Preparation of SBA-15-Supported Metals by Vapor-Phase Infiltration
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.B.; Lyu, F.L.; Yin, Y.D. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.V.N. Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A-Gen. 2018, 559, 57–74. [Google Scholar] [CrossRef]
- Lyu, X.; Wu, X.; Liu, Y.Z.; Huang, W.Y.; Lee, B.Y.D.; Li, T. Synthesis and Characterization of Mesoporous Silica Nanoparticles Loaded with Pt Catalysts. Catalysts 2022, 12, 183. [Google Scholar] [CrossRef]
- Naik, P.J.; Chatterjee, P.; Chen, S.J.; Huang, W.Y.; Slowing, I.I. Regulating the Catalytic Activity of Pd Nanoparticles by Confinement in Ordered Mesoporous Supports. ChemCatChem 2021, 13, 539–542. [Google Scholar] [CrossRef]
- Cejka, J.; Mintova, S. Perspectives of micro/mesoporous composites in catalysis. Catal. Rev.-Sci. Eng. 2007, 49, 457–509. [Google Scholar] [CrossRef]
- Pump, E.; Cao, Z.; Samantaray, M.K.; Bendjeriou-Sedjerari, A.; Cavallo, L.; Basset, J.M. Exploiting Confinement Effects to Tune Selectivity in Cyclooctane Metathesis. ACS Catal. 2017, 7, 6581–6586. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Iglesia, E. Stabilization of active, selective, and regenerable Ni-based dimerization catalysts by condensation of ethene withinordered mesopores. J. Catal. 2017, 352, 505–514. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Iglesia, E. Mechanistic insights and consequences of intrapore liquids in ethene, propene, and butene dimerization on isolated Ni2+ sites grafted within aluminosilicate mesopores. J. Catal. 2020, 389, 690–705. [Google Scholar] [CrossRef]
- Zhang, H.C.; Xiao, Z.R.; Yang, M.; Zou, J.J.; Liu, G.Z.; Zhang, X.W. Highly dispersible cerium-oxide modified Ni/SBA-15 for steam reforming of bio-mass based JP10. Chin. J. Chem. Eng. 2022, 43, 255–265. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, K.R.; Ren, W.X.; He, H.L.; Liang, H.C.; Zhai, Y.P.; Li, W. Recent Advances in the Marriage of Catalyst Nanoparticles and Mesoporous Supports. Adv. Mater. Interfaces 2022, 9, 2101528. [Google Scholar] [CrossRef]
- Chytil, S.; Glomm, W.R.; Blekkan, E.A. Characterization of Pt/SBA-15 prepared by the deposition-precipitation method. Catal. Today 2009, 147, 217–223. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, A.; Pereniguez, R.; Caballero, A. Understanding the differences in catalytic performance for hydrogen production of Ni and Co supported on mesoporous SBA-15. Catal. Today 2018, 307, 224–230. [Google Scholar] [CrossRef]
- Jung, S.W.; Lu, C.; He, H.P.; Ahn, K.Y.; Gorte, R.J.; Vohs, J.M. Influence of composition and Cu impregnation method on the performance of Cu/CeO2/YSZ SOFC anodes. J. Power Source 2006, 154, 42–50. [Google Scholar] [CrossRef]
- Yang, C.M.; Sheu, H.S.; Chao, K.J. Templated synthesis and structural study of densely packed metal nanostructures in MCM-41 and MCM-48. Adv. Funct. Mater. 2002, 12, 143–148. [Google Scholar] [CrossRef]
- Jiao, L.; Regalbuto, J.R. The synthesis of highly dispersed noble and base metals on silica via strong electrostatic adsorption: II. Mesoporous silica SBA-15. J. Catal. 2008, 260, 342–350. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Q.F.; Wei, H.M.; Wang, J.Q.; Cho, M.Y.; Cho, H.S.; Terasaki, O.; Wan, Y. Aggregation-Free Gold Nanoparticles in Ordered Mesoporous Carbons: Toward Highly Active and Stable Heterogeneous Catalysts. J. Am. Chem. Soc. 2013, 135, 11849–11860. [Google Scholar] [CrossRef]
- Zhu, J.J.; Wang, T.; Xu, X.L.; Xiao, P.; Li, J.L. Pt nanoparticles supported on SBA-15: Synthesis, characterization and applications in heterogeneous catalysis. Appl. Catal. B-Environ. 2013, 130, 197–217. [Google Scholar] [CrossRef]
- Sietsma, J.R.A.; Meeldijk, J.D.; den Breejen, J.P.; Versluijs-Helder, M.; van Dillen, A.J.; de Jongh, P.E.; de Jong, K.P. The preparation of supported NiO and Co3O4 nanoparticles by the nitric oxide controlled thermal decomposition of nitrates. Angew. Chem.-Int. Edit. 2007, 46, 4547–4549. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, A.; Chirieac, A.; Ciotonea, C.; Mazilu, I.; Catrinescu, C.; Petit, S.; Marceau, E.; Royer, S.; Dumitriu, E. Enhancement of the dispersion and catalytic performances of copper in the hydrogenation of cinnamaldehyde by incorporation of aluminium into mesoporous SBA-15 silica. Appl. Catal. A-Gen. 2020, 598, 117615. [Google Scholar] [CrossRef]
- Huang, R.J.; Kwon, O.; Lin, C.; Gorte, R.J. The effects of SMSI on m-Cresol hydrodeoxygenation over Pt/Nb2O5 and Pt/TiO2. J. Catal. 2021, 398, 102–108. [Google Scholar] [CrossRef]
- Cao, T.; Wang, C.-Y.; Shen, K.; Vohs, J.M.; Gorte, R.J. Investigation into support effects for Pt and Pd on LaMnO3. Appl. Catal. A Gen. 2022, 646, 118873. [Google Scholar] [CrossRef]
- Lin, C.; Foucher, A.C.; Ji, Y.C.; Stach, E.A.; Gorte, R.J. Investigation of Rh-titanate (ATiO3) interactions on high-surface-area perovskite thin films prepared by atomic layer deposition. J. Mater. Chem. A 2020, 8, 16973–16984. [Google Scholar] [CrossRef]
- Wang, C.Y.; Kwon, O.; Gorte, R.J.; Vohs, J.M. Synthesis of high-surface area tungstated zirconia by atomic layer deposition on mesoporous silica. Microporous Mesoporous Mat. 2022, 335, 111821. [Google Scholar] [CrossRef]
- Kwon, O.; Huang, R.J.; Cao, T.Y.; Vohs, J.M.; Gorte, R.J. Dry reforming of methane over Ni supported on LaMnO3 thin films. Catal. Today 2021, 382, 142–147. [Google Scholar] [CrossRef]
- Onn, T.M.; Zhang, S.Y.; Arroyo-Ramirez, L.; Chung, Y.C.; Graham, G.W.; Pan, X.Q.; Gorte, R.J. Improved Thermal Stability and Methane-Oxidation Activity of Pd/Al2O3 Catalysts by Atomic Layer Deposition of ZrO2. ACS Catal. 2015, 5, 5696–5701. [Google Scholar] [CrossRef]
- Lin, C.; Foucher, A.C.; Ji, Y.C.; Curran, C.D.; Stach, E.A.; McIntosh, S.; Gorte, R.J. “Intelligent” Pt Catalysts Studied on High-Surface-Area CaTiO3 Films. ACS Catal. 2019, 9, 7318–7327. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- Ke, W.; Liu, Y.; Wang, X.L.; Qin, X.D.; Chen, L.M.; Palomino, R.M.; Simonovis, J.P.; Lee, I.; Waluyo, I.; Rodriguez, J.A.; et al. Nucleation and Initial Stages of Growth during the Atomic Layer Deposition of Titanium Oxide on Mesoporous Silica. Nano Lett. 2020, 20, 6884–6890. [Google Scholar] [CrossRef] [PubMed]
- Soled, S. Case Studies of Nobel-Metal Catalysts. In Synthesis of Solid Catalysts; de Jong, K.P., Ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2009; pp. 353–367. [Google Scholar]
- Huang, R.J.; Cheng, Y.; Ji, Y.C.; Gorte, R.J. Atomic Layer Deposition for Preparing Isolated Co Sites on SiO2 for Ethane Dehydrogenation Catalysis. Nanomaterials 2020, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Onn, T.M.; Monai, M.; Dai, S.; Arroyo-Ramirez, L.; Zhang, S.Y.; Pan, X.Q.; Graham, G.W.; Fornasiero, P.; Gorte, R.J. High-surface-area, iron-oxide films prepared by atomic layer deposition on gamma-Al2O3. Appl. Catal. A-Gen. 2017, 534, 70–77. [Google Scholar] [CrossRef]
- Lee, S.; Lin, C.; Kim, S.; Mao, X.Y.; Kim, T.; Kim, S.J.; Gorte, R.J.; Jung, W. Manganese Oxide Overlayers Promote CO Oxidation on Pt. ACS Catal. 2021, 11, 13935–13946. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
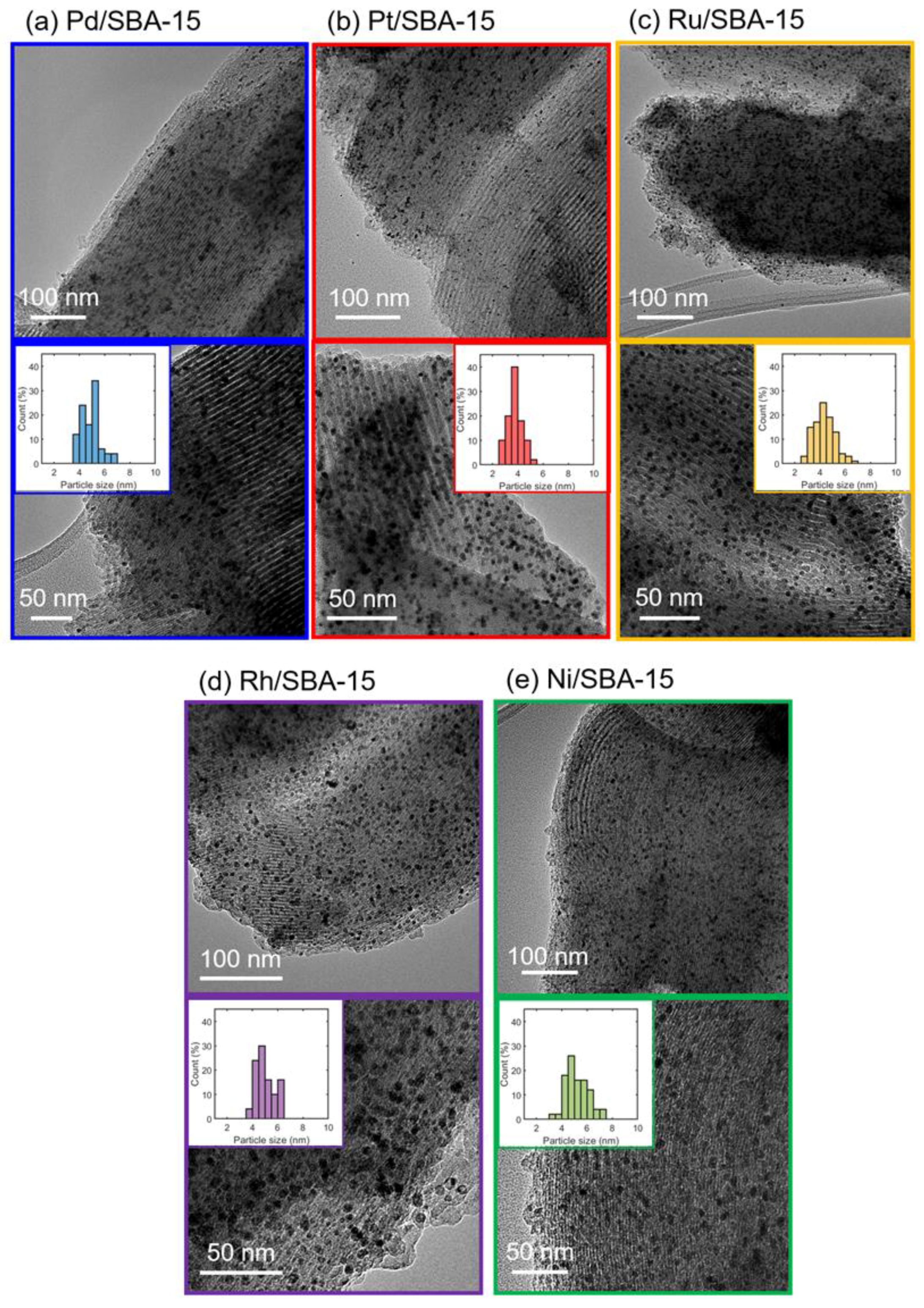
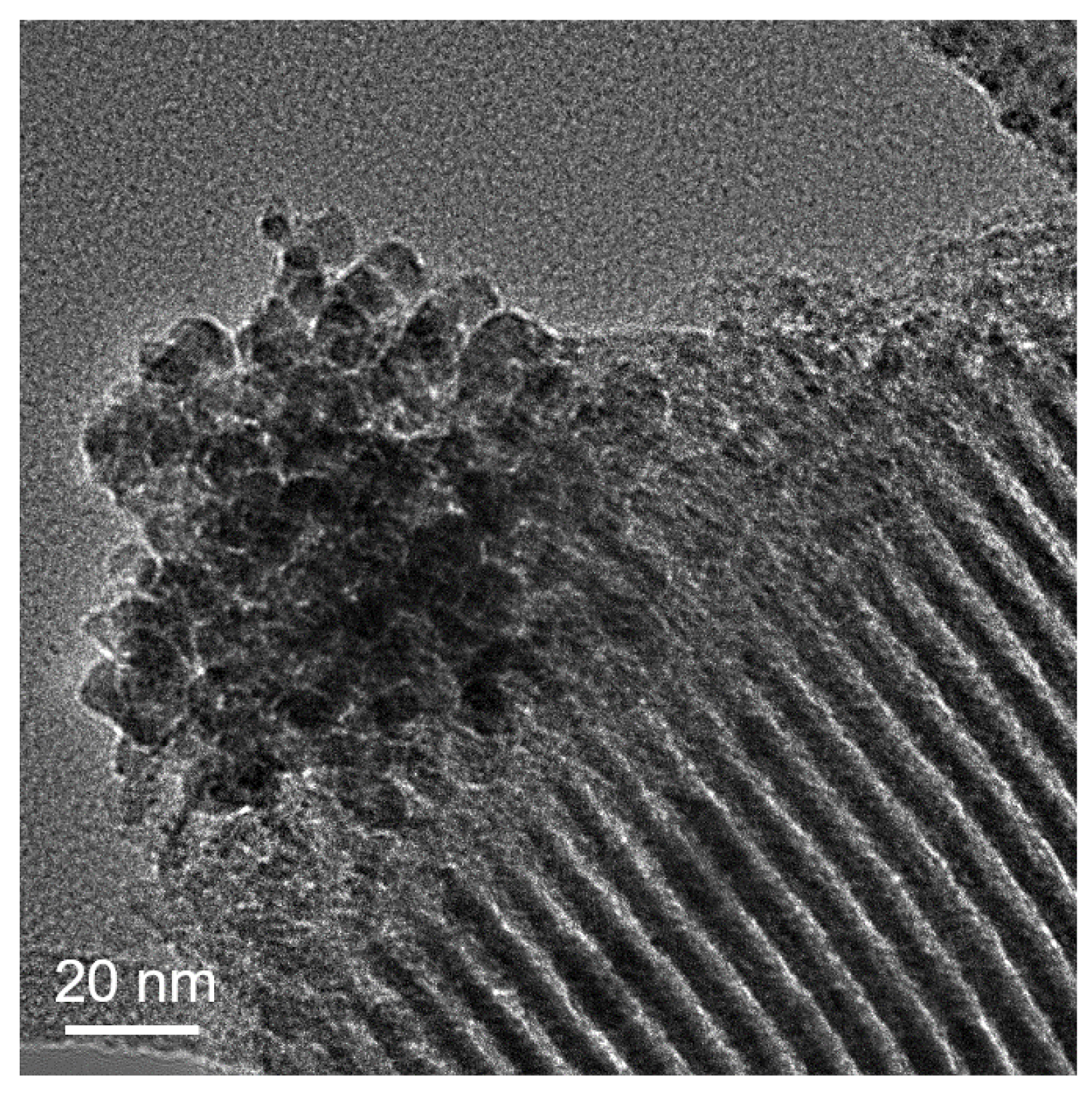
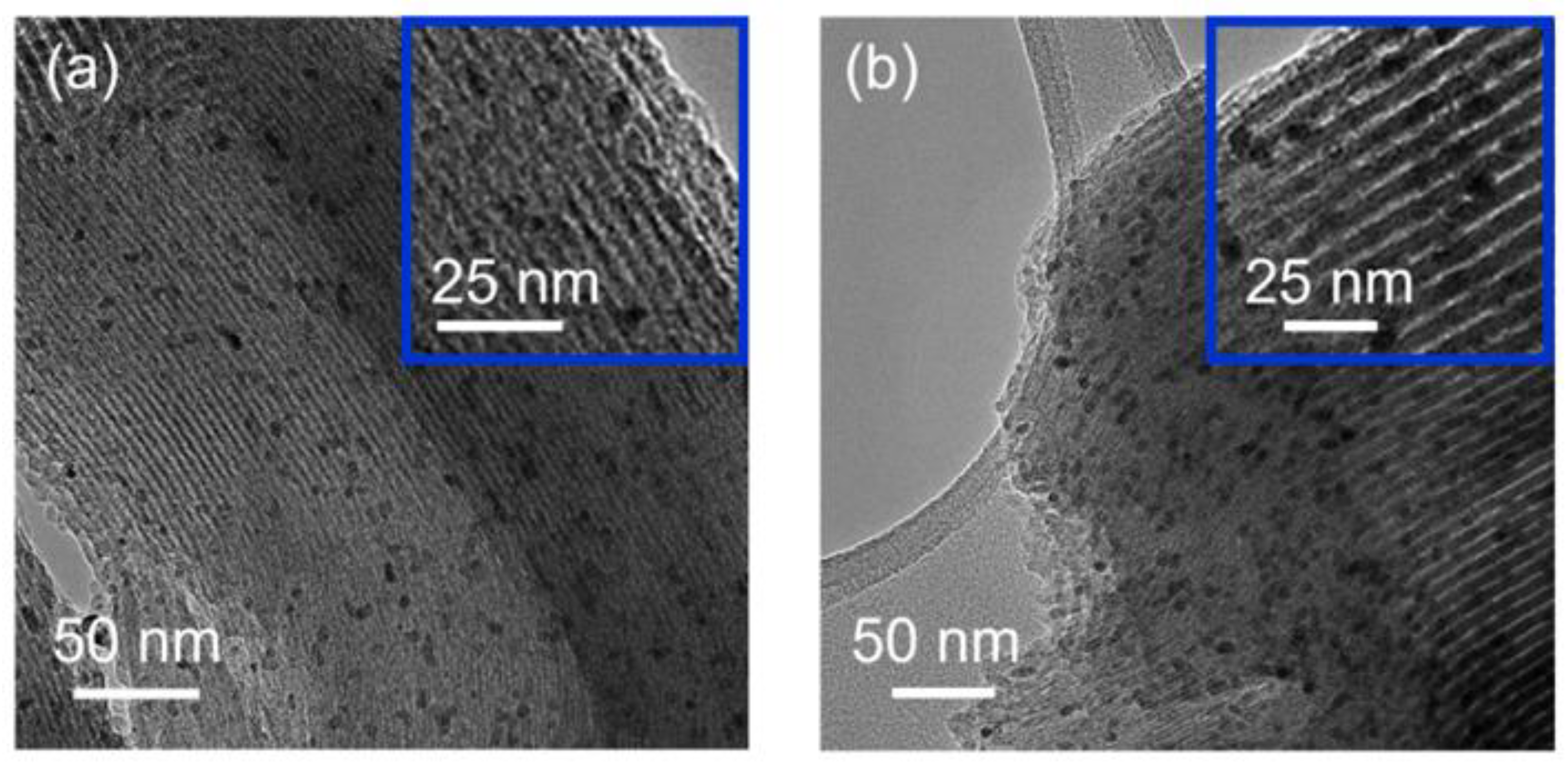
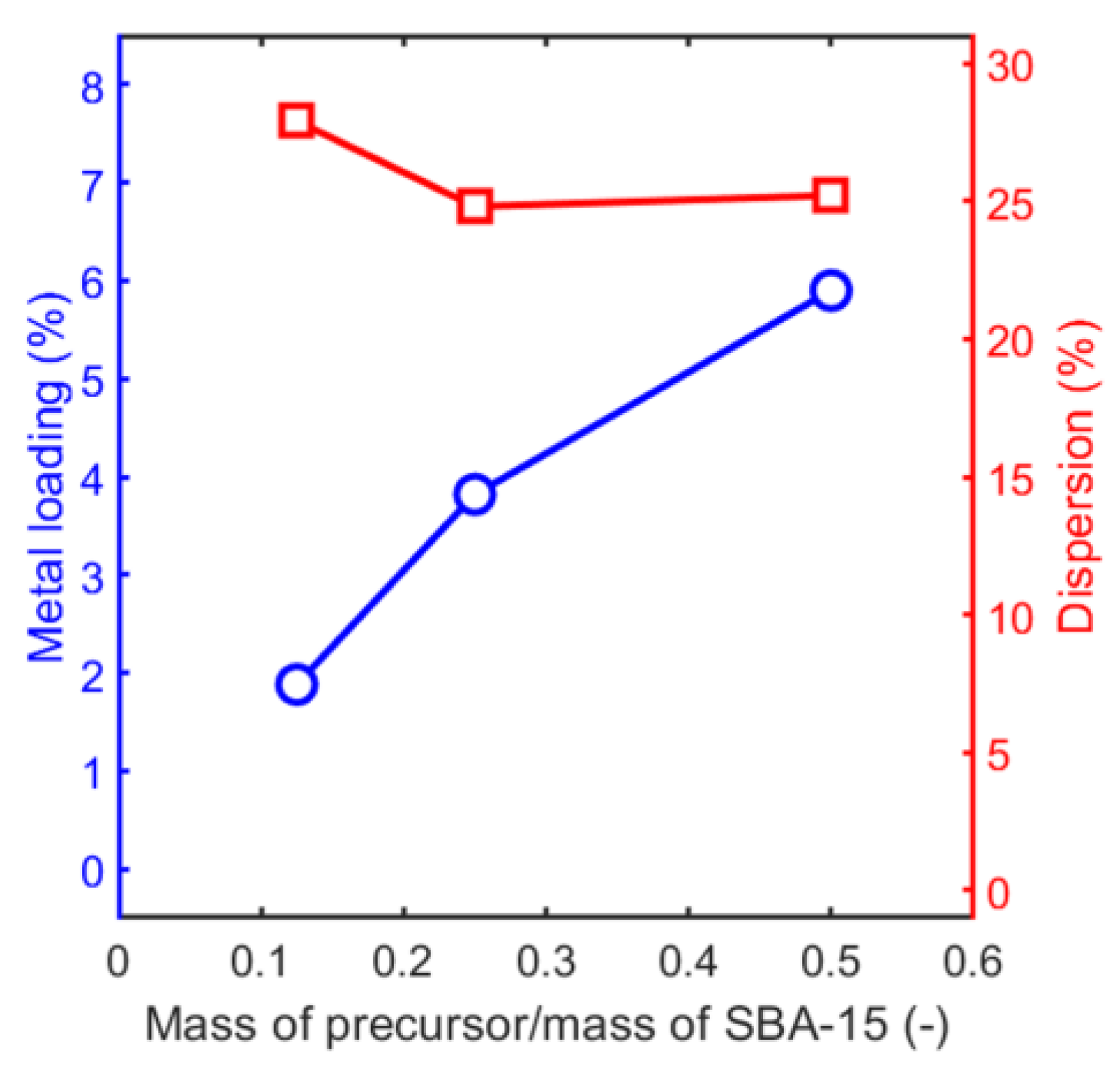

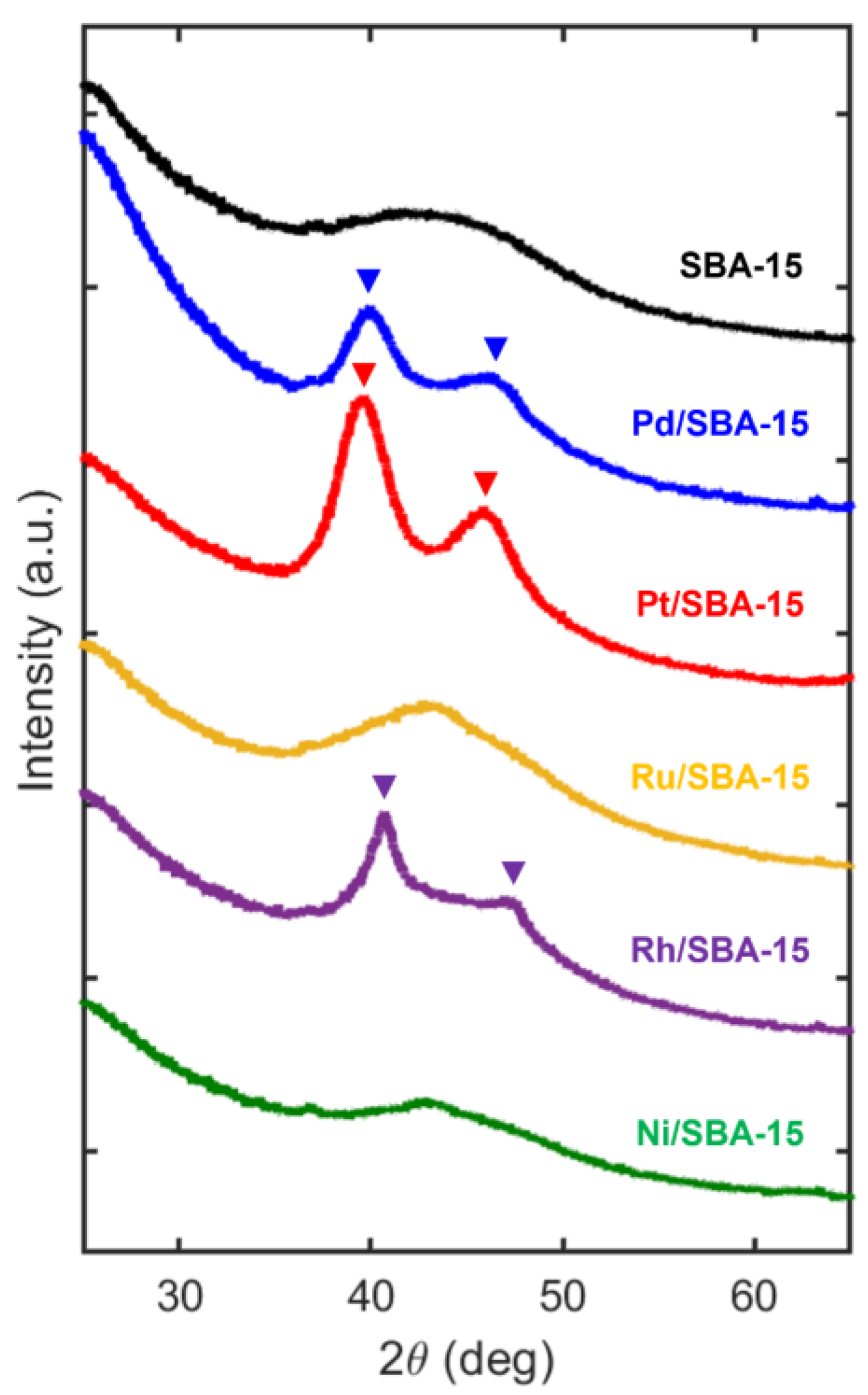

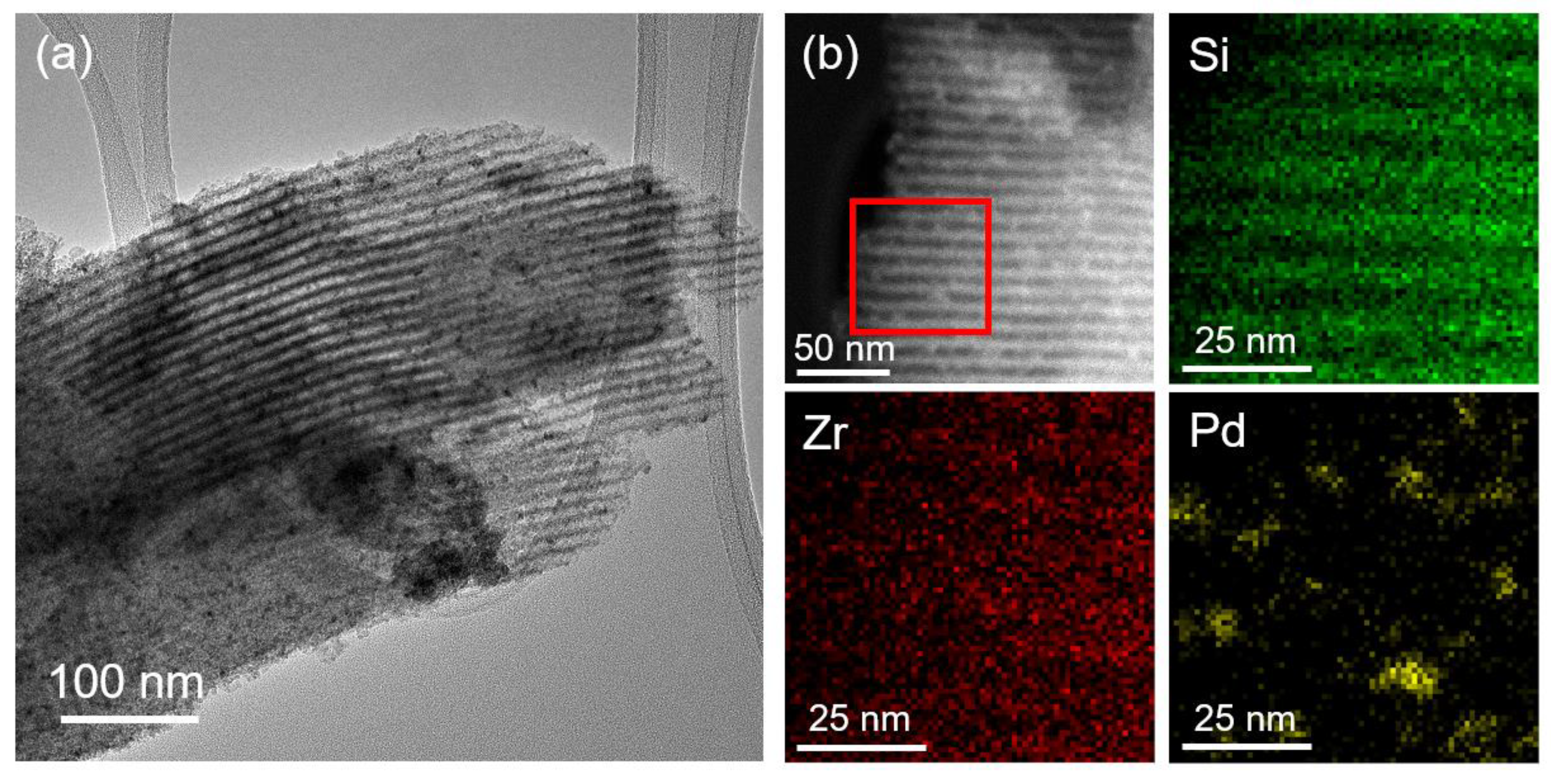
| Sample | Metal Loading (%) | Metal Coverage (Metal Atoms/m2) | Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| SBA-15 | - | - | 785 | 0.81 |
| Pd/SBA-15 | 5.9 | 4.3 × 1017 | 719 | 0.69 |
| Pt/SBA-15 | 9.1 | 3.6 × 1017 | 637 | 0.63 |
| Ru/SBA-15 | 6.7 | 5.1 × 1017 | 748 | 0.69 |
| Rh/SBA-15 | 6.0 | 4.5 × 1017 | 703 | 0.77 |
| Ni/SBA-15 | 4.1 | 5.4 × 1017 | 694 | 0.79 |
| Pd/SBA-15(med) | 3.8 | 2.8 × 1017 | 686 | 0.67 |
| Pd/SBA-15(low) | 1.9 | 1.4 × 1017 | 660 | 0.66 |
| Pd/ZrO2/SBA-15 | Pd: 5.1 ZrO2: 45.6 | 1.2 × 1018 | 224 | 0.30 |
| WI-Pd/SBA-15 | 1.0 | - | 563 | 0.62 |
| Sample | Dispersion Estimated from CO Chemisorption (%) | Particle Size 1 (nm) | Particle Size 2 (nm) | Particle Size 3 (nm) |
|---|---|---|---|---|
| Pd/SBA-15 | 25.2 | 4.4 | 3.3 | 4.9 |
| Pt/SBA-15 | 24.1 | 4.7 | 3.0 | 3.8 |
| Ru/SBA-15 | 11.3 | 11.4 | n.m. | 4.3 |
| Rh/SBA-15 | 21.2 | 5.1 | 4.8 | 5.0 |
| Ni/SBA-15 | n.m. | n.m. | n.m. | 5.2 |
| Pd/SBA-15(med) | 24.8 | 4.5 | 3.2 | 5.2 |
| Pd/SBA-15(low) | 27.9 | 4.0 | 2.8 | 5.0 |
| Pd/ZrO2/SBA-15 | 32.1 | 3.5 | n.m. | 3.3 |
| WI-Pd/SBA-15 | 3.2 | 34.8 | n.m. | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-Y.; Shen, K.; Gorte, R.J.; Vohs, J.M. Preparation of SBA-15-Supported Metals by Vapor-Phase Infiltration. Inorganics 2022, 10, 215. https://doi.org/10.3390/inorganics10110215
Wang C-Y, Shen K, Gorte RJ, Vohs JM. Preparation of SBA-15-Supported Metals by Vapor-Phase Infiltration. Inorganics. 2022; 10(11):215. https://doi.org/10.3390/inorganics10110215
Chicago/Turabian StyleWang, Ching-Yu, Kai Shen, Raymond J. Gorte, and John M. Vohs. 2022. "Preparation of SBA-15-Supported Metals by Vapor-Phase Infiltration" Inorganics 10, no. 11: 215. https://doi.org/10.3390/inorganics10110215
APA StyleWang, C.-Y., Shen, K., Gorte, R. J., & Vohs, J. M. (2022). Preparation of SBA-15-Supported Metals by Vapor-Phase Infiltration. Inorganics, 10(11), 215. https://doi.org/10.3390/inorganics10110215







