Green Synthesis of Fe3O4 Nanoparticles and Its Applications in Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Preparation of Citrus aurantium (CA) Juice
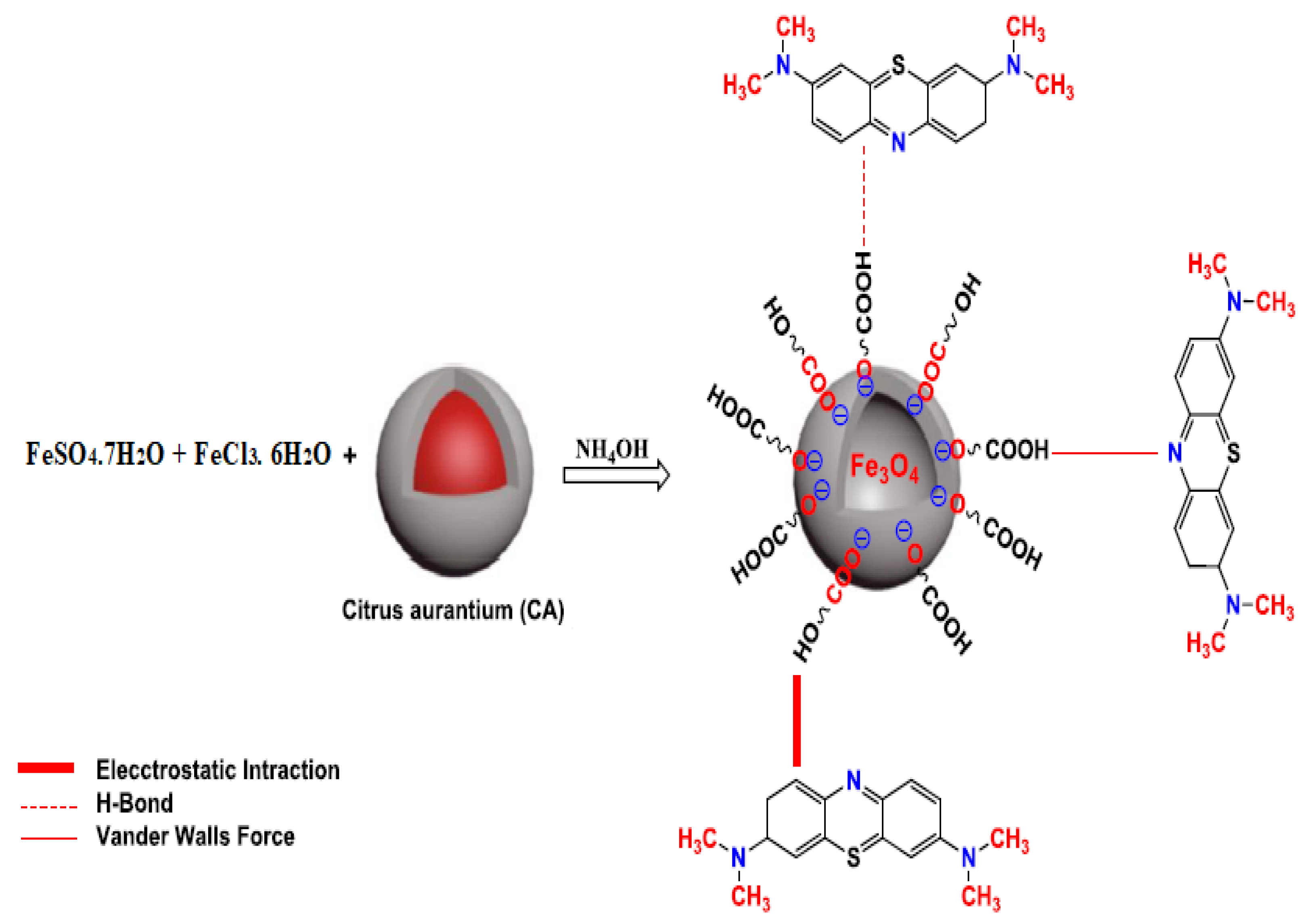
2.1.3. Preparation of Fe3O4
2.1.4. Characterization
2.2. Experimental Design
2.3. Batch Adsorption Studies
3. Results and Discussion
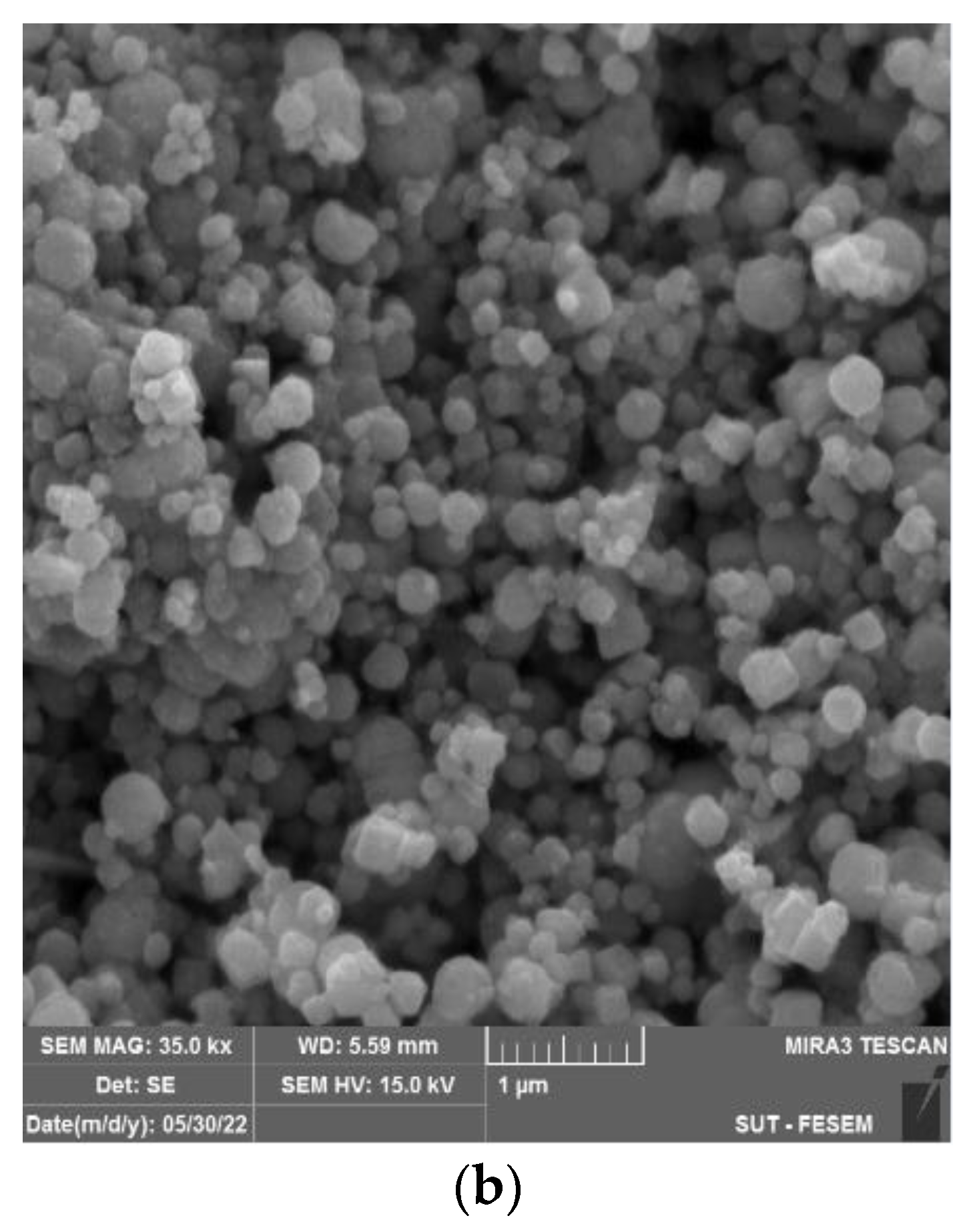
3.1. Characterization of Fe3O4 NPs/CA
3.2. Regression Model Equation
3.3. One Factor Plot
3.4. Variables Combined Influence on the MB Dye Removal
3.5. Optimization and Model Validation
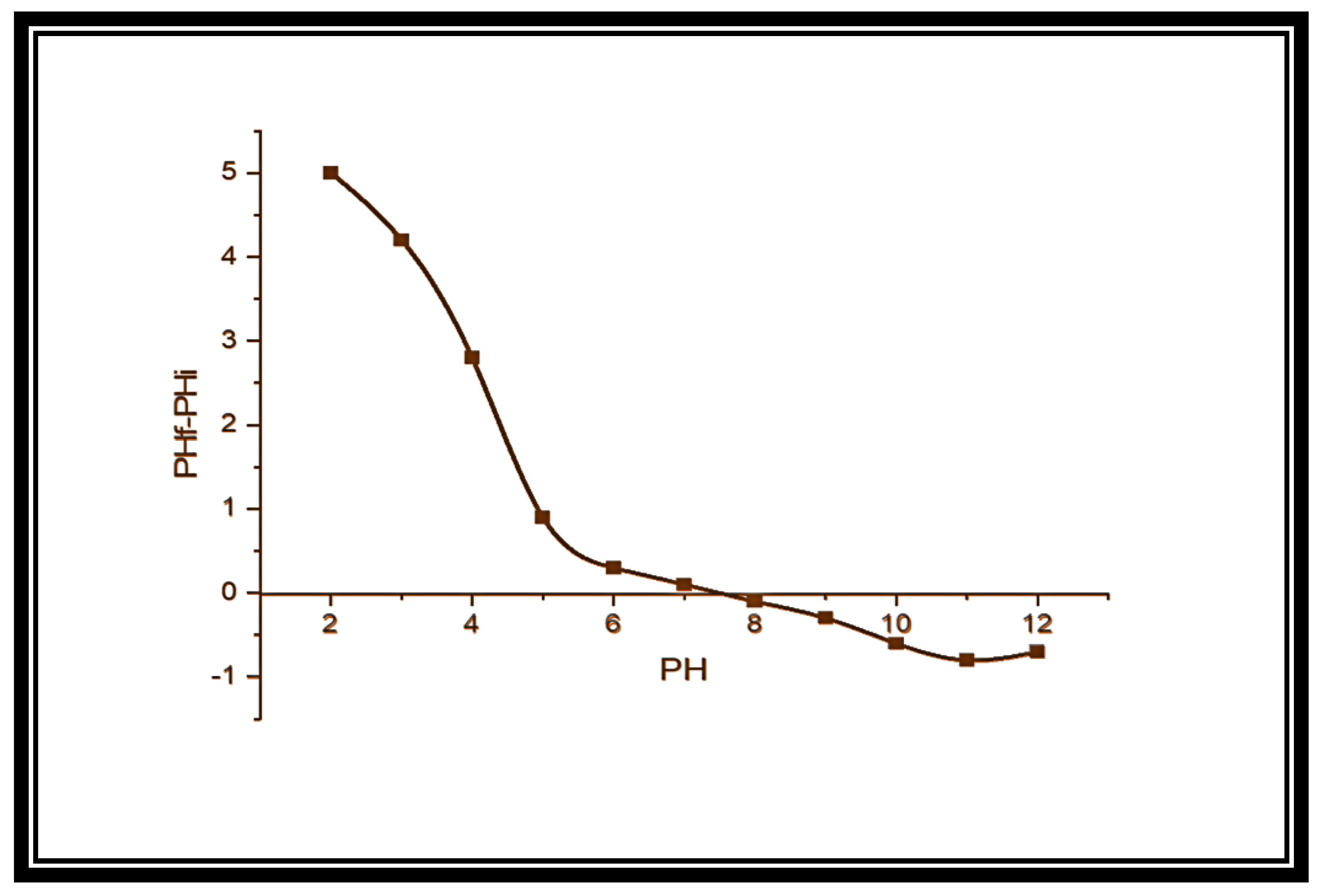
3.6. Mechanism of Adsorption
3.7. Influence of Temperature
3.8. Recycled Adsorption
3.9. Comparative Study of MB Dye Removal with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalaf, I.H.; Al-Sudani, F.T.; AbdulRazak, A.A.; Aldahri, T.; Rohani, S. Optimization of Congo red dye adsorption from wastewater by a modified commercial zeolite catalyst using response surface modeling approach. Water Sci. Technol. 2021, 83, 369–1383. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Abdul Razak, A.A.; Hussein Al-Timimi, D.A. Modified multiwalled carbon nanotubes for treatment of some organic dyes in wastewater. Adv. Mater. Sci. Eng. 2014, 2014, 201052. [Google Scholar] [CrossRef]
- Qasem NA, A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Haounati, R.; Alakhras, F.; Ouachtak, H.; Saleh, T.A.; Al-Mazaideh, G.; Alhajri, E.; Jada, A.; Hafid, N.; Addi, A.A. Synthesized of Zeolite@Ag2O Nanocomposite as Superb Stability Photocatalysis Toward Hazardous Rhodamine B Dye from Water. Arab J. Sci. Eng. 2022. [Google Scholar] [CrossRef]
- Ouachtak, H.; El Guerdaoui, A.; Haounati, R.; Akhouairi, S.; El Haouti, R.; Hafid, N.; Addi, A.A.; Šljukić, B.; Santos DM, F.; Taha, M.L. Highly efficient and fast batch adsorption of orange G dye from polluted water using superb organo-montmorillonite: Experimental study and molecular dynamics investigation. J. Mol. Liq. 2021, 335, 116560. [Google Scholar] [CrossRef]
- Ahmed, F.S.; Alsaffar, M.A.; AbdulRazak, A.A. One-step synthesis of magnetic fly ash composites for methylene blue removal: Batch and column study. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Garg, V.K. Green chemistry for dyes removal from waste water. Green Process. Synth. 2015, 4, 507–508. [Google Scholar] [CrossRef]
- Abdulrazak, A.A.; Shakor, Z.M.; Rohani, S. Optimizing Biebrich Scarlet removal from water by magnetic zeolite 13X utilizing response surface method. J. Environ. Chem. Eng. 2018, 6, 6175–6183. [Google Scholar] [CrossRef]
- Al-Dahri, T.; Abdulrazak, A.A.; Khalaf, I.H.; Rohani, S. Response surface modeling of the removal of methyl orange dye from its aqueous solution using two types of zeolite synthesized from coal fly ash. Mater. Express 2018, 8, 234–244. [Google Scholar] [CrossRef]
- Al-Dahri, T.; Abdulrazak, A.A.; Rohani, S. Preparation and characterization of Linde-type A zeolite (LTA) from coal fly ash by microwave assisted synthesis method: Its application as adsorbent for removal of anionic dyes. Int. J. Coal Prep. Util. 2022, 42, 2064–2077. [Google Scholar] [CrossRef]
- Abdulrazak, A.A.; Rohani, S. Sodium dodecyl sulfate modified Fe2O3/molecular sieves for removal of rhodamine B dyes. Adv. Mater. Sci. Eng. 2018, 2018, 3849867. [Google Scholar] [CrossRef]
- Majid, Z.; Abdulrazak, A.A.; Noori, W.A.H. Modification of zeolite by magnetic nanoparticles for organic dye removal. Arab J. Sci. Eng. 2019, 44, 5457–5474. [Google Scholar] [CrossRef]
- Ahmed, F.S.; Abdulrazak, A.A.; Alsaffar, M.A. Modelling and optimization of methylene blue adsorption from wastewater utilizing magnetic marble dust adsorbent: A response surface methodology approach. Mater. Today Proc. 2022, 60, 1676–1688. [Google Scholar] [CrossRef]
- Babay, S.; Mhiri, T.; Toumi, M. Synthesis, structural and spectroscopic characterizations of maghemite y—Fe203 pre pared by one—Step coprecipitation route. J. Mol. Struct. 2015, 1085, 286–293. [Google Scholar] [CrossRef]
- Gubin, S.P.; Koksharov, Y.A.; Khomutov, G.B.; Yurkov, G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev. 2005, 74, 489–520. [Google Scholar] [CrossRef]
- Belachew, N.; Rama Devi, D.; Basavaiah, K. Facile green synthesis of L-Methionine capped magnetite nanoparticles for adsorption of pollutant Rhodamine B. J. Mol. Liq. 2016, 224, 713–720. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Dias HV, R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Shejawal, K.P.; Randive, D.S.; Bhinge, S.D.; Bhutkar, M.A.; Wadkar, G.H.; Jadhav, N.R. Green synthesis of silver and iron nanoparticles of isolated proanthocyanidin: Its characterization, antioxidant, antimicrobial, and cytotoxic activities against COLO320DM and HT29. J. Genet. Eng. Biotechnol. 2020, 18, 43. [Google Scholar] [CrossRef]
- Horst, M.F.; Coral, D.F.; van Raap, M.B.F.; Alvarez, M.; Lassalle, V. Hybrid nanomaterials based on gum Arabic and magnetite for hyperthermia treatments. Mater. Sci. Eng. C 2007, 74, 443. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Alagar, S.; Mariappan, R.; Piraman, S. Green one-pot synthesis of flowers-like Fe3O4/rGO hybrid nanocomposites for effective electrochemical detection of riboflavin and low-cost supercapacitor applications. Sens. Actuat. B Chem. 2017, 253, 879–892. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green synthesis and characterization of Fe3O4 nanoparticles using Chlorella-K01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci. Rep. 2021, 11, 21996. [Google Scholar] [CrossRef]
- Ramesh, A.V.; Devi, D.R.; Botsa, S.M.; Basavaiah, K. Facile green synthesis of Fe3O4 nanoparticles using aqueous leaf extract of Zanthoxylum armatum DC. for efficient adsorption of methylene blue. J. Asian Ceram. 2018, 6, 145–155. [Google Scholar] [CrossRef]
- Pepe, G.; Pagano, F.; Adesso, S.; Sommella, E.; Ostacolo, C.; Manfra, M.; Chieppa, M.; Sala, M.; Russo, M.; Marzocco, S.; et al. Bioavailable Citrus sinensis Extract: Polyphenolic Composition and Biological Activity. Molecules 2017, 22, 623. [Google Scholar] [CrossRef]
- Khan, U.M.; Sameen, A.; Aadil, R.M.; Shahid, M.; Sezen, S.; Zarrabi, A.; Ozdemir, B.; Sevindik, M.; Kaplan, D.N.; Selamoglu, Z.; et al. Citrus Genus and Its Waste Utilization: A Review on Health-Promoting Activities and Industrial Application. Evid. Based Complement. Alternat. Med. 2021, 2021, 2488804. [Google Scholar] [CrossRef]
- Karadeniz, F. Main organic acid distribution of authentic citrus juices in Turkey. Turk. J. Agric. For. 2004, 28, 267–271. [Google Scholar]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Waldron, R.D. Infrared Spectra of Ferrites. Phys. Rev. 1955, 99, 1727–1735. [Google Scholar] [CrossRef]
- Patrikiadou, E.; Patrikidou, A.; Hatzidaki, E.; Papandreou, C.N.; Zaspalis, V.; Nalbandian, L. Magnetic nanoparticles in Medical Diagnostic applications. Synthesis, characterization. functionalization and proteins conjugation. Curr. Nanosci. 2016, 12, 455–468. [Google Scholar]
- Yan, P.; He, M.; Chen, B.; Hu, B. Restricted accessed nanoparticles for direct magnetic solid phase extraction of trace metal ions from human fluids followed by inductively coupled plasma mass spectrometry detection. Analyst 2015, 140, 4298–4306. [Google Scholar] [CrossRef]
- Dovbeshko, G.I.; Gridina, N.Y.; Kruglova, E.B.; Pashchuk, O.P. FTIR spectroscopy studies of nucleic acid damage. Talanta 2000, 53, 233–246. [Google Scholar] [CrossRef]
- Ragab, S.; El Nemr, A. Zirconyl chloride as a novel and efficient green Lewis acid catalyst for direct acetylation of cotton cellulose in the presence and absence of solvent. J. Polym. Res. 2019, 26, 156. [Google Scholar] [CrossRef]
- He, H.; Gao, C. Supraparamagnetic, conductive, and processable multifunctional graphene nanosheets coated with high-density Fe3O4 nanoparticle. ACS Appl. Mater. Interfaces 2010, 2, 3201–3210. [Google Scholar] [CrossRef]
- Smilgies, D.M. Scherrer grain-size analysis adapted to grazing-incidence scattering with area detectors. J. Appl. Cryst. 2009, 42, 1030–1034. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef]
- Altman, I.S.; Agranovski, I.E.; Choi, M. Mechanism of Nanoparticle Agglomeration during the Combustion Synthesis. Appl. Phys. Lett. 2005, 87, 053104. [Google Scholar] [CrossRef]
- Chen, F.; Xie, S.; Zhang, J.; Liu, R. Synthesis of Spherical Fe304 Magnetic Nanoparticles by Co-Precipitation in Choline Chloride/Urea Deep Eutectic Solvent. Mater. Lett. 2013, 112, 177–179. [Google Scholar] [CrossRef]
- Moosav, S.; Lai, C.W.; Gan, S.; Zamiri, G.; Pivehzhani, O.A.; Johan, M.R. Application of Efficient Magnetic Particles and Activated Carbon for Dye Removal from Wastewater. ACS Omega 2020, 5, 20684–20697. [Google Scholar] [CrossRef]
- Shakor, Z.M.; Abdulrazak, A.A.; Shuhaib, A.A. Optimization of process variables for hydrogenation of cinnamaldehyde to cinnamyl alcohol over a Pt/SiO2 catalyst using response surface methodology. Chem. Eng. Commun. 2022, 209, 827–843. [Google Scholar] [CrossRef]
- Borah, L.; Goswami, M.; Phukan, P. Adsorption of methylene blue and eosin yellow using porous carbon prepared from tea waste: Adsorption equilibrium, kinetics and thermodynamics study. J. Environ. Chem. Eng. 2015, 3, 1018–1028. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Ramachandran, M. Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm. Process. Saf. Environ. Prot. 2015, 95, 215–225. [Google Scholar] [CrossRef]
- Hamdy, A.; Mostafa, M.K.; Nasr, M. Zero-valent iron nanoparticles for methylene blue removal from aqueous solutions and textile wastewater treatment, with cost estimation. Water Sci. Technol. 2018, 78, 367–378. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Amari, A.; Katubi, K.M.; Alzahrani, F.M.; Rebah, F.B.; Tahoon, M.A. Innovative Magnetite Based Polymeric Nanocomposite for Simultaneous Removal of Methyl Orange and Hexavalent Chromium from Water. Process 2021, 9, 576. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Du, Y.; Li, C.; Wei, K.; Lu, J.; Chen, W.; Yang, L. Preparation and Characterization of Cationic Water—Soluble Pillar [ 5] arene Modified Zeolite for Adsorption of Methyl Orange. ACS Omega 2019, 4, 17741–17751. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Ou, L. Kinetic, isotherm, and thermodynamic studies of the adsorption of methyl orange from aqueous solution by chitosan/alumina composite. J. Chem. Eng. Data 2012, 57, 412–419. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Mohmmadi, L.; Ahmadi, S.; Rahdar, A.; Khadkhodaiy, D.; Dehghani, R.; Rahdar, S. Modeling of adsorption of Methylene Blue dye on Ho-CaWO4 nanoparticles using Response Surface Methodology (RSM) and Artificial Neural Network (ANN) techniques. MethodsX 2019, 6, 1779–1797. [Google Scholar] [CrossRef]
- Tounsi, M.S.; Wannes, W.A.; Ouerghemmi, I.; Jegham, S.; Njima, Y.B.; Hamdaoui, G.; Zemni, H.; Marzouk, B. Juice components and antioxidant capacity of four Tunisian Citrus varieties. J. Sci. Food. Agric. 2011, 91, 142–151. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López, F. A Organic Dyes versus Adsorption Processing. Molecules 2021, 26, 5440. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Mahmoudi, A.; Amiri, S. Methylene blue removal using grape leaves waste: Optimization and modeling. Appl. Water Sci. 2022, 12, 112. [Google Scholar] [CrossRef]
- Sharii, S.H.; Shoja, H. Optimization of process variables by response surface methodology for methylene blue dye removal using Spruce sawdust/MgO nano-biocomposite. J. Water. Environ. Nanotechnol. 2018, 3, 157–172. [Google Scholar]
- Pajaie SH, S.; Archin, S.; Asadpou, G. Optimization of Process Parameters by Response Surface Methodology for Methylene Blue Removal Using Cellulose Dusts. Civ. Eng. J. 2018, 4, 620. [Google Scholar] [CrossRef]
- Subramaniam, R.; Ponnusamy, S.K. Novel adsorbent from agricultural waste (cashew NUT shell) for methylene blue dye removal: Optimization by response surface methodology. Water Resour. Ind. 2015, 11, 64–70. [Google Scholar] [CrossRef]
- Alam, Z.; Bari, N.; Kawsari, S. Statistical optimization of Methylene Blue dye removal from a synthetic textile wastewater using indigenous adsorbents. Environ. Sustain. Indic. 2022, 14, 100176. [Google Scholar] [CrossRef]


















| Variables | Levels and Range | ||
|---|---|---|---|
| (+1) | (0) | (+1) | |
| Initial dye concontration (A) (mg/L) | 10 | 30 | 50 |
| Solution pH (B) | 3 | 6 | 9 |
| Adsorbent dose (C) (mg/L) | 200 | 600 | 1000 |
| Contact time (D) (min) | 30 | 60 | 90 |
| Run No. | A: Initial Con. (mg/L) | B: pH | C: Adsorbent Dose (mg/L) | D: Time (min) | Dye Removal (%) |
|---|---|---|---|---|---|
| 1 | 30.00 | 6.00 | 600.00 | 90.00 | 66.1 |
| 2 | 30.00 | 9.00 | 600.00 | 60.00 | 80.2 |
| 3 | 30.00 | 6.00 | 600.00 | 60.00 | 61.99 |
| 4 | 50.00 | 3.00 | 1000.00 | 30.00 | 55.5 |
| 5 | 10.00 | 3.00 | 200.00 | 90.00 | 35.6 |
| 6 | 50.00 | 3.00 | 1000.00 | 90.00 | 75 |
| 7 | 30.00 | 6.00 | 200.00 | 60.00 | 48.45 |
| 8 | 30.00 | 6.00 | 600.00 | 60.00 | 61.99 |
| 9 | 50.00 | 9.00 | 1000.00 | 90.00 | 87.6 |
| 10 | 30.00 | 6.00 | 600.00 | 30.00 | 53.66 |
| 11 | 10.00 | 9.00 | 200.00 | 30.00 | 89.4 |
| 12 | 30.00 | 6.00 | 600.00 | 60.00 | 61.99 |
| 13 | 10.00 | 9.00 | 1000.00 | 30.00 | 89.73 |
| 14 | 50.00 | 6.00 | 600.00 | 60.00 | 56.34 |
| 15 | 30.00 | 6.00 | 600.00 | 60.00 | 61.99 |
| 16 | 30.00 | 6.00 | 600.00 | 60.00 | 61.99 |
| 17 | 50.00 | 9.00 | 200.00 | 30.00 | 62.6 |
| 18 | 10.00 | 3.00 | 1000.00 | 90.00 | 68.7 |
| 19 | 30.00 | 6.00 | 600.00 | 60.00 | 61.99 |
| 20 | 30.00 | 3.00 | 600.00 | 60.00 | 45.36 |
| 21 | 50.00 | 9.00 | 1000.00 | 30.00 | 65.78 |
| 22 | 10.00 | 3.00 | 1000.00 | 30.00 | 56.21 |
| 23 | 10.00 | 9.00 | 1000.00 | 90.00 | 93 |
| 24 | 10.00 | 6.00 | 600.00 | 60.00 | 68.8 |
| 25 | 30.00 | 6.00 | 1000.00 | 60.00 | 78 |
| 26 | 10.00 | 3.00 | 200.00 | 30.00 | 20.4 |
| 27 | 50.00 | 3.00 | 200.00 | 90.00 | 40.74 |
| 28 | 50.00 | 9.00 | 200.00 | 90.00 | 69.54 |
| 29 | 50.00 | 3.00 | 200.00 | 30.00 | 23.99 |
| 30 | 10.00 | 9.00 | 200.00 | 90.00 | 91.3 |
| Fe3O4 | Fe3O4/CA | |
|---|---|---|
| Surface area (m2/g) | 6.67 | 32.656 |
| Pore volume (cm3/g) | 0.00472 | 0.02658 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | ||||
|---|---|---|---|---|---|---|---|---|---|
| Model | 9668.99 | 9 | 1074.33 | 116.16 | <0.0001 | Significant | |||
| A: Initial con. (mg/L) | 321.31 | 1 | 321.31 | 34.74 | <0.0001 | ||||
| B: pH | 5928.76 | 1 | 5928.76 | 641.01 | <0.0001 | ||||
| C: Adsorbent dose (mg/L) | 1071.29 | 1 | 1071.29 | 115.83 | <0.0001 | ||||
| D: Time (min) | 676.02 | 1 | 676.02 | 73.09 | <0.0001 | ||||
| AB | 531.65 | 1 | 531.65 | 57.48 | <0.0001 | ||||
| AD | 64.60 | 1 | 64.60 | 6.98 | 0.0156 | ||||
| BC | 775.76 | 1 | 775.76 | 83.87 | <0.0001 | ||||
| BD | 56.29 | 1 | 56.29 | 6.09 | 0.0228 | ||||
| C2 | 31.99 | 1 | 31.99 | 3.46 | 0.0777 | ||||
| Residual | 184.98 | 20 | 9.25 | ||||||
| Lack of Fit | 184.98 | 15 | 12.33 | ||||||
| Pure Error | 0.000 | 5 | 0.000 | ||||||
| Cor. Total | 9853.97 | 29 | |||||||
| Std. Dev. | 3.04 | R-Squared | 0.9812 | ||||||
| Mean | 63.13 | Adj. R-Squared | 0.9728 | ||||||
| C.V.% | 4.82 | Pred. R-Squared | 0.9346 | ||||||
| PRESS | 644.30 | Adeq. Precision | 43.126 | ||||||
| Terms | A | B | C | D | AB | AD | BC | BD | C2 |
|---|---|---|---|---|---|---|---|---|---|
| PC% | 3.4 | 62.7 | 11.3 | 7.1 | 5.6 | 0.7 | 8.2 | 0.6 | 0.3 |
| Adsorbents | Optimum Conditions | % MB Dye Removal | Ref. |
|---|---|---|---|
| Activated carbon (AC) from grape leaves | pH (11), adsorbent dosage (12.5 g/L), MB initial concentration (100 mg/L), contact time (90 min) | 97.4 | [48] |
| Spruce sawdust (SD) coated by magnesium oxide (MgO) | pH (11), adsorbent dosage (3.50 g/L) | 94.05 | [49] |
| Cellulose dusts (CD) | pH (9.84), adsorbent dosage (4.38 g/L), MB concentration (75.50 mg/L), contact time (208.13 min) | 98.05 | [50] |
| Agricultural waste, cashew nut shell (CNS) | pH (10), adsorbent dose (2.1846 g/L), initial dye concentration (50 mg/L), contact time (62.8693 min) | 100 | [51] |
| Banana leaf ash (BLA) | Adsorbent dose (23.9 mg/100 mL), shaking time (3 h), shaking speed (356 rpm) | 93.75 | [52] |
| Zeolites 13X modified by magnetic nanoparticles | pH (8.93), adsorbent dose (1198 mg/L), temperature (53.39 °C), initial MB concentration (10.05 mg/L) | 96 | [12] |
| Fe3O4/CA | pH (8.98), adsorbent dose (997.99 mg/L), initial concentration of the dye (10.02 mg/L), contact time (43.7 min) | 93.14 | This work |
| Fe3O4 | 72.2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassim, S.; Mageed, A.K.; AbdulRazak, A.A.; Majdi, H.S. Green Synthesis of Fe3O4 Nanoparticles and Its Applications in Wastewater Treatment. Inorganics 2022, 10, 260. https://doi.org/10.3390/inorganics10120260
Bassim S, Mageed AK, AbdulRazak AA, Majdi HS. Green Synthesis of Fe3O4 Nanoparticles and Its Applications in Wastewater Treatment. Inorganics. 2022; 10(12):260. https://doi.org/10.3390/inorganics10120260
Chicago/Turabian StyleBassim, Shahnaz, Alyaa K. Mageed, Adnan A. AbdulRazak, and Hasan Sh. Majdi. 2022. "Green Synthesis of Fe3O4 Nanoparticles and Its Applications in Wastewater Treatment" Inorganics 10, no. 12: 260. https://doi.org/10.3390/inorganics10120260
APA StyleBassim, S., Mageed, A. K., AbdulRazak, A. A., & Majdi, H. S. (2022). Green Synthesis of Fe3O4 Nanoparticles and Its Applications in Wastewater Treatment. Inorganics, 10(12), 260. https://doi.org/10.3390/inorganics10120260






