Uranyl Analogue Complexes—Current Progress and Synthetic Challenges
Abstract
:1. Introduction
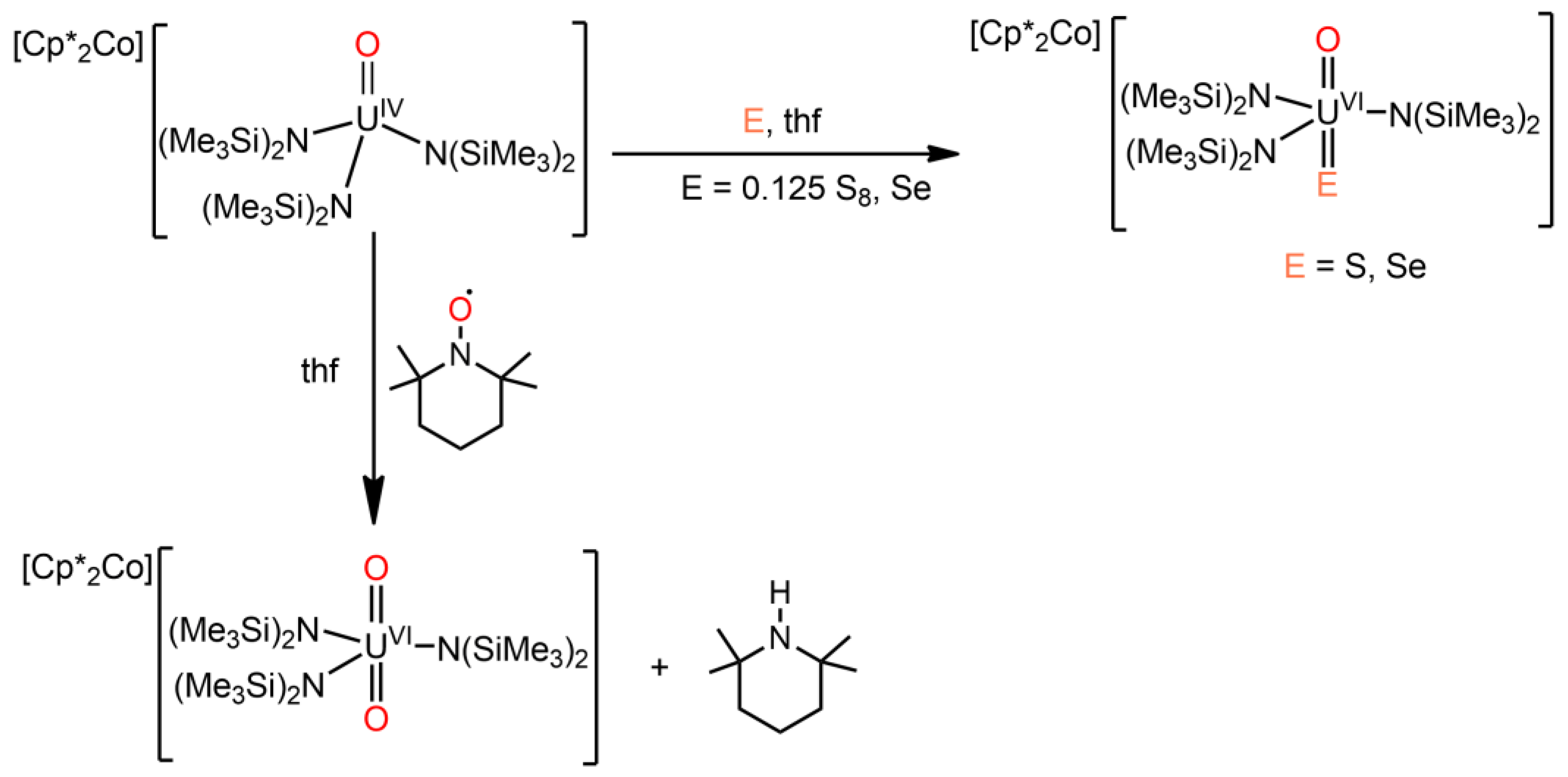
2. Heavy Chalcogenido Complexes
3. Uranium Pnictogenido Complexes
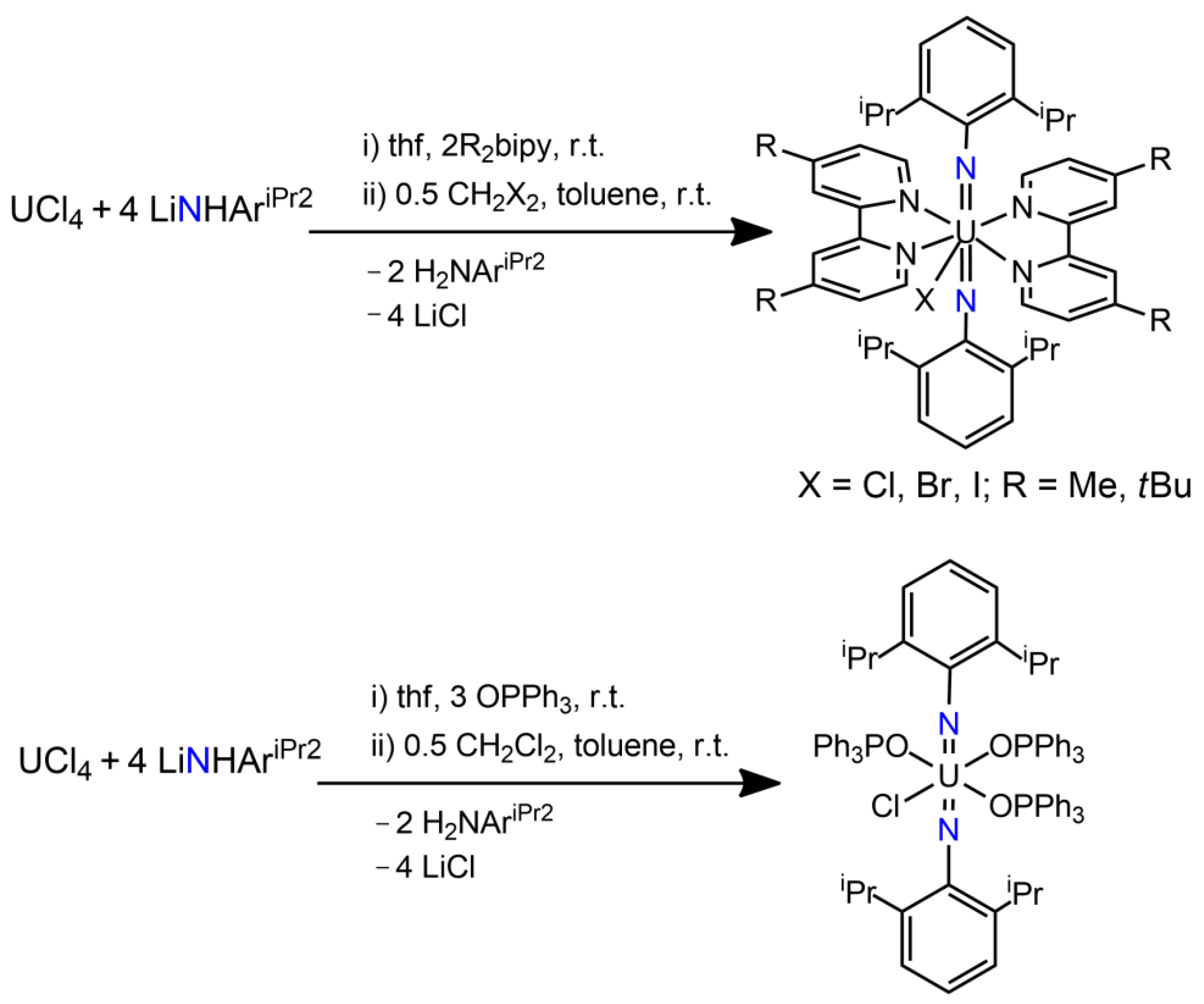
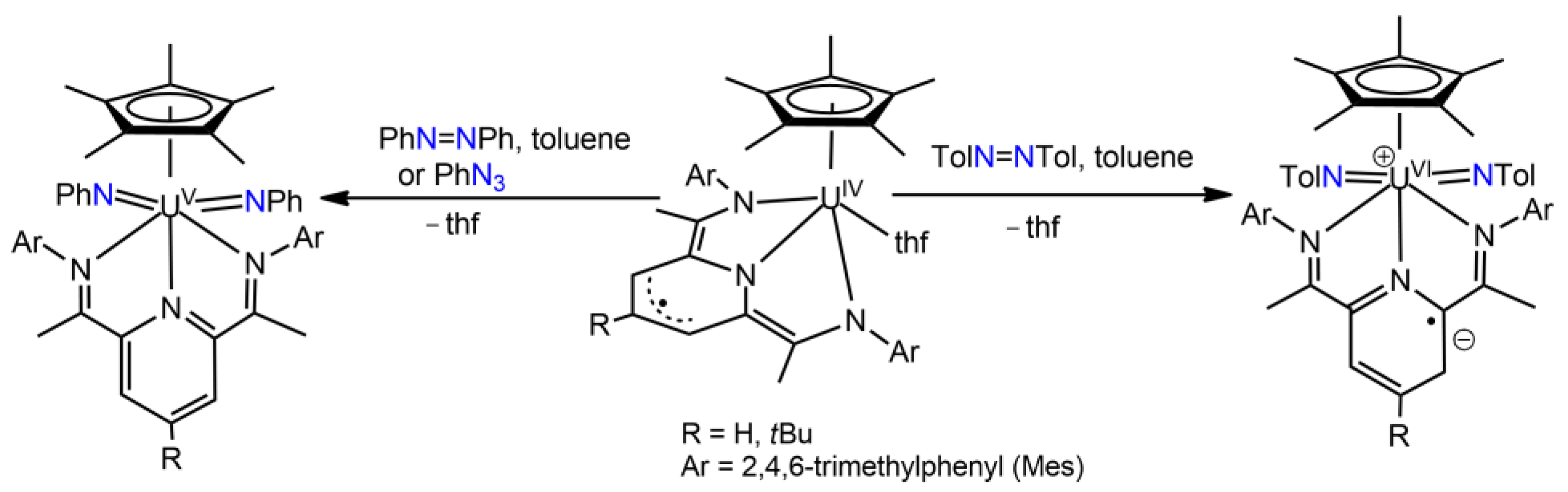
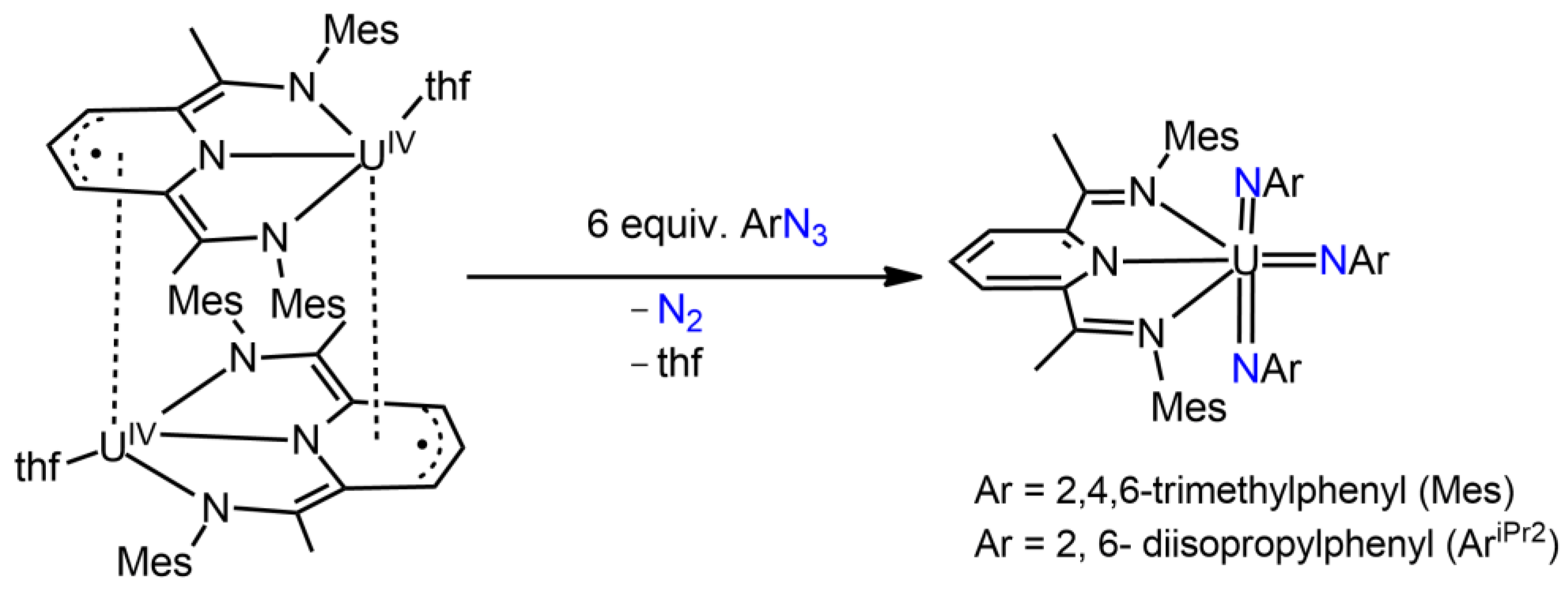
3.1. Uranium Bis(imido) Complexes
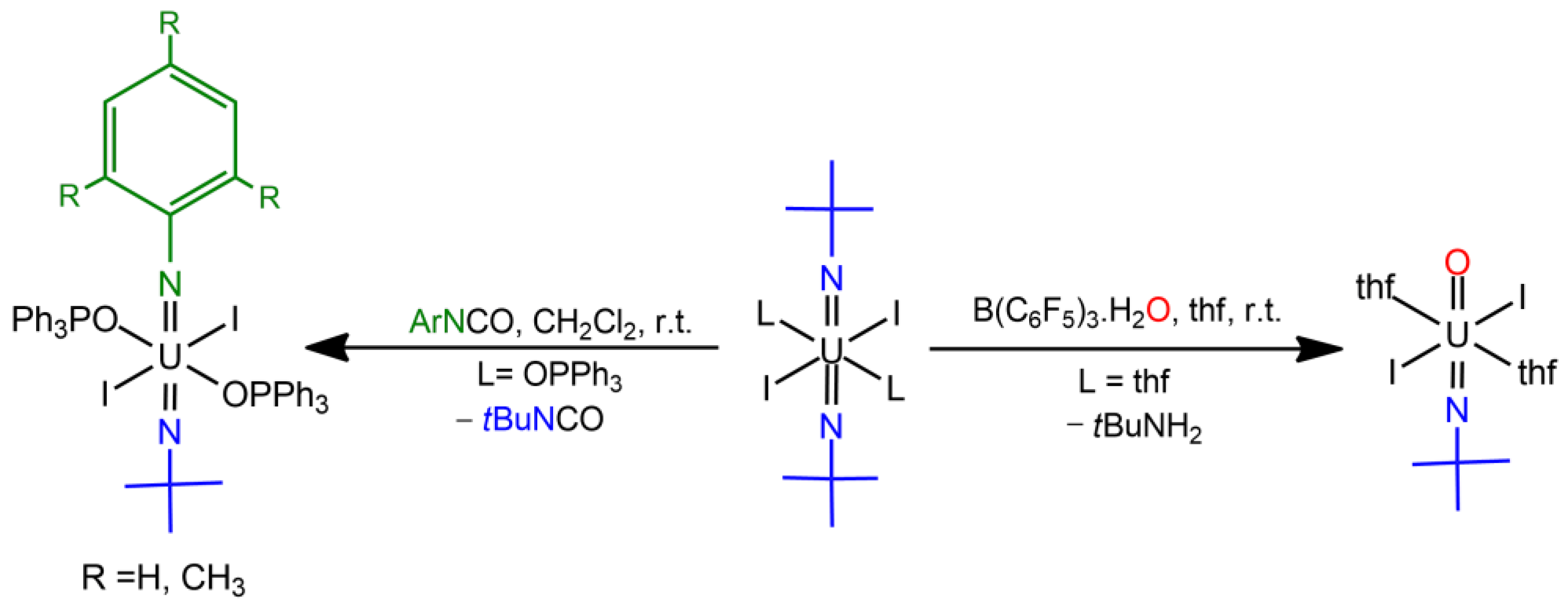
3.2. Uranium Oxido—Imido Complexes
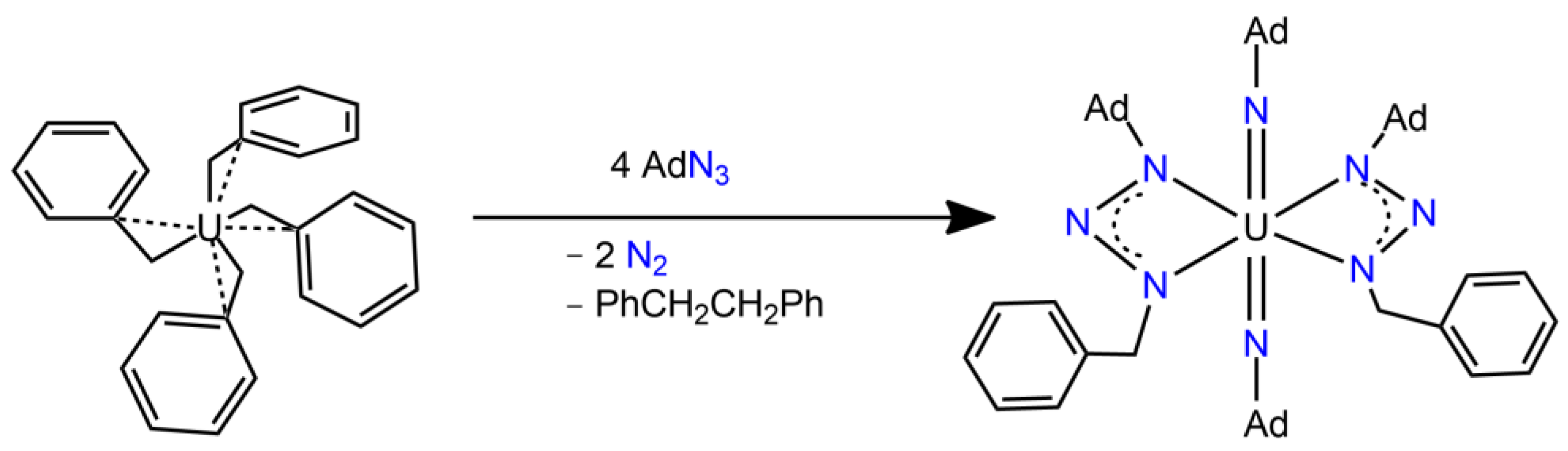
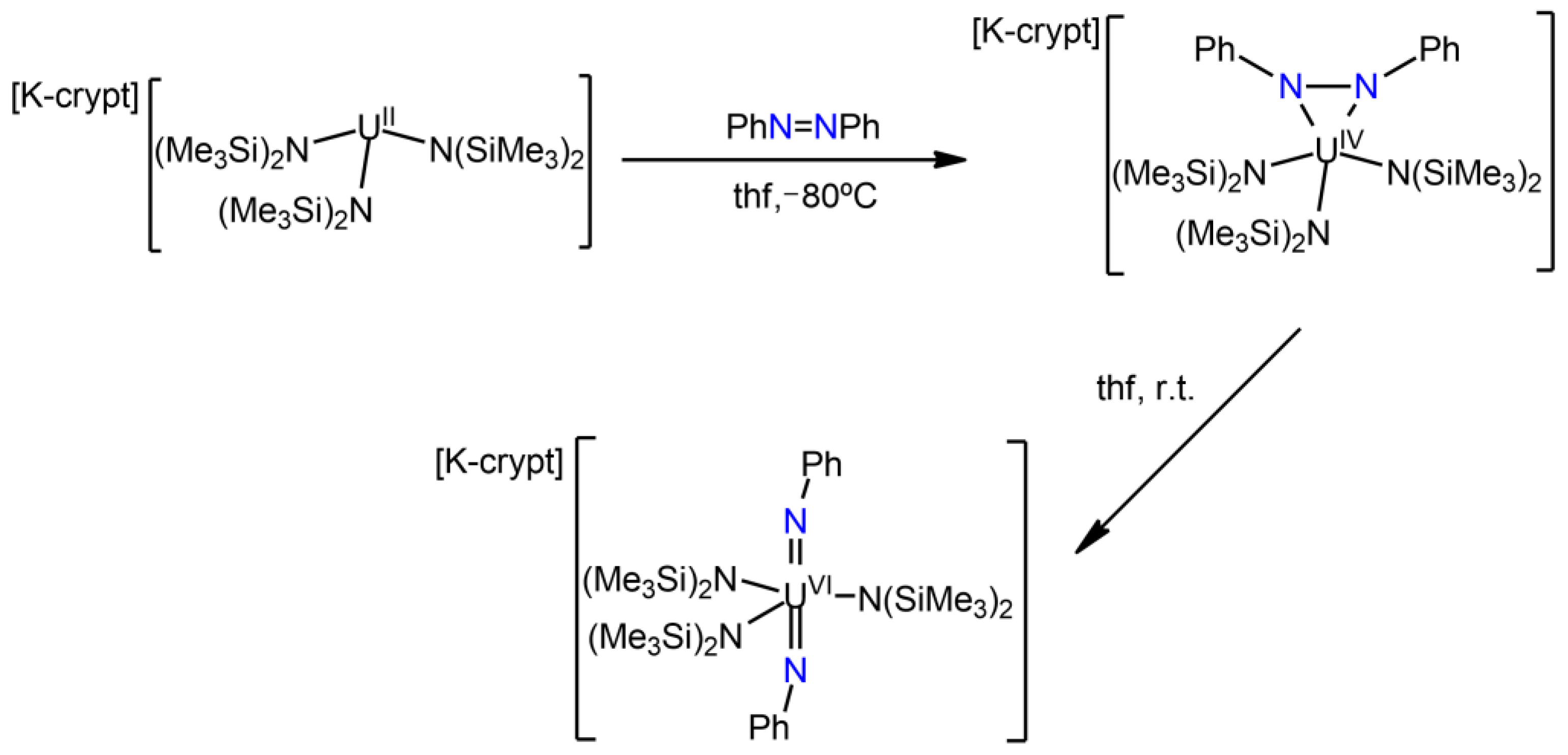
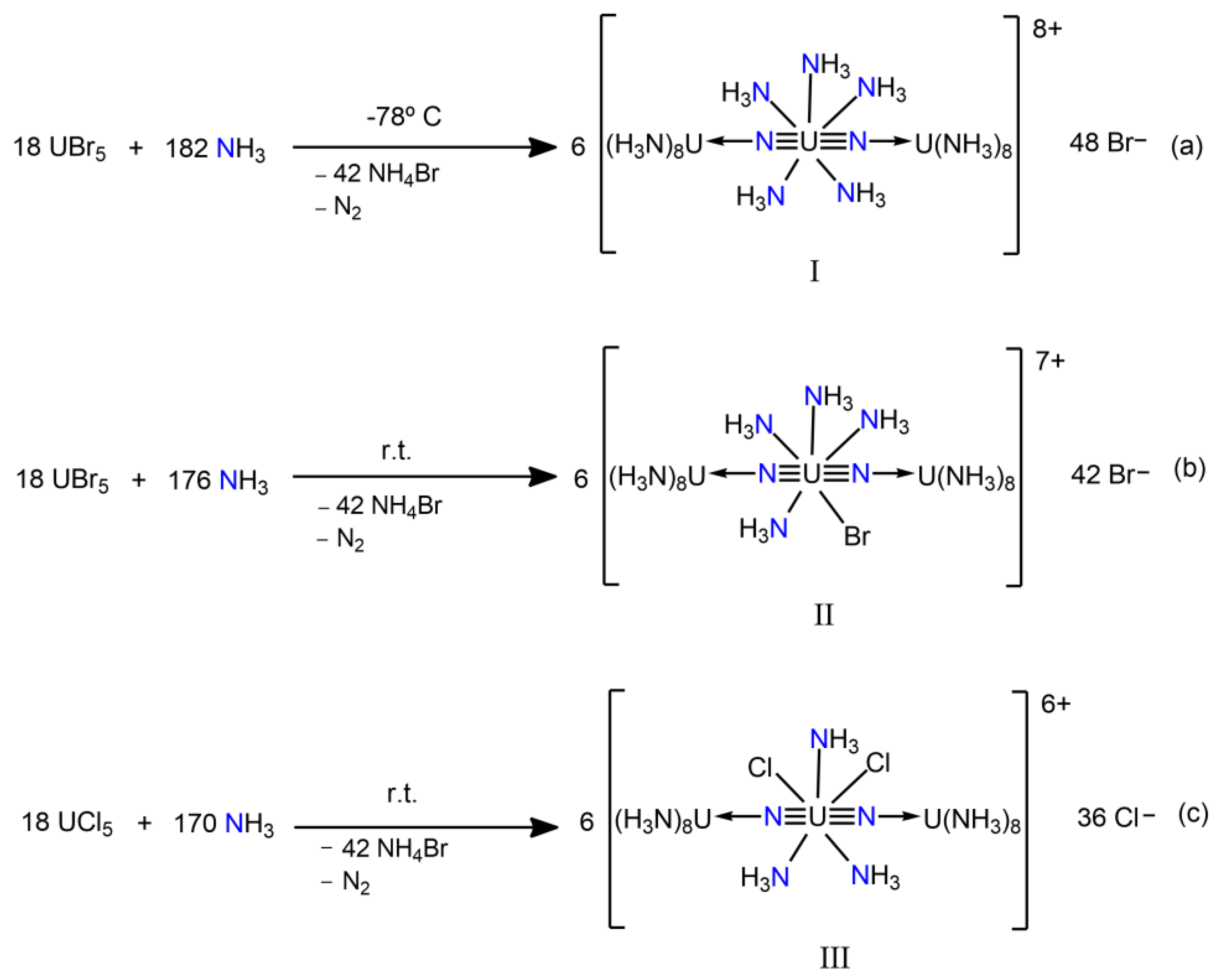
3.3. Uranium Nitrido Complexes
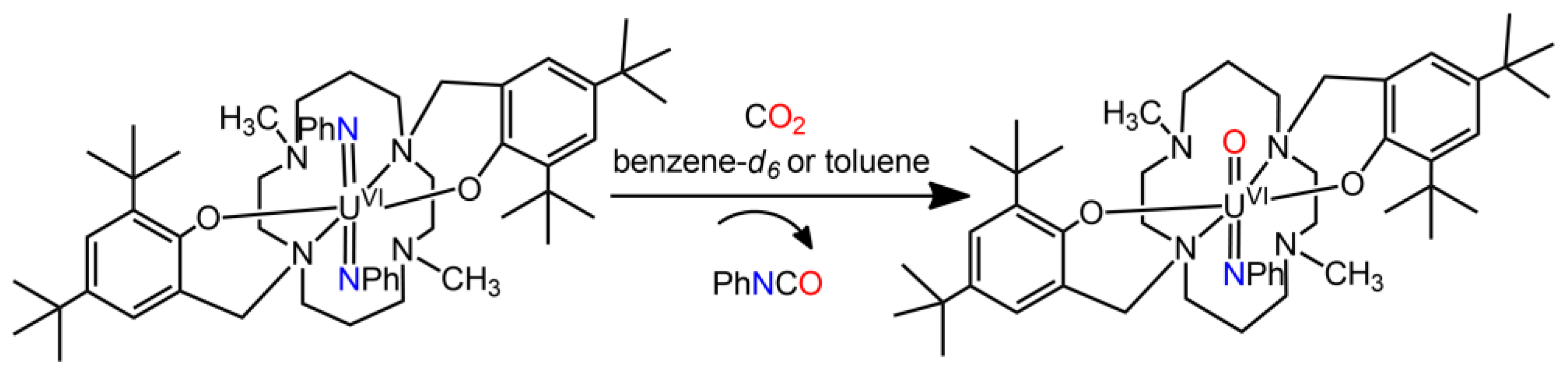
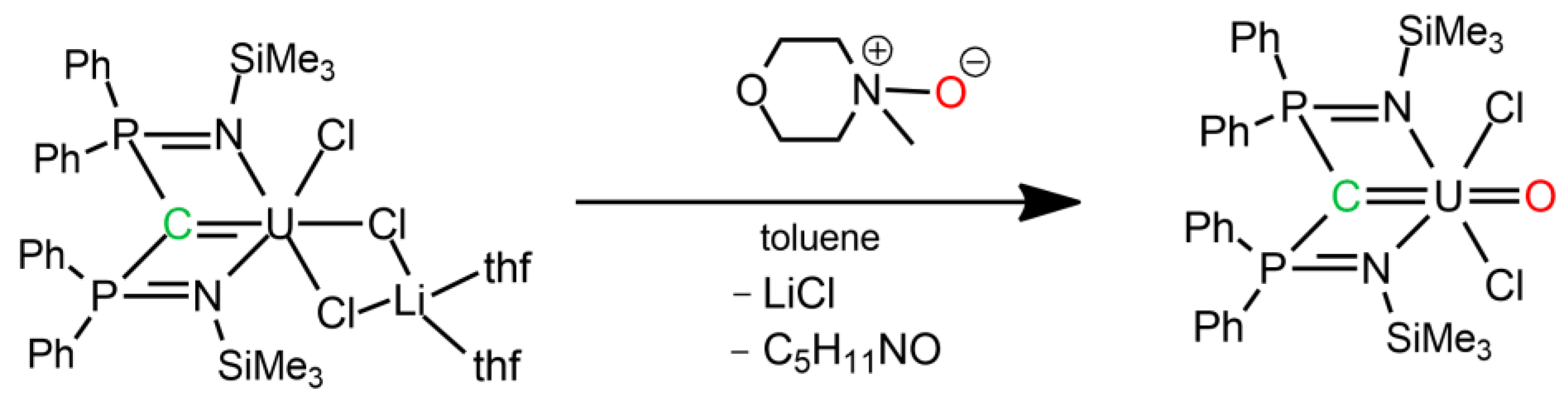
4. Uranium Carbene Complexes
5. Uranium-Transition Metal Complexes
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ad. | adamantyl |
| ArCF3 | 3,5-Bis(trifluoromethyl)phenyl |
| AriPr2 | 2,6-diisopropylphenyl |
| bpy | 2,2′-bipyridine |
| {BIPMTMS}2− | bis-iminophosphoranomethanediide |
| dmap | 4-dimethylaminopyridine |
| dme | 1,2-dimethoxy-ethane |
| dmpe | 1,2-Bis(dimethylphosphino)ethane |
| dpm | 5,5′-dimethylpyrrolylmethane |
| dpmMes | 2,2′-bis(mesityl)-5,5-dimethyldipyrrolylmethane |
| Cp | cyclopentadienyl |
| Cp* | pentamethylcyclopentadienyl |
| Me2-C4H2N | 2,5-dimethylpyrrolyl |
| Me | methyl |
| Mes | 2,4,6-trimethylphenyl |
| Me2bpy | 4,4′-dimethyl-2,2′-bipyridine |
| py | pyridine |
| {N(Me)(SO2Ar’)} | N,4-dimethylbenzenesulfonamido |
| OPPh3 | triphenylphosphane oxide |
| tBubpy | 4,4′-di-tert-butyl-2,2′-bipyridine |
| TEMPO | tetramethylamine-N-oxide |
| thf | tetrahydrofuran |
References
- Jones, M.B.; Gaunt, A.J. Recent Developments in Synthesis and Structural Chemistry of Nonaqueous Actinide Complexes. Chem. Rev. 2013, 113, 1137–1198. [Google Scholar] [CrossRef] [PubMed]
- Liddle, S.T. The Renaissance of Non-Aqueous Uranium Chemistry. Angew. Chem. Int. Ed. 2015, 54, 8604–8641. [Google Scholar] [CrossRef] [PubMed]
- Boreen, M.A.; Arnold, J. The Synthesis and Versatile Reducing Power of Low-Valent Uranium Complexes. Dalton Trans. 2020, 49, 15124–15138. [Google Scholar] [CrossRef] [PubMed]
- Hayton, T.W. Metal–Ligand Multiple Bonding in Uranium: Structure and Reactivity. Dalton Trans. 2010, 39, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Hayton, T.W. Recent Developments in Actinide–Ligand Multiple Bonding. Chem. Comm. 2013, 49, 2956–2973. [Google Scholar] [CrossRef] [PubMed]
- Boreen, M.A.; Arnold, J. Multiple Bonding in Actinide Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Schädle, D.; Anwander, R. Rare-Earth Metal and Actinide Organoimide Chemistry. Chem. Soc. Rev. 2019, 48, 5752–5805. [Google Scholar] [CrossRef]
- Denning, R.G. Electronic Structure and Bonding in Actinyl Ions and their Analogs. J. Phys. Chem. A 2007, 111, 4125–4143. [Google Scholar] [CrossRef]
- Marçalo, J.; Gibson, J.K. Gas-Phase Energetics of Actinide Oxides: An Assessment of Neutral and Cationic Monoxides and Dioxides from Thorium to Curium. J. Phys. Chem. A 2009, 113, 12599–12606. [Google Scholar] [CrossRef]
- Arnold, P.L.; Love, J.B.; Patel, D. Pentavalent Uranyl Complexes. Coord. Chem. Rev. 2009, 253, 1973–1978. [Google Scholar] [CrossRef]
- Faizova, R.; Scopelliti, R.; Chauvin, A.-S.; Mazzanti, M. Synthesis and Characterization of a Water Stable Uranyl(V) Complex. J. Am. Chem. Soc. 2018, 140, 13554–13557. [Google Scholar] [CrossRef]
- Faizova, R.; Fadaei-Tirani, F.; Chauvin, A.-S.; Mazzanti, M. Synthesis and Characterization of Water Stable Uranyl(V) Complexes. Angew. Chem. Int. Ed. 2021, 60, 8227–8235. [Google Scholar] [CrossRef] [PubMed]
- Denning, R.G. Electronic Structure and Bonding in Actinyl Ions. In Complexes, Clusters and Crystal Chemistry; Springer: Berlin/Heidelberg, Germany, 1992; pp. 215–276. [Google Scholar]
- Kaltsoyannis, N. Recent Developments in Computational Actinide Chemistry. Chem. Soc. Rev. 2003, 32, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hayton, T.W. Understanding the Origins of Oyl–U–Oyl Bending in the Uranyl (UO2)2+ Ion. Dalton Trans. 2018, 47, 1003–1009. [Google Scholar] [CrossRef]
- La Pierre, H.S.; Meyer, K. Uranium–Ligand Multiple Bonding in Uranyl Analogues, [L═U═L]n+, and the Inverse Trans Influence. Inorg. Chem. 2013, 52, 529–539. [Google Scholar] [CrossRef]
- de Wet, J.F.; du Preez, J.G.H. The Chemistry of Uranium. Part 20. Tetraphenylphosphonium Pentachloro-oxouranate(VI): Crystal Structure and Bonding Characteristics. J. Chem. Soc. Dalton Trans. 1978, 6, 592–597. [Google Scholar] [CrossRef]
- Rudel, S.S.; Deubner, H.L.; Müller, M.; Karttunen, A.J.; Kraus, F. Complexes Featuring a Linear [N≡U≡N] Core Isoelectronic to the Uranyl Cation. Nat. Chem. 2020, 12, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Fortier, S.; Wu, G.; Kaltsoyannis, N.; Hayton, T.W. Synthesis and Spectroscopic and Computational Characterization of the Chalcogenido-Substituted Analogues of the Uranyl Ion, [OUE]2+ (E = S, Se). J. Am. Chem. Soc. 2013, 135, 5352–5355. [Google Scholar] [CrossRef]
- Smiles, D.E.; Wu, G.; Hrobárik, P.; Hayton, T.W. Use of 77Se and 125Te NMR Spectroscopy to Probe Covalency of the Actinide-Chalcogen Bonding in [Th(En){N(SiMe3)2}3]− (E = Se, Te; n = 1, 2) and their Oxo-Uranium(VI) Congeners. J. Am. Chem. Soc. 2016, 138, 814–825. [Google Scholar] [CrossRef]
- Hunt, R.D.; Yustein, J.T.; Andrews, L. Matrix Infrared Spectra of NUN Formed by the Insertion of Uranium Atoms into Molecular Nitrogen. J. Chem. Phys. 1993, 98, 6070–6074. [Google Scholar] [CrossRef]
- Liang, B.; Andrews, L.; Ismail, N.; Marsden, C.J. The First Infrared Spectra and Quasirelativistic DFT Studies of the US, US2, and US3 Molecules. Inorg. Chem. 2002, 41, 2811–2813. [Google Scholar] [CrossRef]
- Wang, X.; Andrews, L.; Marsden, C.J. Infrared Spectra and Density Functional Calculations of the SUO2 Molecule. Inorg. Chem. 2009, 48, 6888–6895. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Chen, X.; Gong, Y. Sulfur-Substituted Uranyl Stabilized by Fluoride Ligands: Matrix Preparation of U(O)(S)F2 via Oxidation of U(0) by SOF2. Chem. Comm. 2020, 56, 6782–6785. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.C.L.; Michelini, M.C.; Marçalo, J.; Gong, Y.; Gibson, J.K. Synthesis and Properties of Uranium Sulfide Cations. An Evaluation of the Stability of Thiouranyl, {S═U═S}2+. Inorg. Chem. 2013, 52, 14162–14167. [Google Scholar] [CrossRef] [PubMed]
- Lucena, A.F.; Bandeira, N.A.G.; Pereira, C.C.L.; Gibson, J.K.; Marçalo, J. Synthesis, Structure and Bonding of Actinide Disulphide Dications in the Gas Phase. Phys. Chem. Chem. Phys. 2017, 19, 10685–10694. [Google Scholar] [CrossRef] [PubMed]
- Smiles, D.E.; Wu, G.; Hayton, T.W. Reversible Chalcogen-Atom Transfer to a Terminal Uranium Sulfide. Inorg. Chem. 2014, 53, 12683–12685. [Google Scholar] [CrossRef]
- Platts, J.A.; Baker, R.J. A Computational Investigation of Orbital Overlap Versus Energy Degeneracy Covalency in [UE2]2+ (E = O, S, Se, Te) Complexes. Dalton Trans. 2020, 49, 1077–1088. [Google Scholar] [CrossRef]
- Tarlton, M.L.; Fajen, O.J.; Kelley, S.P.; Kerridge, A.; Malcomson, T.; Morrison, T.L.; Shores, M.P.; Xhani, X.; Walensky, J.R. Systematic Investigation of the Molecular and Electronic Structure of Thorium and Uranium Phosphorus and Arsenic Complexes. Inorg. Chem. 2021, 60, 10614–10630. [Google Scholar] [CrossRef]
- Vlaisavljevich, B.; Gagliardi, L.; Wang, X.; Liang, B.; Andrews, L.; Infante, I. U and P4 Reaction Products: A Quantum Chemical and Matrix Isolation Spectroscopic Investigation. Inorg. Chem. 2010, 49, 9230–9235. [Google Scholar] [CrossRef]
- Arney, D.S.J.; Burns, C.J.; Smith, D.C. Synthesis and Structure of the First Uranium(VI) Organometallic Complex. J. Am. Chem. Soc. 1992, 114, 10068–10069. [Google Scholar] [CrossRef]
- Hayton, T.W.; Boncella, J.M.; Scott, B.L.; Palmer, P.D.; Batista, E.R.; Hay, P.J. Synthesis of Imido Analogs of the Uranyl Ion. Science 2005, 310, 1941–1943. [Google Scholar] [CrossRef]
- Hayton, T.W.; Boncella, J.M.; Scott, B.L.; Batista, E.R.; Hay, P.J. Synthesis and Reactivity of the Imido Analogues of the Uranyl Ion. J. Am. Chem. Soc. 2006, 128, 10549–10559. [Google Scholar] [CrossRef]
- Spencer, L.P.; Yang, P.; Scott, B.L.; Batista, E.R.; Boncella, J.M. Imido Exchange in Bis(imido) Uranium(VI) Complexes with Aryl Isocyanates. J. Am. Chem. Soc. 2008, 130, 2930–2931. [Google Scholar] [CrossRef]
- Spencer, L.P.; Yang, P.; Scott, B.L.; Batista, E.R.; Boncella, J.M. Uranium(VI) Bis(imido) Disulfonamide and Dihalide Complexes: Synthesis Density Functional Theory Analysis. Comptes Rendus Chim. 2010, 13, 758–766. [Google Scholar] [CrossRef]
- Swartz Ii, D.L.; Spencer, L.P.; Scott, B.L.; Odom, A.L.; Boncella, J.M. Exploring the Coordination Modes of Pyrrolyl Ligands in Bis(imido) Uranium(VI) Complexes. Dalton Trans. 2010, 39, 6841–6846. [Google Scholar] [CrossRef]
- Jilek, R.E.; Tomson, N.C.; Shook, R.L.; Scott, B.L.; Boncella, J.M. Preparation and Reactivity of the Versatile Uranium(IV) Imido Complexes U(NAr)Cl2(R2bpy)2 (R = Me, tBu) and U(NAr)Cl2(tppo)3. Inorg. Chem. 2014, 53, 9818–9826. [Google Scholar] [CrossRef]
- Spencer, L.P.; Yang, P.; Scott, B.L.; Batista, E.R.; Boncella, J.M. Uranium(VI) Bis(imido) Chalcogenate Complexes: Synthesis and Density Functional Theory Analysis. Inorg. Chem. 2009, 48, 2693–2700. [Google Scholar] [CrossRef]
- Spencer, L.P.; Yang, P.; Scott, B.L.; Batista, E.R.; Boncella, J.M. Oxidative Addition to U(V)−U(V) Dimers: Facile Routes to Uranium(VI) Bis(imido) Complexes. Inorg. Chem. 2009, 48, 11615–11623. [Google Scholar] [CrossRef]
- Spencer, L.P.; Schelter, E.J.; Yang, P.; Gdula, R.L.; Scott, B.L.; Thompson, J.D.; Kiplinger, J.L.; Batista, E.R.; Boncella, J.M. Cation–Cation Interactions, Magnetic Communication, and Reactivity of the Pentavalent Uranium Ion [U(NtBu)2]+. Angew. Chem. Int. Ed. 2009, 48, 3795–3798. [Google Scholar] [CrossRef]
- Spencer, L.P.; Gdula, R.L.; Hayton, T.W.; Scott, B.L.; Boncella, J.M. Synthesis and Reactivity of Bis(imido) Uranium(VI) Cyclopentadienyl Complexes. Chem. Comm. 2008, 40, 4986–4988. [Google Scholar] [CrossRef]
- Seaman, L.A.; Fortier, S.; Wu, G.; Hayton, T.W. Comparison of the Redox Chemistry of Primary and Secondary Amides of U(IV): Isolation of a U(VI) Bis(imido) Complex or a Homoleptic U(VI) Amido Complex. Inorg. Chem. 2011, 50, 636–646. [Google Scholar] [CrossRef]
- Spencer, L.P.; Yang, P.; Minasian, S.G.; Jilek, R.E.; Batista, E.R.; Boland, K.S.; Boncella, J.M.; Conradson, S.D.; Clark, D.L.; Hayton, T.W.; et al. Tetrahalide Complexes of the [U(NR)2]2+ Ion: Synthesis, Theory, and Chlorine K-Edge X-ray Absorption Spectroscopy. J. Am. Chem. Soc. 2013, 135, 2279–2290. [Google Scholar] [CrossRef]
- Tomson, N.C.; Anderson, N.H.; Tondreau, A.M.; Scott, B.L.; Boncella, J.M. Oxidation of Uranium(IV) Mixed Imido–Amido Complexes with PhEEPh and to Generate Uranium(VI) Bis(imido) Dichalcogenolates, U(NR)2(EPh)2(L)2. Dalton Trans. 2019, 48, 10865–10873. [Google Scholar] [CrossRef]
- Camp, C.; Pécaut, J.; Mazzanti, M. Tuning Uranium–Nitrogen Multiple Bond Formation with Ancillary Siloxide Ligands. J. Am. Chem. Soc. 2013, 135, 12101–12111. [Google Scholar] [CrossRef]
- Kraft, S.J.; Fanwick, P.E.; Bart, S.C. Exploring the Insertion Chemistry of Tetrabenzyluranium Using Carbonyls and Organoazides. Organometallics 2013, 32, 3279–3285. [Google Scholar] [CrossRef]
- Anderson, N.H.; Odoh, S.O.; Yao, Y.; Williams, U.J.; Schaefer, B.A.; Kiernicki, J.J.; Lewis, A.J.; Goshert, M.D.; Fanwick, P.E.; Schelter, E.J.; et al. Harnessing Redox Activity for the Formation of Uranium Tris(imido) Compounds. Nat. Chem. 2014, 6, 919–926. [Google Scholar] [CrossRef]
- Kiernicki, J.J.; Ferrier, M.G.; Lezama Pacheco, J.S.; La Pierre, H.S.; Stein, B.W.; Zeller, M.; Kozimor, S.A.; Bart, S.C. Examining the Effects of Ligand Variation on the Electronic Structure of Uranium Bis(imido) Species. J. Am. Chem. Soc. 2016, 138, 13941–13951. [Google Scholar] [CrossRef]
- Maria, L.; Santos, I.C.; Sousa, V.R.; Marçalo, J. Uranium(III) Redox Chemistry Assisted by a Hemilabile Bis(phenolate) Cyclam Ligand: Uranium–Nitrogen Multiple Bond Formation Comprising a trans-{RN═U(VI)═NR}2+ Complex. Inorg. Chem. 2015, 54, 9115–9126. [Google Scholar] [CrossRef]
- Maria, L.; Bandeira, N.A.G.; Marçalo, J.; Santos, I.C.; Gibson, J.K. CO2 Conversion to Phenyl Isocyanates by Uranium(VI) Bis(imido) Complexes. Chem. Comm. 2020, 56, 431–434. [Google Scholar] [CrossRef]
- Modder, D.K.; Palumbo, C.T.; Douair, I.; Scopelliti, R.; Maron, L.; Mazzanti, M. Single Metal Four-electron Reduction by U(II) and Masked “U(II)” Compounds. Chem. Sci. 2021, 12, 6153–6158. [Google Scholar] [CrossRef]
- Jilek, R.E.; Spencer, L.P.; Lewis, R.A.; Scott, B.L.; Hayton, T.W.; Boncella, J.M. A Direct Route to Bis(imido)uranium(V) Halides via Metathesis of Uranium Tetrachloride. J. Am. Chem. Soc. 2012, 134, 9876–9878. [Google Scholar] [CrossRef]
- Cladis, D.P.; Kiernicki, J.J.; Fanwick, P.E.; Bart, S.C. Multi-Electron Reduction Facilitated by a Trianionic Pyridine(diimine) Ligand. Chem. Comm. 2013, 49, 4169–4171. [Google Scholar] [CrossRef]
- Kiernicki, J.J.; Higgins, R.F.; Kraft, S.J.; Zeller, M.; Shores, M.P.; Bart, S.C. Elucidating the Mechanism of Uranium Mediated Diazene N═N Bond Cleavage. Inorg. Chem. 2016, 55, 11854–11866. [Google Scholar] [CrossRef]
- Brown, J.L.; Batista, E.R.; Boncella, J.M.; Gaunt, A.J.; Reilly, S.D.; Scott, B.L.; Tomson, N.C. A Linear trans-Bis(imido) Neptunium(V) Actinyl Analog: NpV(NDipp)2(tBu2bipy)2Cl (Dipp = 2,6-iPr2C6H3). J. Am. Chem. Soc. 2015, 137, 9583–9586. [Google Scholar] [CrossRef]
- Minasian, S.G.; Keith, J.M.; Batista, E.R.; Boland, K.S.; Clark, D.L.; Conradson, S.D.; Kozimor, S.A.; Martin, R.L.; Schwarz, D.E.; Shuh, D.K.; et al. Determining Relative f and d Orbital Contributions to M–Cl Covalency in MCl62– (M = Ti, Zr, Hf, U) and UOCl5– Using Cl K-Edge X-ray Absorption Spectroscopy and Time-Dependent Density Functional Theory. J. Am. Chem. Soc. 2012, 134, 5586–5597. [Google Scholar] [CrossRef]
- Warner, B.P.; Scott, B.L.; Burns, C.J. A Simple Preparative Route to Bis(imido)-uranium(VI) Complexes by the Direct Reductions of Diazenes and Azides. Angew. Chem. Int. Ed. 1998, 37, 959–960. [Google Scholar] [CrossRef]
- Ryan, A.J.; Angadol, M.A.; Ziller, J.W.; Evans, W.J. Isolation of U(II) Compounds Using Strong Donor Ligands, C5Me4H and N(SiMe3)2, Including a Three-coordinate U(II) Complex. Chem. Comm. 2019, 55, 2325–2327. [Google Scholar] [CrossRef]
- Kiernicki, J.J.; Cladis, D.P.; Fanwick, P.E.; Zeller, M.; Bart, S.C. Synthesis, Characterization, and Stoichiometric U–O Bond Scission in Uranyl Species Supported by Pyridine(diimine) Ligand Radicals. J. Am. Chem. Soc. 2015, 137, 11115–11125. [Google Scholar] [CrossRef]
- Raghavan, A.; Anderson, N.H.; Tatebe, C.J.; Stanley, D.A.; Zeller, M.; Bart, S.C. Insight into geometric preferences in uranium(VI) mixed tris(imido) systems. Chem. Commun. 2020, 56, 11138–11141. [Google Scholar] [CrossRef]
- Hayton, T.W.; Boncella, J.M.; Scott, B.L.; Batista, E.R. Exchange of an Imido Ligand in Bis(imido) Complexes of Uranium. J. Am. Chem. Soc. 2006, 128, 12622–12623. [Google Scholar] [CrossRef]
- Schmidt, A.-C.; Heinemann, F.W.; Maron, L.; Meyer, K. A Series of Uranium (IV, V, VI) Tritylimido Complexes, their Molecular and Electronic Structures and Reactivity with CO2. Inorg. Chem. 2014, 53, 13142–13153. [Google Scholar] [CrossRef]
- Maria, L.; Bandeira, N.A.G.; Marçalo, J.; Santos, I.C.; Ferreira, A.S.D.; Ascenso, J.R. Experimental and Computational Study of a Tetraazamacrocycle Bis(aryloxide) Uranyl Complex and of the Analogues {E═U═NR}2+ (E = O and NR). Inorg. Chem. 2022, 61, 346–356. [Google Scholar] [CrossRef]
- Staun, S.L.; Sergentu, D.-C.; Wu, G.; Autschbach, J.; Hayton, T.W. Use of 15N NMR Spectroscopy to Probe Covalency in a Thorium Nitride. Chem. Sci. 2019, 10, 6431–6436. [Google Scholar] [CrossRef]
- Du, J.; Seed, J.A.; Berryman, V.E.J.; Kaltsoyannis, N.; Adams, R.W.; Lee, D.; Liddle, S.T. Exceptional Uranium(VI)-Nitride Triple Bond Covalency from 15N Nuclear Magnetic Resonance Spectroscopy and Quantum Chemical Analysis. Nat. Comm. 2021, 12, 5649. [Google Scholar] [CrossRef]
- Mullane, K.C.; Lewis, A.J.; Yin, H.; Carroll, P.J.; Schelter, E.J. Anomalous One-Electron Processes in the Chemistry of Uranium Nitrogen Multiple Bonds. Inorg. Chem. 2014, 53, 9129–9139. [Google Scholar] [CrossRef]
- Lewis, A.J.; Carroll, P.J.; Schelter, E.J. Reductive Cleavage of Nitrite to Form Terminal Uranium Mono-Oxo Complexes. J. Am. Chem. Soc. 2013, 135, 511–518. [Google Scholar] [CrossRef]
- Lu, E.; Cooper, O.J.; McMaster, J.; Tuna, F.; McInnes, E.J.L.; Lewis, W.; Blake, A.J.; Liddle, S.T. Synthesis, Characterization, and Reactivity of a Uranium(VI) Carbene Imido Oxo Complex. Angew, Chem. Int. Ed. 2014, 53, 6696–6700. [Google Scholar] [CrossRef]
- O’Grady, E.; Kaltsoyannis, N. On the Inverse Trans Influence. Density Functional Studies of [MOX5]n− (M = Pa, n = 2; M = U, n = 1; M = Np, n = 0; X = F, Cl or Br). J. Chem. Soc. Dalton Trans. 2002, 6, 1233–1239. [Google Scholar] [CrossRef]
- Duval, P.B.; Burns, C.J.; Buschmann, W.E.; Clark, D.L.; Morris, D.E.; Scott, B.L. Reaction of the Uranyl(VI) Ion (UO22+) with a Triamidoamine Ligand: Preparation and Structural Characterization of a Mixed-Valent Uranium(V/VI) Oxo−Imido Dimer. Inorg. Chem. 2001, 40, 5491–5496. [Google Scholar] [CrossRef]
- Cobb, P.J.; Wooles, A.J.; Liddle, S.T. A Uranium(VI)–Oxo-Imido Dimer Complex Derived from a Sterically Demanding Triamidoamine. Inorg. Chem. 2020, 59, 10034–10041. [Google Scholar] [CrossRef]
- Crawford, M.-J.; Ellern, A.; Karaghiosoff, K.; Mayer, P.; Nöth, H.; Suter, M. Synthesis and Characterization of Heavier Dioxouranium(VI) Dihalides. Inorg. Chem. 2004, 43, 7120–7126. [Google Scholar] [CrossRef] [PubMed]
- Kushto, G.P.; Souter, P.F.; Andrews, L. An Infrared Spectroscopic and Quasirelativistic Theoretical Study of the Coordination and Activation of Dinitrogen by Thorium and Uranium Atoms. J. Chem. Phys. 1998, 108, 7121–7130. [Google Scholar] [CrossRef]
- Zhou, M.; Andrews, L. Infrared Spectra and Pseudopotential Calculations for NUO+, NUO, and NThO in Solid Neon. J. Chem. Phys. 1999, 111, 11044–11049. [Google Scholar] [CrossRef]
- Wang, X.; Andrews, L.; Vlaisavljevich, B.; Gagliardi, L. Combined Triple and Double Bonds to Uranium: The N≡U═N−H Uranimine Nitride Molecule Prepared in Solid Argon. Inorg. Chem. 2011, 50, 3826–3831. [Google Scholar] [CrossRef]
- Heinemann, C.; Schwarz, H. NUO+, a New Species Isoelectronic to the Uranyl Dication UO22+. Chem. Eur. J. 1995, 1, 7–11. [Google Scholar] [CrossRef]
- Van Stipdonk, M.J.; Michelini, M.C.; Plaviak, A.; Martin, D.; Gibson, J.K. Formation of Bare UO22+ and NUO+ by Fragmentation of Gas-Phase Uranyl–Acetonitrile Complexes. J. Phys. Chem. A 2014, 118, 7838–7846. [Google Scholar] [CrossRef]
- Dau, P.D.; Armentrout, P.B.; Michelini, M.C.; Gibson, J.K. Activation of Carbon Dioxide by a Terminal Uranium–Nitrogen Bond in the Gas-Phase: A Demonstration of the Principle of Microscopic Reversibility. Phys. Chem. Chem. Phys. 2016, 18, 7334–7340. [Google Scholar] [CrossRef]
- Pyykkö, P.; Li, J.; Runeberg, N. Quasirelativistic Pseudopotential Study of Species Isoelectronic to Uranyl and the Equatorial Coordination of Uranyl. J. Phys. Chem. 1994, 98, 4809–4813. [Google Scholar] [CrossRef]
- Kaltsoyannis, N. Computational Study of Analogues of the Uranyl Ion Containing the −NUN− Unit: Density Functional Theory Calculations on UO22+, UON+, UN2, UO(NPH3)3+, U(NPH3)24+, [UCl4{NPR3}2] (R = H, Me), and [UOCl4{NP(C6H5)3}]-. Inorg. Chem. 2000, 39, 6009–6017. [Google Scholar] [CrossRef]
- Wei, F.; Wu, G.; Schwarz, W.H.E.; Li, J. Geometries, Electronic Structures, and Excited States of UN2, NUO+, and UO22+: A Combined CCSD(T), RAS/CASPT2 and TDDFT study. Theor. Chem. Acc. 2011, 129, 467–481. [Google Scholar] [CrossRef]
- Tecmer, P.; Gomes, A.S.P.; Ekström, U.; Visscher, L. Electronic Spectroscopy of UO22+, NUO+ and NUN: An Evaluation of Time-Dependent Density Functional Theory for Actinides. Phys. Chem. Chem. Phys. 2011, 13, 6249–6259. [Google Scholar] [CrossRef]
- Zhao, J.; Chi, C.-X.; Meng, L.-Y.; Jiang, X.-L.; Grunenberg, J.; Hu, H.-S.; Zhou, M.; Li, J.; Schwarz, W.H.E. Cis- and Trans-Binding Influences in [NUO · (N2)n]+. J. Chem. Phys. 2022, in press. [Google Scholar] [CrossRef]
- Fortier, S.; Wu, G.; Hayton, T.W. Synthesis of a Nitrido-Substituted Analogue of the Uranyl Ion, [N═U═O]+. J. Am. Chem. Soc. 2010, 132, 6888–6889. [Google Scholar] [CrossRef]
- Andrews, L.; Wang, X.; Gong, Y.; Kushto, G.P.; Vlaisavljevich, B.; Gagliardi, L. Infrared Spectra and Electronic Structure Calculations for NN Complexes with U, UN, and NUN in Solid Argon, Neon, and Nitrogen. J. Phys. Chem. A 2014, 118, 5289–5303. [Google Scholar] [CrossRef]
- King, D.M.; Tuna, F.; McInnes, E.J.L.; McMaster, J.; Lewis, W.; Blake, A.J.; Liddle, S.T. Isolation and Characterization of a Uranium(VI)–Nitride Triple Bond. Nat. Chem. 2013, 5, 482–488. [Google Scholar] [CrossRef]
- Evans, W.J.; Miller, K.A.; Ziller, J.W.; Greaves, J. Analysis of Uranium Azide and Nitride Complexes by Atmospheric Pressure Chemical Ionization Mass Spectrometry. Inorg. Chem. 2007, 46, 8008–8018. [Google Scholar] [CrossRef]
- Schrock, R.R. Recent Advances in High Oxidation State Mo and W Imido Alkylidene Chemistry. Chem. Rev. 2009, 109, 3211–3226. [Google Scholar] [CrossRef]
- Benedikter, M.J.; Ziegler, F.; Groos, J.; Hauser, P.M.; Schowner, R.; Buchmeiser, M.R. Group 6 Metal Alkylidene and Alkylidyne N-Heterocyclic Carbene Complexes for Olefin and Alkyne Metathesis. Coord. Chem. Rev. 2020, 415, 213315. [Google Scholar] [CrossRef]
- Liddle, S.T.; Mills, D.P.; Wooles, A.J. Early Metal Bis(phosphorus-stabilised)carbene Chemistry. Chem. Soc. Rev. 2011, 40, 2164–2176. [Google Scholar] [CrossRef]
- Gregson, M.; Wooles, A.J.; Cooper, O.J.; Liddle, S.T. Covalent Uranium Carbene Chemistry. Comments Inorg. Chem. 2015, 35, 262–294. [Google Scholar] [CrossRef]
- Lyon, J.T.; Andrews, L.; Hu, H.-S.; Li, J. Infrared Spectra and Electronic Structures of Agostic Uranium Methylidene Molecules. Inorg. Chem. 2008, 47, 1435–1442. [Google Scholar] [CrossRef]
- Lyon, J.T.; Andrews, L. Formation and Characterization of the Uranium Methylidene Complexes CH2UHX (X = F, Cl, and Br). Inorg. Chem. 2006, 45, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.T.; Andrews, L.; Malmqvist, P.-Å.; Roos, B.O.; Yang, T.; Bursten, B.E. Infrared Spectrum and Bonding in Uranium Methylidene Dihydride, CH2UH2. Inorg. Chem. 2007, 46, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Van Stipdonk, M.J.; Tatosian, I.J.; Iacovino, A.C.; Bubas, A.R.; Metzler, L.J.; Sherman, M.C.; Somogyi, A. Gas-Phase Deconstruction of UO22+: Mass Spectrometry Evidence for Generation of [OUVICH]+ by Collision-Induced Dissociation of [UVIO2(C≡CH)]+. J. Am. Soc. Mass. Spectrom. 2019, 30, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Metzler, L.J.; Farmen, C.T.; Corcovilos, T.A.; Van Stipdonk, M.J. Intrinsic Chemistry of [OUCH]+: Reactions with H2O, CH3CN and O2. Phys. Chem. Chem. Phys. 2021, 23, 4475–4479. [Google Scholar] [CrossRef]
- Metzler, L.J.; Farmen, C.T.; Fry, A.N.; Seibert, M.P.; Massari, K.A.; Corcovilos, T.A.; Van Stipdonk, M.J. Intrinsic Reactivity of [OUCH]+: Apparent Synthesis of [OUS]+ by Reaction with CS2. Rapid Commun. Mass Spectrom. 2022, 36, e9260. [Google Scholar] [CrossRef]
- Zhou, M.; Andrews, L.; Li, J.; Bursten, B.E. Reaction of Laser-Ablated Uranium Atoms with CO: Infrared Spectra of the CUO, CUO-, OUCCO, (η2-C2)UO2, and U(CO)x (x = 1−6) Molecules in Solid Neon. J. Am. Chem. Soc. 1999, 121, 9712–9721. [Google Scholar] [CrossRef]
- Wang, X.; Andrews, L.; Malmqvist, P.-Å.; Roos, B.O.; Gonçalves, A.P.; Pereira, C.C.L.; Marçalo, J.; Godart, C.; Villeroy, B. Infrared Spectra and Quantum Chemical Calculations of the Uranium Carbide Molecules UC and CUC with Triple Bonds. J. Am. Chem. Soc. 2010, 132, 8484–8488. [Google Scholar] [CrossRef]
- Li, J.; Bursten Bruce, E.; Liang, B.; Andrews, L. Noble Gas-Actinide Compounds: Complexation of the CUO Molecule by Ar, Kr, and Xe Atoms in Noble Gas Matrices. Science 2002, 295, 2242–2245. [Google Scholar] [CrossRef]
- Andrews, L.; Liang, B.; Li, J.; Bursten, B.E. Noble Gas−Actinide Complexes of the CUO Molecule with Multiple Ar, Kr, and Xe Atoms in Noble-Gas Matrices. J. Am. Chem. Soc. 2003, 125, 3126–3139. [Google Scholar] [CrossRef]
- Yang, T.; Tyagi, R.; Zhang, Z.; Pitzer, R.M. Configuration Interaction Studies on the Electronic States of the CUO Molecule. Mol. Phys. 2009, 107, 1193–1195. [Google Scholar] [CrossRef]
- Tecmer, P.; van Lingen, H.; Gomes, A.S.P.; Visscher, L. The Electronic Spectrum of CUONg4 (Ng = Ne, Ar, Kr, Xe): New Insights in the Interaction of the CUO Molecule with Noble Gas Matrices. J. Chem. Phys. 2012, 137, 084308. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-S.; Qiu, Y.-H.; Xiong, X.-G.; Schwarz, W.H.E.; Li, J. On the Maximum Bond Multiplicity of Carbon: Unusual C≣U Quadruple Bonding in Molecular CUO. Chem. Sci. 2012, 3, 2786–2796. [Google Scholar] [CrossRef]
- Mills, D.P.; Cooper, O.J.; Tuna, F.; McInnes, E.J.L.; Davies, E.S.; McMaster, J.; Moro, F.; Lewis, W.; Blake, A.J.; Liddle, S.T. Synthesis of a Uranium(VI)-Carbene: Reductive Formation of Uranyl(V)-Methanides, Oxidative Preparation of a [R2C═U═O]2+ Analogue of the [O═U═O]2+ Uranyl Ion (R = Ph2PNSiMe3), and Comparison of the Nature of UIV═C, UV═C, and UVI═C Double Bonds. J. Am. Chem. Soc. 2012, 134, 10047–10054. [Google Scholar] [CrossRef] [PubMed]
- Cooper, O.J.; Mills, D.P.; McMaster, J.; Moro, F.; Davies, E.S.; Lewis, W.; Blake, A.J.; Liddle, S.T. Uranium–Carbon Multiple Bonding: Facile Access to the Pentavalent Uranium Carbene [U{C(PPh2NSiMe3)2}(Cl)2(I)] and Comparison of UV═C and UIV═C Bonds. Angew. Chem. Int. Ed. 2011, 50, 2383–2386. [Google Scholar] [CrossRef] [PubMed]
- Fryer-Kanssen, I.; Kerridge, A. Elucidation of the Inverse Trans Influence in Uranyl and its Imido and Carbene Analogues via Quantum Chemical Simulation. Chem. Commun. 2018, 54, 9761–9764. [Google Scholar] [CrossRef]
- Gagliardi, L.; Pyykkö, P. Theoretical Search for Very Short Metal–Actinide Bonds: NUIr and Isoelectronic Systems. Angew. Chem. Int. Ed. 2004, 43, 1573–1576. [Google Scholar] [CrossRef]
- Bursten, B.E.; Ozin, G.A. Xα-SW Calculations for Naked Actinide Dimers: Existence of ϕ Bonds between Metal Atoms. Inorg. Chem. 1984, 23, 2910–2911. [Google Scholar] [CrossRef]
- Pyykkö, P.; Patzschke, M.; Suurpere, J. Calculated Structures of [Au=C=Au]2+ and Related Systems. Chem. Phys. Lett. 2003, 381, 45–52. [Google Scholar] [CrossRef]
- Santos, M.; Marçalo, J.; Pires de Matos, A.; Gibson, J.K.; Haire, R.G. Actinide-Transition Metal Heteronuclear Ions and Their Oxides: {IrUO}+ as an Analogue to Uranyl. Eur. J. Inorg. Chem. 2006, 2006, 3346–3349. [Google Scholar] [CrossRef]
- Feng, R.; Glendening, E.D.; Peterson, K.A. Coupled Cluster Studies of Platinum–Actinide Interactions. Thermochemistry of PtAnOn+ (n = 0–2 and An = U, Np, Pu). J. Phys. Chem. A 2021, 125, 5335–5345. [Google Scholar] [CrossRef]
- Vieira, M.C.; Marçalo, J.; Pires de Matos, A. Gas-Phase Reactivity of Lanthanide and Actinide Cations with the Archetypal Organometallic Complexes Fe(CO)5 and Fe(C5H5)2. J. Organomet. Chem. 2001, 632, 126–132. [Google Scholar] [CrossRef]
- Chi, C.; Wang, J.-Q.; Qu, H.; Li, W.-L.; Meng, L.; Luo, M.; Li, J.; Zhou, M. Preparation and Characterization of Uranium–Iron Triple-Bonded UFe(CO)3− and OUFe(CO)3− Complexes. Angew. Chem. Int. Ed. 2017, 56, 6932–6936. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Fang, W.; Maron, L.; Zhu, C. Heterometallic Clusters with Uranium–Metal Bonds Supported by Double-Layer Nitrogen–Phosphorus Ligands. Acc. Chem. Res. 2022, 55, 1718–1730. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Liddle, S.T. f-Element-Metal Bond Chemistry. Rev. Inorg. Chem. 2012, 32, 1–22. [Google Scholar] [CrossRef]



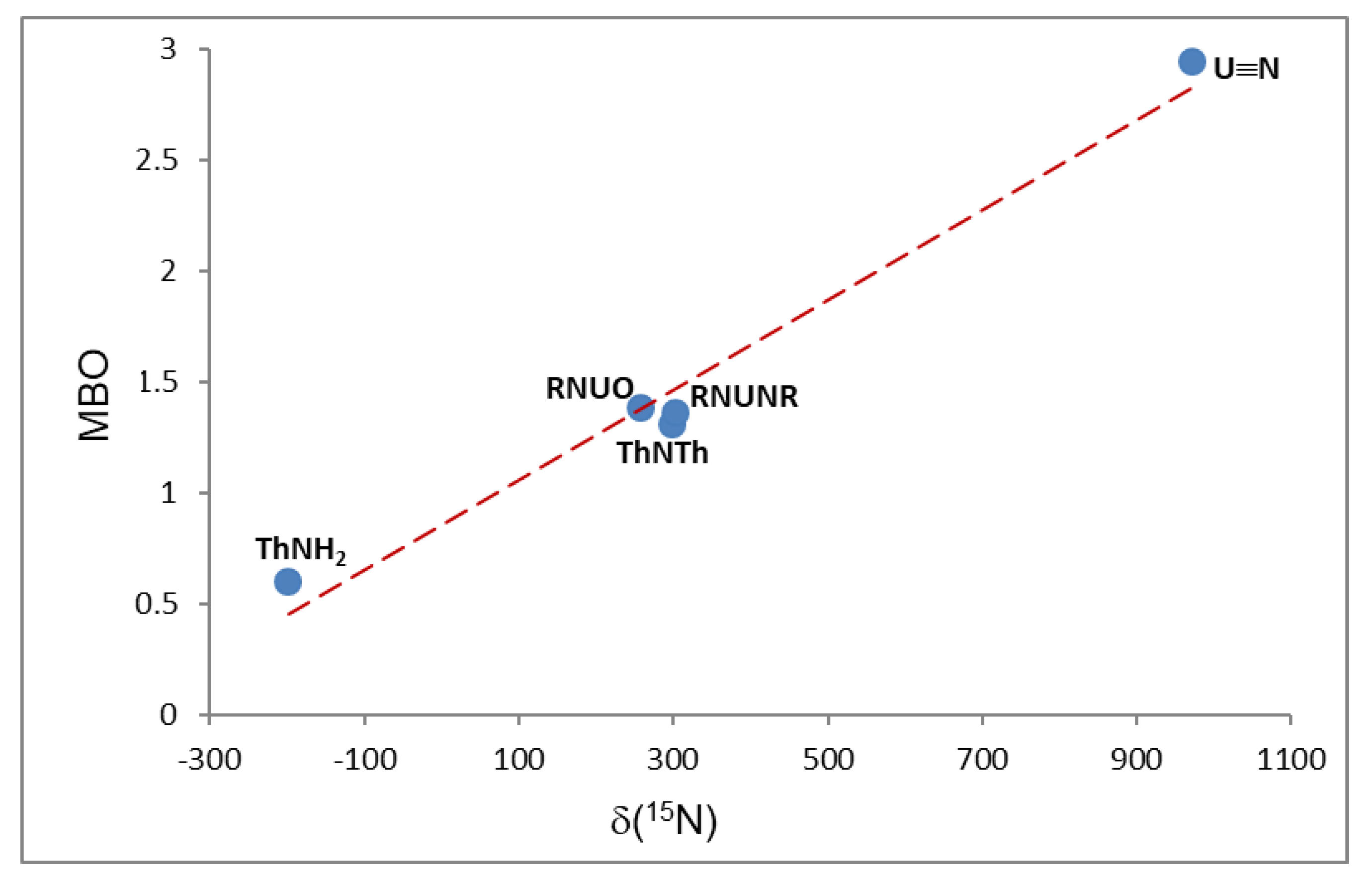
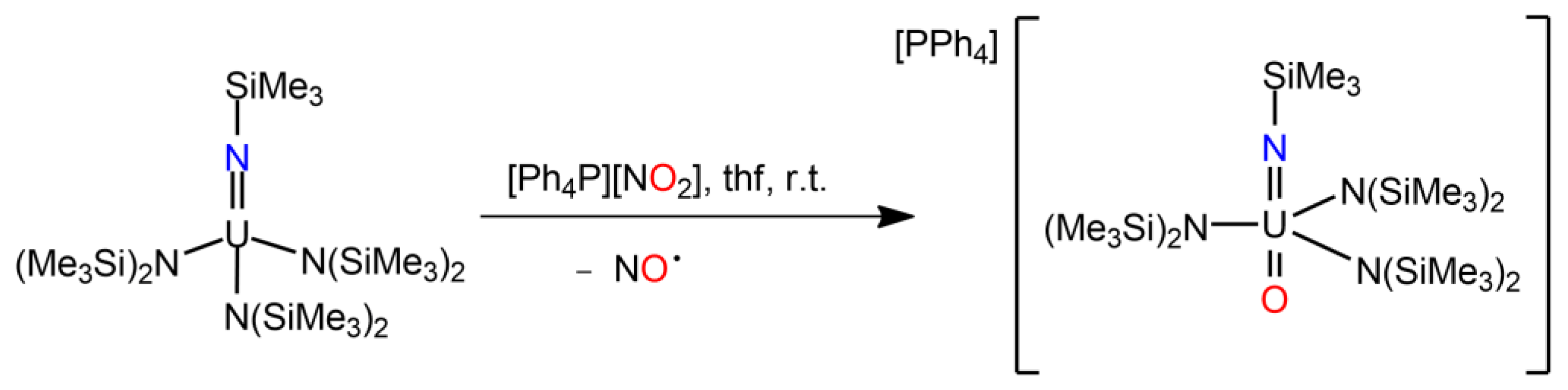

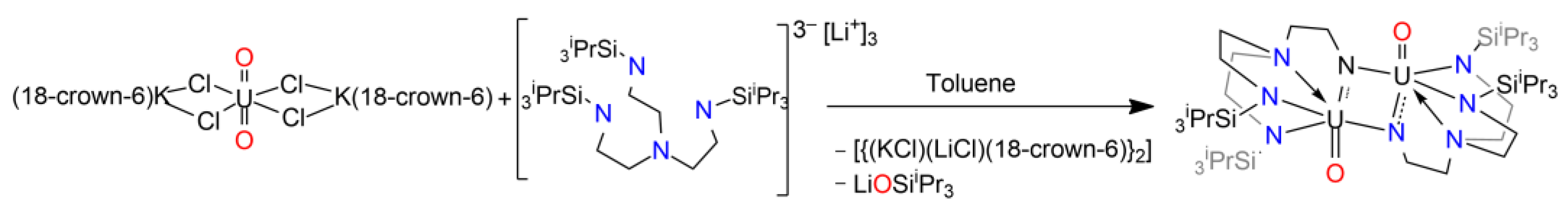
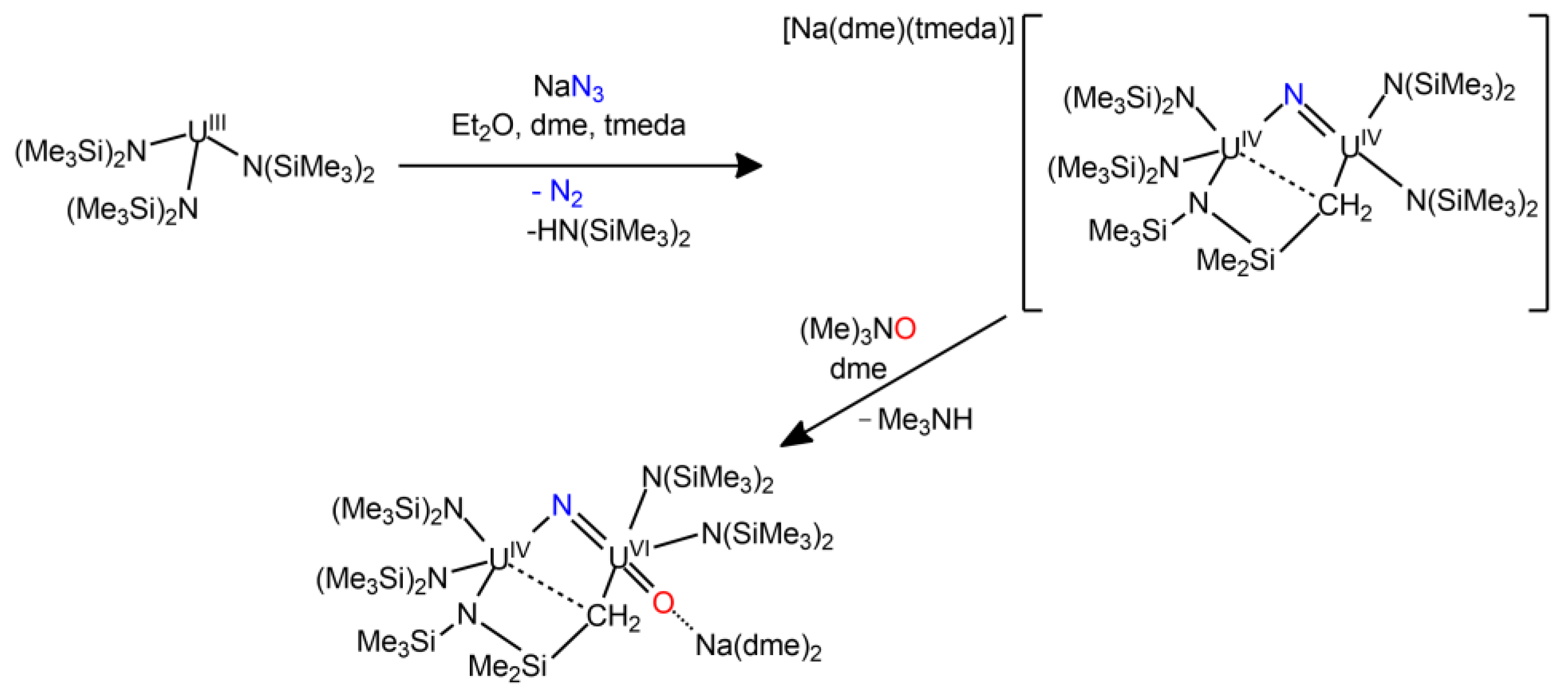
| Compound | Average U-N Bond Lengths (Å) | N-U-N Angle (Deg) | Average U-N-C Angle (Deg) | Ref. |
|---|---|---|---|---|
| [U(NtBu)2I2(thf)2] | 1.844(8) | 175.4(2) | 168.3(9) | [32,33] |
| [U(NtBu)2I2(thf)3] | 1.855(2) | 175.6(1) | 166.3(2) | [33] |
| [U(NtBu)2I2(py)2] | 1.835(2) | 180 | 174.5(2) | [33] |
| [U(NPh)2I2(thf)3] | 1.862(4) | 177.4(9) | 177.0(9) | [32,33] |
| [U(AriPr2)2I2(THF)3] | 1.887(4) | 169.3(1) | 175.6(16) | [33] |
| [U(NarCF3)2I2(thf)3] | 1.88(3) | 176.8(4) | 168.6(14) | [33] |
| [U(NtBu)2I2(OPPh3)2] | 1.840(4) | 177.8(2) | 170.9(8) | [33] |
| [U(NtBu)(NPh)I2(OPPh3)2] | 1.832(8) | 177.4(4) | 170.5(8) | [34] |
| 1.841(8) | 172.9(9) | |||
| [U(Nmes)2I2(OPPh3)2] | 1.867(5) | 180.0(5) | 175.4(3) | [34] |
| [U(NtBu)2Cl2(OPPh3)2] | 1.848(4) | 180.0(2). | 170.0(4) | [35] |
| [U(NtBu)2Br2(OPPh3)2] | 1.828(9) | 180.0(3) | 167.0(8) | [35] |
| [U(NtBu)2{N(Me)(SO2Ar’)}2(Me2bpy)] | 1.843(29) | 167.0(3) | 166.8(12) | [35] |
| [U(NtBu)2(η1-Me2-C4H2N)2(OPPh3)2] | 1.842(3) | 171.4(2) | 156.4(3) | [36] |
| [U(NtBu)2(dpm)(thf)2] | 1.856(6) | 172.8(3) | 162(5) | [36] |
| [U(NtBu)2(dpmMes)(dmpe)] | 1.856(18) | 165.9(6) | 170.0(85) | [36] |
| [U(NtBu)2Br2(Me2bpy)] | 1.838(5) | 169.8(1) | 171.6(36) | [35] |
| [U(NtBu)2Cl(μ-Cl)(Me2bpy)]2 | 1.834(18) | 172.3(3) | 164.1(37) | [35] |
| [U(Nmes)2I2(tBu2bpy)] | 1.867(3) | 173.7(2) | 171.7(3) | [37] |
| [U(NariPr2)2I2(tBu2bpy)] | 1.868(4) | 168.4(1) | 176.4(23) | [37] |
| [U(NtBu)2(O-2-tBuC6H4)2(OPPh3)2] | 1.870(6) | 180.0 | 180 | [38] |
| [U(NtBu)2(SPh)2(OPPh3)2] | 1.840(7) | 180 | 170.3(6) | [38] |
| [U(NtBu)2(SePh)2(OPPh3)2] | 1.861(6) | 180 | 169.4(4) | [38] |
| [U(NtBu)2I2(Me2bpy)] | 1.816(17) | 171.2(5) | 173(2) | [38] |
| [U(NtBu)2(SPh)2(tBu2bpy)] | 1.840(8) | 178.2(4) | 172.25(21) | [38] |
| [U(NtBu)2(SePh)2(Me2bpy)] | 1.844(5) | 175.9(2) | 174.6(31) | [38] |
| [U(NtBu)2(TePh)2(tBu2bpy)] | 1.828(12) | 177.4(3) | 173(5) | [38] |
| [U(NtBu)2(Cl)(I)(tBu2bpy)] | 1.822(6) | 175.2(3) | 171(7) | [39] |
| [U(NtBu)2(SePh)(I)(tBu2bpy)] | 1.823(12) | 174.7(4) | 174.7(16) | [39] |
| [(U(NtBu)2(I)(tBu2bpy))2(μ-O)] | 1.848(24) | 165.6(6) | 173(6) | [39,40] |
| [U(NtBu)2(I)(tBu2bpy)]2(μ-η2:η2-Se4)] | 1.833(8) | 172.4(2) | 173.9(43) | [39] |
| [Ucp2(NtBu)2(dmpe)] | 1.936(6) | 154.4(2) | 166.8(3) | [41] |
| [Ucp(NtBu)2I(dmpe)] | 1.886(8) | 161.1(2) | 166.1(26) | [41] |
| [Li(thf)]2[U(NtBu)2(HNtBu)4] | 1.915(5) | 180 | 164.8(4) | [42] |
| [U(NR)2Cl4][PPh4]2 (R = tBu, Ph) | 1.816(8) | 180.0(5) | 163.9(7) | [43] |
| Ph: 1.842(8) | 180.0(1) | 158.7(7) | ||
| [U(Nme)2Cl4][Net4]2 | 1.828(6) | 180.0(5) | 155.6(5) | [43] |
| [U(NtBu)2Br4][Net4]2 | 1.825(4) | 180.0 | 169.1(3) | [43] |
| [U(NtBu)2(SPh)2(py)2] | 1.862(2) | 180.0 | 172.9(1) | [43] |
| [U(NariPr2)2(SePh)2(py)2] | 1.886(1) | 180.0(1) | 164.6(1) | [44] |
| [U(NtBu)2(TePh)2(py)2] | 1.858(3) | 180.0(1) | 174.4(2) | [44] |
| [U2(Nad)4(OSi(OtBu)3)4] | 1.89(5) (U-Nterm.) | 171.4(27) | 167.5(8) | [45] |
| [U(Nad)2{CH2(Ph)N3(Ad)-κ2N1,3}] | 1.907(14) | 173.4(3) | 162.6(8) | [46] |
| [U(MesPDI)I(Nmes)3] | 1.992(5) | 166.6(2) | 170.8(3) | [47] |
| 2.024(5) (eq) | 180.0(1) | |||
| [Ucp*(Ntol)2(MesPDIMe)] | 1.950(7) | 155.9(3) | 167.8(5) | [48] |
| [Ucp*(Ntol)2(MesPDIMe)][SbF6] | 1.927(5) | 155.4(2) | 173.1(14) | [48] |
| [Ucp*(Ntol)2(tBu-MesPDIMe)] | 1.942(10) | 155.5(3) | 168.8(8) | [48] |
| [Ucp*(Ntol)2(tBu-MesPDIMe)][BPh4] | 1.950(10) | 153.1(2) | 169.6(18) | [48] |
| [U{(tBu2ArO)2Me2-cyclam}(NPh)2] | 1.901(8) | 172.95(8) | 154.9(2) | [49] |
| [U{(tBu2ArO)2Me2-cyclam}(NPh)(Ntol)] | 1.909(6) | 173.7(4) | 152.9(7) | [50] |
| 1.911(7) | 152.1(7) | |||
| [K-(crypt)][U{N(SiMe3)2}3(NPh)2] | 1.932(11) | 178.8(3) | 163.8(1.8) | [51] |
| Compound | Average U-N Bond Lengths (Å) | N-U-N Angle (Deg) | Average U-N-C Angle (Deg) | Ref. |
|---|---|---|---|---|
| [{U(μ-NtBu)(NtBu)I(tBu2bpy)}2] | 1.898(5) | 170.1(2) | 168(2) | [40] |
| 2.072(5) | 130.2(4) | |||
| [U(NAriPr2)2Cl(tBubpy)2] | 1.978(6) | 166.0(2) | 168(4) | [52] |
| [U(NAriPr2)2Br(tBubpy)2] | 1.974(12) | 165.9(2) | 167(4) | [52] |
| [U(NAriPr2)2I(tBubpy)2] | 1.958(22) | 174.9(2)° | 165.9(8) | [52] |
| [U(NAriPr2)2Cl(OPPh3)3] | 1.972(8) | 177.0(2) | 178.2(8) | [52] |
| [U(MesPDIMe)I(NMes)2(thf)] | 2.012(16) | 167.0(4) | 175(4) | [47] |
| [UCp*(MesPDIMe)(NPh)2] | 2.015(16) | 154.2(2) | 169.3(14) | [53] |
| [UCp*(MesPDIMe)](NPh)(NTol)] | 2.012(8) | 154.8(3) | 168.4(7) | [54] |
| 2.005(7) | 175.4(6) |
| Compound. | U-N Bond Lengths (Å) | U-O Bond Lengths (Å) | E-U-E Angle (Deg) | U-N-C Angle (Deg) | Ref. |
|---|---|---|---|---|---|
| [U(NtBu)(O)I2(OPPh3)2] | 1.821(7) | 1.764(5) | 178.4(3) | 172.3(7) | [61] |
| [U(NtBu)(O)I2(thf)(NH2Ph)2] | 1.823(4) | 1.781(4) | 178.6(2) | 170.4(4) | [61] |
| [UO2I2(OPPh3)3] | - | 1.760(4) | 180 | - | [72] |
| [U(NariPr2)(O)I2(OPPh3)2] | 1.847(3) | 1.778(2) | 178.9(1) | 170.3(2) | [37] |
| [U(BIPMTMS)(Nmes)(O)(dmap)2] | 1.921(2) | 1.814(2) | 167.1(1) | 174.2(2) | [68] |
| [U(BIPMTMS)(O)2(dmap)2] | - | 1.794(2) 1.785(2) | 167.2(1) | - | [68] |
| [U(κ4-{(tBu2ArO)2Me2-cyclam})(NPh)(O)] | 1.879(3) | 1.787(3) | 176.4(3) | 156.9(3) | [50] |
| [U(κ4-{(tBu2ArO)2Me2-cyclam})(O)2] | - | 1.775(5) | 177.6(3) | - | [63] |
| [{UO(μ-NCH2CH2N{CH2CH2NsiPri3}2)}2] | 2.057(14) | 1.805(13) | 159.9(6) | - | [71] |
| 2.052(15) | 1.811(14) | 162.3(6) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maria, L.; Marçalo, J. Uranyl Analogue Complexes—Current Progress and Synthetic Challenges. Inorganics 2022, 10, 121. https://doi.org/10.3390/inorganics10080121
Maria L, Marçalo J. Uranyl Analogue Complexes—Current Progress and Synthetic Challenges. Inorganics. 2022; 10(8):121. https://doi.org/10.3390/inorganics10080121
Chicago/Turabian StyleMaria, Leonor, and Joaquim Marçalo. 2022. "Uranyl Analogue Complexes—Current Progress and Synthetic Challenges" Inorganics 10, no. 8: 121. https://doi.org/10.3390/inorganics10080121
APA StyleMaria, L., & Marçalo, J. (2022). Uranyl Analogue Complexes—Current Progress and Synthetic Challenges. Inorganics, 10(8), 121. https://doi.org/10.3390/inorganics10080121







