Effect of the Precursor on the Synthesis of ZnO and Its Photocatalytic Activity
Abstract
:1. Introduction
2. Results
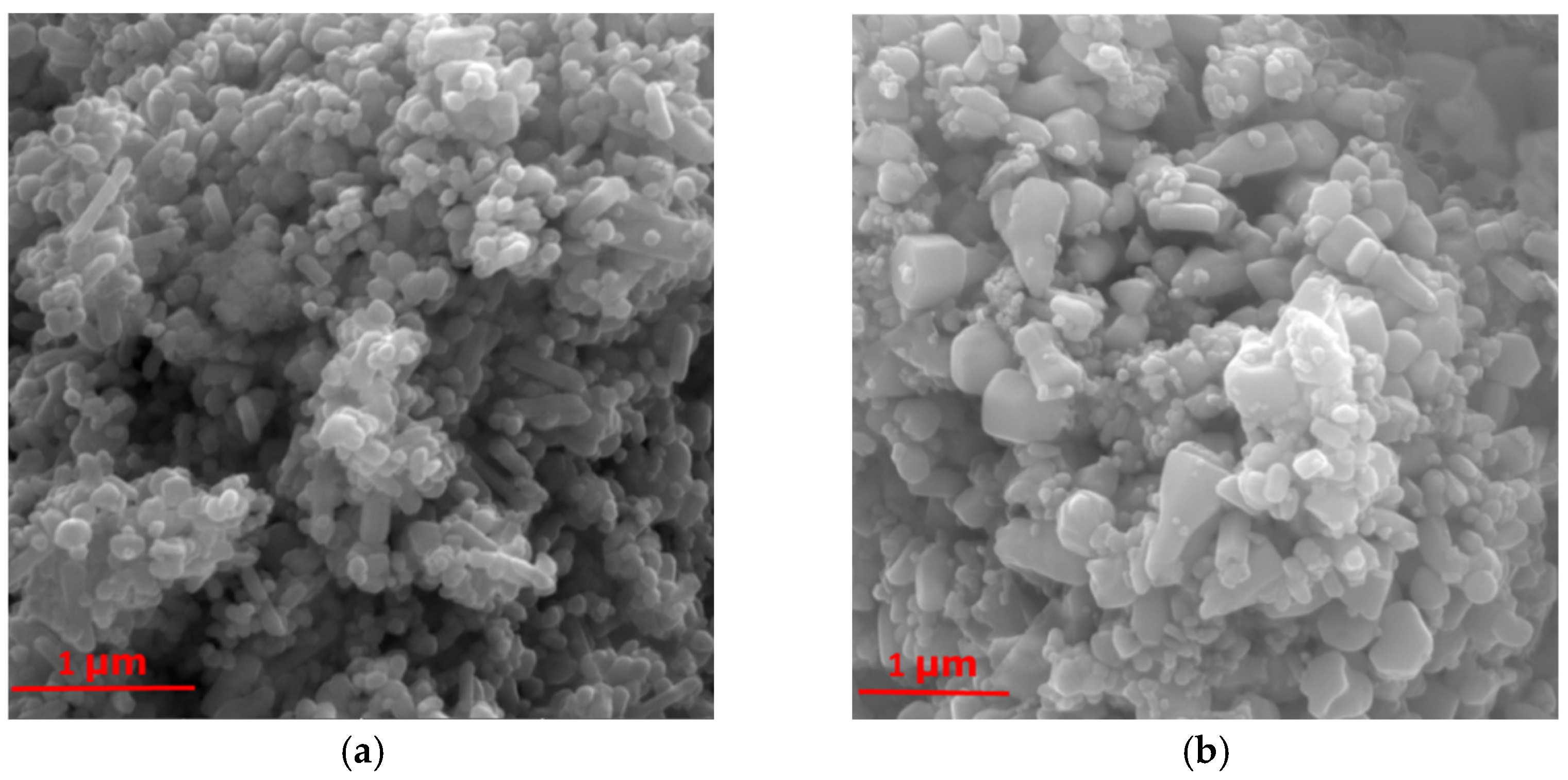
2.1. Scanning Electron Microscopy (SEM) Analysis
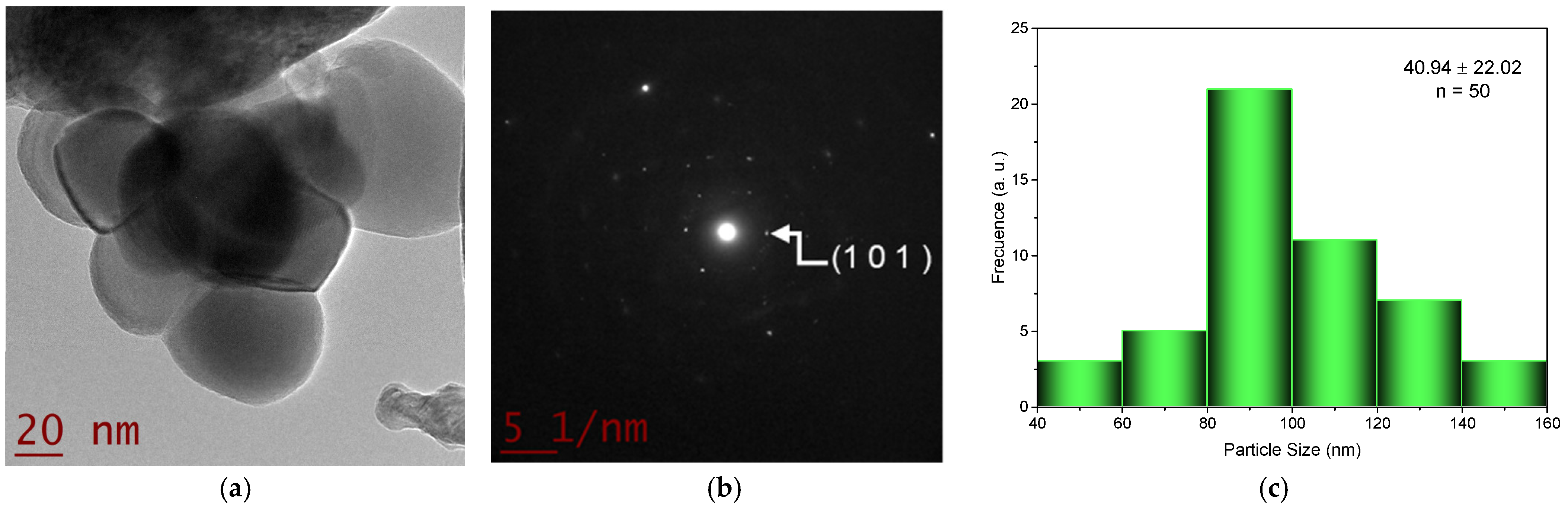
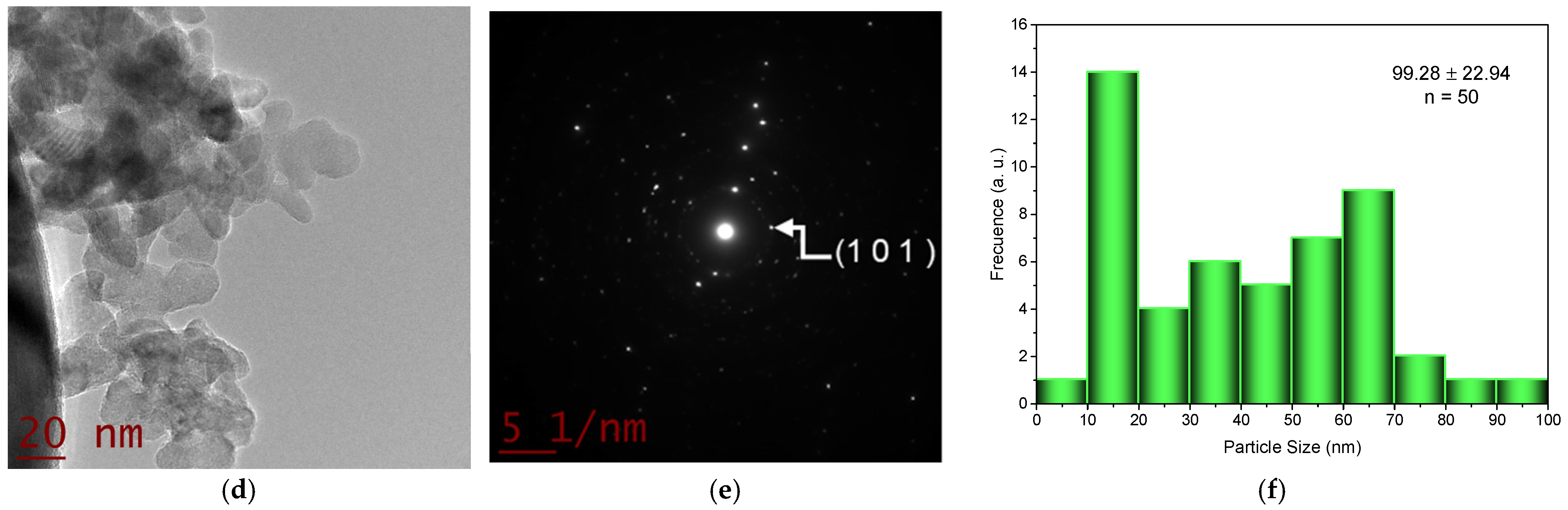
2.2. Transmision Electron Microscopy (TEM) Studies
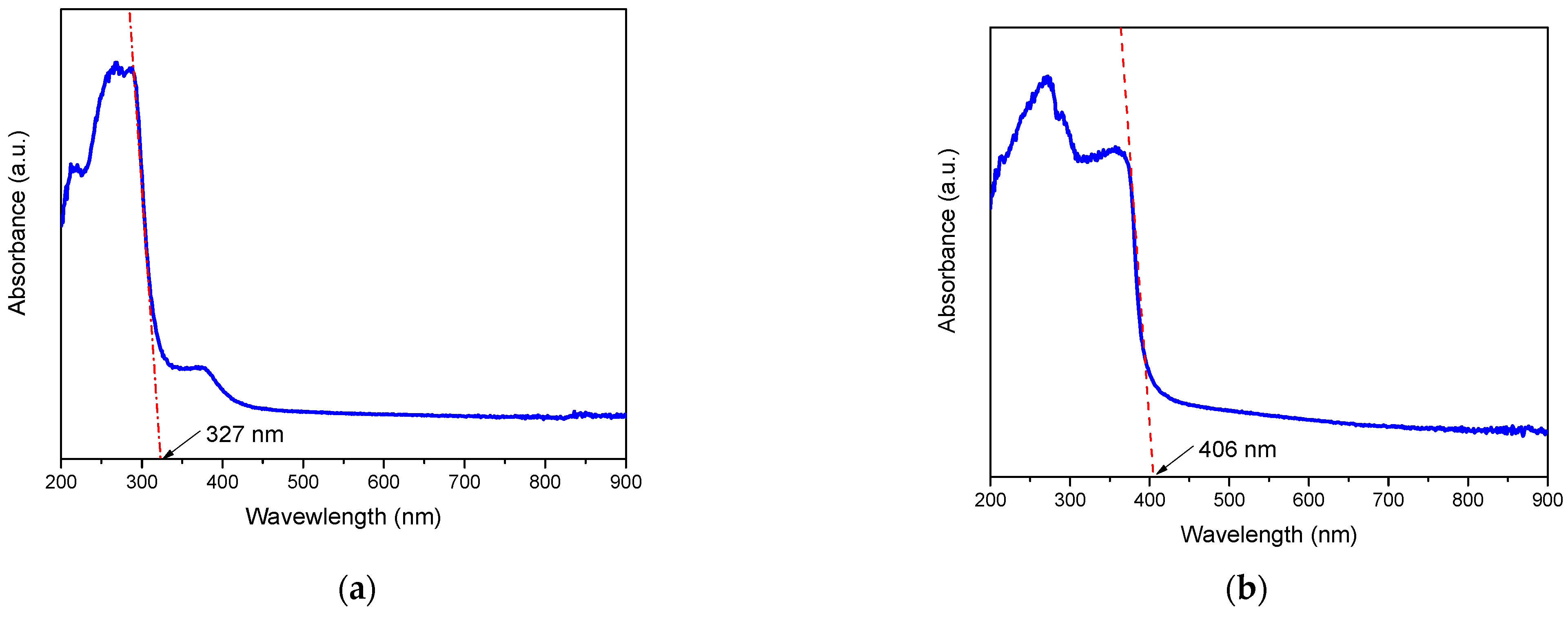
2.3. UV–VIS by Diffuse Reflectance Spectroscopy
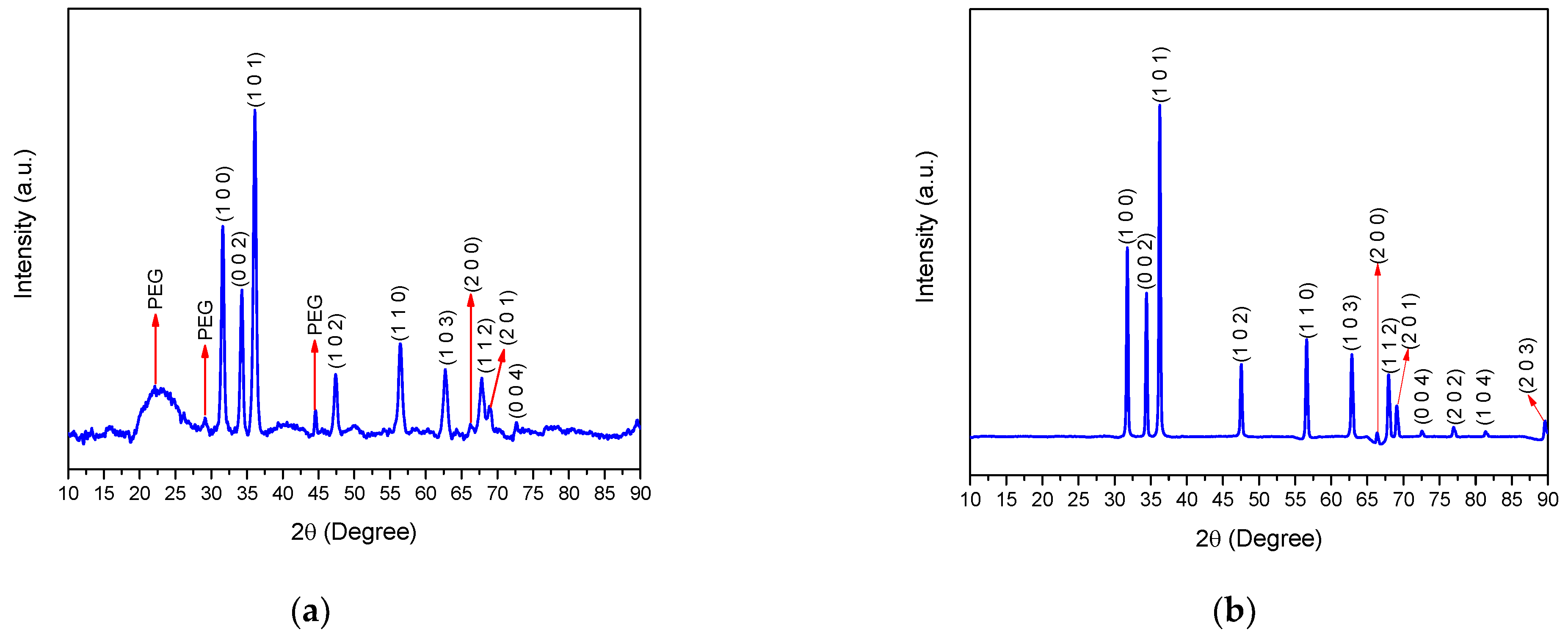
2.4. X-ray Diffraction
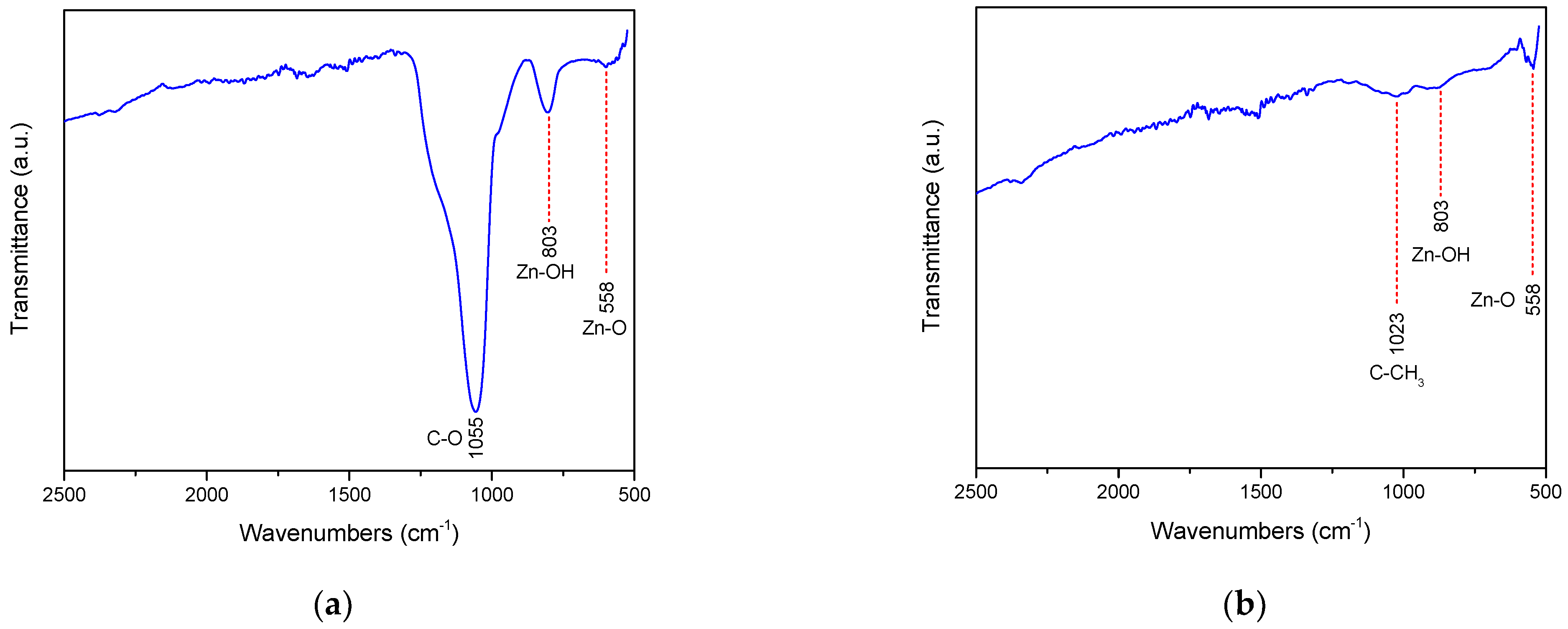
2.5. FT-IR Results
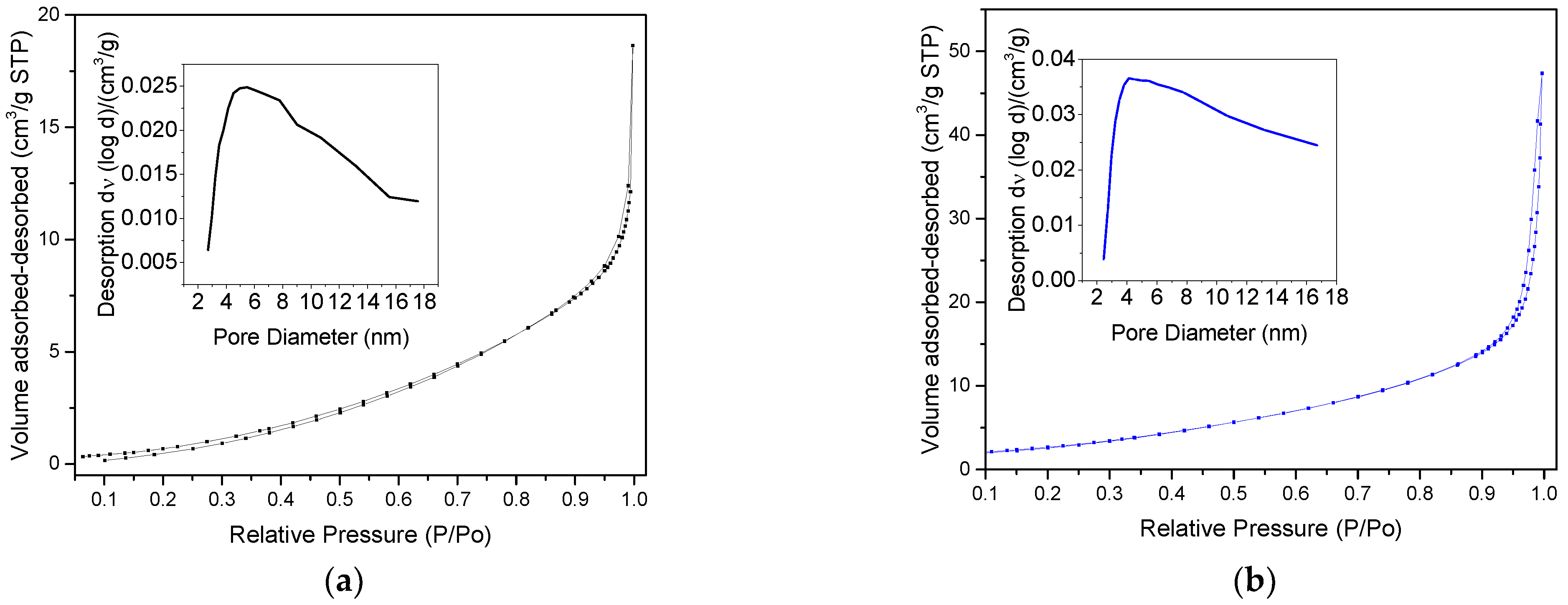
2.6. Nitrogen Physisorption Analysis
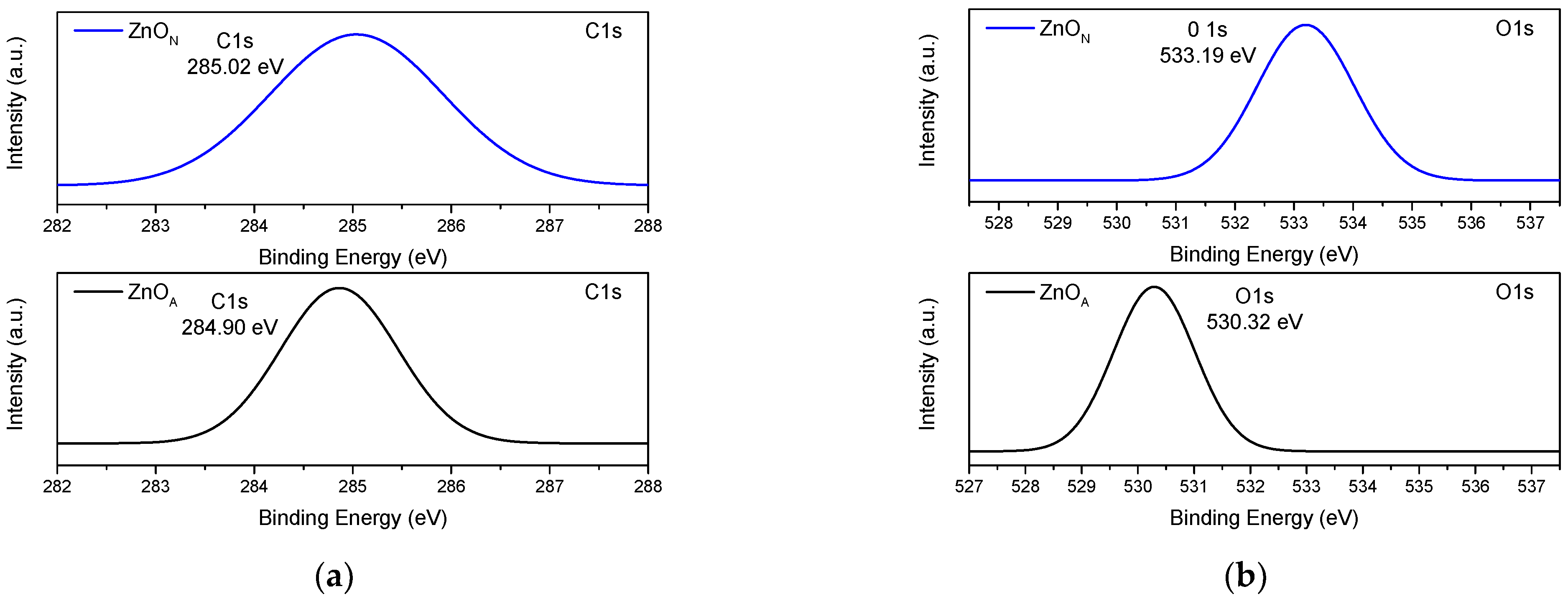
2.7. XPS Spectroscopy
2.8. Degradation of Organic Compounds
Mineralization of Organic Compounds
3. Materials and Methods
3.1. Chemical Reagents
3.2. ZnO Nanoparticles Synthesis
3.3. Sample Characterization
3.3.1. Scanning Electron Microscopy
3.3.2. Transmission Electron Microscopy
3.3.3. UV–Vis Diffuse Reflactance Spectroscopy (UV–Vis DRS)
3.3.4. X-ray Diffraction
3.3.5. FTIR Spectroscopy
3.3.6. Nitrogen Physisorption Analysis
3.3.7. XPS Spectroscopy
3.4. Photocatalytic Activity
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garba, Z.N.; Zhou, W.; Lawan, I.; Xiao, W.; Zhang, M.; Wang, L.; Chen, L.; Yuan, Z. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: A review. J. Environ. Manag. 2019, 241, 59–75. [Google Scholar] [CrossRef]
- Zada, A.; Khan, M.; Khan, M.A.; Khan, Q.; Habibi-Yangjeh, A.; Dang, A.; Maqbool, M. Review on the hazardous applications and photodegradation mechanisms of chlorophenols over different photocatalysts. Environ. Res. 2021, 195, 110742. [Google Scholar] [CrossRef]
- Cruz González, G.; Julcour, C.; Chaumat, H.; Jáuregui-Haza, U.; Delmas, H. Degradation of 2,4-dichlorophenoxyacetic acid by photolysis and photo-Fenton oxidation. J. Environ. Chem. Eng. 2018, 6, 874–882. [Google Scholar] [CrossRef]
- Bejarano González, F. Los Plagicidas Altamente Peligrosos en México, 1st ed.; RAPAM: Texcoco, Mexico, 2017; pp. 1–365. [Google Scholar]
- Centro de Estudios para el Desarrollo Rural Sustentable y la Soberanía Alimentaria. Uso y Regulación de Herbicidas en México, 1st ed.; CEDRSSA: Mexico City, Mexico, 2020; pp. 1–43. [Google Scholar]
- Ramos-Ramírez, E.; Gutiérrez-Ortega, N.L.; Tzompantzi-Morales, F.; Barrera-Rodríguez, A.; Castillo-Rodríguez, J.C.; Tzompantzi-Flores, C.; Santolalla-Vargas, C.E.; del Pilar Guevara-Hornedo, M. Photocatalytic degradation of 2,4-Dichlorophenol on NiAl-mixed oxides derivatives of activated layered double hydroxides. Top. Catal. 2020, 63, 546–563. [Google Scholar] [CrossRef]
- Diaz, E.; Mohedano, A.F.; Casas, J.A.; Calvo, L.; Gilarranz, M.A.; Rodriguez, J.J. Deactivation of a Pd/AC catalyst in the hydrodechlorination of chlorinated herbicides. Catal. Today 2015, 241, 86–91. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Samy, M.; Ookawara, S.; Ohno, T. Immobilization of S-TiO2 on reusable aluminum plates by polysiloxane for photocatalytic degradation of 2,4-dichlorophenol in water. J. Water Process Eng. 2018, 26, 329–335. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes. Chem. Eng. J. 2017, 320, 436–447. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Films 2016, 605, 2–19. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Investigation on visible-light photocatalytic degradation of 2,4-dichlorophenoxyacetic acid in the presence of MoO3/ZnO nanorod composites. J. Mol. Catal. A Chem. 2013, 370, 123–131. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Mun, L.K.; Zainal, Z.; Hussein, M.Z. Photodegradation of Chlorophenoxyacetic Acids by ZnO/r-Fe2O3 Nanocatalysts: A Comparative Study. Int. J. Chem. 2013, 5, 56–65. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Lyubchik, E.; Pai, S.; Pugazhendhi, A.; Vinayagam, R.; Selvaraj, R. Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J. Photochem. Photobiol. B Biol. 2019, 199, 111621. [Google Scholar] [CrossRef]
- Xu, J.; Cui, Z.; Liu, Z.; Xu, F.; Chen, Y. Organic-inorganic nanohybrid electrochemical sensors from multi-walled carbon nanotubes decorated with zinc oxide nanoparticles and insitu wrapped with poly(2-methacryloyloxyethyl-ferrocenecarboxylate) for detection of food additives. Nanomaterials 2019, 9, 1388. [Google Scholar] [CrossRef] [Green Version]
- Singh, O.; Kohli, N.; Singh, R.C. Precursor controlled morphology of zinc oxide and its sensing behaviour. Sens. Actuators B Chem. 2013, 178, 149–154. [Google Scholar] [CrossRef]
- Koutu, V.; Shastri, L.; Malik, M.M. Effect of NaOH concentration on optical properties of zinc oxide nanoparticles. Mater. Sci. Pol. 2016, 34, 819–827. [Google Scholar] [CrossRef] [Green Version]
- Changsong, L.; Zhiwen, L.; Yanfeng, G.; Qifeng, Z. Microstructral evolution of well-aligned ZnO nanorods array films in aqueous solution. J. Wuhan Univ. Technol. 2007, 22, 603–606. [Google Scholar] [CrossRef]
- Kenanakis, G.; Vernardou, D.; Koudoumas, E.; Katsarakis, N. Growth of c-axis oriented ZnO nanowires from aqueous solution: The decisive role of a seed layer for controlling the wires’ diameter. J. Cryst. Growth 2009, 311, 4799–4804. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, V.; Shrivastava, J.; Solanki, A. Preparation and characterization of nanostructured ZnO thin films for photoelectrochemical splitting of water. Bull. Mater. Sci. 2009, 32, 23–30. [Google Scholar] [CrossRef]
- Bazli, L.; Siavashi, M.; Shiravi, A. A review of Carbon nanotube/TiO2 composite prepared via Sol-Gel method. J. Compos. Compd. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Fardood, S.T.; Ramazani, A.; Joo, S.W. Synthesis and characterization of Zinc oxide nanoparticles using Black Tea extract. J. Appl. Chem. Res. 2017, 11, 8–17. [Google Scholar]
- Yan, X.B.; Tay, B.K.; Yang, Y. Dispersing and functionalizing multiwalled carbon nanotubes in TiO2 Sol. J. Phys. Chem. B 2006, 110, 25844–25849. [Google Scholar] [CrossRef]
- Siahpoosh, S.; Salahi, E.; Hessari, F.; Mobasherpour, I. Synthesis of γ-Alumina nanoparticles with high-surface-area via Sol-Gel method and their performance for the removal of Nickel from aqueous solution. Bull. Société R. Sci. Liège 2016, 85, 912–934. [Google Scholar] [CrossRef]
- Alwan, R.M.; Kadhim, Q.A.; Sahan, K.M.; Ali, R.A.; Roaa, J.; Mahdi, N.A.K.; Jassim, A.N. Synthesis of Zinc oxide nanoparticles via Sol—Gel route and their characterization. Nanosci. Nanotechnol. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Tseng, Y.K.; Chuang, M.H.; Chen, Y.C.; Wu, C.H. Synthesis of 1D, 2D, and 3D ZnO polycrystalline nanostructures using the sol-gel method. J. Nanotechnol. 2012, 2012, 712850. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures: Via a sol-gel synthesis of solvent driven shape-controlled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef] [Green Version]
- El Mragui, A.; Daou, I.; Zegaoui, O. Influence of the preparation method and ZnO(ZnO+TiO2) weight ratio on the physicochemical and photocatalytic properties of ZnO-TiO2 nanomaterials. Catal. Today 2018, 321–322, 41–51. [Google Scholar] [CrossRef]
- Ba-Abbad, M.; Kadhum, A.A.; Mohamad, A.B.; Takriff, M.; Sopian, K. Solar photocatalytic degradation of environmental pollutants using ZnO prepared by sol-gel: 2,4-Dichlorophenol as case study. Int. J. Therm. Environ. Eng. 2010, 1, 37–42. [Google Scholar] [CrossRef]
- Rodriguez-Mata, A.E.; Tzompantzi, F.J.; Amabilis-Sosa, L.E.; Diaz-Peña, I.; Bustos-Terrones, Y.; Rangel-Peraza, J.G. Characterization of SO42−/ZnO and photodegradation kinetics of 2,4-Dichlorophenoxyacetic acid (2,4-D). Kinet. Catal. 2018, 59, 720–726. [Google Scholar] [CrossRef]
- Sahu, K.; Kar, A.K. Counterion-induced tailoring of energy transfer in hydrothermally grown nanostructured ZnO for photocatalysis. Cryst. Growth Des. 2021, 21, 3656–3667. [Google Scholar] [CrossRef]
- Mohammed, R.; Ali, M.E.M.; Gomaa, E.; Mohsen, M. Highly stable, reusable, and MW-assisted prepared ZnO nanorods for wastewater decontamination: Precursors ratios effect and insights on matrix and pollutants mineralization. J. Environ. Chem. Eng. 2021, 9, 104630. [Google Scholar] [CrossRef]
- Lin, C.-C.; You, Y.-C. Mass-production of ZnO nanoparticles by precipitation in a rotating packed bed: Effect of zinc salt. J. Mater. Res. Technol. 2020, 9, 8451–8458. [Google Scholar] [CrossRef]
- Mohammed, R.; Ali, M.E.M.; Gomaa, E.; Mohsen, M. Green ZnO nanorod material for dye degradation and detoxification of pharmaceutical wastes in water. J. Environ. Chem. Eng. 2020, 8, 104295. [Google Scholar] [CrossRef]
- Abdulrahman, A.F. Study the optical properties of the various deposition solutions of ZnO nanorods grown on glass substrate using chemical bath deposition technique. J. Ovonic Res. 2020, 16, 181–188. [Google Scholar]
- Mayekar, J.; Radha, S.; Dhar, V. Role of salt precursor in the synthesis of zinc oxide role of salt precursor in the synthesis of zinc oxide. Int. J. Res. Eng. Technol. 2014, 3, 43–45. [Google Scholar] [CrossRef]
- Ramzuz, N.I.M.; Zakaria, N.; Zain, Z.M. Synthesis and characterization of zinc oxide nanostructures in biosensor application. Int. J. Biosens. Bioelectron. 2020, 6, 48–54. [Google Scholar] [CrossRef]
- Kaur, M.; Kalia, A. Role of salt precursors for the synthesis of zinc oxide nanoparticles and in imparting variable antimicrobial activity. J. Appl. Nat. Sci. 2016, 8, 1039–1048. [Google Scholar] [CrossRef]
- Dahiya, A.S.; Boubenia, S.; Franzo, G.; Mirabella, S.; Alquier, D. Photoluminescence study of the influence of additive Ammonium hydroxide in hydrothermally grown ZnO nanowires. Nanoscale Res. Lett. 2018, 13, 249. [Google Scholar] [CrossRef]
- Darroudi, M.; Sabouri, Z.; Kazemi Oskuee, R.; Khorsand Zak, A.; Kargar, H.; Hamid, M.H.N.A. Sol-gel synthesis, characterization, and neurotoxicity effect of zinc oxide nanoparticles using Gum Tragacanth. Ceram. Int. 2013, 39, 9195–9199. [Google Scholar] [CrossRef]
- Thorat, J.H.; Kanade, K.G.; Nikam, L.K.; Chaudhari, P.D.; Kale, B.B. Nanostructured ZnO hexagons and optical properties. J. Mater. Sci. Mater. Electron. 2011, 22, 394–399. [Google Scholar] [CrossRef]
- Wu, G.S.; Xie, T.; Yuan, X.Y.; Li, Y.; Yang, L.; Xiao, Y.H.; Zhang, L.D. Controlled synthesis of ZnO nanowires or nanotubes via sol-gel template process. Solid State Commun. 2005, 134, 485–489. [Google Scholar] [CrossRef]
- Veriansyah, B.; Kim, J.D.; Min, B.K.; Shin, Y.H.; Lee, Y.W.; Kim, J. Continuous synthesis of surface-modified zinc oxide nanoparticles in supercritical methanol. J. Supercrit. Fluids 2010, 52, 76–83. [Google Scholar] [CrossRef]
- Bhatia, S.; Verma, N. Photocatalytic Activity of ZnO Nanoparticles with Optimization of Defects. Mater. Res. Bull. 2017, 95, 468–476. [Google Scholar] [CrossRef]
- Benhebal, H.; Chaib, M.; Salmon, T.; Geens, J.; Leonard, A.; Lambert, S.D.; Crine, M.; Heinrichs, B. Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol-gel process. Alex. Eng. J. 2013, 52, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Meenakshi, G.; Sivasamy, A. Synthesis and characterization of zinc oxide nanorods and its photocatalytic activities towards degradation of 2,4-D. Ecotoxicol. Environ. Saf. 2017, 135, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.H.; Duan, G.; Li, Y.; Yang, S.; Xu, X. Blue Luminescence of ZnO nanoparticles based on non-equilibrium processes: Defect origins and emission controls. J. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, L.; Alkan, G.; Milošević, O.; Rabanal, M.E.; Friedrich, B. Synthesis and characterisation of spherical core-shell Ag/ZnO nanocomposites using single and two-steps ultrasonic spray pyrolysis (USP). Catal. Today 2019, 321–322, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Sun, F.; Wang, X.; Huang, Z.; Keshvardoostchokami, M.; Kumar, P.; Liu, B. Synthesis of ZnO hierarchical structures and their gas sensing properties. Nanomaterials 2019, 9, 1277. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, J.; Larbot, A.; Persin, M. Preparation and characterization of nano-zinc oxide. J. Mater. Process. Technol. 2007, 189, 379–383. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.S.; Hadavifar, M.; Ghasemi, S.S.; Chamjangali, M.A. Synthesis of ZnO nanostructure using activated carbon for photocatalytic degradation of methyl orange from aqueous solutions. Appl. Water Sci. 2018, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Ghica, D.; Vlaicu, I.D.; Stefan, M.; Nistor, L.C.; Nistor, S.V. On the agent role of Mn2+ in redirecting the synthesis of Zn(OH)2 towards nano-ZnO with variable morphology. RSC Adv. 2016, 6, 106732–106741. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, R.; Ray, S.K. Removal of congo red and methyl violet from water using nano clay filled composite hydrogels of poly acrylic acid and polyethylene glycol. Chem. Eng. J. 2015, 260, 269–283. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Reddy, G.V.S.; Reddy, A.B.; Sudhakar, K.; Sadiku, E.R. Preparation and characterization of poly(ethylene glycol) stabilized nano silver particles by a mechanochemical assisted ball mill process. J. Appl. Polym. Sci. 2016, 133, 43027. [Google Scholar] [CrossRef]
- Pholnak, C.; Sirisathitkul, C.; Suwanboon, S.; Harding, D.J. Effects of precursor concentration and reaction time on sonochemically synthesized ZnO nanoparticles. Mater. Res. 2014, 17, 405–411. [Google Scholar] [CrossRef]
- Osman, D.A.M.; Mustafa, M.A. Synthesis and characterization of Zinc oxide nanoparticles using zinc acetate dihydrate and sodium hydroxide. J. Nanosci. Nanoeng. 2015, 1, 248–251. Available online: https://www.aiscience.org/diario/jnn (accessed on 14 October 2021).
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [PubMed]
- Al-dhahir, T.A.; Al-obodi, E.E.; Al-saadi, T.M.; Issam, L. ZnO nanoparticles: Synthesis and crystal structure study. Wasit J. Sci. Med. 2014, 7, 87–95. [Google Scholar]
- Mesaros, A.; Vasile, B.S.; Toloman, D.; Lelia, P.O.; Marinca, T.; Unguresan, M.; Perhaita, I.; Filip, M.; Iordache, F.; Ciontea, L. Towards understanding the enhancement of antibacterial activity in manganese doped ZnO nanoparticles. Appl. Surf. Sci. 2018, 471, 960–972. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.; Abdulkareem, A.S.; Oladejo, J.T.; Shuaib, D.T.; Mohammed, A.K.; Sumaila, A. Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045013. [Google Scholar] [CrossRef]
- Hakim, A.A.N.; Rashid, A.R.A.; Arsad, N.; Surani, A.H. Zinc oxide thin film synthesized by Sol-Gel method. Solid State Phenom. 2020, 307, 51–57. [Google Scholar] [CrossRef]
- Gayathri, S.; Ghosh, O.; Sathishkumar, S.; Sudhakara, P.; Jayaramudu, J.; Ray, S.; Viswanath, A. Investigation of physicochemical properties of Ag doped ZnO nanoparticles prepared by chemical route. Appl. Sci. Lett. 2015, 1, 8–13. [Google Scholar]
- Balogun, S.W.; James, O.O.; Sanusi, Y.K.; Olayinka, O.H. Green synthesis and characterization of zinc oxide nanoparticles using bashful (Mimosa pudica), leaf extract: A precursor for organic electronics applications. SN Appl. Sci. 2020, 2, 504. [Google Scholar] [CrossRef] [Green Version]
- Koodziejczak-Radzimska, A.; Markiewicz, E.; Jesionowski, T. Structural characterisation of ZnO particles obtained by the emulsion precipitation method. J. Nanomater. 2012, 2012, 656353. [Google Scholar] [CrossRef]
- Da Silva-Neto, M.L.; de Oliveira, M.C.A.; Dominguez, C.T.; Lins, R.E.M.; Rakov, N.; de Araújo, C.B.; de Souza Menezes, L.; de Oliveira, H.P.; Gomes, A.S.L. UV random laser emission from flexible ZnO-Ag-enriched electrospun cellulose acetate fiber matrix. Sci. Rep. 2019, 9, 11765. [Google Scholar] [CrossRef]
- Rodriguez Martinez, C.; Joshi, P.; Vera, J.L.; Ramirez-Vick, J.E.; Perales, O.; Singh, S.P. Cytotoxic Studies of PEG Functionalized ZnO Nanoparticles on MCF-7 Cancer Cells. In Proceedings of the NSTI Nanotechnology Conference and Expo, NSTI-Nanotech 2011, Boston, MA, USA, 13–16 June 2011; Volume 3, pp. 420–423. [Google Scholar]
- Chithra, M.J.; Sathya, M.; Pushpanathan, K. Effect of pH on crystal size and photoluminescence property of zno nanoparticles prepared by chemical precipitation method. Acta Metall. Sin. 2015, 28, 394–404. [Google Scholar] [CrossRef]
- Phoohinkong, W.; Foophow, T.; Pecharapa, W. Synthesis and characterization of copper zinc oxide nanoparticles obtained via metathesis process. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 035003. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for Gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Q.; Lian, J.; Zhong, J.; Wang, F.; Li, J.; He, Y.; Wang, R. Bovine serum albumin modified ZnO to degrade organic dyes under ultraviolet. New J. Chem. 2016, 6, 5604–5610. [Google Scholar] [CrossRef]
- Hamrouni, A.; Moussa, N.; Parrino, F.; Di Paola, A.; Houas, A.; Palmisano, L. Sol–gel synthesis and photocatalytic activity of ZnO–SnO2 nanocomposites. J. Mol. Catal. A Chem. 2014, 390, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Kuang, P.; Cheng, B.; Yu, J.; Jiang, C. ZnO Hierarchical Microsphere for Enhanced Photocatalytic Activity; Elsevier B.V.: Amsterdam, The Netherlands, 2018; ISBN 0086278787102. [Google Scholar]
- Jiang, J.; Zhang, K.; Chen, X.; Zhao, F.; Xie, T.; Wang, D.; Lin, Y. Porous Ce-doped ZnO hollow sphere with enhanced photodegradation activity for artificial waste water. J. Alloys Compd. 2017, 699, 907–913. [Google Scholar] [CrossRef]
- Podyacheva, O.Y.; Lisitsyn, A.S.; Kibis, L.S.; Stadnichenko, A.I.; Boronin, A.I.; Slavinskaya, E.M.; Stonkus, O.A.; Yashnik, S.A.; Ismagilov, Z.R. Influence of the nitrogen-doped carbon nanofibers on the catalytic properties of supported metal and oxide nanoparticles. Catal. Today 2018, 301, 125–133. [Google Scholar] [CrossRef]
- Barrera, A.; Tzompantzi, F.; Campa-Molina, J.; Casillas, J.E.; Pérez-Hernández, R.; Ulloa-Godinez, S.; Velásquez, C.; Arenas-Alatorre, J. Photocatalytic activity of Ag/Al2O3-Gd2O3 photocatalysts prepared by the sol-gel method in the degradation of 4-chlorophenol. RSV Adv. 2018, 8, 3108–3119. [Google Scholar] [CrossRef] [Green Version]
- Lavand, A.B.; Malghe, Y.S. Synthesis, characterization and visible light photocatalytic activity of Carbon and Iron modified ZnO. J. King Saud Univ. Sci. 2018, 30, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.-D.; Dastan, D.; Li, J.; Yin, X.-T.; Wang, Q. Discriminable sensing response behavior to homogeneous gases based on n-ZnO/p-NiO Composites. Nanomaterials 2020, 10, 785. [Google Scholar] [CrossRef] [Green Version]
- Yousef, A.; Barakat, N.A.M.; Amna, T.; Unnithan, A.R.; Al-Deyab, S.S.; Yong Kim, H. Influence of CdO-doping on the photoluminescence properties of ZnO nanofibers: Effective visible light photocatalyst for waste water treatment. J. Lumin. 2012, 132, 1668–1677. [Google Scholar] [CrossRef]
- Rambu, A.P.; Nica, V.; Dobromir, M. Influence of Fe-doping on the optical and electrical properties of ZnO films. Superlattices Microstruct. 2013, 59, 87–96. [Google Scholar] [CrossRef]
- Shimizu, K.; Shchukarev, A.; Boily, J.F. X-ray photoelectron spectroscopy of fast-frozen hematite colloids in aqueous solutions. 3. Stabilization of ammonium species by surface (Hydr)oxo groups. J. Phys. Chem. C 2011, 115, 6796–6801. [Google Scholar] [CrossRef]
- Nawawi, W.I.; Zaharudin, R.; Azlan, M.; Ishak, M.; Ismail, K. The preparation and characterization of immobilized TiO2/PEG by using DSAT as a support binder. Appl. Sci. 2017, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Ba-abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Photocatalytic degradation of chlorophenols under direct solar radiation in the presence of ZnO catalyst. Res. Chem. Intermed. 2013, 39, 1981–1996. [Google Scholar] [CrossRef]
- Burbano, J.; Cruz, I.; Colina-Márquez, J.; López-vásquez, A.; Machuca, F. Evaluation of Zinc oxide-based photocatalytic degradation of a commercial pesticide. J. Adv. Oxid. Technol. 2008, 11, 49–55. [Google Scholar] [CrossRef]
- Kundu, S.; Pal, A.; Dikshit, A.K. UV induced degradation of herbicide 2,4-D: Kinetics, mechanism and effect of various conditions on the degradation. Sep. Purif. Technol. 2005, 44, 121–129. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Mantilla, A.; Bañuelos, F.; Fernández, J.L.; Gómez, R. Improved photocatalytic degradation of phenolic compounds with ZnAl mixed oxides obtained from LDH materials. Top. Catal. 2011, 54, 257–263. [Google Scholar] [CrossRef]
- Melián, E.P.; Díaz, O.G.; Rodríguez, J.M.D.; Araña, J.; Peña, J.P. Adsorption and photocatalytic degradation of 2,4-dichlorophenol in TiO2 suspensions. Effect of hydrogen peroxide, sodium peroxodisulphate and ozone. Appl. Catal. A Gen. 2013, 455, 227–233. [Google Scholar] [CrossRef]
- Macías-Sánchez, J.J.; Hinojosa-Reyes, L.; Caballero-Quintero, A.; De La Cruz, W.; Ruiz-Ruiz, E.; Hernández-Ramírez, A.; Guzmán-Mar, J.L. Synthesis of nitrogen-doped ZnO by sol-gel method: Characterization and its application on visible photocatalytic degradation of 2,4-D and picloram herbicides. Photochem. Photobiol. Sci. 2015, 14, 536–542. [Google Scholar] [CrossRef]
- Barik, A.J.; Gogate, P.R. Degradation of 2,4-dichlorophenol using combined approach based on ultrasound, ozone and catalyst. Ultrason. Sonochem. 2017, 36, 517–526. [Google Scholar] [CrossRef]
- Sánchez-Cantú, M.; Barcelos-Santiago, C.; Gomez, C.M.; Ramos-Ramírez, E.; Ruiz Peralta, M.D.L.; Tepale, N.; González-Coronel, V.J.; Mantilla, A.; Tzompantzi, F. Evaluation of Hydrocalumite-like compounds as catalyst precursors in the photodegradation of 2,4-Dichlorophenoxyacetic acid. Int. J. Photoenergy 2016, 5256941. [Google Scholar] [CrossRef] [Green Version]
- Djebbar, K.; Sehili, T. Kinetics of heterogeneous photocatalytic decomposition of 2,4-Dichlorophenoxyacetic acid over titanium dioxide and zinc oxide in aqueous solution. Pestic. Sci. 1998, 54, 269–276. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Montalvo-González, E.; González-Silva, N.; Méndez-Robles, M.D.; Romero-Toledo, R.; Yahia, E.M.; Pérez-Larios, A. Synthesis and characterization of TiO2-ZnO-MgO mixed oxide and their antibacterial activity. Materials 2019, 12, 698. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jin, Z.; Li, W.; Qiu, J. Preparation of ZnO porous thin films by sol–gel method using PEG template. Mater. Lett. 2005, 59, 3620–3625. [Google Scholar] [CrossRef]
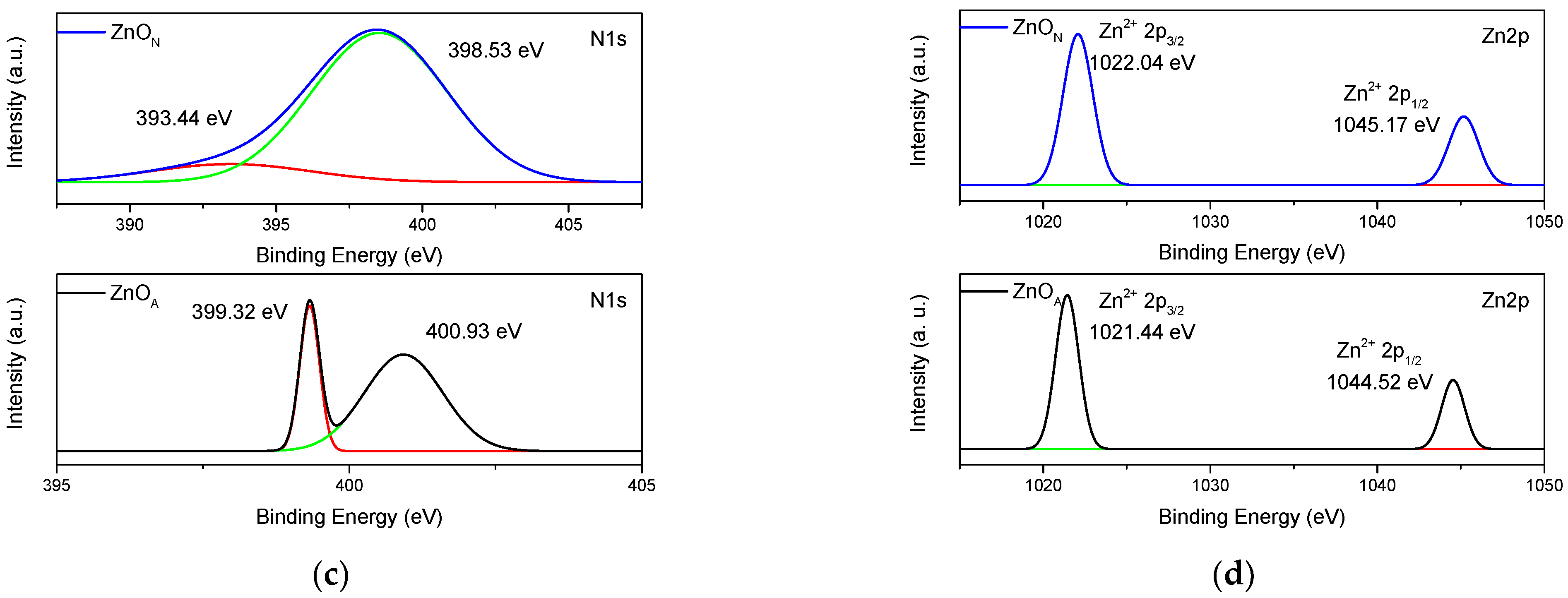
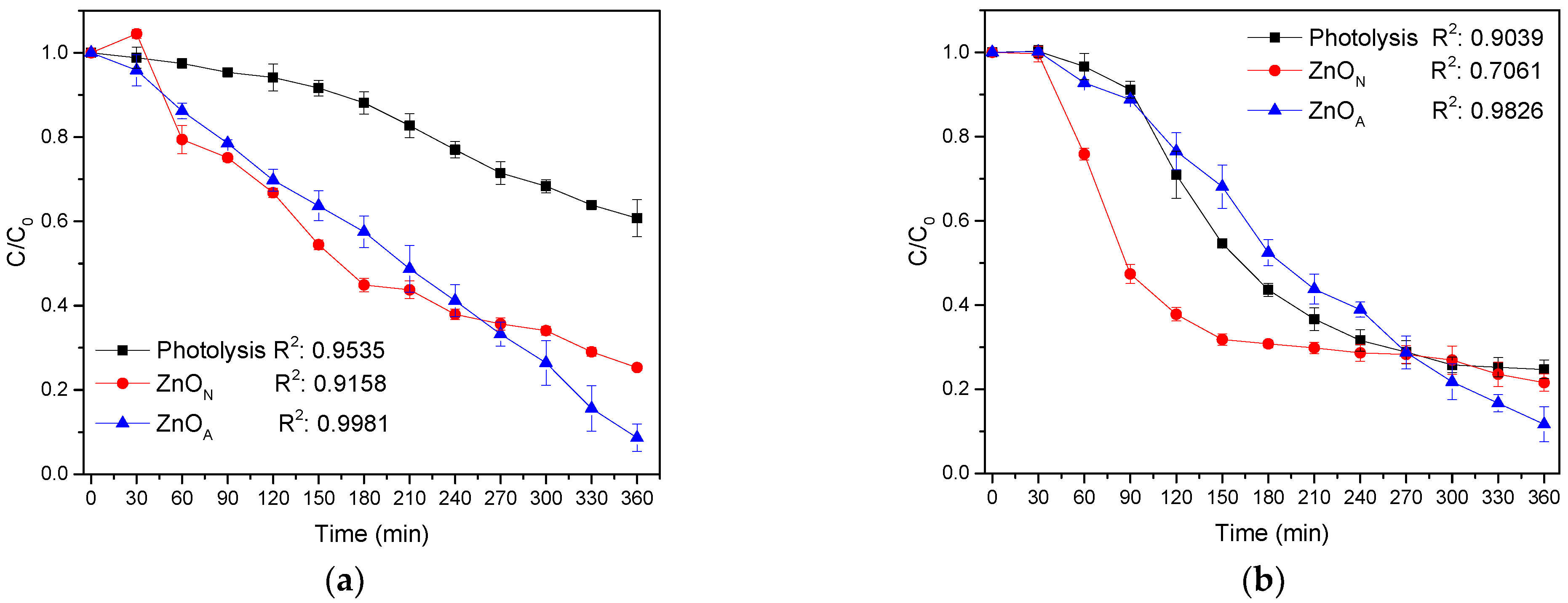
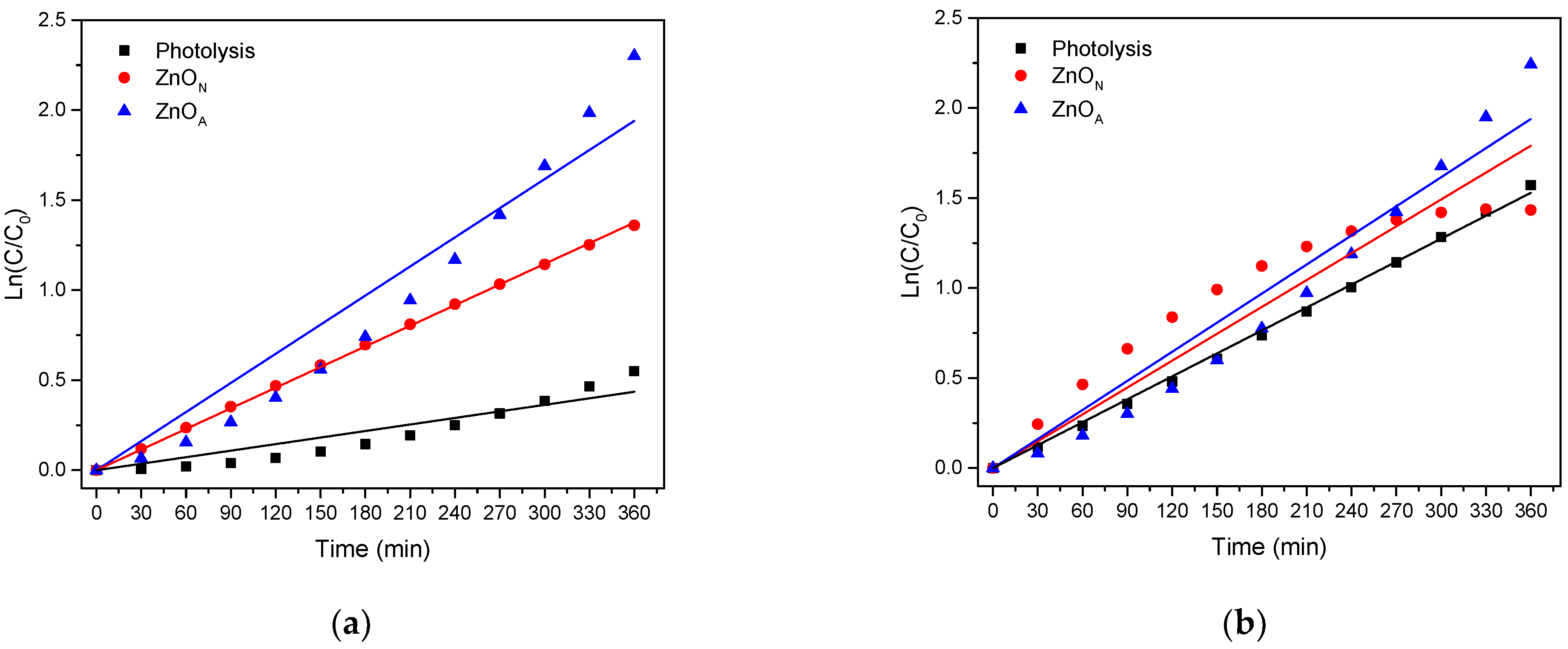
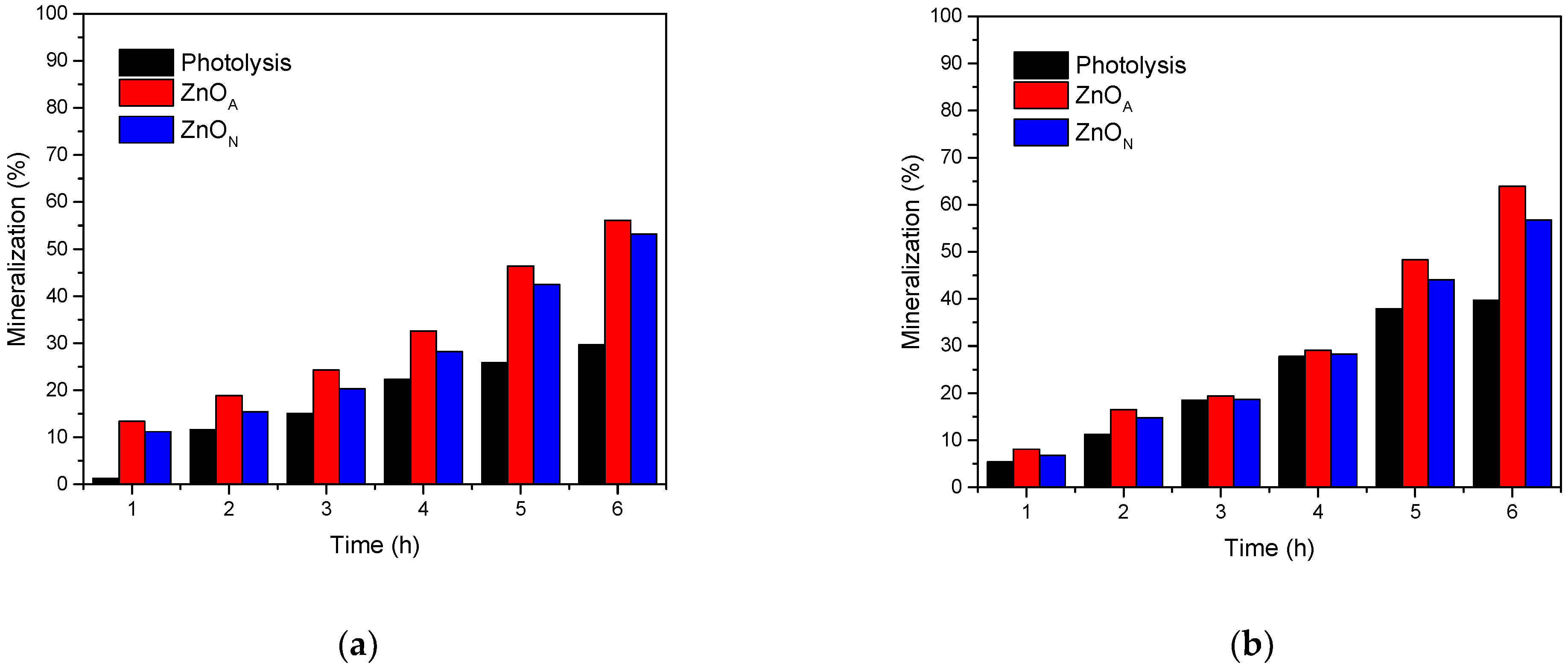
| Catalyst | Eg (eV) | dc (nm) | d-Spacing (nm) | SBET (m2g−1) | VP (cm3g−1) | dP (nm) |
|---|---|---|---|---|---|---|
| ZnON | 3.79 | 17.2 | 0.2480 | 0.85 | 0.01 | 6.02 |
| ZnOA | 3.05 | 31.3 | 0.2472 | 2.24 | 0.02 | 5.78 |
| Name | ZnON | ZnOA | ||
|---|---|---|---|---|
| Atomic % | Weight % | Atomic % | Weight % | |
| Zn2p | 28.16 | 45.09 | 31.58 | 67.42 |
| C1s | 13.85 | 8.35 | 27.39 | 10.74 |
| O1s | 57.56 | 46.09 | 40.45 | 21.25 |
| N1s | 0.43 | 0.47 | 0.58 | 0.59 |
| Compound | 2,4-D | 2,4-DCP |
|---|---|---|
| Photolysis | 40.1% c | 73.6% b |
| ZnON | 74.7% b | 78.4% b |
| ZnOA | 90.9% a | 86.7% a |
| Compound | k (min−1) (10−3) | t½ (min) | ||||
|---|---|---|---|---|---|---|
| Photolysis | ZnON | ZnOA | Photolysis | ZnON | ZnOA | |
| 2,4-D | 0.3 | 0.4 | 2 | 394.5 | 181.6 | 18.5 |
| 2,4-DCP | 0.3 | 0.4 | 0.5 | 241.7 | 162.7 | 113.8 |
| 2,4-D | 2,4-DCP | |||||
|---|---|---|---|---|---|---|
| Photolysis | ZnON | ZnOA | Photolysis | ZnON | ZnOA | |
| Kinetic zero order | 0.8678 | 0.9846 | 0.9200 | 0.9379 | 0.8040 | 0.9446 |
| Kinetic first order | 0.8674 | 0.9847 | 0.9184 | 0.9377 | 0.8066 | 0.9432 |
| Kinetic L-M Model | 0.9177 | 0.9794 | 0.9477 | 0.9499 | 0.8329 | 0.9715 |
| Compound | 2,4-D | 2,4-DCP |
|---|---|---|
| Photolysis | 30% b | 40% c |
| ZnON | 53% a | 57% b |
| ZnOA | 56% a | 64% a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limón-Rocha, I.; Guzmán-González, C.A.; Anaya-Esparza, L.M.; Romero-Toledo, R.; Rico, J.L.; González-Vargas, O.A.; Pérez-Larios, A. Effect of the Precursor on the Synthesis of ZnO and Its Photocatalytic Activity. Inorganics 2022, 10, 16. https://doi.org/10.3390/inorganics10020016
Limón-Rocha I, Guzmán-González CA, Anaya-Esparza LM, Romero-Toledo R, Rico JL, González-Vargas OA, Pérez-Larios A. Effect of the Precursor on the Synthesis of ZnO and Its Photocatalytic Activity. Inorganics. 2022; 10(2):16. https://doi.org/10.3390/inorganics10020016
Chicago/Turabian StyleLimón-Rocha, Isaias, C. A. Guzmán-González, Luis M. Anaya-Esparza, R. Romero-Toledo, J. L. Rico, O. A. González-Vargas, and A. Pérez-Larios. 2022. "Effect of the Precursor on the Synthesis of ZnO and Its Photocatalytic Activity" Inorganics 10, no. 2: 16. https://doi.org/10.3390/inorganics10020016
APA StyleLimón-Rocha, I., Guzmán-González, C. A., Anaya-Esparza, L. M., Romero-Toledo, R., Rico, J. L., González-Vargas, O. A., & Pérez-Larios, A. (2022). Effect of the Precursor on the Synthesis of ZnO and Its Photocatalytic Activity. Inorganics, 10(2), 16. https://doi.org/10.3390/inorganics10020016







