Simultaneous HPLC Determination of Clindamycin Phosphate, Tretinoin, and Preservatives in Gel Dosage Form Using a Novel Stability-Indicating Method
Abstract
:1. Introduction
2. Materials and Method
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Preparation of Solutions
2.3.1. Mobile Phase Preparation
2.3.2. Standard Stock Solution I (Tretinoin)
2.3.3. Standard Stock Solution II (Methyl Paraben and Imidazolidinyl Urea)
2.3.4. Preparation of Standard Solution
2.3.5. Preparation of Sample Solution
2.3.6. Stability/Stress Testing Studies
3. Results and Discussion
3.1. Method Development
- a.
- temperature selection
- b.
- mobile phase composition
- c.
- elution rate
- d.
- wavelength selection
3.1.1. Optimized Chromatographic Conditions
3.1.2. Formulation Analysis
3.2. System Suitability
3.3. Method Validation
3.3.1. Specificity
3.3.2. Linearity
3.3.3. Range
3.3.4. Precision
3.3.5. Repeatability
3.3.6. Intermediate Precision
3.3.7. Accuracy
3.3.8. Robustness
3.3.9. Limit of Detection
3.3.10. Limit of Quantification
3.4. Stress Testing Analysis
3.4.1. Temperature Stress Analysis
3.4.2. Sunlight Stress Analysis
3.5. Evaluation of Suggested Analytical Approach in Clindamycin Phosphate/Tretinoin Assay in Commercially Available Topical Gel Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Collier, C.N.; Harper, J.C.; Cantrell, W.C.; Wang, W.; Foster, K.W.; Elewski, B.E. The prevalence of acne in adults 20 years and older. J. Am. Acad. Dermatol. 2008, 58, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Hedden, S.L.; Davidson, S.; Smith, C.B. Cause and effect: The relationship between acne and self-esteem in the adolescent years. J. Nurse Pract. 2008, 4, 595–600. [Google Scholar] [CrossRef]
- Thiboutot, D.; Gollnick, H.; Bettoli, V.; Dréno, B.; Kang, S.; Leyden, J.J.; Shalita, A.R.; Lozada, V.T.; Berson, D.; Finlay, A. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne group. J. Am. Acad. Dermatol. 2009, 60, S1–S50. [Google Scholar] [CrossRef]
- Berson, D.S.; Shalita, A.R. The treatment of acne: The role of combination therapies. J. Am. Acad. Dermatol. 1995, 32, S31–S41. [Google Scholar] [CrossRef]
- Rietschel, R.L.; Duncan, S.H. Clindamycin phosphate used in combination with tretinoin in the treatment of acne. Int. J. Dermatol. 1983, 22, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.L.; Qi, J.; Rainer, B.; Sachs, D.L.; Helfrich, Y.R. Treatment of acne in pregnancy. J. Am. Board Fam. Med. 2016, 29, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Desai, C. Meyler’s side effects of drugs: The international encyclopedia of adverse drug reactions and interactions. Indian J. Pharmacol. 2016, 48, 224. [Google Scholar]
- Ye, Y.R.; Bektic, E.; Buchta, R.; Houlden, R.; Hunt, B. Simultaneous determination of tretinoin and clindamycin phosphate and their degradation products in topical formulations by reverse phase HPLC. J. Sep. Sci. 2004, 27, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, S. Tramadol hydrochloride. In Martindale: The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Dedić, M.; Bečić, E.; Imamović, B.; Žiga, N. Determination of clindamycin hydrochloride content in 1% clindamycin lotion. Bull. Chem. Technol. Bosnia Herzeg. 2018, 50, 49–54. [Google Scholar]
- Mifsud, M.; Vella Szijj, J.; Ferrito, V.; Serracino-Inglott, A.; Azzopardi, L.M.; Sammut Bartolo, N.; LaFerla, G.; Sammut, C. A simple HPLC-UV method for the determination of clindamycin in human plasma. J. Chem. Pharm. Res. 2014, 6, 696–704. [Google Scholar]
- Stanković, M.; Savić, V.; Marinković, V. Determination of clindamycin phosphate in different vaginal gel formulations by reverse phase high performance liquid chromatography. Acta Fac. Med. Naissensis 2013, 30, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Leelarasamee, A.; Tatong, W.; Kasattut, N.; Sriboonruang, T.; Ayudhya, D.P.N. Bioequivalence study of clindamycin phosphate injection (Clinott-P®) in Thai healthy volunteers. J.-Med. Assoc. Thail. 2006, 89, 683. [Google Scholar]
- Hamada, S.; Nakajima, M.; Kaszynski, R.H.; Otaka, S.; Goto, H.; Matsui, H.; Fushimi, K.; Yamaguchi, Y.; Yasunaga, H. Association between adjunct clindamycin and in-hospital mortality in patients with necrotizing soft tissue infection due to group A Streptococcus: A nationwide cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.S. An overview of the retinoids. Am. J. Med. 1989, 86, 568–574. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Retinoids—Which dermatological indications will benefit in the near future? Ski. Pharmacol. Physiol. 2001, 14, 303–315. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Katsambas, A.D. The role of topical retinoids in the treatment of photoaging. Drugs 2005, 65, 1061–1072. [Google Scholar] [CrossRef]
- Vo, T.T.; Jung, E.-M.; Choi, K.-C.; Frank, H.Y.; Jeung, E.-B. Estrogen receptor α is involved in the induction of calbindin-D9k and progesterone receptor by parabens in GH3 cells: A biomarker gene for screening xenoestrogens. Steroids 2011, 76, 675–681. [Google Scholar] [CrossRef]
- Guadarrama, P.; Fomine, S.; Salcedo, R.; Martínez, A. Construction of simplified models to simulate estrogenic disruptions by esters of 4-hydroxy benzoic acid (parabens). Biophys. Chem. 2008, 137, 1–6. [Google Scholar] [CrossRef]
- Hossaini, A.; Larsen, J.-J.; Larsen, J.C. Lack of oestrogenic effects of food preservatives (parabens) in uterotrophic assays. Food Chem. Toxicol. 2000, 38, 319–323. [Google Scholar] [CrossRef]
- Soni, M.; Taylor, S.; Greenberg, N.; Burdock, G. Evaluation of the health aspects of methyl paraben: A review of the published literature. Food Chem. Toxicol. 2002, 40, 1335–1373. [Google Scholar] [CrossRef]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Cela, R. Evaluation of the occurrence and biodegradation of parabens and halogenated by-products in wastewater by accurate-mass liquid chromatography-quadrupole-time-of-flight-mass spectrometry (LC-QTOF-MS). Water Res. 2011, 45, 6770–6780. [Google Scholar] [CrossRef]
- Jong, C.T.; Statham, B.N.; Green, C.M.; King, C.M.; Gawkrodger, D.J.; Sansom, J.E.; English, J.S.; Wilkinson, S.M.; Ormerod, A.D.; Chowdhury, M.M. Contact sensitivity to preservatives in the UK, 2004–2005: Results of multicentre study. Contact Dermat. 2007, 57, 165–168. [Google Scholar] [CrossRef]
- Rietschel, R.L.; Warshaw, E.M.; Sasseville, D.; Fowler Jr, J.F.; DeLeo, V.A.; Belsito, D.V.; Taylor, J.S.; Storrs, F.J.; Mathias, T.C.; Maibach, H.I. Sensitivity of petrolatum and aqueous vehicles for detecting allergy to imidazolidinylurea, diazolidinylurea, and DMDM hydantoin: A retrospective analysis from the North American Contact Dermatitis Group. Dermatitis 2007, 18, 155–162. [Google Scholar] [CrossRef]
- Soni, M.; Carabin, I.; Burdock, G. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Buchholz, H.J.; Belsito, D.V.; Maibach, H.I.; Fowler, J.F., Jr.; Rietschel, R.L.; Zug, K.A.; Mathias, C.T.; Pratt, M.D.; Sasseville, D. Allergic patch test reactions associated with cosmetics: Retrospective analysis of cross-sectional data from the North American Contact Dermatitis Group, 2001–2004. J. Am. Acad. Dermatol. 2009, 60, 23–38. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Yiannias, J.A. Contact dermatitis to medications and skin products. Clin. Rev. Allergy Immunol. 2019, 56, 41–59. [Google Scholar] [CrossRef]
- García-Gavín, J.; González-Vilas, D.; Fernández-Redondo, V.; Toribo, J. Allergic contact dermatitis in a girl due to several cosmetics containing diazolidinyl-urea or imidazolidinyl-urea. Contact Dermat. 2010, 1, 49–50. [Google Scholar] [CrossRef]
- Bordbar, M.; Yeganeh-Faal, A.; Ghasemi, J.; Ahari-Mostafavi, M.; Sarlak, N.; Baharifard, M. Simultaneous spectrophotometric determination of minoxidil and tretinoin by the H-point standard addition method and partial least squares. Chem. Pap. 2009, 63, 336–344. [Google Scholar] [CrossRef]
- Brown, L.W. High-pressure liquid chromatographic assays for clindamycin, clindamycin phosphate, and clindamycin palmitate. J. Pharm. Sci. 1978, 67, 1254–1257. [Google Scholar] [CrossRef]
- Kipp, J.E.; Hlavaty, J.J. Nonisothermal stability assessment of stable pharmaceuticals: Testing of a clindamycin phosphate formulation. Pharm. Res. 1991, 8, 570–575. [Google Scholar] [CrossRef]
- Procedures, A. Method Validation Chemistry, Guidance for Industry, SIC Manufacturing and Controls Documentation; US Department of Health and Human Services, FDA: Silver Spring, MD, USA, 2000.
- Tashtoush, B.M.; Jacobson, E.L.; Jacobson, M.K. A rapid HPLC method for simultaneous determination of tretinoin and isotretinoin in dermatological formulations. J. Pharm. Biomed. Anal. 2007, 43, 859–864. [Google Scholar] [CrossRef]
- Nataraj, K.; Raju, G.; Narasimha Surya, A.B. UV spectrophotometric method development for estimation of clindamycin phosphate in bulk and dosage form. Int. J. Pharm. Biol. Sci. 2013, 3, 164e7. [Google Scholar]
- Gupta, A.; Gulati, M.; Pandey, N.K. A validated UV spectrophotometric method for simultaneous estimation of tretinoin and benzoyl peroxide in bulk and semisolid dosage form. Rasayan J. Chem. 2009, 2, 649–654. [Google Scholar]
- Borman, P.; Elder, D. Q2 (R1) validation of analytical procedures. In ICH Quality Guidelines; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 5, pp. 127–166. [Google Scholar]
- ICH Expert Working Group. Stability Testing of New Drug Substances and Products; Q1A (R2), current step; John Wiley & Sons: Hoboken, NJ, USA, 2003; Volume 4, pp. 1–24. [Google Scholar]
- Modi, P.B.; Shah, N.J. Novel stability-indicating RP-HPLC method for the simultaneous estimation of clindamycin phosphate and adapalene along with preservatives in topical gel formulations. Sci. Pharm. 2014, 82, 799–814. [Google Scholar] [CrossRef] [Green Version]
- Gruvberger, B.; Bruze, M.; Tammela, M. Preservatives in moisturizers on the Swedish market. Acta Derm.-Stockh. 1998, 78, 52–56. [Google Scholar]
- Rastogi, S.C. Analytical control of preservative labelling on skin creams. Contact Dermat. 2000, 43, 339–343. [Google Scholar] [CrossRef]







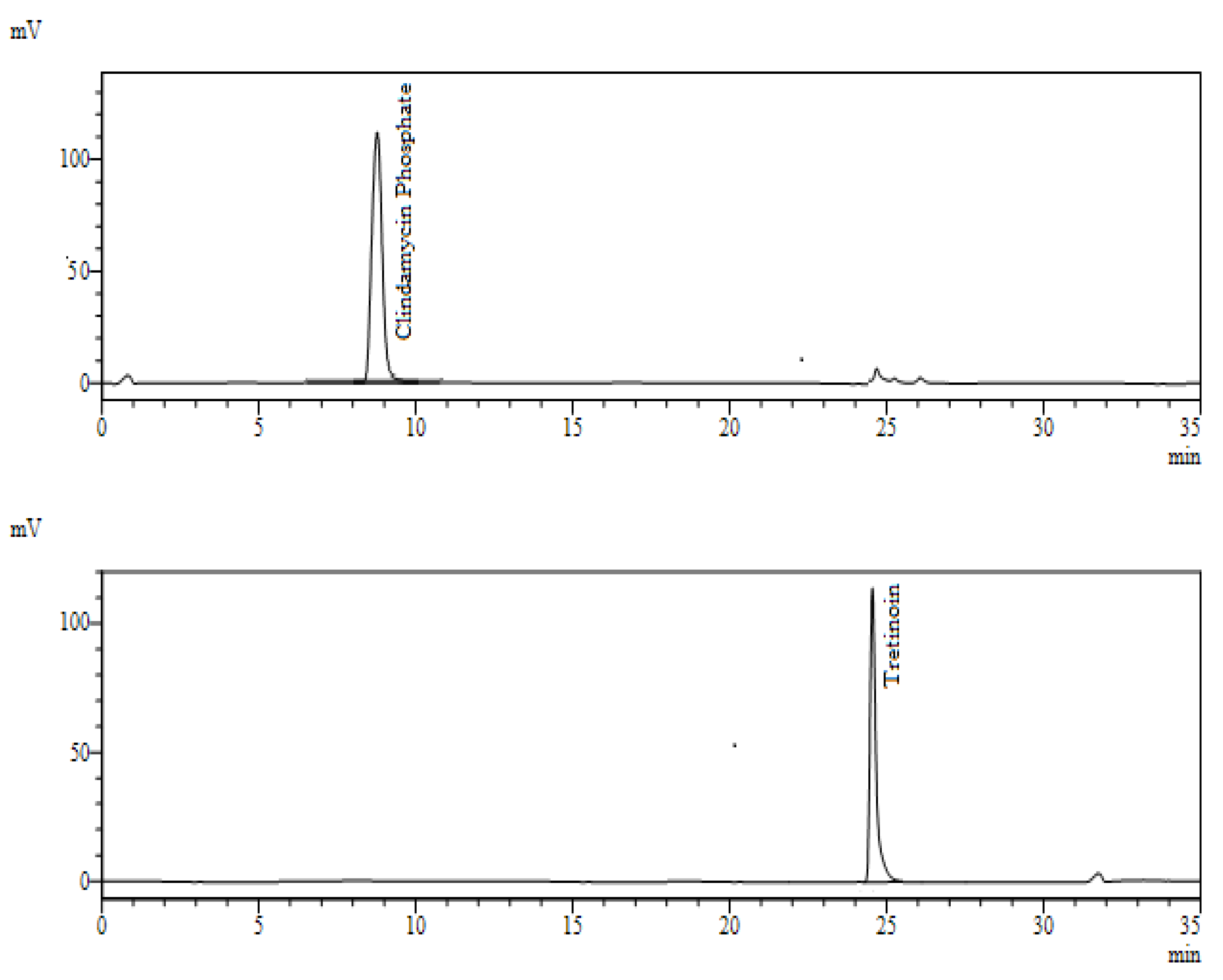
| API/Preservative | tR (min) | Parameters and Acceptance Criteria | |||
|---|---|---|---|---|---|
| Instrument Precision (RSD ≤ 2%) | Theoretical Plates (≥2000) | Tailing Factor (<2.0) | Resolution (>2) | ||
| Imidazolidinyl urea | 2.2 | 0.32% | 21,482.500 | 1.128 | - |
| Methyl paraben | 7.3 | 0.64% | 21,247.500 | 1.205 | 4.7 |
| Clindamycin Phosphate | 8.9 | 0.78% | 23,318.500 | 1.105 | 2.4 |
| Tretinoin | 24.8 | 0.73% | 40,487.000 | 1.083 | - |
| API/Preservative | Validation Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Linearity | Repeatability | Range (µg/mL) | Accuracy | LOD (Limit of Detection) (µg/mL) | LOQ (Limit of Quantitation) (µg/mL) | |||
| Regression Equation | R | Mean Recovery (%) | RSD | |||||
| Clindamycin Phosphate | y = 8543.2x + 238.4 | 1.00 | 0.52% | 144–336 | 100.35 | 0.50 | 0.351 | 1.063 |
| Methyl Paraben | y = 6952.6x − 504.55 | 1.00 | 0.26% | 31.2–72.8 | 99.79 | 0.27 | 0.047 | 0.141 |
| Imidazolidinyl Urea | y = 1175.6x + 2563.7 | 0.999 | 0.31% | 51.6–120.4 | 100.15 | 0.17 | 1.101 | 3.335 |
| Tretinoin | y = 252086.4x − 40937.5 | 0.999 | 0.33% | 3.0–7.0 | 100.48 | 0.57 | 0.144 | 0.436 |
| Parameters | Clindamycin Phosphate | Tretinoin | Methyl Paraben | Imidazolidinyl Urea |
|---|---|---|---|---|
| Analyst 1 | 99.88 | 100.95 | 100.29 | 100.05 |
| Analyst 2 | 99.98 | 100.39 | 100.12 | 100.07 |
| RSD ≤ 2% | 0.07% | 0.39% | 0.12% | 0.01% |
| Day 1 | 100.13 | 100.31 | 100.24 | 100.30 |
| Day 2 | 100.06 | 100.77 | 100.28 | 100.06 |
| RSD ≤ 2% | 0.05% | 0.32% | 0.03% | 0.17% |
| APIs | % Recovery (%) | RSD (%) | LOD (µg/mL) | LOQ (µg/mL) | Reference |
|---|---|---|---|---|---|
| PHE | 98.8 | 1.72 | 0.3 | 1.0 | [38] |
| MP | 101.7 | 0.91 | 0.2 | 0.6 | |
| CP | 99.0 | 1.67 | 7.0 | 20.0 | |
| ADA | 101.3 | 0.49 | 0.2 | 0.5 | |
| CP | 99.2 | 0.6 | - | - | [39] |
| Tretinoin | 100.3 | 0.8 | - | - |
| API/Preservative | Temperature 40 °C, 3 Months | Sunlight, 10 h (25–30 °C) | Remarks |
|---|---|---|---|
| Clindamycin Phosphate | 99.6% | 99.7% | No major degradation products formed |
| Methyl Paraben | 99.2% | 99.4% | No major degradation products formed |
| Imidazolidinyl Urea | 98.6% | 99.8% | No major degradation products formed |
| Tretinoin | 87.2% | 65.2% | One major degradation product was formed |
| API/Preservative | Clinacin-T Gel | Acdermin Gel | Cleret Gel | Clinda-T Gel | ||||
|---|---|---|---|---|---|---|---|---|
| Labeled (mg/gm) | Found (mg/gm) | Labeled (mg/gm) | Found (mg/gm) | Labeled (mg/gm) | Found (mg/gm) | Labeled (mg/gm) | Found (mg/gm) | |
| Clindamycin Phosphate | 12 | 12.10 | 12 | 12.05 | 12 | 12.04 | 12 | 12.05 |
| Methyl Paraben | Not declared | 2.28 | Not declared | 2.14 | Not declared | 2.00 | Not declared | Not found |
| Imidazolidinyl Urea | Not declared | 4.32 | Not declared | Not found | Not declared | Not found | Not declared | Not found |
| Tretinoin | 0.25 | 0.29 | 0.25 | 0.26 | 0.25 | 0.26 | 0.25 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarfraz, S.; Hussain, S.; Javed, M.; Raza, A.; Iqbal, S.; Alrbyawi, H.; Aljazzar, S.O.; Elkaeed, E.B.; Somaily, H.H.; Pashameah, R.A.; et al. Simultaneous HPLC Determination of Clindamycin Phosphate, Tretinoin, and Preservatives in Gel Dosage Form Using a Novel Stability-Indicating Method. Inorganics 2022, 10, 168. https://doi.org/10.3390/inorganics10100168
Sarfraz S, Hussain S, Javed M, Raza A, Iqbal S, Alrbyawi H, Aljazzar SO, Elkaeed EB, Somaily HH, Pashameah RA, et al. Simultaneous HPLC Determination of Clindamycin Phosphate, Tretinoin, and Preservatives in Gel Dosage Form Using a Novel Stability-Indicating Method. Inorganics. 2022; 10(10):168. https://doi.org/10.3390/inorganics10100168
Chicago/Turabian StyleSarfraz, Sadaf, Shahid Hussain, Mohsin Javed, Ali Raza, Shahid Iqbal, Hamad Alrbyawi, Samar O. Aljazzar, Eslam B. Elkaeed, Hamoud H. Somaily, Rami Adel Pashameah, and et al. 2022. "Simultaneous HPLC Determination of Clindamycin Phosphate, Tretinoin, and Preservatives in Gel Dosage Form Using a Novel Stability-Indicating Method" Inorganics 10, no. 10: 168. https://doi.org/10.3390/inorganics10100168
APA StyleSarfraz, S., Hussain, S., Javed, M., Raza, A., Iqbal, S., Alrbyawi, H., Aljazzar, S. O., Elkaeed, E. B., Somaily, H. H., Pashameah, R. A., Alzahrani, E., & Farouk, A.-E. (2022). Simultaneous HPLC Determination of Clindamycin Phosphate, Tretinoin, and Preservatives in Gel Dosage Form Using a Novel Stability-Indicating Method. Inorganics, 10(10), 168. https://doi.org/10.3390/inorganics10100168








