤ 13, –53 ≤ l ≤ 53 | –12 ≤ h ≤ 0, 0 ≤ k ≤ 13, 0 ≤ l ≤ 54 * |
| Reflections collected/independent | 12026/1019 | 25228 **/1082 |
| Rint/Rσ | 0.0438/0.0196 | 0.0371/0.0106 |
| Data/restraints/parameters | 1019/0/34 | 1082/0/34 |
| Goodness-of-fit on F2 | 1.116 | 1.112 |
| R1/wR2 for I ≥ 2σ(I) | 0.0179/0.0388 | 0.0206/0.0376 |
| for all data | 0.0197/0.0394 | 0.0282/0.0401 |
| Largest diff. peak/hole/e Å−3 | 0.23/–0.36 | 0.41/–0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobeynikov, N.A.; Usoltsev, A.N.; Abramov, P.A.; Komarov, V.Y.; Sokolov, M.N.; Adonin, S.A. Trimethylammonium Sn(IV) and Pb(IV) Chlorometalate Complexes with Incorporated Dichlorine. Inorganics 2023, 11, 25. https://doi.org/10.3390/inorganics11010025
Korobeynikov NA, Usoltsev AN, Abramov PA, Komarov VY, Sokolov MN, Adonin SA. Trimethylammonium Sn(IV) and Pb(IV) Chlorometalate Complexes with Incorporated Dichlorine. Inorganics. 2023; 11(1):25. https://doi.org/10.3390/inorganics11010025
Chicago/Turabian StyleKorobeynikov, Nikita A., Andrey N. Usoltsev, Pavel A. Abramov, Vladislav Yu. Komarov, Maxim N. Sokolov, and Sergey A. Adonin. 2023. "Trimethylammonium Sn(IV) and Pb(IV) Chlorometalate Complexes with Incorporated Dichlorine" Inorganics 11, no. 1: 25. https://doi.org/10.3390/inorganics11010025
APA StyleKorobeynikov, N. A., Usoltsev, A. N., Abramov, P. A., Komarov, V. Y., Sokolov, M. N., & Adonin, S. A. (2023). Trimethylammonium Sn(IV) and Pb(IV) Chlorometalate Complexes with Incorporated Dichlorine. Inorganics, 11(1), 25. https://doi.org/10.3390/inorganics11010025









