Abstract
Condensation of the reaction between enrofloxacin and ethylenediamine in the existence of glacial acetic acid produced a new N,N-ethylene (bis 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-1,4-dihydroquinoline-3-carboxylic acid Schiff base (H2Erx-en). H2Erx-en was used as a tetra-dentate ligand to produce novel complexes by interacting with metal ions iron(III), yttrium(III), zirconium(IV), and lanthanum(III). The synthetic H2Erx-en and its chelates had been detected with elemental analysis, spectroscopic methods, mass spectrometry, thermal studies, conductometric and magnetic measurements experiments. The calculated molar conductance of the complexes in 1 × 10−3 M DMF solution shows that iron(III), yttrium(III) and lanthanum(III) are 1:1 electrolytes, however the zirconium(IV) complex is non-electrolyte. The infrared spectra of H2Erx-en chelates indicated that the carboxylic group is deprotonated and H2Erx-en is associated with metals as a tetra-dentate through nitrogen and oxygen atoms. The disappearance of the carboxylic proton in all complexes corroborated information concerning H2Erx-en deprotonation and complexation with metal ions, according to 1H NMR data. Thermal analysis revealed the abundance of H2O particles in the chelates’ entrance and outlet spheres, indicating the disintegration pattern of H2Erx-en and their chelates. The Coats–Redfern and Horowitz–Metzeger approaches were utilized to calculate the thermodynamic items (Ea, ΔS *, ΔH *, and ΔG *) at n = 1 and n ≠ 1. The resulting data reveal better organized chelate building activation. Density functional theory (DFT) was created to properly grasp the optimal architecture of the molecules. The chelates are softer than H2Erx-en, with estimates varying between 95.23 eV to 400.00 eV, compared to 31.47 eV for H2Erx-en. The disc diffusion technique was utilized to assess H2Erx-en and their chelates in an antimicrobial assay against various food and phytopathogens. The zirconium(IV) chelate has the most potent antibacterial action and is particularly efficient against Salmonella typhi.
1. Introduction
Schiff base ligands occupy a dominant position in the coordination compound domain. They can be implemented as bidentate, tridentate, tetradendate, and polydentate ligands that are entirely recognized be assorted with most metal ions, and their complexes have been documented [1,2,3,4]. Furthermore, Schiff bases offer a number of distinguishing characteristics, including high synthetic versatility, strong discrimination for the central metal atom, and structural resemblance to surely circulating biological techniques [2,3]. Schiff bases and metal complexes provide a diverse variety of uses in chemistry, as well as comprehensive industrial, agricultural, and medicinal applications [4]. They also have the capacity to perform as catalysts and corrosion protection agents [5]. Schiff base ligands and their chelates just lately gained a well-deserved and critical relevance due to their diverse spectroscopic properties as well as their remarkable antibacterial, antifungal, and antitumor properties [6]. The broad-spectrum antibiotic drug family quinolone is an artefact. One of the quinolone family’s members is fluoroquinolone. The antibiotics block bacteria from entering the target cell, demonstrating their antibacterial activities. Enrofloxacin is a second-generation quinolone antibiotic that is effective against both Gram-positive and Gram-negative bacteria, particularly those that are sensitive to sulfonamides and b-lactam antibiotics [5,6]. The first antibiotic approved for use by veterinarians, enrofloxacin, may be useful in treating infectious diseases of the urinary bladder, respiratory tract, and skins in animals and pets [7,8,9,10]. Enrofloxacin reacts with Ti(IV), Y(III), Zr(IV), Pd(II), and Ce(IV) to create chelates [11]. Compared to free enrofloxacin, these complexes are more bactericidal agents against the majority of common bacterial strains. This study presents a very intriguing collection of prospective bacterial inhibitors, which might increase their applications as promising clinical treatments with a broad spectrum and open the door to the development of practical compounds with specialized biological applications [11]. According to their special electronic configuration and biological relevance of lanthanide elements, a detailed literature research has shown that a variety of lanthanide Schiff base complexes have been proven to be very good antibacterial, anti-inflammatory, antiviral, anticoagulant, and antitumor agents and used as sensors for detecting sugars in neutral aqueous media. The reactivity of the Schiff base complexes depends on the stereochemistry of the complexes, the atomic radii of the metals and the bending constants (Kb) values [12,13,14]. In particular, there is considerable bibliographic research reporting the significant biological activity of zinc and cerium metal complexes with the Schiff base ligand [15]. In the literature survey there is not any study on the enrofloxacin Schiff base. With the continuing interest in the chemistry of the potentially useful Schiff base generated from fluoroquinolone that have biological action [16,17,18], the aim of the current research was to study the effect of changing atomic radii, atomic mass, or oxidation state of Fe(III), Y(III), Zr(IV) and La(III) with Cl− as a counter ion on the efficacy or stability of the biological properties of enrofloxacin as antibacterial agent in its new form as a H2Erx-en Schiff base (Scheme 1) to determine the target sites of novel synthesized complexes at the bacterial cells. The resulting complexes were characterized using elemental analyses, molar conductivity, magnetic susceptibility assessments, FT-IR, UV-Vis, 1HNMR, mass spectra, and thermal behavior and DFT. The antimicrobial activity of the H2Erx-en and its metal complexes has been checked against a wide range of bacteria and fungi.
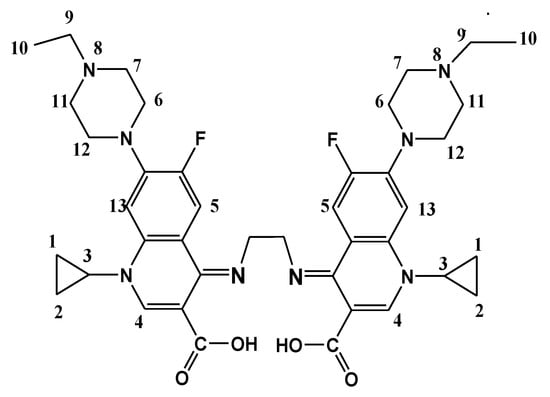
Scheme 1.
N,N-ethylene (bis 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-1,4-dihydroquinoline-3-carboxylic acid (H2Erx-en).
2. Results and Discussion
2.1. Elemental and Molar Conductance
Table 1 shows the findings of elemental analyses (C, H, and N) with the percentage contents of the metal and chloride together with the molecular formula, molecular weight, melting points, color, yield percentage and molar conductivity. The stoichiometry of the current complexes was revealed to be 1:1 (H2Erx-en: Metal), which was consistent with the predictions of the ML-type formula over all complexes, as evidenced by elemental analyses results. All of the complexes mentioned here are hydrates in various states of hydration. The compounds are accessible in DMF and DMSO mediums, and their melting points are high and abrupt, demonstrating their purity. The molar conductivity values of the synthetic compounds obtained at room temperature in DMSO as a solvent are demonstrated Table 1. The molar conductivity values of the chelates (1), (2), (3) and (4) were found to be 90.25, 88.37, 14.67, 89.12 Ω−l mol−1 cm2, respectively. These data clearly showed the chelates of Fe(III), Y(III) and La(III) were ionic in character and electrolytes, while the Zr(IV) complex was a non-electrolyte [15,16,19].

Table 1.
Elemental analysis and physico-analytical data for H2Erx-en and its metal complexes.
2.2. IR Spectra and Mode of Bonding
Infrared spectral analysis is the most reliable method for supporting the site of ligand attachment to metal ions. The positions or intensities of the peaks should change after coordination. The infrared spectrum of H2Erx-en manifests the obscurity of the bands’ pertinence to the v(-NH2) group of ethylenediamine and of the ν(C=O) of pyridone group for enrofloxacin. Instead, a new, very strong band at 1626 cm−1 for ν(C=N) Table 2 is acquired showing the complete condensation of NH2 groups with the pyridone group forming H2Erx-en (Figure S1) [15,16,20]. The IR spectra of complexes display discrete differences when compared to the H2Erx-en spectrum, signifying complexation. The IR spectra of all complexes containing hydration and or coordination water molecules reveal bands at 3437–3410 cm−1, related to the v(O-H) vibration mode of the water molecules [21,22,23]. The prominence of bands in the spectra of all complexes approximately 3350, 840, and 600 cm−1 was ascribed to v(O-H), the rocking and wagging vibration for coordinated water molecules [24,25]. These data were assured by thermal analysis, mass spectrometry as well as 1H NMR. The v(COOH) and v (C=N) have been associated with the two bands found in the spectrum of H2Erx-en at 1735 and 1626 cm−1, correspondingly (Scheme 2) [26,27,28]. The disappearance of the band at 1735 cm−1 in all complexes and a lower value for the v(C=N) of the characteristic azomethine group’s band demonstrate that the H2Erx-en molecule is chelated to metal ions through N2 and O2 atoms [29,30]. In the case of monodentate carboxylate ligand in our complexes, with an average of v > 200 cm−1, the anti-symmetric and symmetric (COO)- stretches will be relocated to higher and lower frequencies, correspondingly [31,32,33]. The subsistence of vas (COO−) in the 1629–1627 cm−1 domain and vs(COO−) in the 1388–1307 cm−1 range with ∆ν >200 cm−1 demonstrates that the COO− interacts as monodentate through one of the oxygen atoms [18,34]. The characteristic band at 807 cm−1 in ZrO(II) complex has been assigned to the v(Zr=O) vibration [29]. The bands observed in the spectra of complexes in the domain 636–507 cm−1 were ascribed to v(M-O) and v(M-N), correspondingly [24,25,26,27,28,29,35].

Table 2.
Selected infrared wave numbers (cm−1) for H2Erx-en and its metal complexes.
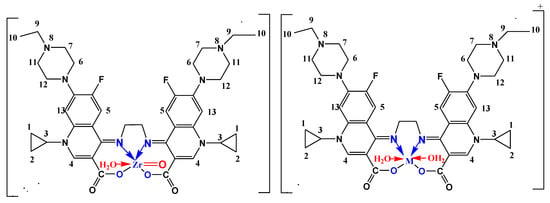
Scheme 2.
Coordination mode of Fe (III), Y (III), Zr (IV) and La (III) with H2Erx-en.
2.3. Electronic Absorption Studies and Magnetic Moment Measurements
To comprehend the electronic structure of our complexes, UV–Vis spectra were registered in dimethyl sulfoxide in the range 200–800 nm (Table 3). The intra ligand transitions (π-π * and n-π *) for H2Erx-en were accountable at 286 and 328 nm (Figure S2) [36,37]. In the comparison of the UV–Vis spectra of the current metal chelates with that of the free ligand, we showed the persistence of the ligand bands in all complexes. However, the bands were slightly shifted to blue or red regions of the spectrum in all complexes [38,39]. The absorption and amplitude variations in the spectra of metal chelates are most probably triggered by metal ions, which amplifies the conjugation and delocalization of the entire electronic system, culminating in an energy change in the conjugated chromospheres’ π-π * and n-π * transition [40]. The consequences distinctly articulate that the ligand interacts with Fe(III), Y(III), Zr(IV) and La(III) ions in stratification with other spectral datum, moreover, as expressed by modern molecular orbital theory [41]. The complexes manifested new bands in the range of 458–488 nm, which may be ascribed to a combination of ligand–metal charge transfer [42,43]. The electronic spectrum of Fe(III) complex reveals an absorption band at 17,793 cm−1, which equates to 6A1-4T2 (G) transitions, and a noticeable magnetic moment estimate of 1.80 B.M, demonstrating that the complex seems to have a low octahedral spin and the other complexes (2), (3) and (4) are diamagnetic [15]. The molar absorptivity (ε) of complexes specified from their electronic spectra was documented (Table 3) through by means of relation: A = εcl, where A = absorbance, c = 1 × 10−3 M, l = length of cell (1 cm).

Table 3.
UV-Vis. spectra for H2Erx-en and its metal complexes.
2.4. 1H NMR Spectra
The 1H NMR spectra of H2Erx-en and its chelates were restricted in (DMSO-d6) solution applying tetramethyl silane (TMS) as an internal standard. 1H NMR spectrum of H2Erx-en: δ (1.03–1.32) (1,2) (d, J = 0.87, 4H, -CH2 cyclopropane), (1.42–1.46) (10) (m, J = 0.12, 3H, -CH3 piperazine), 1.64 (3) (s, J =4.92, 1H, -CH cyclopropane), 2.39 (9) (s, J= 7.17, 6H, -CH2 piperazine), 2.59–3.62 (8) (s, J = 3.09, 2H, -NH piperazine), 7.55–8.65 (4,5, 6,7, 11 and 13) (d, J = 3.30, 2H, HAr) (Table 4) [44]. Figure S3 demonstrates the distinguishing singlet (COOH) at δ: 11.13 ppm to the proton of carboxylic acid. The carboxylic proton (COOH) is not detectable in spectra of the complexes, implying that H2Erx-en is coordinated through its carboxylate oxygen atom [18,45]. Furthermore, the 1HNMR spectra for complexes demonstrate a new peak at 4.55–5.25 ppm, attributable to the subsistence of H2O in the complexes [18]. When the principal peaks of H2Erx-en are matched to the spectra of its complexes, it is discovered that all of H2Erx-en peaks are preserved in the spectra of the complexes with chemical alteration upon attachment of the H2Erx-en foundation to the metal ion [45].

Table 4.
1H NMR values (ppm) and tentative assignments for H2Erx-en and its metal complexes.
2.5. Mass Spectrometry
Under ionization conditions, the ligand, metal ion, counter ions, solvent, temperature, concentration, and other parameters all inspire the structure and consistency of coordination complexes. The mass spectrum of H2Erx-en endorses the proposed structure (Figure S4). The H2Erx-en exhibited (M.+) at m/z = 742 (55.12%) and the molecular ion peak [a] eliminates C3H5, which provides fragment [b] at m/z = 701 (80.32%). It moreover misses C6H10 to afford [c] at m/z = 660 (64.51%), and furthermore it misses C6H13N2 to afford [d] at m/z = 633 (69.78%) and it eliminates C12H26N4, C8H12O4, C14H28N4O4 and C20H38N4O4 to produce [e] at m/z = 524 (58.34%), [f] at m/z = 570 (85.89%), [g] at m/z = 434 (56.29%) and [h] at m/z = 352 (66.37%) (Scheme 3). The mass spectrometry was utilized to mark the fragmentation characteristics of our thoughtful complexes. The mass spectrometry of complexes demonstrated molecular peaks at m/z = 957 (83.23%), 1008 (78.31%), 956 (85.98%) 1058 (75.14%) for Fe(III), Y(III), Zr(IV), and La(III), respectively. The attributable products’ molecular weights coincided with observation data of the elemental and thermogravimetric research. Fragmentation design of complex (4) is specified as a model (Scheme 4). The molecular ion peak [a] seemed to be at m/z = 1058 (75.14%) misses H2O to give [b] at m/z = 1040 (85.42%) and eliminates 3H2O to provide [c] at m/z = 1004 (60.00%). The [a] misses Cl·6H2O to give [d] at m/z = 914 (45.20%) and it misses C9H19O4Cl to afford [e] at m/z = 909 (84.37%). The molecular ion peak [a] losses C6H22O6 to afford [f] at m/z = 868(74.96%) and it misses C6H22O6Cl and to produce fragments [g] at m/z = 833 (84.47%).
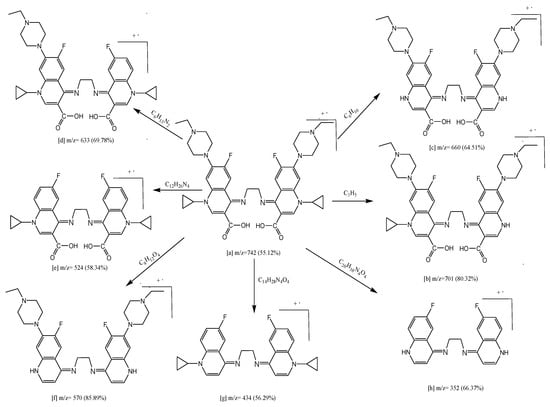
Scheme 3.
Fragmentation pattern of H2Erx-en.
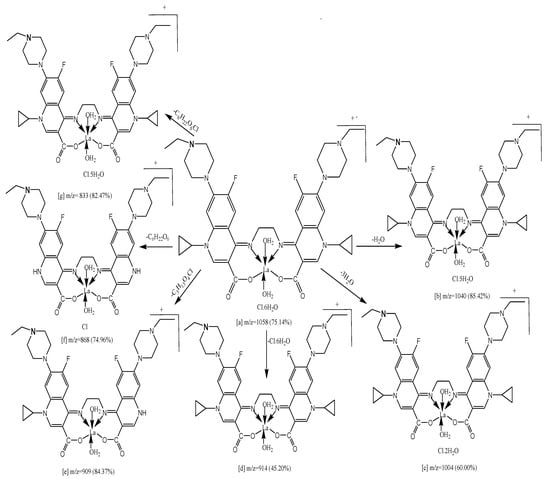
Scheme 4.
Fragmentation pattern of complex (4).
2.6. Thermal Analysis Studies (TG and DTG)
According to the research, the constituents of solid complexes decompose in the following order: lattice, coordinated H2O, anion, chelating agent and sediments, which correlate to either metal oxide or free metal. The simultaneous TG/DTG analysis of H2Erx-en, Fe(III), Y(III), Zr(IV), and La(III) metal chelates was studied from ambient temperature to 1000 °C under a nitrogen atmosphere using α- Al2O3 as the reference. The TG of H2Erx-en progressed in one phase with an estimated mass loss of 100.00% (calc. 100.00%) at one maximum temperature (357 °C), which may be accredited to the loss of 20 C2H2 + 2 HF + 2 NH3 + 4 NO + N2. TG of complex (1) has been split at two stages: the first stage, at one maximum temperature 60 °C accompanying of 9.30% (calc. 9.39 %), represents the loss of five hydration H2O. The second phase, at 237 and 308 °C maxima with a mass loss of 82.25% (calc. 82.28 %), may contribute to the consequent decrease of coordinated H2O and H2Erx-en. The TG of complex (2) underwent three steps of decomposition: the first step at a maximum temperature of 71 °C with a loss of 10.60 % (calc. 10.70%) attributed to the elimination of six hydration H2O. Two successive steps within the maxima temperatures at 224, 323 and 416, 688, and 765 °C are related to loss of 18 C2H2 + 2.5 H2O and CO + 2 HF + HCl + NO + 2 NH3 + 2.5 N2 with a mass loss of 74.56 % (calc. 74.55%), leaving 0.5 Y2O3 + 3 C as residue. The complexes (3) and (4) showed liberation of hydrated, coordinated water and the non-coordinated part of the ligand in the first and second steps. The third step is accounted for by the decomposition of the ligand and the leaving of ZrO2 + C and 0.5 La2O3 + 3 C residues (Table 5). According to the thermogravimetric analysis, this indicates that the adsorbed water molecules (lattice of water) on the surface of our complexes was desorbed at Tmax from 50–100 °C. Meanwhile, the coordinated oxygen atoms of H2O and H2Erx-en decomposed from 190–390 °C (Table 5); these data indicate the high stability of our complexes in the solid and solution states.

Table 5.
The maximum temperature Tmax (°C) and weight loss values of the decomposition stages for H2Erx-en and its metal complexes.
2.7. Calculation of Activation Thermodynamic Parameters
The kinetic and thermodynamic parameters including (Ea) the activation energy, (ΔS *) the entropy of activation, (ΔH *) the enthalpy of activation, and (ΔG *) free energy change, together with correlation coefficients of Arrhenius plots (r) and standard derivation (SD), have been determined by Coats–Redfern [46] and Horowitz–Metzger [47] models (Figure S6). Coats–Redfern equation:
Horowitz–Metzger equation:
The higher (Ea) values specified in Table 6 demonstrate the complexes’ thermo stability. The mounting of ΔG* value in sequential decomposition stages means that the rate of removal of chelated Erx-en will be slower than the prior H2Erx-en, and thus will excess from one phase to the next. Comparative to the anterior complex that requires further energy, this competence is attributable to the constitutional residual stiffness complex subsidiary exemption of one or more H2Erx-en for its reorganization previously approaching any amendment. The (ΔS *) over all complexes had been set to be a negative value that further defined their stability [48]. The positive values in (ΔH *) denote an endothermic degradation process.

Table 6.
Thermal behavior and kinetic parameters determined using Coats–Redfern (CR) and Horowitz–Metzger (HM) methods operated for H2Erx-en and its metal complexes.
2.8. Antimicrobial Investigation
The antimicrobial effectiveness of H2Erx-en and its metal complexes was evaluated against four species of bacteria Bacillus cereus (B. cereus) GST4, Staphylococcus aureus (S. aureus) ATCC6538 (G+ve), Escherichia coli (E. coli) ATCC11229 and Salmonella typhi (Salm. typhi) ATCC14028(G-ve) and two fungi, Candida albicans (C. albicans) OC10 and Penicillium vulpinum (P. vulpinum) CM1(Figure 1). Ciprofloxacin, amikacin, cefuroxime, cefpodexime and ceftazidime were utilized as positive standards against the respective bacteria. Ethylenediamine and DMF were utilized as the control. The antimicrobial potency was established by the magnitude of inhibition and the zone generated around the wells in the plates’ compounds. H2Erx-en is quite effective against S. aureus, B. cereus and Salm. typhi with no growth inhibition against E. coli, C. albicans and P. vulpinum (Table S1). Complexes (1), (2) and (3) demonstrated high significant activity against S. aureus and B. cereus, and complexes (3) and (4) revealed very high significant activity against Salm. typhi and B. cereus, respectively. The compounds showed that there is no growth inhibition against the two fungi species. The lowest MIC for Salm. typhi was observed in the case of complex (4) at 0.50 μg/mL, followed by H2Erx-en. The MIC for S. aureus was 0.75 μg/mL for complexes (1), (2) and (3), whereas it was 1.00 μg/mL in case of H2Erx-en at. The lowest MIC for B. cereus was observed in case of complex (1) at 0.50 μg/mL, followed by complex (4) at 0.75 μg/mL (Figure 2). Such amplified effectiveness of the metal complexes can be elucidated on the foundation of chelation theory [49]. This would imply that chelation could aid a complex’s capacity to permeate the microbe cell membrane, as suggested by Tweedy’s chelation theory [50,51,52]. Pathogens that release a large number of enzymes responsible for activity breakdown appear to be particularly prone to sophisticated ion deactivation. The metal complexes make it easier to diffuse through the spore membrane’s lipid lattice to the action site. The performance of metal complexes versus distinct organisms differs due to the impermeability of microorganism cells or variances in microbial cell ribosomes [49,50]. The activity index for the complexes was calculated using Equation (5) and is shown in the Figure 3.
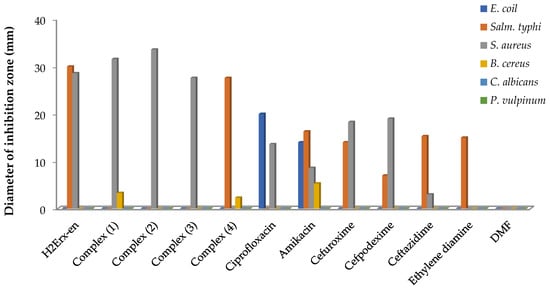
Figure 1.
Antimicrobial activity of H2Erx-en and its metal complexes.
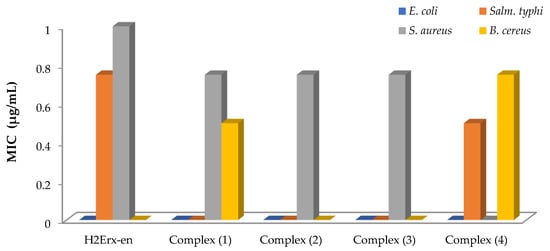
Figure 2.
MIC for the sensitive bacteria for H2Erx-en and its metal complexes.
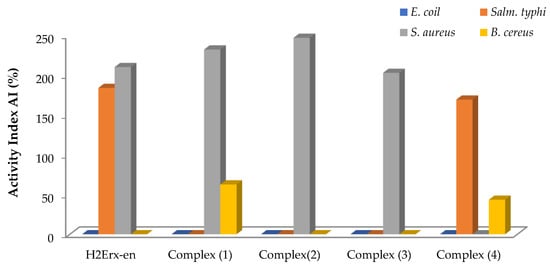
Figure 3.
Activity index of H2Erx-en and its metal complexes.
The current obtained data shown in Table 7 demonstrated that the inhibition zone of [ZrO(Erx-en)(H2O)]·5H2O is very highly significant in the case of S. aureus, while Zr(IV)/Erx [11] are very highly significant in the case of E. coli. Additionally, in case of S. aureus, the complex [Fe(Erx-en)(H2O)2]Cl·5H2O is more effective than previous work [53].

Table 7.
The comparison between Zr(IV) and Fe(III) complexes in our complexes and some previous works.
2.9. Structural Parameters and Models
2.9.1. Structural Parameters and Model of H2Erx-en
The optimized geometric parameters for H2Erx-en as determined by B3LYP/CEP-31G calculations are provided in Table S2. The cyclopropyl ring of H2Erx-en does not lie in the same plane as the quinolone ring (Scheme 5), and the dihedral angles of C6N9C13C15 and C6N9C13C14 are 61.16° and 126.91°, respectively. The calculated dihedral angles for C4C1N20C25, C1N20C25C24, and C21N20C1C4 are 90.62°, 136.88°, and −81.72°, respectively, supporting this observation. The values are not 0 or 180 degrees. O11 and O17 are in a cis configuration and C8O11 is in the same plane of C16O17, while O11 and O18 are in a trans configuration, and C8O11 is not in the same plane of C16O18. The dihedral angles O11C8C12C16 are 7.46° (almost 0.0), C8C12C16O17 is −4.02° (almost 0.0), and C10C12C16O18 [54]. The charge density is built up on the O atoms and is distributed over H2Erx-en, which reacted as a tetradentate ligand with a highly dipole µ = 11.991 with energy value −32,133.449 k [54]; the optimized geometrical structure reflects that the three donating atoms (N1, N4 and O10) lie in the same plane while O9 is lying on the other plane. The equilibrium geometric parameters data reflect an sp2 hybridization [54,55,56].
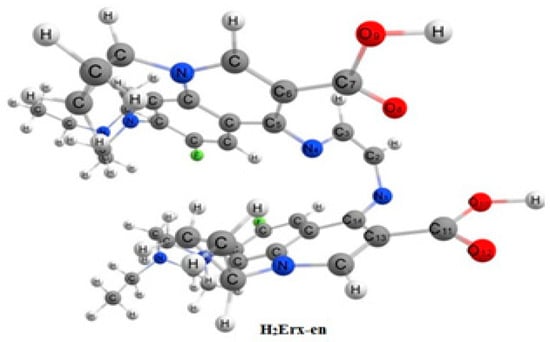
Scheme 5.
Optimized geometrical structure of H2Erx-en by using DFT calculations.
2.9.2. Molecular Modeling of Complexes
Complex (1) consists of one unit of Erx-en molecule bonded with Fe(III) through O9, O10, N1 and N4. The Fe(III) completes the distorted octahedral structure by O16 and O15 (Scheme S1). The atoms N1, N4, O9, and O15 are the only ones that occupy the equatorial plane. O10 and O16 are located in the axial plane. For O15-Fe-O16, N1-Fe-O9, N4-Fe-O15, O10-Fe-O16, N4-Fe-O10, and O15-Fe-O16, the bond angles varied between 76.69° and 166.07° (Table S3); these values were a little off from an ordinary octahedron. The measurements of the oxygen and nitrogen bonds with Fe(III) (O9, O10, O15, O16, N1 and N4) are respectively 1.935, 1.930, 1.999, 2.111 1.982, and 1.9739 Å [57,58,59]. These values demonstrated that the two Erx-en terminals are perpendicular to each other and O15 and O16 are lying in cis form. The total energy value of this complex is −347,128.827 kcal/mol and this complex has a lower dipole moment at 12.852 D.
The Y(III) was chelated with one molecule of Erx-en and two water molecules to complete the coordination sphere of the octahedral structure (Scheme S2). O9, O10, N1, and N4 are the only four atoms that occupy the equatorial plane of Erx-en and the axial plane containing O15, O16. The bond angles varied between 70.37° to 151.06° for N1-Y-O15, N1-Y-O16, N4-Y-O16 and O15-Y-O16 (Table S3) [60,61,62,63,64]. The bond lengths between Y(III) and their coordinated atoms were calculated (Table S3). The total energy value of this complex is −333986.825 kcal/mol and this complex has a slightly higher dipole moment at 14.597D.
For complex (3), the Zr(IV) coordinated with Erx-en through O9, O10, N1, N4 and O15 (Scheme S3). The equatorial plane contains N1, N4, O9 and O16 while the axial plane is occupied by O10 and O15. The bond lengths, bond angles, dipole moment and the calculated energy are recorded in (Table S3) [65,66,67].
For complex (4) the equilibrium geometric parameters are listed in (Table S3). The findings indicated that Erx-en molecules are not lying parallel to each other but rather perpendicular to each other, with (O9, O10), N1 of Erx-en and O16 occupying the equatorial plane [68,69]. N4 and O15, the remaining two coordinated atoms, are found in the axial position.
2.9.3. Charge Distribution Analysis
Natural population analysis (NPA) served as the foundation for the charge distribution analysis of H2Erx-en and its compounds. N1, N4, O9, and O10 have charge distributions of −0.285, −0.087, −0.362, and −0.391, respectively (Table S4). When donations were made through N1 and N4, rather than O9 and O10, these negative values were changed to lower ones (Table S4). Additionally, the two terminal carbonyl groups of H2Erx-en have charges on O8 and O10 of −0.454 and −0.429, respectively, which show a large increase in charge density on N1 and N4 as well as O9 and O10. These values suggest that there are total negative and positive poles on H2Erx-en with a dipole of μ = 10.56 D. Each charge density of H2Erx-en and its chelate shows a high charge density on Y(III) and La(III) complexes with charge accumulations of 0.448 and 0.406, respectively, while Fe(III) and Zr(IV) ions have a lower charge density (0.089 and 0.307), indicating a strong donation from H2Erx-en donor sites. The minus charge is distributed on N1, N4, O9 and O10 of H2Erx-en, except for C6 and C13, which are immediately connected to the two carboxylate groups; all carbon atoms in every chelate have a positive charge. The charge on N1 and N4 of H2Erx-en was decreased from −0.285 and −0.087 to inferior appreciates in each metal chelate, whereas the charge on O9 and O10 was transferred to greater negative values (−0.362 and −0.391) in Table S4. The nitrogen atoms N1 and N4 connected to the carbons C5 and C14 exhibit larger positive values, indicating back-donation from the metal ions to the Erx-en π * orbitals. The distribution of atomic charges, which is influenced by the locations of the positive and negative charge centers, determines the direction of the dipole moment vector in compounds.
2.9.4. Frontier Molecular Orbitals
The value of ΔE (HOMO-LUMO gap), which varies depending on the type of metal ion, is closely related to the executed molecule’s chemical reactivity and stability. All complexes (Table S4) have lower ∆E values than H2Erx-en, making them more reactive than H2Erx-en. A more reactive electronic system may exist than one with a smaller value of ∆E. [70,71]. A smaller value La(III) complex is 0.005 eV more reactive than other complexes. The iso-density surface plots of HOMO and LUMO for H2Erx-en and its complexes are shown in Figure 4. Ionization energy (I) and electron affinity (A) are two variables that determine the hardness (η), which is defined as (η = (I−A)/2). On the other hand, between the HOMO and LUMO, (I−A) equals ∆E. As a result, the H2Erx-en ligand and its complexes’ hardness can be determined using the formula (η = ∆E/2). Soft molecules have a smaller ∆E while hard molecules have a higher ∆E [72]. Table S4 provides the values for η and E. The table clearly shows that each complex is soft, with values ranging from 0.0025 for the La(III) complex to 0.0105 for the Y(III) complex, while for the H2Erx-en it is = 0.0315. Complexes’ values for ∆E are consistent with those for stable transition metal complexes. Quantum chemical parameters such as global softness (S), electro negativity (χ), absolute softness (σ), chemical potential (ω), global electrophilicity (Pi), and additional electronic charge (Nmax) of the free ligand and investigated complexes were calculated based on the energies of HOMO and LUMO. According to these values, the Y(III) complex is considered to be a hard complex (σ = 95.238 eV), whereas the La(III) complex is considered to be absolutely soft (σ = 400 eV). The (σ) of (H2Erx-en) is 31.476 eV, and all investigated complexes are regarded as soft complexes with respect to the free ligands.
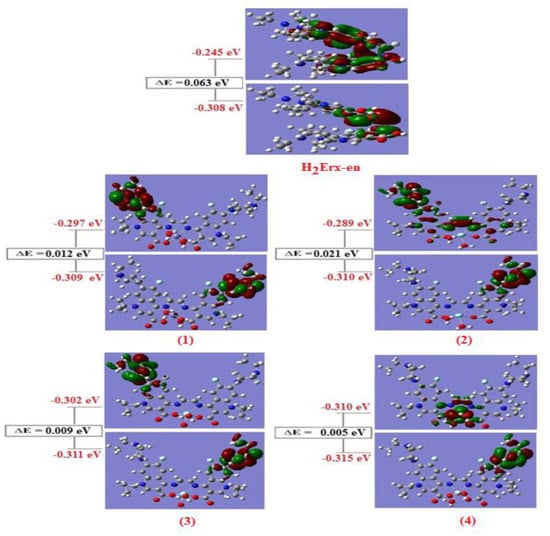
Figure 4.
Molecular orbital surfaces and energy levels of studied complexes by using DFT calculations.
2.9.5. Excited State
The G03W program produces an appropriate version of the UV-visible Spectroscopy when used with the TD-DFT at the B3LYP tier [73,74,75]. In order to calculate discrete transition energies and oscillator strengths, time-dependent density functional response theory (TD-DFT) has lately been recast and applied on a range of different particles and atoms. The hybrid functional method suggested by Bauernschmitt and Aldrich’s [76] was taken into account in the excitation energies computation. Compared to traditional Hatree-Fock (HF) based approaches, these hybrid methods are often a substantial advancement. In this work, the wave functions of SCF MOs were extensively explored, and the optimal architecture was derived and implemented in all subsequent computations. The calculated wave functions of the different MOs show and infer the percentage of privileged chelate fragments that contribute to the overall wave functions of different levels. The results reveal that there is some electron delocalization in the different chemical subshells. The HOMO and LUMO may function as electron donors and acceptors, respectively, in a reaction pattern. The HOMO electron density for the H2Erx-en ligand is concentrated on the central ethylenediamine by 62.3 percent, with the remaining 27.7 percent concentrated on the two pyridine rings of the two terminal Enrofloxacin molecules, as seen in Table S5. With the exception of the two groups of ethyl–piperazin-1-yl rings at the two terminal ends, the LUMO electron density is scattered and distributes across all H2Erx-en atoms. Both complexes have a localized electronic density for the HOMO state that ranges from 99.7% for the Fe(III) chelate to 100% for the Y(III), Zr(IV), and La(III) chelates. All complexes have a localized electronic density for the HOMO state that ranges from 99.7% for the Fe(III) complex to 100% for the Y(III), Zr(IV), and La(III) complexes. With the exception of the La(III) chelate, where there is delocalization of electron density that spreads over all atoms of the complexes with a high percentage of metal ions above 66.8 percent, the placement of electron density in the case of LUMO is also placed on Erx-en with a percentage that varies between 72.4 percent in the case of Y(III) complex and 100 percent in the case of Fe(III) and Zr(IV) complexes (Table S5). Based on DFT calculations, Frontier molecular orbitals, excited states, softness, and ∆E in addition to the Mülikan charge accumulated over all involved atoms on these complexes, the total energies of the optimized geometrical structures at ground state for the complexes under study were measured and used to construct thermal stability for all complexes. According to the dipole moment values and optimum energy values shown in Table S4 and stated in the text, the relationship between the positions of oxygen atoms in water molecules has a significant impact on how stable the complexes under study are. Thermal stability for all complexes was built on the measured total energies of the optimized geometrical structures at ground state for the studied complexes using DFT calculations, frontier molecular orbitals, excited states, softness and E, in addition to the Mülikan charge accumulated over all atoms involved in these complexes. The position of the oxygen atoms of water molecules with respect to each other plays an important role on the degree of stability of the studied complexes and their stabilities are reflected by the dipole moment values and optimized energy values as reported in the text and listed in Table S4. The order of complexes according to stability is Zr(IV) > Y(III) > La(III) > Fe(III) with the total energies values varying between −347,128.827 kcal/mol for Fe(III) complex to −333,922.301 kcal/mol for Zr(IV) complex.
3. Materials and Methods
3.1. Materials and Reagents
The chemicals and solvents handled were of the analytical reagent grade and we utilized no more additional purification. Enrofloxacin (H2Erx), ethylenediamine (-en), FeCl3, YCl3.6 H2O, ZrOCl2.8 H2O, LaCl3.7 H2O, NaOH, K2CrO4, AgNO3, absolute ethyl alcohol, dimethylformamide (DMF), dimethyl sulfoxide (DMSO), conc HNO3 69%, H2O2 20%, EDTA disodium (C10H14N2Na2O8), NH4OH, NH4Cl, 4-Amino-4′-methoxydiphenylamine (C13H14N2O), Solochrome Black T (C20H12N3NaO7S) and Alizarin violet (C20H12O7) were provided from Obour Pharmaceutical Industrial Company, Obour, Egypt, Sigma-Aldrich Chemicals, Burlington, MA, United states. All glass was immersed in a chromatic combination (K2Cr2O7 + conc. H2SO4) nightly, then scrubbed completely utilizing doubly distilled water, then baked at 100 °C to dry.
3.2. Computational Method
DFT was utilized for estimate design variables and energies at a B3LYP functional model with a Cep-31G basis set via running the Gaussian 98W suite of software [77], The elevated base sequence was used to identify the energy with great precision. The natural atomic orbital populations were used to calculate the atomic charges. The crucial term for the hybrid functional is the B3LYP; it is a linear function of the previously mentioned integral grade [78,79,80,81,82].
3.3. Preparation of H2Erx-en Schiff base (C40H48F2N8O4)
An ethanolic mixed solution produced by H2Erx (4 mmol, 1.42 g) and ethylenediamine (2 mmol, 0.12 mL) was refluxed for 4 h in the subsistence of 1 mL glacial ethanoic acid. The final mixture was placed in a water bath and chilled to 0 °C. The dark red precipitate was filtered out, scrubbed with ethanol three times, and desiccated with suction on CaCl2.
3.4. Preparation of Metal Complexes
The reddish brown [Fe(Erx-en)(H2O)2]Cl·5H2O complex (1) was made by combining 0.5 mmol (0.371 g) H2Erx-en with 1 mmol (0.04 g) NaOH in 50 mL pure ethanol, and 0.5 mmol (0.081 g) FeCl3 in 20 mL ethanol was added. After 3 h of refluxing, the precipitate was filtered and desiccated. The pale brown, yellow, and white solid complexes [Y(Erx-en)(H2O)2]Cl·6H2O (2), [ZrO(Erx-en)(H2O)]·5H2O (3) and [La(Erx-en)(H2O)2]Cl·6H2O (4) were made using a procedure similar to the mentioned above using YCl3·6H2O (303.36 g/mole, 0.5 mmol 0.1516 g/mole), ZrOCl2.8H2O (322.25 g/mole, 0.5 mmol 0.1611 g/mole) and LaCl3·7H2O (371.37 g/mole, 0.5 mmol 0.1856 g/mole), respectively.
3.5. Instruments
The FT-IR 460 PLUS Spectrophotometer was applied to record IR spectra in KBr discs in the ambit of 4000–400 cm−1. The 1H NMR spectra were acquired employing DMSO-d6 as the solvent on a Varian Mercury VX-300 NMR Spectrometer. Electronic spectra were accomplished by a UV-3101PC Shimadzu. The absorption spectra were recorded as solutions in DMSO-d6. Mass spectrometry was achieved in the domain 0–1090 utilizing a GCMS-QP-1000EX Shimadzu (ESI-70ev). The TGA-50H Shimadzu was also applied for TG-DTG measurements in an N2 atmosphere in the temperature range of room temperature to 1000 °C. The M percentage was evaluated using complex metric titration, thermogravimetric analysis (by changing the solid products into metal oxide) and atomic absorption using a spectrometer of type PYE-UNICAM SP 1900 [83,84] fitted with the correspondent lamp was utilized for this intentional [18]. A Perkin Elmer 2400 CHN elemental analyzer was used to accomplish the analyses. On a Buchi apparatus, melting points were acquired. The magnetic susceptibilities of the powdered materials were studied on a Sherwood scientific magnetic scale with a Gouy balance and Hg[Co(CSN)4] as the signing, the molar conductance of 1 × 10−3 M solutions of the ligands and their complexes in DMF was investigated utilizing CONSORT K410. All experiments were performed at room temperature employing freshly prepared solutions.
3.6. Antimicrobial Investigation
3.6.1. Antibacterial Activity Assay
B. cereus GST4, S. aureus ATCC6538 (G+ve), E. coli ATCC11229 and Salm. typhi ATCC14028(G-ve) were identified from a patient suffering from a respiratory illness at the Microbiology Division, Medicine College, Zagazig University, Egypt. It was discovered in a urinary specimen from an infection of the bladder victim at El-Ahrar Hospital, where it was analyzed for Schiff base and its chelates utilizing a previously enhanced reported methodology of Beecher and Wong [85]. The bactericidal nutritional agar medium utilized was Müller Hinton agar [86,87,88]. The liquid was therefore refrigerated to 47 degrees Celsius before the microbes from the analysis were planted into it. The layer of sterile water agar was placed over the produced growing media for microbial pathogens. After gelation, seeded agar plates with 5 mm diameter discs were aseptically placed onto them, and incubated procedures were provided. Utilizing the plate diffusion technique, the inhibitory zone diameters were measured to evaluate the antibacterial activity [89]. After dissolving in DMF at 1.0 × 10−3 M, the examined compounds were inserted into Petri dishes (only 0.1 mL). These culture plates were then cultured for bacteria for 20 h at 37 °C. The activity was marked using the inhibitory zone’s diameter (in millimeters). Amikacin (AK-30 mcg), cefuroxime (CXM-30 mcg), cefpodoxime (CPD-30 mcg), ceftazidime (CAZ-30 mcg) and ciprofloxacin (CIP-5 mcg) were used as positive reference standards.
3.6.2. Antifungal Activity Assay
According to traditional microbiology and bacteriological codes for correspondence of microbes as stock cultures in the microbiology lab, Faculty of Science, Zagazig University, Egypt, the antifungal examination was clarified against C. albicans OC10 and P. vulpinum CM1, which were identified from a patient retinal ulceration case. By measuring the widths of the inhibition zones, the disc diffusion technique was digitized to investigate the Schiff base and its chelates [86,89,90]. As standard values for assessing the sensitivity of fungal pathogens, the antibiotic discs Nystatin (NS-100 U) and Fluconazole (FU-10 mcg) were used. The antibiotic plates were obtained from Hi-media Laboratories Ltd.
To determine the minimum inhibitory concentration (MIC) of H2Erx-en and its chelates versus S. aureus, B. cereus, E. coli, Salm. typhi, C. albicans and P. vulpinum in LB broth, the standard broth micro dilution method was used [91]. Tested compounds were included in concentrations from 0.25 to 1.00 μg/mL.
4. Conclusions
Thermal investigations and different spectroscopic techniques were used to identify H2Erx-en of Fe(III), Y(III), Zr(IV) and La(III) complexes. The practical data support that H2Erx-en reacted with the metal ions through the two nitrogen atoms of azomethine groups and two oxygen atoms of carboxylate groups and completed distorted octahedral with two or one water molecules. The thermodynamic parameters of the compounds were calculated using the Coats–Redfern and Horowitz–Metzger procedures. The data of DFT calculations showed that the computed and experimental geometrical parameters coincide quite well. The La(III) complex with a smaller ΔE value (0.005 eV) is more reactive than other complexes with higher ΔE values Table S4. Complexes Fe(III), Y(III), and Zr(IV) demonstrated very extremely significant antibacterial activity data against S. aureus and B. cereus. Additionally, complexes Zr(IV) and La(III) showed extremely significant antimicrobial activity against Salm. typhi and B. cereus, respectively.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics10110177/s1. Figure S1. Infrared spectra for H2Erx-en and its metal complexes. Figure S2. Electronic absorption spectra for H2Erx-en and its metal complexes. Figure S3. 1H NMR spectra for H2Erx-en and its metal complexes. Figure S4. Mass spectra diagrams for H2Erx-en and its metal complexes. Figure S5. TG and DTG diagrams for H2Erx-en and its metal complexes. Table S1. The inhibition diameter zone (mm) values for H2Erx-en and its metal complexes. Table S2. Equilibrium geometric parameters bond lengths (Å), bond angles (°), dihedral angles (°), total energy (k cal/mol) and dipole moment of H2Erx-en using DFT calculations. Table S3. Equilibrium geometric parameters bond lengths (Å), bond angles (°), dihedral angles (°), Total energy (k cal/mol) and Dipole moment of the studied complexes using DFT calculations. Table S4. Calculated charges on donating sites and energy values (HOMO, LUMO, Energy gap ∆E/eV, hardness (η), global softness (S), electro negativity (χ), absolute softness (σ), chemical potential (Pi), global electrophilicity (ω) and additional electronic charge (∆Nmax) of H2Erx-en and its metal complexes using DFT calculation. Table S5. Composition of the frontier molecular orbital for the obtained stable complexes using DFT/B3LYP/Cep-31G. Scheme S1. Optimized geometrical structure of complex (1) using DFT calculations. Scheme S2. Optimized geometrical structure of complex (2) using DFT calculations. Scheme S3. Optimized geometrical structure of complex (3) using DFT calculations. Scheme S4. Optimized geometrical structure of complex (4) using DFT calculations.
Author Contributions
Conceptualization, A.A.M.; Data curation, W.A.Z., W.H.E.-S. and H.S.E.; Formal analysis, A.A.M. and F.M.A.; Investigation, A.A.M., F.M.A., W.A.Z., W.H.E.-S. and H.S.E.; Methodology, A.A.M. and F.M.A.; Resources, S.A.S.; Software, F.M.A. and W.H.E.-S.; Supervision, W.A.Z., S.A.S. and H.S.E.; Validation, S.A.S.; Writing-original draft, A.A.M., W.A.Z., W.H.E.-S. and S.A.S.; Writing-review & editing, H.S.E. All authors have read and agreed to the published version of the manuscript. Figure S6. The diagrams of kinetic parameters of H2Erx-en and its metal complexes using Coats-Redfern(CR) and Horowitz-Metzger (HM) equations.
Funding
This research received no external funding.
Institutional Review Board Statement
We declare that our research does not involve any human subjects, human materials, human tissues, or human data.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dhar, D.N.P.; Saxena, N.; Kumar, S. Applications of metal complexes of Schiff bases-A review. J. Sci. Ind. Res. 2009, 68, 181–187. [Google Scholar]
- Elshwiniy, W.H.; Ibrahim, A.G.; Sadeek, S.A.; Zordok, W.A. Synthesis, structural elucidation, molecular modeling and antimicrobial studies of 6-(2-hydroxyphenylimine)-2-thioxotetrahydropyrimidin-4(1H)-one (L) Schiff base metal complexes. Appl. Organomet. Chem. 2021, 35, e6174. [Google Scholar]
- Mohamed, A.A.; Elshafie, H.S.; Sadeek, S.A.; Camele, I. Biochemical Characterization, Phytotoxic Effect and Antimicrobial Activity against Some Phytopathogens of New Gemifloxacin Schiff Base Metal Complexes. Chem. Biodivers. 2021, 18, e2100365. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, P.; Gunasekaran, S.; Manikandan, A. Structural, spectral analysis of ambroxol using DFT methods J. Mol. Struct. 2017, 1144, 379–388. [Google Scholar] [CrossRef]
- Shargh, H.; Nasseri, M.A. Schiff-Base Metal (II) Complexes as New Catalysts in the Efficient, Mild and Regioselective Conversion of 1,2-Epoxyethanes to 2-Hydroxyethyl Thiocyanates with Ammonium Thiocyanate. Bull. Chem. Soc. Japan. 2003, 76, 137–142. [Google Scholar] [CrossRef]
- Turel, I.; Leban, I.; Bukovec, N. Crystal structure and characterization of the bismuth(III) compound with quinolone family member (ciprofloxacin). Antibacterial study. J. Inorg. Biochem. 1997, 66, 241–245. [Google Scholar] [CrossRef]
- Efthimiadou, E.; Karaliota, A.; Psomas, G. Mononuclear dioxomolybdenum(VI) complexes with the quinolones enrofloxacin and sparfloxacin: Synthesis, structure, antibacterial activity and interaction with DNA. Polyhedron 2008, 27, 349–356. [Google Scholar] [CrossRef]
- Souza, M.J.e.; Bittencourt, C.F.; Morsch, L.M. LC determination of enrofloxacin. J. Pharm. Biomed. Anal. 2002, 28, 1195–1199. [Google Scholar] [CrossRef]
- Prescott, J.F.; Yielding, K.M. In vitro susceptibility of selected veterinary bacterial pathogens to ciprofloxacin, enrofloxacin and norfloxacin. Can. J. Vet. Res. 1990, 54, 195–197. [Google Scholar]
- Dessus-Babus, S.; Bebear, C.M.; Charron, A.; Bebear, C.; de Barbeyrac, B. Sequencing of Gyrase and Topoisomerase IV Quinolone-Resistance-Determining Regions of Chlamydia trachomatis and Characterization of Quinolone-Resistant Mutants Obtained In Vitro. Antimicrob. Agents Chemother. 1998, 42, 2474. [Google Scholar] [CrossRef]
- Elshwiniy, W.H.; Sadeek, S.A.; El-Attar, M.S. Metal Complexes of Enrofloxacin Part I: Preparation, Spectroscopic, Thermal Analyses Studies and Antimicrobial Evaluation. J. Kor. Chem. Soc. 2013, 57, 574. [Google Scholar]
- Mahmoud, M.A.; Abou-elmagd, M.G. Template Synthesis, Spectral and Thermal Properties of Nd(III) Metformin Schiff-Base Complexes As Potential Hypoglycemic Agents. Egypt. J. Chem. 2020, 63, 1009–1031. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abdel-Salam, E.; Abou-Elmagd, M.; Sallam, S. Template Synthesis, Spectral, Thermal and Glucose Sensing of Pr3+ Complexes of Metformin Schiff-Bases. J. Fluoresc. 2019, 29, 319–333. [Google Scholar] [CrossRef]
- Łyszczek, R. Hydrothermal synthesis, thermal and luminescent investigations of lanthanide(III) coordination polymers based on the 4,4′-oxybis(benzoate) ligand. J. Therm. Anal. Calorim. 2012, 108, 1101–1110. [Google Scholar] [CrossRef]
- Imran, M.; Iqbal, J.; Iqbal, S.; Ijaz, N. In Vitro Antibacterial studies of ciprofloxacin-imines and their complexes with Cu(II), Ni(II), Co(II), and Zn(II). Turk. J. Biol. 2007, 31, 67–72. [Google Scholar]
- El-Attar, M.S.; Ahmed, F.M.; Sadeek, S.A.; Mohammed, S.F.; Zordok, W.A.; El-Shwiniy, W.H. Characterization, DFT, and antimicrobial evaluation of some new N2O2 tetradentate Schiff base metal complexes. Appl. Organomet. Chem. 2022, 36, e00433. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Attar, M.S.; Abd El-Hamid, S.M. Spectral, characterization and antibacterial activity of new some transition metal complexes with ciprofloxacin-imines. Bull. Chem. Soc. Ethiop. 2015, 29, 259. [Google Scholar] [CrossRef][Green Version]
- Elshafie, H.S.; Sadeek, S.A.; Camele, I.; Mohamed, A.A. Biochemical Characterization of New Gemifloxacin Schiff Base (GMFX-o-phdn) Metal Complexes and Evaluation of Their Antimicrobial Activity against Humans and Some Phytopathogens. Int. J. Mol. Sci. 2022, 23, 2110. [Google Scholar] [CrossRef]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterisation of Coordination Compounds Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986; p. 230. [Google Scholar]
- Mahmoud, W.H.; Deghadi, R.G.; Mohamed, G.G. Metal complexes of ferrocenyl-substituted Schiff base: Preparation, characterization, molecular structure, molecular docking studies, and biological investigation. J. Organomet. Chem. 2020, 917, 121113. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Sadeek, S.A. Ligational and biological studies of Fe(III), Co(II), Ni(II), Cu(II) and Zr(IV) complexes with carbamazepine as antiepileptic drug. Appl. Organomet. Chem. 2021, 35, e6178. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Refat, M.S.; Hashem, H.A. Complexation and thermogravimetric investigation on tin(II) and tin(IV) with norfloxacin as antibacterial agent. J. Coord. Chem. 2006, 59, 759–775. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Elshwiniy, W.H. Metal complexes of the fourth generation quinolone antimicrobial drug gatifloxacin: Synthesis, structure and biological evaluation. J. Mol. Struct. 2010, 977, 243–253. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Elshwiniy, W.H. Preparation, structure and microbial evaluation of metal complexes of the second generation quinolone antibacterial drug lomefloxacin. J. Mol. Struct. 2010, 981, 130–138. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Elshwiniy, W.H. Metal complexes of the third generation quinolone antibacterial drug sparfloxacin: Preparation, structure, and microbial evaluation. J. Coord. Chem. 2010, 63, 3471–3482. [Google Scholar] [CrossRef]
- Refat, M.S. Synthesis and characterization of norfloxacin-transition metal complexes (group 11, IB):Spectroscopic, thermal, kinetic measurements and biological activity. Spectrochim. Acta A 2007, 68, 1393–1405. [Google Scholar] [CrossRef]
- Gao, F.; Yang, P.; Xie, J.; Wang, H. Synthesis, characterization and antibacterial activity of novel Fe(III), Co(II), and Zn(II) complexes with norfloxacin. J. Inorg. Biochem. 1995, 60, 61–67. [Google Scholar]
- Robertson, G.B.; Tucker, P.A. Carbonato bis (tri-isopropylphosphine) platinum(II), C19H42O3P2Pt. Acta. Cryst. 1983, 39, 858–860. [Google Scholar]
- Kortsaris, A.E.; Kyriakidis, D.A. Ornithine decarboxylase and phosphatase activity can be stimulated by low concentrations of interferon in human breast cancer cell lines. Microbiologica 1988, 11, 347–353. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1963; p. 232. [Google Scholar]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Omar, M.M.; Hindy, A.M.M. Synthesis, characterization and biological activity of some transition metals with Schiff base derived from 2 thiophenecarboxaldehyde and aminobenzoic acid. Spectrochim. Acta A 2005, 62, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; McCarthy, P.J. Spectroscopy and Structure of Metal Chelate Compounds; John Wiley and Sons, Inc.: New York, NY, USA, 1968. [Google Scholar]
- Liu, Y.T.; Lian, G.D.; Yin, D.W.; Su, B.J. Synthesis, characterization and biological activity of ferrocene-based Schiff base ligands and their metal (II) complexes. Spectrochim. Acta A 2013, 100, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Singh, R.V. Ferrocenyl-substituted Schiff base complexes of boron: Synthesis, structural, physico-chemical and biochemical aspects. Spectrochim. Acta 2011, 78, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wu, S.; Zeng, F.; Zhao, J. Synthesis and fluorescence property of terbium complex with novel schiff-base macromolecular ligand. Eur. Polym. J. 2006, 42, 1670–1675. [Google Scholar] [CrossRef]
- Sultanaa, N.; Nazad, A.; Arayne, M.S.; Mesaikc, M.A. Synthesis, characterization, antibacterial, antifungal and immunomodulating activities of gatifloxacin–metal complexes. J. Mol. Struct. 2010, 969, 17–24. [Google Scholar] [CrossRef]
- Leelavathy, C.; Antony, S.A. Synthesis, spectral characterization and biological activity of metal(II) complexes with 4-aminoantipyrine derivatives. Spectrochim. Acta A 2013, 113, 346–355. [Google Scholar] [CrossRef]
- Psomas, G.; Tarushi, A.; Efthimiadou, E.K. Synthesis, characterization and DNA-binding of the mononuclear dioxouranium(VI) complex with ciprofloxacin. Polyhedron 2008, 27, 133–138. [Google Scholar] [CrossRef]
- Saif, M.; Mashaly, M.M.; Eid, M.F.; Fouad, R. Synthesis, characterization and thermal studies of binary and/or mixed ligand complexes of Cd(II), Cu(II), Ni(II) and Co(III) based on 2-(Hydroxybenzylidene) thiosemicarbazone: DNA binding affinity of binary Cu(II) complex. Spectrochim. Acta 2012, 92, 347–356. [Google Scholar] [CrossRef]
- Lever, A.B.P. The electronic spectra of tetragonal metal complexes analysis and significance. Coord. Chem. Rev. 1968, 3, 119–140. [Google Scholar] [CrossRef]
- Mondal, N.; Dey, D.K.; Mitra, S.K.; Abdul Malik, M. Synthesis and structural characterization of mixed ligand η1-2-hydroxyacetophenone complexes of cobalt(II). Polyhedron 2000, 19, 2707–2711. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Mohamed, G.G.; Elsawy, H.A.; Radwan, M.A. Metal complexes of novel Schiff base derived from the condensation of 2-quinoline carboxaldehyde and ambroxol drug with some transition metal ions. Appl. Organometal. Chem. 2018, 32, e4392. [Google Scholar] [CrossRef]
- Drevenšek, P.; Košmrlj, J.; Giester, G.; Skauge, T.; Sletten, E.; Sepčić, K.; Turela, I. X-Ray crystallographic, NMR and antimicrobial activity studies of magnesium complexes of fluoroquinolones—racemic ofloxacin and its S-form, levofloxacin. J. Inorg. Biochem. 2006, 100, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Horowitz, H.H.; Metzger, G. A New Analysis of Thermogravimetric Traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- El Gammal, O.A. Mononuclear and binuclear complexes derived from hydrazone Schiff base NON donor ligand: Synthesis, structure, theoretical and biological studies. Inorg. Chim. Acta 2015, 435, 73–81. [Google Scholar] [CrossRef]
- Raman, N.; Kulandaisamy, A.; Thangaraj, C.; Jeyasubramanian, K. Redox and antimicrobial studies of transition metal(II) tetradentate Schiff base complexes. Trans. Met. Chem. 2003, 28, 29–36. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Sharaby, C.M. Metal complexes of Schiff base derived from sulphametrole and o-vanilin: Synthesis, spectral, thermal characterization and biological activity. Spectrochim. Acta A 2007, 66, 949–958. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sadeek, S.A.; Zordok, W.A.; Mohamed, A.A. Meloxicam and Study of Their Antimicrobial Effects against Phyto and Human Pathogens. Molecules 2021, 26, 1480. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sadeek, S.A.; Camele, I.; Awad, H.M.; Mohamed, A.A. Biological and Spectroscopic Investigations of New Tenoxicam and 1.10-Phenthroline Metal Complexes. Molecules 2021, 26, 1027. [Google Scholar] [CrossRef]
- Soliman, M.H.; Mohamed, G.G.; Hindy, A.M.M. Biological activity, spectral and thermal characterization of mixed ligand complexes of enrofloxacin and glycine: In vitro antibacterial and antifungal activity studies. Monatsh. Chem. 2015, 146, 259–273. [Google Scholar] [CrossRef]
- Zordok, W.A.; Sadeek, S.A. Synthesis, spectroscopic, structure, thermal analyses, and biological activity evaluation of new norfloxacin vanadium (V) solvates (L) (L = An, DMF, Py, Et3N and o-Tol). J. Therm. Anal. Calorim. 2017, 128, 971–991. [Google Scholar] [CrossRef]
- Greenaway, A.M.; Dasgupta, T.P.; Koshy, K.C.; Sadler, G.G. A correlation between infrared stretching mode absorptions and structural angular distortions for the carbonato ligand in a wide variety of complexes. Spectrochim. Acta A 1986, 42, 949–954. [Google Scholar] [CrossRef]
- Reck, G.; Faust, G.; Dietz, G. X-ray crystallographic studies of diclofenac-sodium--structural analysis of diclofenac-sodium tetrahydrate. Pharmazie 1988, 43, 771–774. [Google Scholar] [PubMed]
- Malhotra, R.C.; Bohra, R. Metal Carboxylates; Academic Press: London, UK, 1983. [Google Scholar]
- Salazar-Medina, A.J.; amez-Corrales, R.G.; Ramírez, J.Z.; Gonz_alez-Aguilar, G.A.; Vel_azquez-Contreras, E.F. Characterization of metal-bound water in bioactive Fe(III)-cyclophane complexes. J. Mol. Struct. 2018, 1154, 225–231. [Google Scholar] [CrossRef]
- Tyagi, N.; Singh, O.; Ghosh, K. Non-heme iron(III) complex with tridentate ligand: Synthesis, structures and catalytic oxidations of alkanes. Catal. Commun. 2017, 95, 83–87. [Google Scholar] [CrossRef]
- Bazargan, M.; Mirzaei, M.; Eshtiagh-Hosseini, H.J.; Mague, T.; Bauza, A.; Frontera, A. Synthesis, X-ray characterization and DFT study of a novel Fe(III)–pyridine-2,6-dicarboxylic acid N-oxide complex with unusual coordination mode. Inorg. Chim. Acta 2016, 449, 44–51. [Google Scholar] [CrossRef]
- Huaqiong, L.; Huiduo, X.; Guoliang, Z. Synthesis and crystal structures of La(III), Y(III) complexes of homoveratric acid with 1,10-phenanthroline. J. Rare Earths 2010, 28, 7–11. [Google Scholar]
- Tamboura, F.B.; Haba, P.M.; Gaye, M.A.; Sall, S.A.; Barry, H.; Jouini, T. Structural studies of bis-(2,6-diacetylpyridine-bis-(phenylhydrazone)) and X-ray structure of its Y(III), Pr(III), Sm(III) and Er(III) complex. Polyhedron 2004, 23, 1191–1197. [Google Scholar] [CrossRef]
- Cai, M.; Chen, J.; Taha, M. Synthesis, structures and antibacterial activities of two complexes of yttrium (III) with 2,6-pyridinedicarboxylate. Inorg. Chem. Commun. 2010, 13, 199–202. [Google Scholar] [CrossRef]
- Karunarathne, M.C.; Baumann, J.W.; Heeg, M.J.; Martin, P.D.; Winter, C.H. Synthesis, structural characterization, and volatility evaluation of zirconium and hafnium amidate complexes. J. Organometallic Chem. 2017, 847, 204–212. [Google Scholar] [CrossRef]
- Sahraei, A.; Kargar, H.; Hakimi, M.; Tahir, M.N. Distorted square-antiprism geometry of new zirconium (IV) Schiff base complexes: Synthesis, spectral characterization, crystal structure and investigation of biological properties. J. Mol. Struct. 2017, 1149, 576–584. [Google Scholar] [CrossRef]
- Yan, L.; Wang, X.; Zhou, M. Synthesis, structural characterization and catalytic properties of a N-functionalized organoamide zirconium complex. Inorg. Chem. Commun. 2016, 65, 32–34. [Google Scholar] [CrossRef]
- Gao, X.S.; Jiang, X.; Yao, C. Two new complexes of Lanthanide(III) ion with the N3O2-donor Schiff base ligand: Synthesis, crystal structure, and magnetic properties. J. Mol. Struct. 2016, 1126, 275–279. [Google Scholar] [CrossRef]
- Change, R. Physical Chemistry for Chemical and Biological Sciences; University Science Books: Mill Valley, CA, USA, 2000. [Google Scholar]
- Leciejewicz, J.; Ptasiewicz-Bąk, H.; Premkumar, T.; Govindarajan, S. Crystal Structure of a Lanthanum(III) Complex with Pyrazine-2-Carboxylate and Water Ligands. J. Coord. Chem. 2004, 57, 97–103. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; Wiley: London, UK, 1976. [Google Scholar]
- Kurtaran, R.; Odabasoglu, S.; Azizoglu, A.; Kara, H.; Atakol, O. Experimental and computational study on [2,6-bis(3,5-dimethylN-pyrazolyl)pyridine]-(dithiocyanato)mercury(II). Polyhedron. 2007, 26, 5069–5074. [Google Scholar] [CrossRef]
- Krogmann, K.; Anorg, Z. Die Kristallstruktur von K2[Pd(C2O4)2]4H2O. Allg. Chem. 1966, 346, 188–202. [Google Scholar] [CrossRef]
- Ciofini, I.; Laine, P.P.; Bedioui, F.; Admo, C. Photoinduced Intramolecular Electron Transfer in Ruthenium and Osmium Polyads:Insights from Theory. J. Am. Chem. Soc. 2004, 126, 10763–11077. [Google Scholar] [CrossRef]
- Ciofini, I.I.; Daul, C.; Adamo, C.J. Phototriggered Linkage Isomerization in Ruthenium–Dimethylsulfoxyde Complexes: Insights from Theory. J. Phys. Chem. A 2003, 107, 11182–11190. [Google Scholar] [CrossRef]
- Casida, F.M. Recent Advances in Density Functional Methods, Part 1; Chong, D.P., Ed.; World Scientific: Singapore, 1995. [Google Scholar]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 98; Revision A.6, Inc.: Pittsburgh, PA, USA, 1998. [Google Scholar]
- Kohn, W. Density Functional and Density Matrix Method Scaling Linearly with the Number of Atoms. Phys. Rev. Lett. 1996, 76, 3168. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Flurry, R.L., Jr. Molecular Orbital Theory of Bonding in Organic Molecules; Marcel Dekker: New York, NY, USA, 1968. [Google Scholar]
- Bergner, A.; Dolg, M.; Kuechle, W.; Stoll, H.; Preuss, H. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys. 1993, 80, 1431–1441. [Google Scholar] [CrossRef]
- Sakr, S.H.; Elshafie, H.S.; Camele, I.; Sadeek, S.A. Synthesis, Spectroscopic, and Biological Studies of Mixed Ligand Complexes of Gemifloxacin and Glycine with Zn(II), Sn(II), and Ce(III). Molecules 2018, 23, 1182. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.H.; Sadeek, S.A.; Camele, I. Biological investigations and spectroscopic studies of new Moxifloxacin/Glycine-Metal complexes. Chem. Biodiver. 2019, 16, e1800633. [Google Scholar] [CrossRef]
- Beecher, D.J.; Wong, A.C. Identification of hemolysin BL-producing Bacillus cereus isolates by a discontinuous hemolytic pattern in blood agar. Appl. Environ. Microbial. 1994, 60, 1646–1651. [Google Scholar] [CrossRef]
- Fallik, E.; Klein, J.; Grinberg, S.; Lomaniee, E.; Lurie, S.; Lalazar, A. Effect of Postharvest Heat Treatment of Tomatoes on Fruit Ripening and Decay Caused by Botrytis cinerea. Plant Dis. 1993, 77, 985–988. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.; Bufo, S.A.; Camele, I. An attempt of biocontrol the tomato-wilt disease caused by Verticillium dahliae using Burkholderia gladioli pv. agaricicola and its bioactive secondary metabolites. Int. J. Plant Biol. 2017, 8, 57–60. [Google Scholar]
- Elshafie, H.S.; Viggiani, L.; Mostafa, M.S.; El-Hashash, M.A.; Bufo, S.A.; Camele, I. Biological activity and chemical identification of ornithine lipid produced by Burkholderia gladioli pv. agaricicola ICMP 11096 using LC-MS and NMR analyses. J. Biol. Res. 2017, 90, 96–103. [Google Scholar]
- Rehman, W.; Badshah, A.; Baloch, M.K.; Ali, S.; Hameed, G.; Khan, K.M. Synthesis Characterization and Biological Screening of Tri-benzyl Tin(IV) Complexes of Some Schiff Bases. Chin. Chem. Soc. 2004, 51, 929. [Google Scholar] [CrossRef]
- Sofo, A.; Elshafie, H.S.; Scopa, A.; Mang, S.M.; Camele, I. Impact of airborne zinc pollution on the antimicrobial activity of olive oil and the microbial metabolic profiles of Zn-contaminated soils in an Italian olive orchard. J. Trace Elem. Med. Biol. 2018, 49, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.T.; Cleirigh, C.O.; Walsh, P.K.; Shea, D.G.O. Development of a robust microtiter plate-based assay method for assessment of bioactivity. J. Microbiol. Meth. 2004, 5, 327–334. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).