Polymeric Copper(II) Complexes with a Newly Synthesized Biphenyldicarboxylic Acid Schiff Base Ligand—Synthesis, Structural and Thermal Characterization
Abstract
1. Introduction
2. Results and Discussion
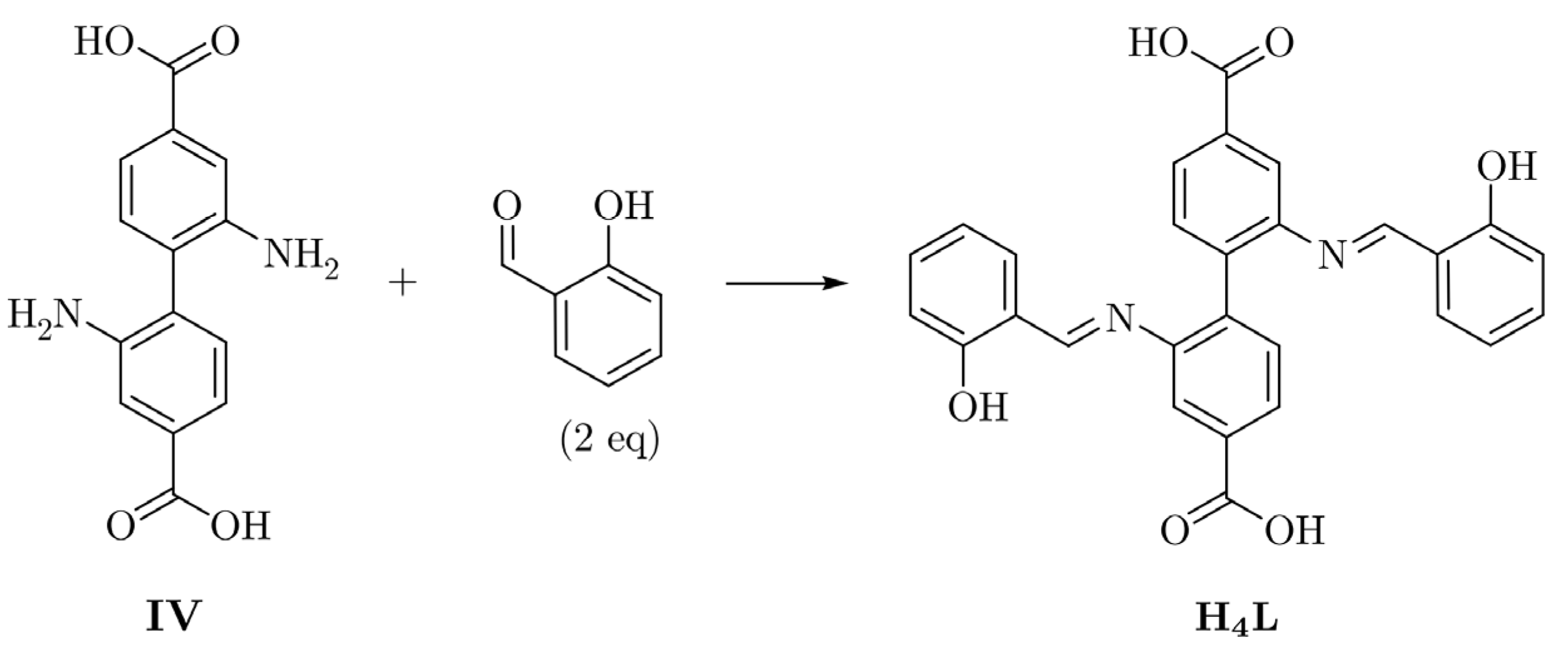
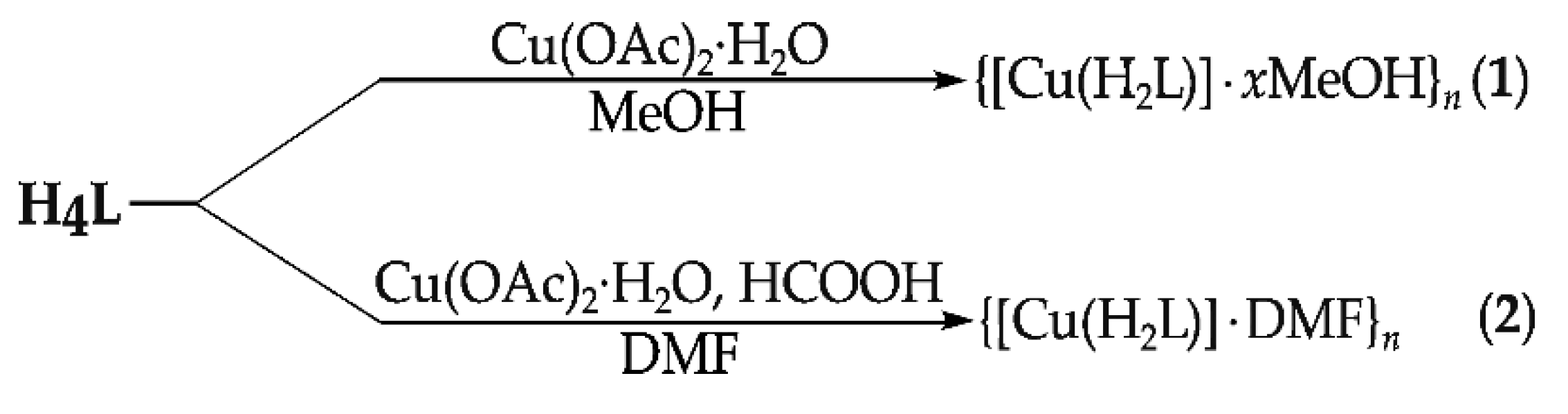
2.1. Syntheses of the Compounds
2.2. Crystal Structures
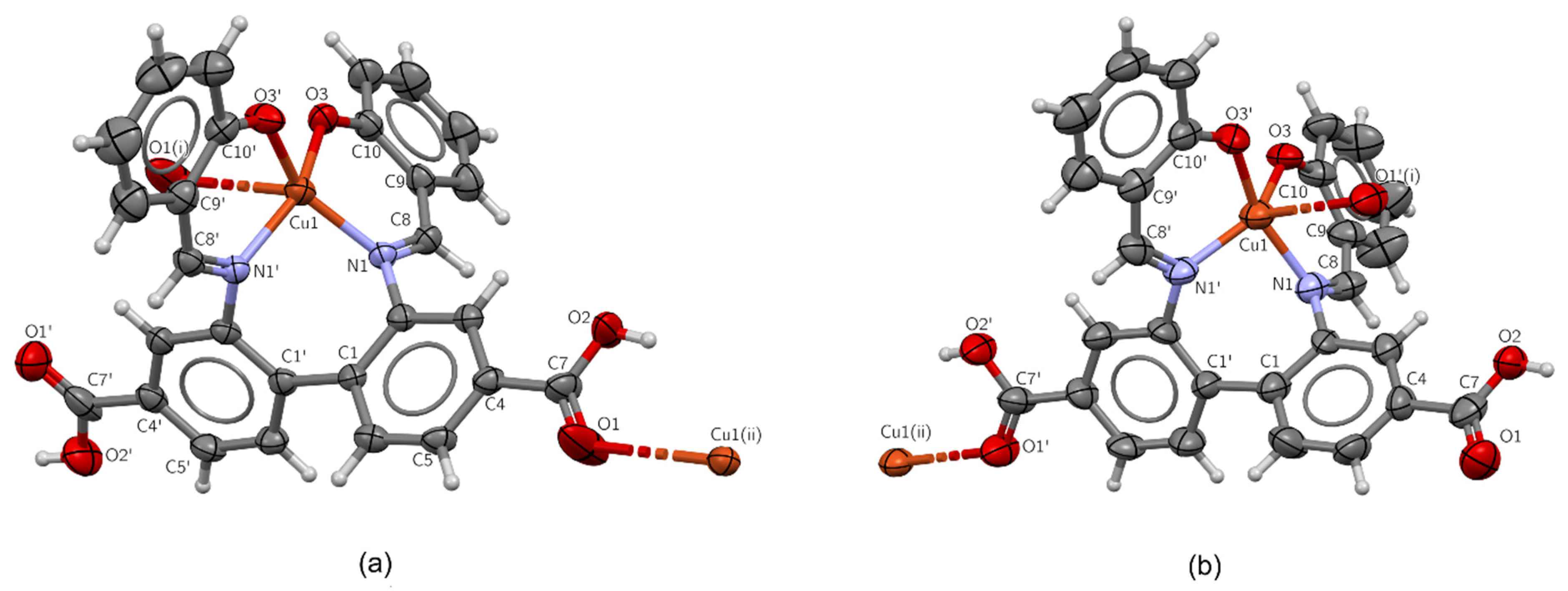
2.2.1. Structure of H4L·3DMF
2.2.2. Crystal Structures of Complexes
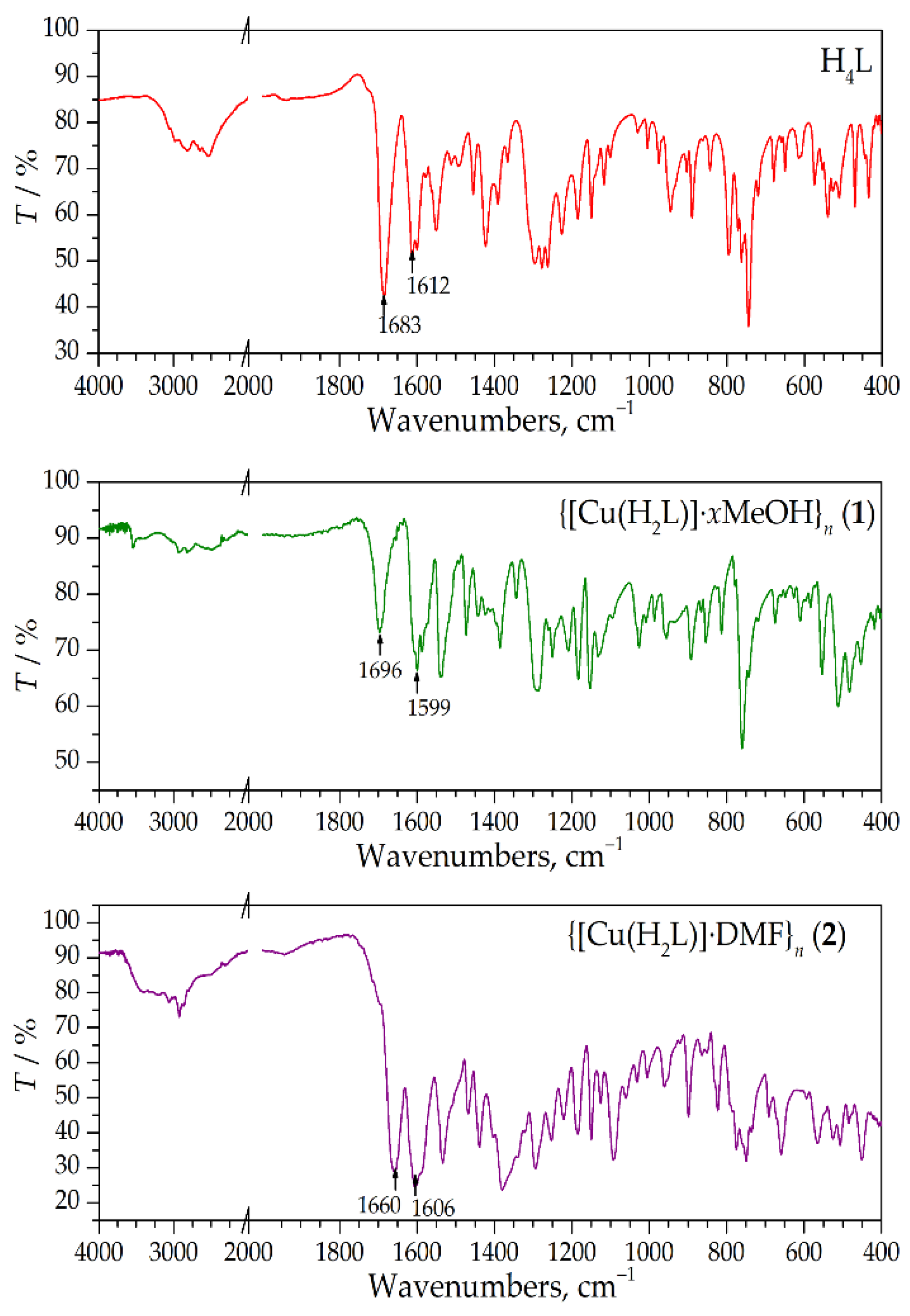
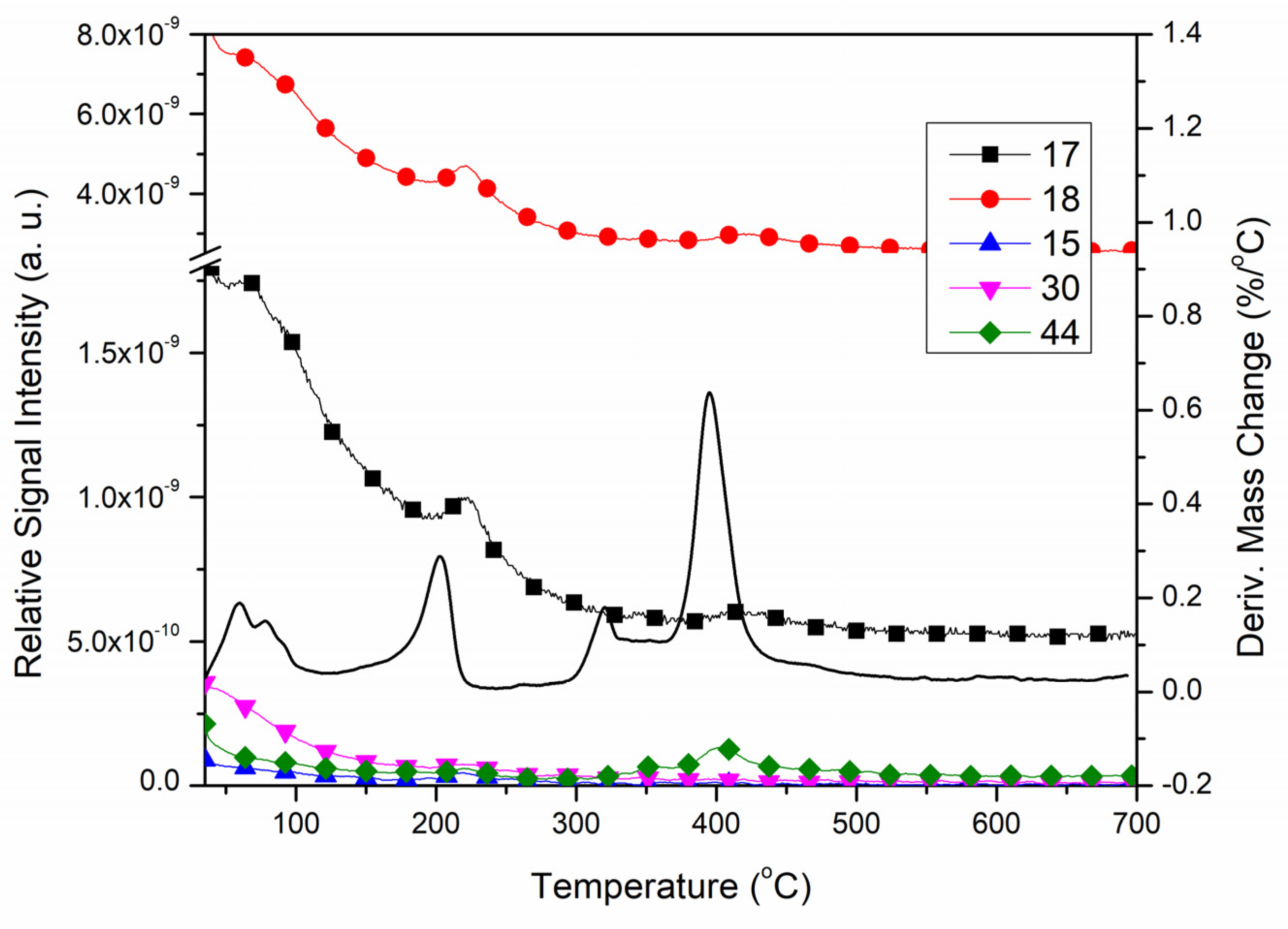
2.3. Thermal Properties of the Ligand and Complexes
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Preparation of the Compounds
3.2.1. H4L
3.2.2. {[Cu(H2L)]·xMeOH}n (1)
3.2.3. {[Cu(H2L)]·DMF}n (2)
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tombesi, A.; Pettinari, C. Metal Organic Frameworks as Heterogeneous Catalysts in Olefin Epoxidation and Carbon Dioxide Cycloaddition. Inorganics 2021, 9, 81. [Google Scholar] [CrossRef]
- Ristić, P.; Filipović, N.; Blagojević, V.; Ćirković, J.; Barta Holló, B.; Đokić, V.R.; Donnard, M.; Gulea, M.; Marjanović, I.; Klisurić, O.R.; et al. 2D and 3D Silver-Based Coordination Polymers with Thiomorpholine-4-Carbonitrile and Piperazine-1,4-Dicarbonitrile: Structure, Intermolecular Interactions, Photocatalysis, and Thermal Behavior. CrystEngComm 2021, 23, 4799–4815. [Google Scholar] [CrossRef]
- Ristić, P.; Todorović, T.R.; Blagojević, V.; Klisurić, O.R.; Marjanović, I.; Barta Holló, B.; Vulić, P.; Gulea, M.; Donnard, M.; Monge, M.; et al. 1D and 2D Silver-Based Coordination Polymers with Thiomorpholine-4-Carbonitrile and Aromatic Polyoxoacids as Coligands: Structure, Photocatalysis, Photoluminescence, and TD-DFT Study. Cryst. Growth Des. 2020, 20, 4461–4478. [Google Scholar] [CrossRef]
- Brezovan, M.; Juráková, J.; Moncol, J.; Dlháň, Ľ.; Korabik, M.; Šalitroš, I.; Pavlik, J.; Segľa, P. The Role of the Bridge in Single-Ion Magnet Behaviour: Reinvestigation of Cobalt(II) Succinate and Fumarate Coordination Polymers with Nicotinamide. Inorganics 2022, 10, 128. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Mironov, V.S.; Yakushev, I.A.; Svetogorov, R.D.; Maximova, O.v; Manakin, Y.v; Kornev, A.B.; Vasiliev, A.N.; Yagubskii, E.B. End-to-End Azido-Bridged Lanthanide Chain Complexes (Dy, Er, Gd, and Y) with a Pentadentate Schiff-Base [N3O2] Ligand: Synthesis, Structure, and Magnetism. Inorg. Chem. 2020, 59, 563–578. [Google Scholar] [CrossRef]
- Khattak, Z.A.K.; Ahmad, N.; Younus, H.A.; Ullah, H.; Yu, B.; Munawar, K.S.; Ashfaq, M.; Ali, S.; Shahadat, H.M.; Verpoort, F. Synthesis of 3D Cadmium(II)-Carboxylate Framework Having Potential for Co-Catalyst Free CO2 Fixation to Cyclic Carbonates. Inorganics 2022, 10, 162. [Google Scholar] [CrossRef]
- Demakov, P.A.; Volynkin, S.S.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. A Selenophene-Incorporated Metal–Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity. Molecules 2020, 25, 4396. [Google Scholar] [CrossRef]
- Kravtsov, V.C.; Lozovan, V.; Siminel, N.; Coropceanu, E.B.; Kulikova, O.v.; Costriucova, N.v.; Fonari, M.S. From 1d to 2d Cd(Ii) and Zn(Ii) Coordination Networks by Replacing Monocarboxylate with Dicarboxylates in Partnership with Azine Ligands: Synthesis, Crystal Structures, Inclusion, and Emission Properties. Molecules 2020, 25, 5616. [Google Scholar] [CrossRef]
- Troyano, J.; Zapata, E.; Perles, J.; Amo-Ochoa, P.; Fernández-Moreira, V.; Martínez, J.I.; Zamora, F.; Delgado, S. Multifunctional Copper(I) Coordination Polymers with Aromatic Mono- and Ditopic Thioamides. Inorg. Chem. 2019, 58, 3290–3301. [Google Scholar] [CrossRef]
- Michaels, H.; Golomb, M.J.; Kim, B.J.; Edvinsson, T.; Cucinotta, F.; Waddell, P.G.; Probert, M.R.; Konezny, S.J.; Boschloo, G.; Walsh, A.; et al. Copper Coordination Polymers with Selective Hole Conductivity. J. Mater. Chem. A Mater. 2022, 10, 9582–9591. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Baimuratova, R.K.; Knerelman, E.I.; Davydova, G.I.; Kudaibergenov, S.E.; Kharissova, O.v.; Zhinzhilo, V.A.; Uflyand, I.E. Synthesis of Copper(II) Trimesinate Coordination Polymer and Its Use as a Sorbent for Organic Dyes and a Precursor for Nanostructured Material. Polymers 2020, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hamon, J.R. Recent Developments in Penta-, Hexa- and Heptadentate Schiff Base Ligands and Their Metal Complexes. Coord. Chem. Rev. 2019, 389, 94–118. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff Base Complexes, Applications in Cancer Diagnosis, Therapy, and Antibacterial Activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- Radanović, M.M.; Barta Holló, B. Some Aromatic Schiff Bases and Their Metal Complexes. In Schiff Base in Organic, Inorganic and Physical Chemistry; IntechOpen Limited: London, UK, 2022; pp. 1–32. [Google Scholar]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent Organic Frameworks Based on Schiff-Base Chemistry: Synthesis, Properties and Potential Applications. Chem. Soc. Rev. 2016, 45, 5635. [Google Scholar] [CrossRef]
- Clarke, R.M.; Storr, T. The Chemistry and Applications of Multimetallic Salen Complexes. Dalton Trans. 2014, 43, 9380. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Bazhin, D.N.; Goryaeva, M.v; Burgart, Y.v; Saloutin, V.I. The Use of 2-(1-Alkoxyalkylidene)-1,3-Dicarbonyl Compounds in Organic Synthesis. Russ. Chem. Rev. 2014, 83, 120–142. [Google Scholar] [CrossRef]
- Dammert, W. Disazomethin-Pigmentfarbstoff Federal Republic of Germany Patent. DE1963403 A, 15 July 1971. [Google Scholar]
- Suthar, B.P.; Patel, S.R. Synthesis and Characterization of Poly Schiff Bases from Benzidine-3,3’-Dicarboxylic Acid. Die Angew. Makromol. Chem. 1981, 93, 189–198. [Google Scholar] [CrossRef]
- Sun, R.; Liu, B.; Li, B.G.; Jie, S. Palladium(II)@Zirconium-Based Mixed-Linker Metal–Organic Frameworks as Highly Efficient and Recyclable Catalysts for Suzuki and Heck Cross-Coupling Reactions. ChemCatChem 2016, 8, 3261–3271. [Google Scholar] [CrossRef]
- Yildiz, C.; Kutonova, K.; Oßwald, S.; Titze-Alonso, A.; Bitzer, J.; Bräse, S.; Kleist, W. Post-Synthetic Modification of DUT-5-Based Metal Organic Frameworks for the Generation of Single-Site Catalysts and Their Application in Selective Epoxidation Reactions. ChemCatChem 2020, 12, 1134–1142. [Google Scholar] [CrossRef]
- Gotthardt, M.A.; Grosjean, S.; Brunner, T.S.; Kotzel, J.; Gänzler, A.M.; Wolf, S.; Bräse, S.; Kleist, W. Synthesis and Post-Synthetic Modification of Amine-, Alkyne-, Azide- and Nitro-Functionalized Metal–Organic Frameworks Based on DUT-5. Dalton Trans. 2015, 44, 16802. [Google Scholar] [CrossRef]
- Qian, W.; Huang, W.; Cong, Y.; Li, F. Selective Oxidation of Benzyl Alcohols Catalyzed by UiO-67-Sal-CuCl2 in Air. Chem. J. Chin. Univ. 2019, 40, 1178–1183. [Google Scholar]
- He, L.; Wang, R.-D.; Wang, S.; Zhu, R.-R.; Li, Z.; Wu, Y.-Y.; Ma, J.; Du, L.; Zhao, Q.-H. An AIE Material with Time-Dependent Luminescence Conversion Obtained by 2D Coordination Polymer Modification via Covalent Post-Synthetic Modification. Dalton Trans. 2021, 50, 16685. [Google Scholar] [CrossRef] [PubMed]
- Olkhovik, V.K.; Vasilevskii, D.A.; Pap, A.A.; Kalechyts, G.v; Matveienko, Y.v; Baran, A.G.; Halinouski, N.A.; Petushok, V.G. Synthesis of New Polyconjugated Molecules with Biphenyl, Dibenzothiophene, Carbazole and Phenanthrene Units. Arkivoc 2008, 9, 69–93. [Google Scholar] [CrossRef]
- Khansari, A.; Bryant, M.R.; Jenkinson, D.R.; Jameson, G.B.; Qazvini, O.T.; Liu, L.; Burrows, A.D.; Telfer, S.G.; Richardson, C. Interpenetration Isomers in Isoreticular Amine-Tagged Zinc MOFs. CrystEngComm 2019, 21, 7498. [Google Scholar] [CrossRef]
- Deshpande, R.K.; Minnaar, J.L.; Telfer, S.G. Thermolabile Groups in Metal-Organic Frameworks: Suppression of Network Interpenetration, Post-Synthetic Cavity Expansion, and Protection of Reactive Functional Groups. Angew. Chem.-Int. Ed. 2010, 49, 4598–4602. [Google Scholar] [CrossRef]
- Dikhtiarenko, A.; Olivos Suarez, A.I.; Pustovarenko, A.; García-Granda, S.; Gascon, J.; Suarez, A.I.O.; Pustovarenko, A.; García-Granda, S.; Gascon, J. Crystal Structure of 2,2′-Diamino-[1,1′-Biphenyl]-4,4′-Dicarboxylic Acid Dihydrate, C14H16N2O6. Z. Krist.-New Cryst. Struct. 2016, 231, 65–67. [Google Scholar] [CrossRef][Green Version]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Hylland, K.T.; Gerz, I.; Wragg, D.S.; Øien-Ødegaard, S.; Tilset, M. The Reactivity of Multidentate Schiff Base Ligands Derived from Bi- and Terphenyl Polyamines towards M(II) (M=Ni, Cu, Zn, Cd) and M(III) (M=Co, Y, Lu). Eur. J. Inorg. Chem. 2021, 2021, 1869–1889. [Google Scholar] [CrossRef]
- Cheeseman, T.P.; Hall, D.; Waters, T.N. The Colour Isomerism and Structure of Some Copper Co-Ordination Compounds. Part XII. The Crystal Structure of NN′-(2{,}2′-Biphenyl)Bis(Salicylaldiminato)Copper(II). J. Chem. Soc. A 1966, 1396–1406. [Google Scholar] [CrossRef]
- Ababneh, T.S.; Al-Shboul, T.M.A.; Jazzazi, T.M.A.; Alomari, M.I.; Görls, H.; Westerhausen, M. Crystallographic and Computational Study of the Structure of Copper(II) 2,2′-Bis(2-Oxidobenzylideneamino)-4,4′-Dimethyl-1,1′-Biphenyl. Transit. Met. Chem. 2020, 45, 435–442. [Google Scholar] [CrossRef]
- Heo, J.; Jeon, Y.-M.; Mirkin, C.A. Reversible Interconversion of Homochiral Triangular Macrocycles and Helical Coordination Polymers. J. Am. Chem. Soc. 2007, 129, 7712–7713. [Google Scholar] [CrossRef] [PubMed]
- Kunert, R.; Philouze, C.; Berthiol, F.; Jarjayes, O.; Storr, T.; Thomas, F. Distorted Copper(Ii) Radicals with Sterically Hindered Salens: Electronic Structure and Aerobic Oxidation of Alcohols. Dalton Trans. 2020, 49, 12990–13002. [Google Scholar] [CrossRef] [PubMed]
- Panther, T.; Baumann, U. Dinukleare Kupfer (II)-Komplexe Mit Chiralen Makrocyclischen Liganden Vom Schiff-Basen-Typ: Synthesen Und Strukturen Complexes with Macrocyclic Ligands. V Dinuclear Copper (II) Complexes with Chiral Macrocyclic Ligands of Schiff-Base Type: Synthes. Synthese 2001, 627, 238–243. [Google Scholar] [CrossRef]
- Che, C.-M.; Kwong, H.-L.; Chu, W.-C.; Cheng, K.-F.; Lee, W.-S.; Yu, H.-S.; Yeung, C.-T.; Cheung, K.-K. Copper Complexes of Chiral Tetradentate Binaphthyl Schiff-Base Ligands: Syntheses, X-Ray Crystal Structures and Activity in Catalytic Asymmetric Cyclopropanation of Alkenes. Eur. J. Inorg. Chem. 2002, 2002, 1456–1463. [Google Scholar] [CrossRef]
- Saito, M.; Sato, H.; Mori, Y.; Fukuda, Y. Structure and Enantio-Differentiating Behaviors of Nickel(Ii) Complexes with Chiral Schiff Base Ligands Derived from 1, 1’ Binaphthyl-2,2’-Diamine. Bull. Chem. Soc. Jpn. 2009, 82, 1266–1273. [Google Scholar] [CrossRef]
- Wang, Y.; Stack, T.D.P. Galactose Oxidase Model Complexes: Catalytic Reactivities. J. Am. Chem. Soc. 1996, 118, 13097–13098. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Society. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Holmes, R.R. Structure of Cyclic Pentacoordinated Molecules of Main Group Elements. Acc. Chem. Res. 1979, 12, 257–265. [Google Scholar] [CrossRef]
- Holmes, R.R. Five-Coordinated Structures. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1984; Volume 32, pp. 119–235. ISBN 9780470166338. [Google Scholar]
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. 5. The Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Foundations and Advances SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Crystallogr. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Biological Crystallography Structure Validation in Chemical Crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Momerwell, W.D.S.; Purkis, L.H.; Smith, B.R.; Taylor, R.; Cooper, R.I.; Harris, S.E.; et al. Retrieval of Crystallographically-Derived Molecular Geometry Information. J. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]





| Bond | Distances (Å) | Bond | Distances (Å) |
|---|---|---|---|
| N1–C8 | 1.273(2) | O1–C7 | 1.217(3) |
| N1′–C8′ | 1.277(3) | O1′–C7′ | 1.201(3) |
| O3–C10 | 1.334(3) | O2–C7 | 1.289(3) |
| O3′–C10′ | 1.344(3) | O2′–C7′ | 1.316(3) |
| Bond | Distances (Å) | Angles (°) | ||
|---|---|---|---|---|
| D–H···A | D–H | H···A | D···A | D–H···A |
| O2–H2···O5 | 0.82 | 1.76 | 2.574(3) | 170.4 |
| O2′–H2′···O4 | 0.82 | 1.78 | 2.596(2) | 177.8 |
| O3–H3O···N1 | 0.90(4) | 1.78(4) | 2.611(2) | 154(3) |
| O3′–H3O′···N1′ | 0.92(3) | 1.74(3) | 2.611(2) | 158(3) |
| Bond | Distances (Å) | |
|---|---|---|
| 1 | 2 | |
| Cu1–O3 | 1.924(5) | 1.912(3) |
| Cu1–O3′ | 1.902(5) | 1.899(4) |
| Cu1–N1 | 1.941(6) | 1.976(4) |
| Cu1–N1′ | 1.995(6) | 1.954(4) |
| Cu1–O1′(i) | 2.626(5) | 2.774(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanović, M.G.; Radnović, N.D.; Barta Holló, B.; Radanović, M.M.; Kordić, B.B.; Raičević, V.N.; Vojinović-Ješić, L.S.; Rodić, M.V. Polymeric Copper(II) Complexes with a Newly Synthesized Biphenyldicarboxylic Acid Schiff Base Ligand—Synthesis, Structural and Thermal Characterization. Inorganics 2022, 10, 261. https://doi.org/10.3390/inorganics10120261
Bogdanović MG, Radnović ND, Barta Holló B, Radanović MM, Kordić BB, Raičević VN, Vojinović-Ješić LS, Rodić MV. Polymeric Copper(II) Complexes with a Newly Synthesized Biphenyldicarboxylic Acid Schiff Base Ligand—Synthesis, Structural and Thermal Characterization. Inorganics. 2022; 10(12):261. https://doi.org/10.3390/inorganics10120261
Chicago/Turabian StyleBogdanović, Milica G., Nikola D. Radnović, Berta Barta Holló, Mirjana M. Radanović, Branko B. Kordić, Vidak N. Raičević, Ljiljana S. Vojinović-Ješić, and Marko V. Rodić. 2022. "Polymeric Copper(II) Complexes with a Newly Synthesized Biphenyldicarboxylic Acid Schiff Base Ligand—Synthesis, Structural and Thermal Characterization" Inorganics 10, no. 12: 261. https://doi.org/10.3390/inorganics10120261
APA StyleBogdanović, M. G., Radnović, N. D., Barta Holló, B., Radanović, M. M., Kordić, B. B., Raičević, V. N., Vojinović-Ješić, L. S., & Rodić, M. V. (2022). Polymeric Copper(II) Complexes with a Newly Synthesized Biphenyldicarboxylic Acid Schiff Base Ligand—Synthesis, Structural and Thermal Characterization. Inorganics, 10(12), 261. https://doi.org/10.3390/inorganics10120261










