Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Preparation and Functionalization of Ordered Hierarchical Porous Silica Materials
3. Results and Discussion
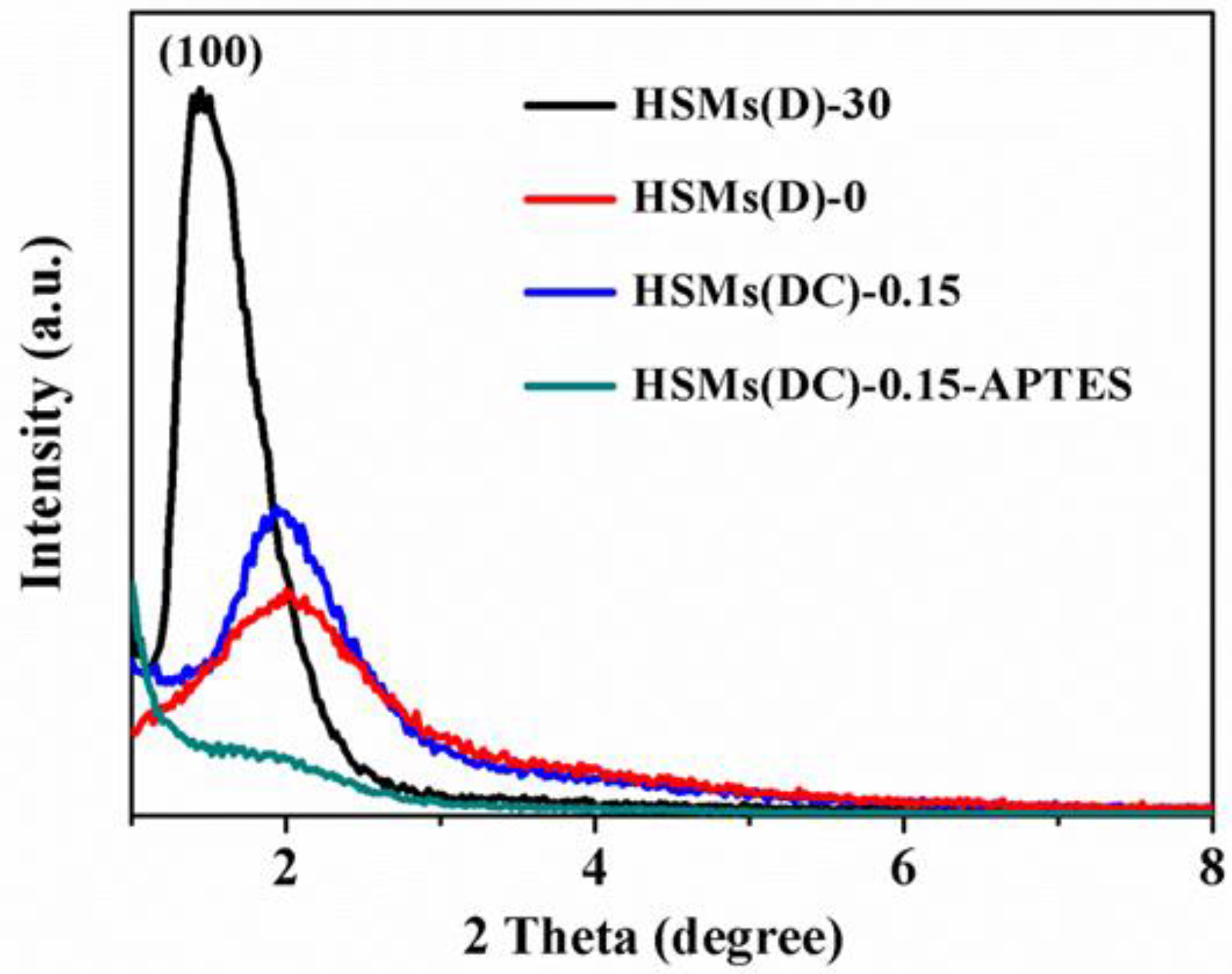
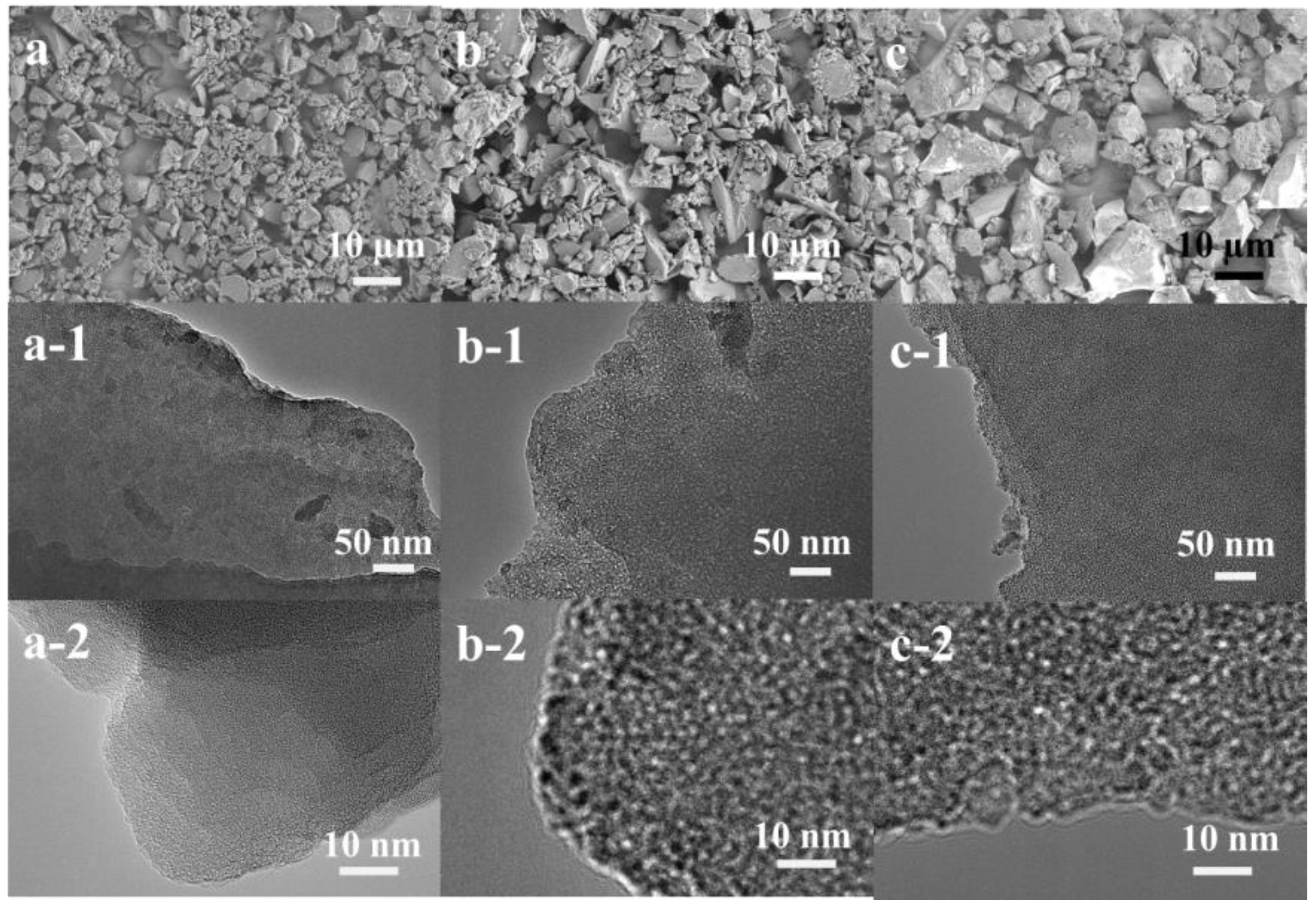
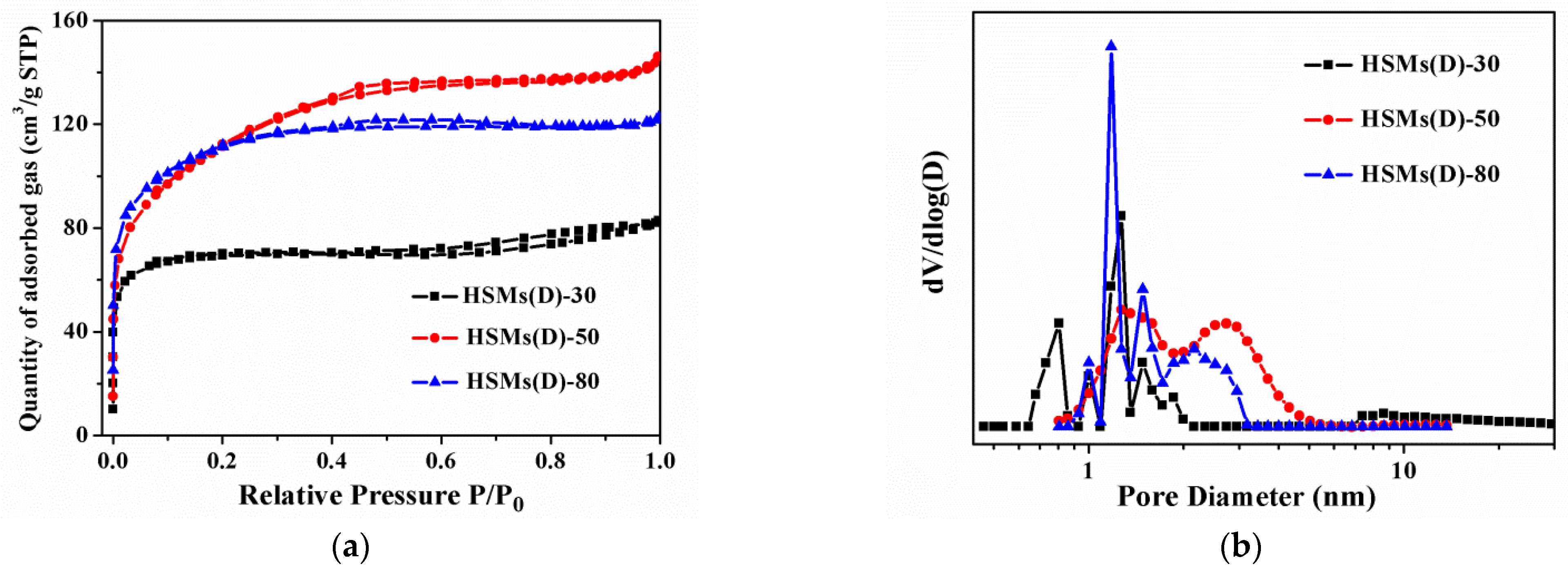
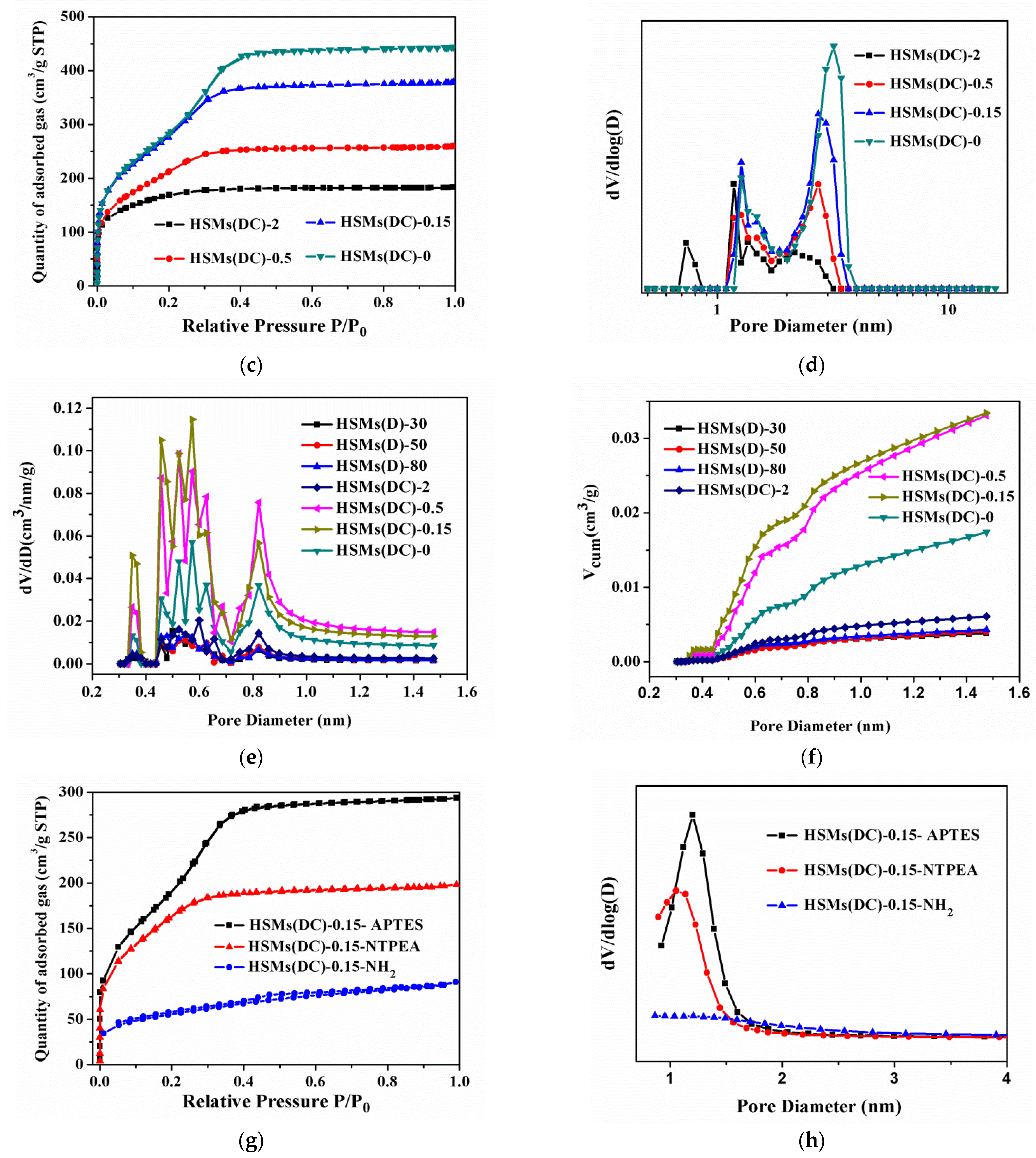
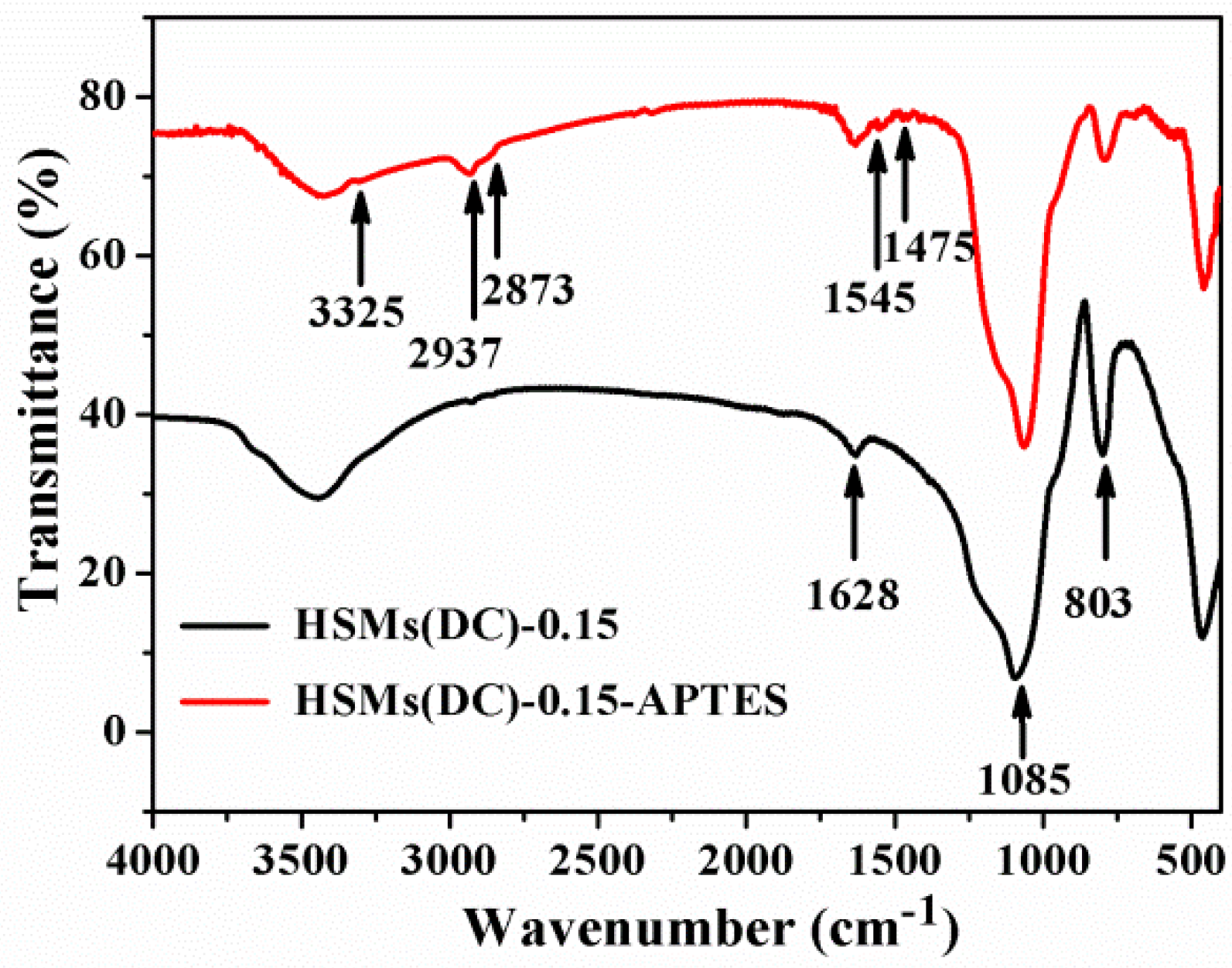
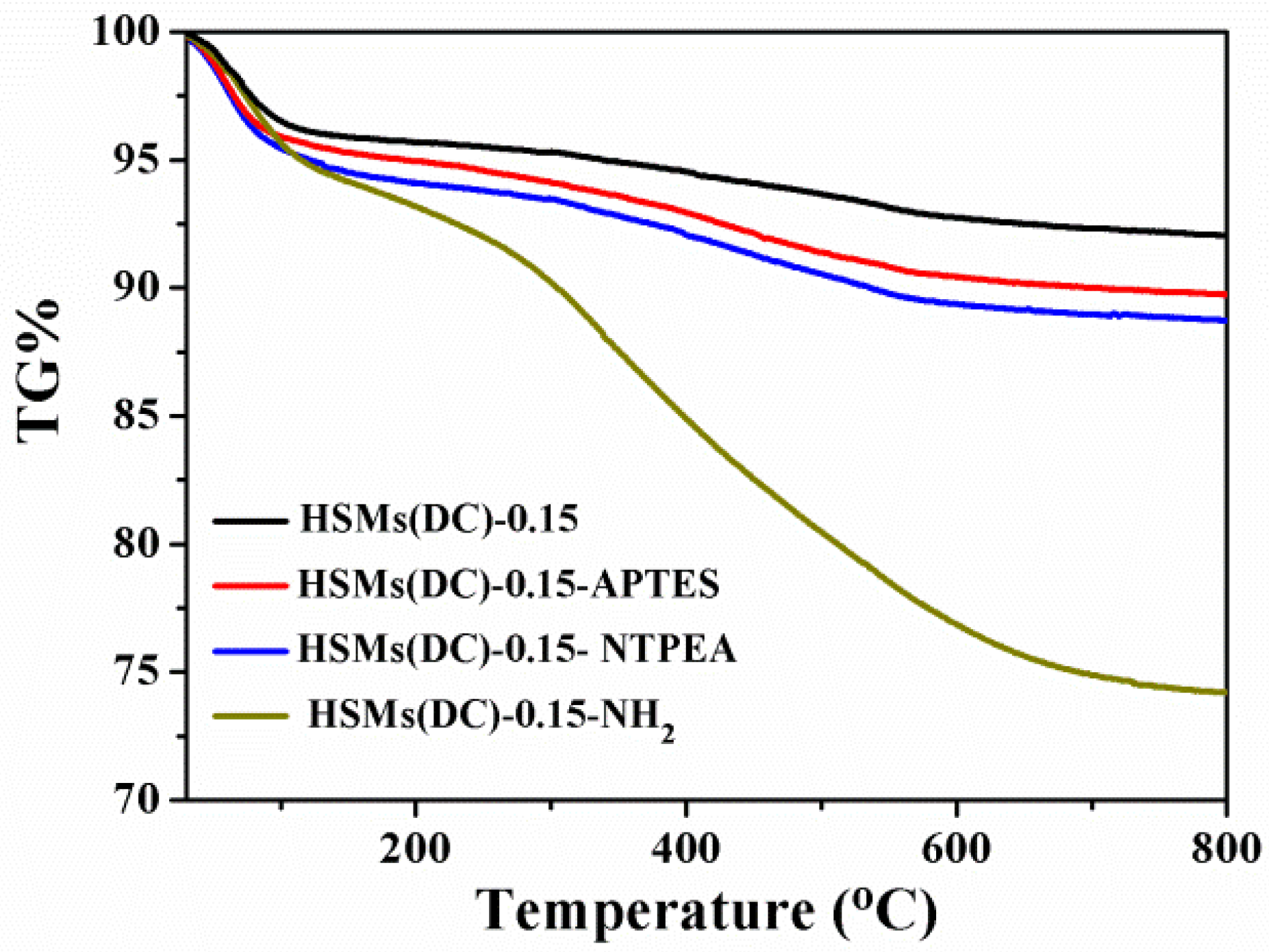
3.1. Structural and Textural Features
3.2. CO2 Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia, M.; Knuutila, H.K.; Aronu, U.E. Influence of substitution of water by organic solvents in amine solutions on absorption of CO2. Int. J. Greenh. Gas Control 2018, 78, 286–305. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R. Preparation of a dual pore structure activated carbon from rice husk char as an adsorbent for CO2 capture. Fuel Process. Technol. 2019, 186, 35–39. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Zhang, G.Y.; Gao, M.M.; Huang, L.; Li, L.; Liu, S.W.; Xie, C.X.; Zhang, Y.H.; Yu, S.T. N-doped porous carbon from different nitrogen sources for high-performance supercapacitors and CO2 adsorption. J. Alloys Compd. 2019, 786, 826–838. [Google Scholar] [CrossRef]
- Carta, D.; Montini, T.; Casula, M.F.; Monai, M.; Bullita, S.; Fornasiero, P.; Corrias, A. The water gas shift reaction over Pt-CeO2 nanoparticles confined within mesoporous SBA-16. J. Mater. Chem. A 2017, 5, 20024–20034. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.Y.; Zhang, G.J.; Xu, Y.; Zhang, Q.Q.; Liu, J.; Li, G.Q.; Zhao, Y.; Wang, Y.; Zhang, Y. High CO2 adsorption on amine-functionalized improved macro-/mesoporous multimodal pore silica. Fuel 2022, 315, 12319. [Google Scholar] [CrossRef]
- Liang, W.Q.; Huang, J.H.; Xiao, P.; Singh, R.; Guo, J.; Dehdari, L.; Li, G.K. Amine-immobilized HY zeolite for CO2 capture from hot flue gas. Chin. J. Chem. Eng. 2022, 43, 335–342. [Google Scholar] [CrossRef]
- Hiremath, V.; Shavi, R.; Seo, J.G. Controlled oxidation state of Ti in MgO-TiO2 composite for CO2 capture. Chem. Eng. J. 2017, 308, 177–183. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal-organic frameworks for CO2 capture regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances in the synthesis of covalent organic frameworks for CO2 capture. J. CO2 Util. 2017, 17, 137–161. [Google Scholar] [CrossRef]
- Loganathan, S.; Ghoshal, A.K. Amine tethered pore-expanded MCM-41: A promising adsorbent for CO2 capture. Chem. Eng. J. 2017, 308, 827–839. [Google Scholar] [CrossRef]
- Qian, X.C.; Yang, J.H.; Fei, Z.Y.; Liu, Q.; Zhang, Z.X.; Chen, X.; Tang, J.H.; Cui, M.F.; Qiao, X. A Simple Strategy to Improve PEI Dispersion on MCM-48 with Long-Alkyl Chains Template for Efficient CO2 Adsorption. Ind. Eng. Chem. Res. 2019, 58, 10975–10983. [Google Scholar] [CrossRef]
- Sujan, A.R.; Kumar, D.R.; Sakwa-Novak, M.; Ping, E.W.; Hu, B.; Park, S.J.; Jones, C.W. Poly(glycidyl amine)-Loaded SBA-15 Sorbents for CO2 Capture from Dilute and Ultradilute Gas Mixtures. ACS Appl. Polym. Mater. 2019, 1, 3137–3147. [Google Scholar] [CrossRef]
- Jiao, J.; Cao, J.; Lv, P.P. Amine-immobilized Three-dimensional Wormhole Mesostructured MSU-J Silica for CO2 Adsorption: Effect of Amine Loading and Temperature on the Adsorption Capacity. Chem. Lett. 2015, 44, 928–930. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.; Arencibia, A.; Calleja, G.; Sanz, R. Tuning the textural properties of HMS mesoporous silica. Functionalization towards CO2 adsorption. Microporous Mesoporous Mater. 2018, 260, 235–244. [Google Scholar] [CrossRef]
- Zelěnák, V.; Badaničová, M.; Halamová, D.; Čejka, J.; Zukal, A.; Murafa, N.; Goerigk, G. Amine-modified ordered mesoporous silica: Effect of pore size on carbon dioxide capture. Chem. Eng. J. 2008, 144, 336–342. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.X.; Fujii, M.; Yang, L.J.; Song, C.S. CO2 capture over molecular basket sorbents: Effects of SiO2 supports and PEG additive. J. Energy Chem. 2017, 26, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Regufe, M.J.; Ferreira, A.F.P.; Loureiro, J.M.; Shi, Y.X.; Rodrigues, A.; Ribeiro, A.M. New hybrid composite honeycomb monolith with 13X zeolite and activated carbon for CO2 capture. Adsorption 2018, 24, 249–265. [Google Scholar] [CrossRef]
- Qasem, N.; Ben-Mansour, R. Energy and productivity efficient vacuum pressure swing adsorption process to separate CO2 from CO2/N2 mixture using Mg-MOF-74: A CFD simulation. Appl. Energy 2018, 209, 190–202. [Google Scholar] [CrossRef]
- Shen, Z.F.; Cai, Q.; Yin, C.C.; Xia, Q.; Cheng, J.; Li, X.; Wang, Y.G. Facile synthesis of silica nanosheets with hierarchical pore structure and their amine-functionalized composite for enhanced CO2 capture. Chem. Eng. Sci. 2020, 217, 115528. [Google Scholar] [CrossRef]
- Qu, H.Q.; Ma, Y.R.; Li, B.; Wang, L. Hierarchical zeolites: Synthesis, structural control, and catalytic applications. Emergent Mater. 2020, 3, 25–245. [Google Scholar] [CrossRef]
- Santos, S.C.G.; Pedrosa, A.M.M.G.; Souza, J.B.; Cecilia, J.A.; Rodríguez-Castellón, E. Carbon dioxide adsorption on micro-mesoporous composite materials of ZSM-12/MCM-48 type: The role of the contents of zeolite and functionalized amine. Mater. Res. Bull. 2015, 70, 663–672. [Google Scholar] [CrossRef]
- Jia, Y.F.; Wei, J.W.; Yuan, Y.; Geng, L.L.; Chen, S.Q.; Liao, L. Tetraethylenepentamine impregnated composite material ZSM-5/SBA-16 for CO2 adsorption. J. Mater. Res. 2022, 37, 543–553. [Google Scholar] [CrossRef]
- Yuan, Y.; Wei, J.W.; Geng, L.L.; Mei, D.J.; Liao, L. An amine-bifunctionalization strategy with Beta/KIT-6 composite as a support for CO2 adsorbent preparation. RSC Adv. 2020, 10, 34187–34196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.H.; Zhou, M.X.; He, G.H. Equilibrium and Diffusion of CO2 Adsorption on Micro-Mesoporous NaX/MCM-41 via Molecular Simulation. Ind. Eng. Chem. Res. 2019, 58, 14380–14388. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Han, Y. Direct Observation of Nanorange Ordered Microporosity within Mesoporous Molecular Sieves. Chem. Mater. 2002, 14, 2536–2540. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, G.; Wang, X. Direct synthesis of disordered micro-mesoporous molecular sieve. Microporous Mesoporous Mater. 2009, 123, 221–227. [Google Scholar] [CrossRef]
- Mori, H.; Uota, M.; Fujikawa, D.; Yoshimura, T.; Kuwahara, T.; Sakai, G.; Kijima, T. Synthesis of micro-mesoporous bimodal silica nanoparticles using lyotropic mixed surfactant liquid-crystal templates. Microporous Mesoporous Mater. 2006, 91, 172–180. [Google Scholar] [CrossRef]
- Zhao, S.; He, M.; Zhou, Y.M.; Sheng, X.L.; Fu, X.Q.; Zhang, Y.W. Synthesis of micro/mesoporous silica material by dual-template method as a heterogeneous catalyst support for alkylation. RSC Adv. 2015, 5, 28124–28132. [Google Scholar] [CrossRef]
- Voronova, M.I.; Surov, O.V.; Kraev, A.S.; Isaeva, D.A.; Mityukhina, I.S.; Zakharov, A.G. Template Synthesis of Mesoporous Silicas with the Use of Nanocrystalline Cellulose. Colloid J. 2017, 79, 18–25. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Koo, W.T.; Jang, J.S.; Kim, D.H.; Cho, H.J.; Kim, I.D. Chitosan-templated Pt nanocatalyst loaded mesoporous SnO2 nanofibers: A superior chemiresistor toward acetone molecules. Nanoscale 2018, 10, 13713–13721. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.X.; Li, N.; Liu, S.B.; Wang, L.; Wang, Z.G.; Zhang, Z.; Ding, B.Q. Site-Specific Synthesis of Silica Nanostructures on DNA Origami Templates. Adv. Mater. 2020, 32, 2000294.1–2000294.5. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Kulak, A.; Aoki, K.; Ikoma, T.; Tanaka, J.; Mann, S. Preparation of Higher-Order Zeolite Materials by Using Dextran Templating. Angew. Chem. Int. Ed. 2004, 116, 6691–6695. [Google Scholar] [CrossRef] [PubMed]
- Gaweł, B.; Øye, G. Hierarchical g-alumina monoliths with macro- and meso porosity prepared by using cross-linked dextran gel beads as the template. Mater. Lett. 2013, 95, 86–88. [Google Scholar] [CrossRef]
- DasP, T.K.; Ilaiyaraja, P.; Sudakar, C. Template assisted nanoporous TiO2 nanoparticles: The effect of oxygen vacancy defects on photovoltaic performance of DSSC and QDSSC. Sol. Energy 2018, 159, 920–929. [Google Scholar]
- Roy, A.; Bhandari, S.; Ghosh, A.; Sundaram, S.; Mallick, T.K. Incorporating Solution-Processed Mesoporous WO3 as an Interfacial Cathode Buffer Layer for Photovoltaic Applications. J. Phys. Chem. A 2020, 124, 5709–5719. [Google Scholar] [CrossRef]
- Priya, R.; Michalska-Doma, M.; Kumar, S.; Pandey, O.P. Morphological and optical studies of Gd2O3:Eu nanostructures synthesized via sacrificial template directed co-precipitation route. Opt. Laser Technol. 2021, 143, 107357. [Google Scholar] [CrossRef]
- Wei, M.M.; Zhang, L.; Xiong, Y.Q. Nanopore structure characterization for organic-rich shale using the non-local-density functional theory by a combination of N2 and CO2 adsorption. Microporous Mesoporous Mater. 2016, 227, 88–94. [Google Scholar] [CrossRef]
- He, Y.Q.; Chen, Q.; Tian, Y.Y.; Yan, C.H.; He, Y.F.; Li, K. Estimation of shale pore-size-distribution from N2 adsorption characteristics employing modified BJH algorithm. Pet. Sci. Technol. 2021, 39, 843–859. [Google Scholar] [CrossRef]
- Wang, L.X.; Wu, J.J.X.; Wang, X.; Cheng, Q.; Zheng, L.; Zhang, J.L.; Li, W. Effects of CTAB on porous silica templated by chitosan. J. Mater. Sci. 2010, 45, 4470–4479. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Ma, X.C.; Wei, D.; Yang, Y.H.; Zeng, Z.; Li, L.Q. Development of ultramicropore-mesopore interconnected pore architectures for boosting carbon dioxide capture at low partial pressure. Carbon 2022, 192, 41–49. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Salem, J.K.; Al-Agha, A. Silver-NPs functionalized hexagonal SBA-15 and lamellar SiO2-L81 mesoporous silica, synthesis and structural characterization. J. Sol-Gel Sci. Technol. 2020, 93, 175–184. [Google Scholar] [CrossRef]
- Ge, L.; Wei, J.W.; Geng, L.L.; Chen, S.Q.; Liao, L. Amine-bifunctionalized ZSM-5/SBA-16 composite for CO2 adsorption. J. Porous Mater. 2022, 29, 19–31. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kim, S.; Yoon, M.; Bae, T.H. Hierarchical Zeolites with Amine-Functionalized Mesoporous Domains for Carbon Dioxide Capture. Chem. Sus. Chem. 2016, 9, 455–461. [Google Scholar] [CrossRef]
- Jin, X.Q.; Xiong, M.M.; Zhu, L.L.; Zhang, L.Y.; Wu, Z. Influence of particle size of mesoporous silica composite nanoparticles coated with pH/temperature responsive copolymer on ibuprofen release behaviors. J. Dispers. Sci. Technol. 2022, 43, 501–516. [Google Scholar] [CrossRef]
- Xu, C.; Hedin, N. Ultramicroporous CO2 adsorbents with tunable mesopores based on polyimines synthesized under off-stoichiometric conditions. Microporous Mesoporous Mater. 2016, 222, 80–86. [Google Scholar] [CrossRef]
- Zhang, G.J.; Zhao, P.Y.; Xu, Y. Development of amine-functionalized hierarchically porous silica for CO2 capture. J. Ind. Eng. Chem. 2017, 54, 59–68. [Google Scholar] [CrossRef] [Green Version]
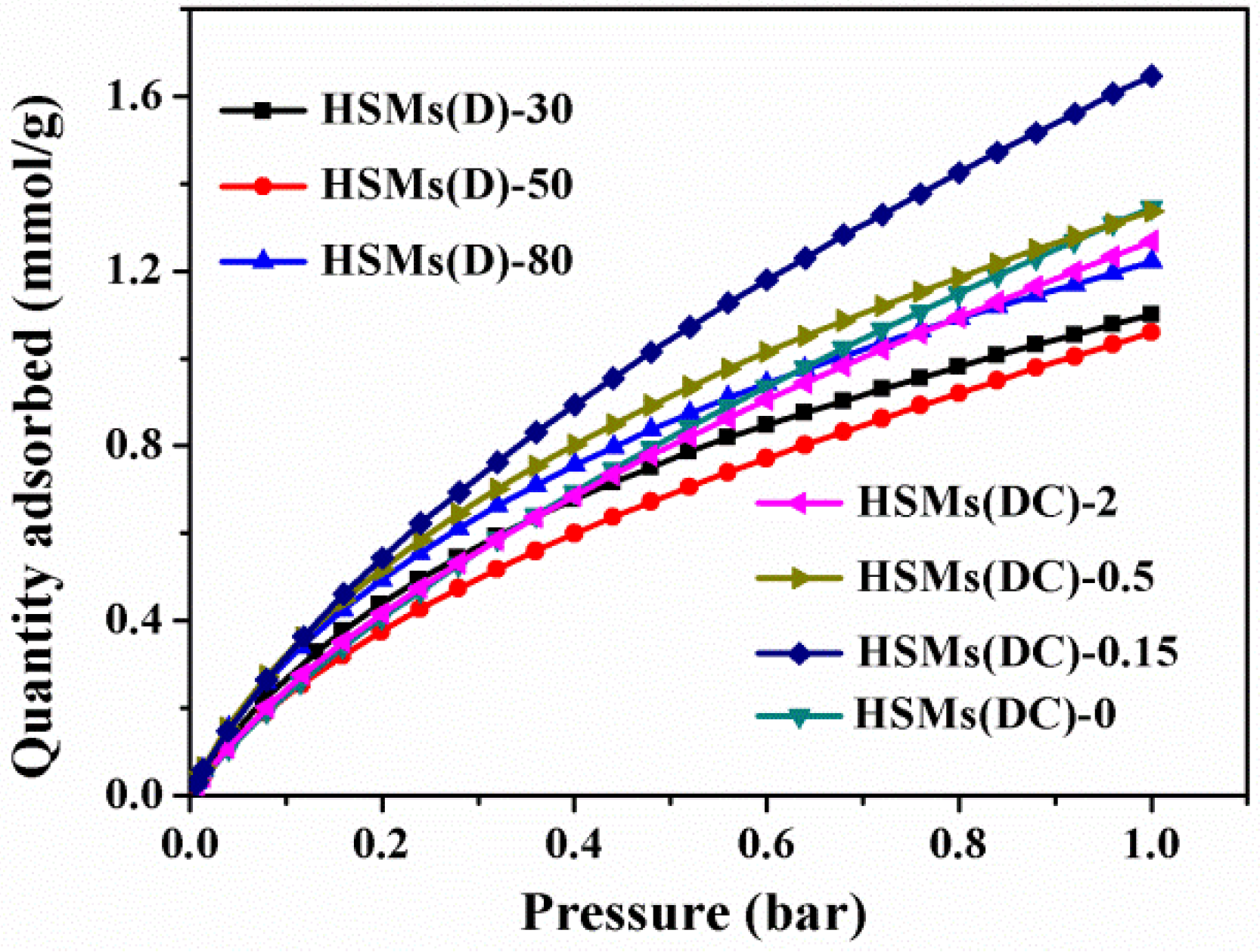
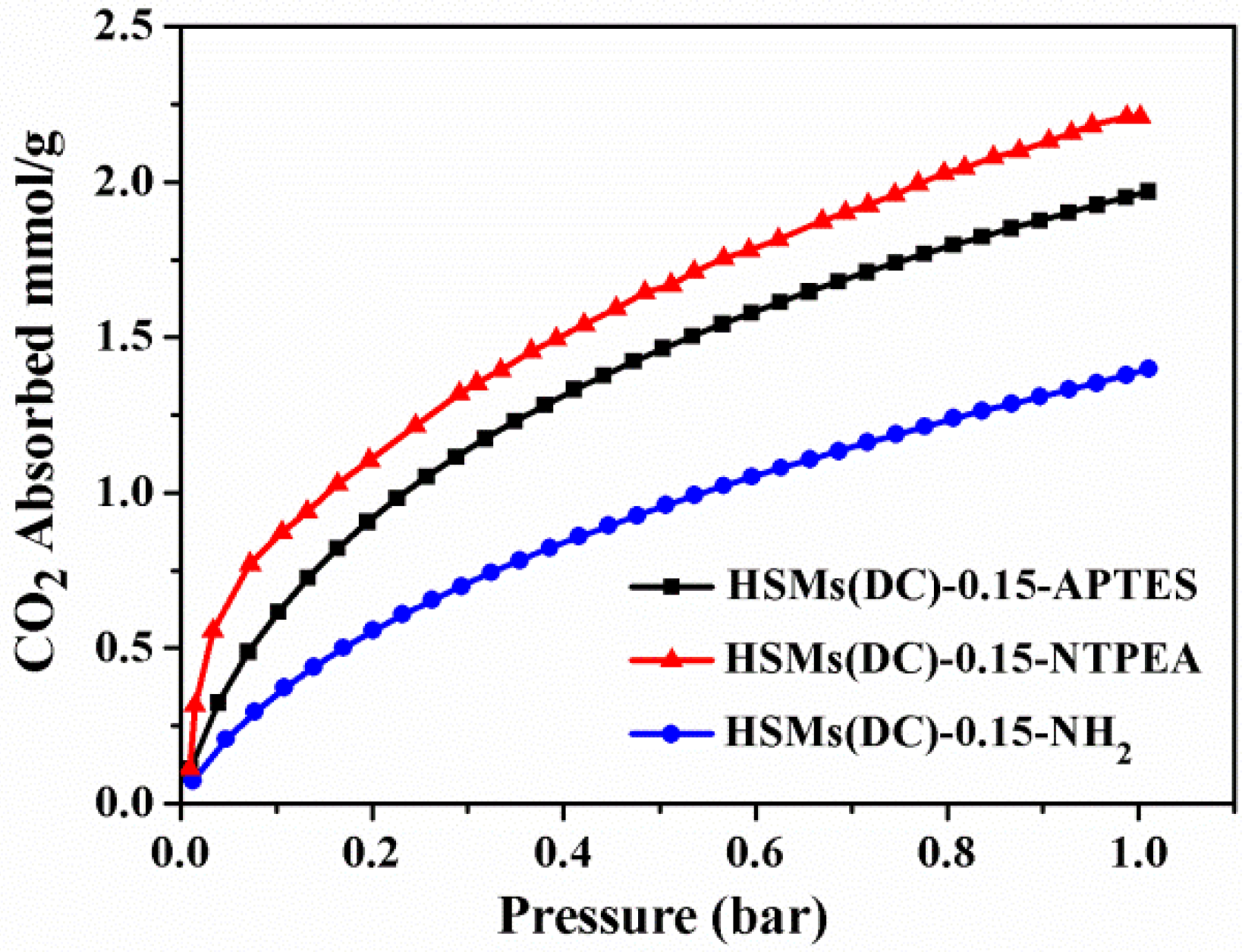
| Samples | SBET a/Smi b (m2/g) | Vt/Vmi b/Vnarrow micro c (cm3/g) | Carbon Dioxide Uptake d 273/298 K (mmol/g) |
|---|---|---|---|
| HSMs(D)-30 | 269/218 | 0.13/0.09/0.003 | 1.10/0.65 |
| HSMs(D)-50 | 401/70 | 0.23/0.03/0.003 | 1.00/0.57 |
| HSMs(D)-80 | 406/175 | 0.19/0.08/0.004 | 1.22/0.74 |
| HSMs(DC)-2 | 601/172 | 0.28/0.07/0.006 | 1.26/0.70 |
| HSMs(DC)-0.5 | 801/751 | 0.42/0.37/0.033 | 1.33/0.74 |
| HSMs(DC)-0.15 | 1045/953 | 0.58/0.53/0.034 | 1.64/0.86 |
| HSMs(DC)-0 | 1052/972 | 0.68/0.61/0.017 | 1.34/0.74 |
| HSMs(DC)-0.15-APTES | 781/703 | 0.45/0.39/– | 1.98/– |
| HSMs(DC)-0.15-NTPEA | 603/521 | 0.31/0.27/– | 2.22/– |
| HSMs(DC)-0.15-NH2 | 120/68 | 0.12/0.06/– | 1.38/– |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Ge, J.; Zhang, L.; Wu, Z.; Zhu, L.; Xiong, M. Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine. Inorganics 2022, 10, 87. https://doi.org/10.3390/inorganics10070087
Jin X, Ge J, Zhang L, Wu Z, Zhu L, Xiong M. Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine. Inorganics. 2022; 10(7):87. https://doi.org/10.3390/inorganics10070087
Chicago/Turabian StyleJin, Xiaoqi, Jinlong Ge, Liyuan Zhang, Zhong Wu, Linlin Zhu, and Mingwen Xiong. 2022. "Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine" Inorganics 10, no. 7: 87. https://doi.org/10.3390/inorganics10070087
APA StyleJin, X., Ge, J., Zhang, L., Wu, Z., Zhu, L., & Xiong, M. (2022). Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine. Inorganics, 10(7), 87. https://doi.org/10.3390/inorganics10070087





