Oxygen Ion and Proton Transport in Alkali-Earth Doped Layered Perovskites Based on BaLa2In2O7
Abstract
:1. Introduction
2. Results and Discussion
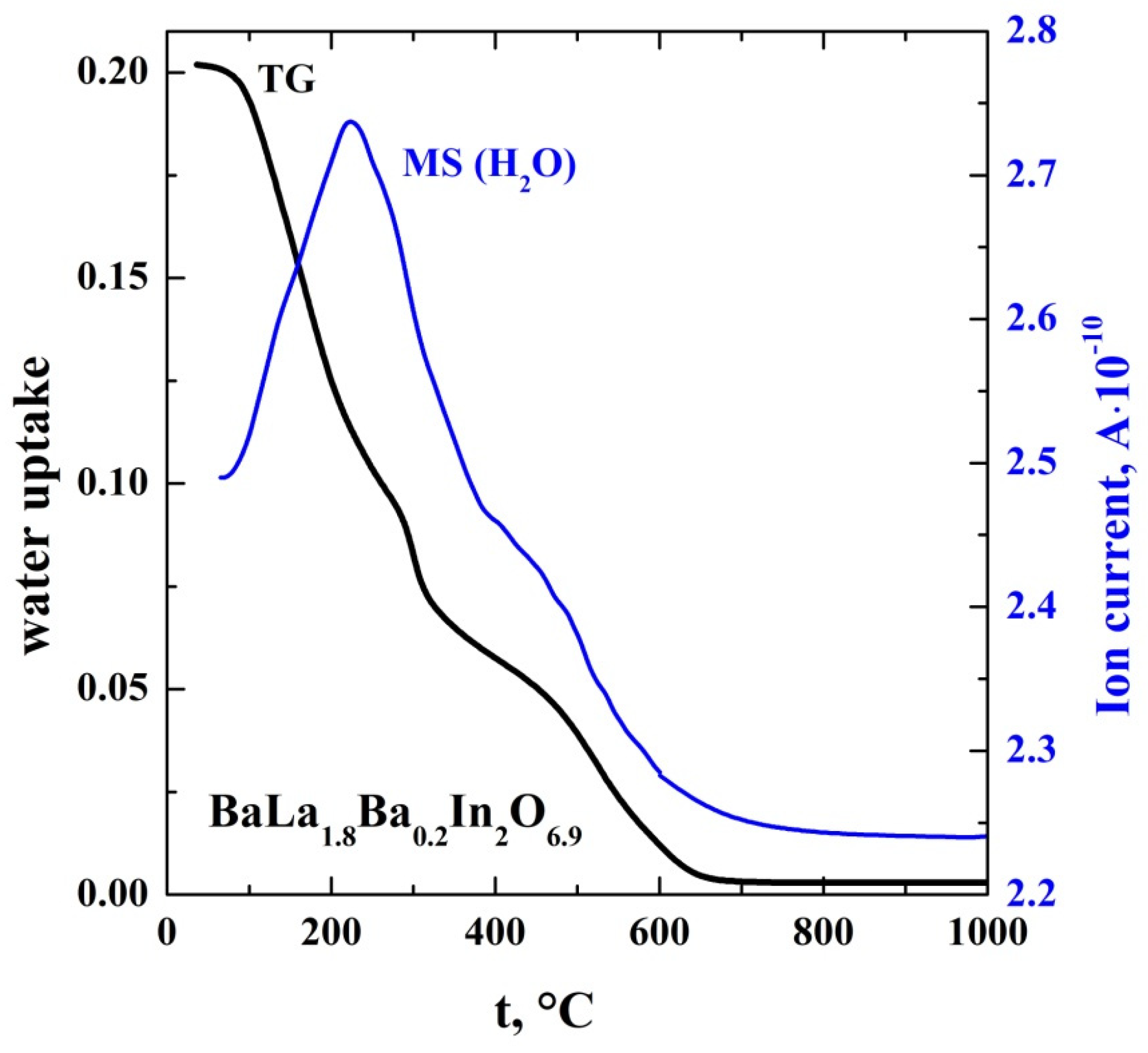
2.1. XRD and TG Investigations
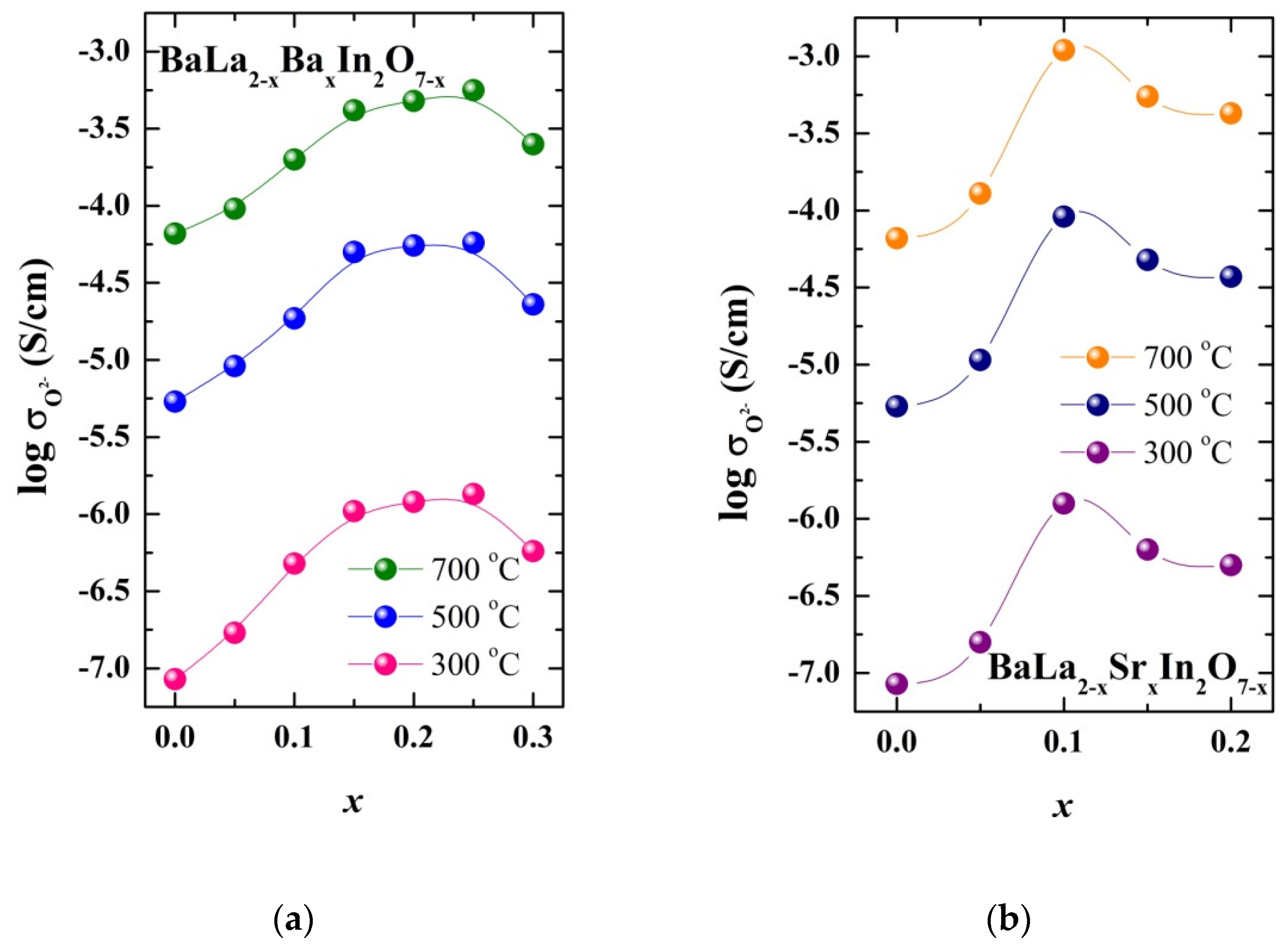
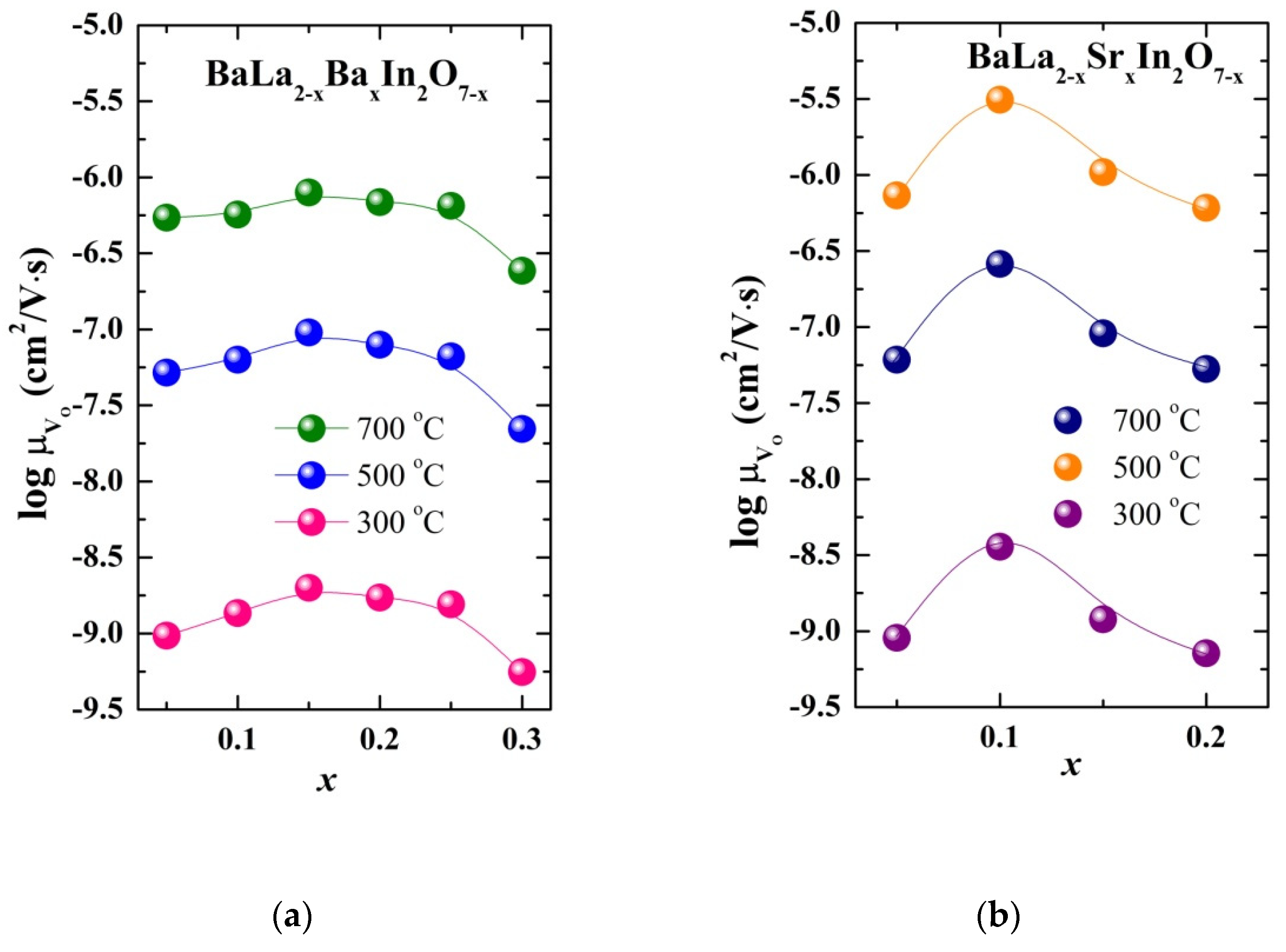
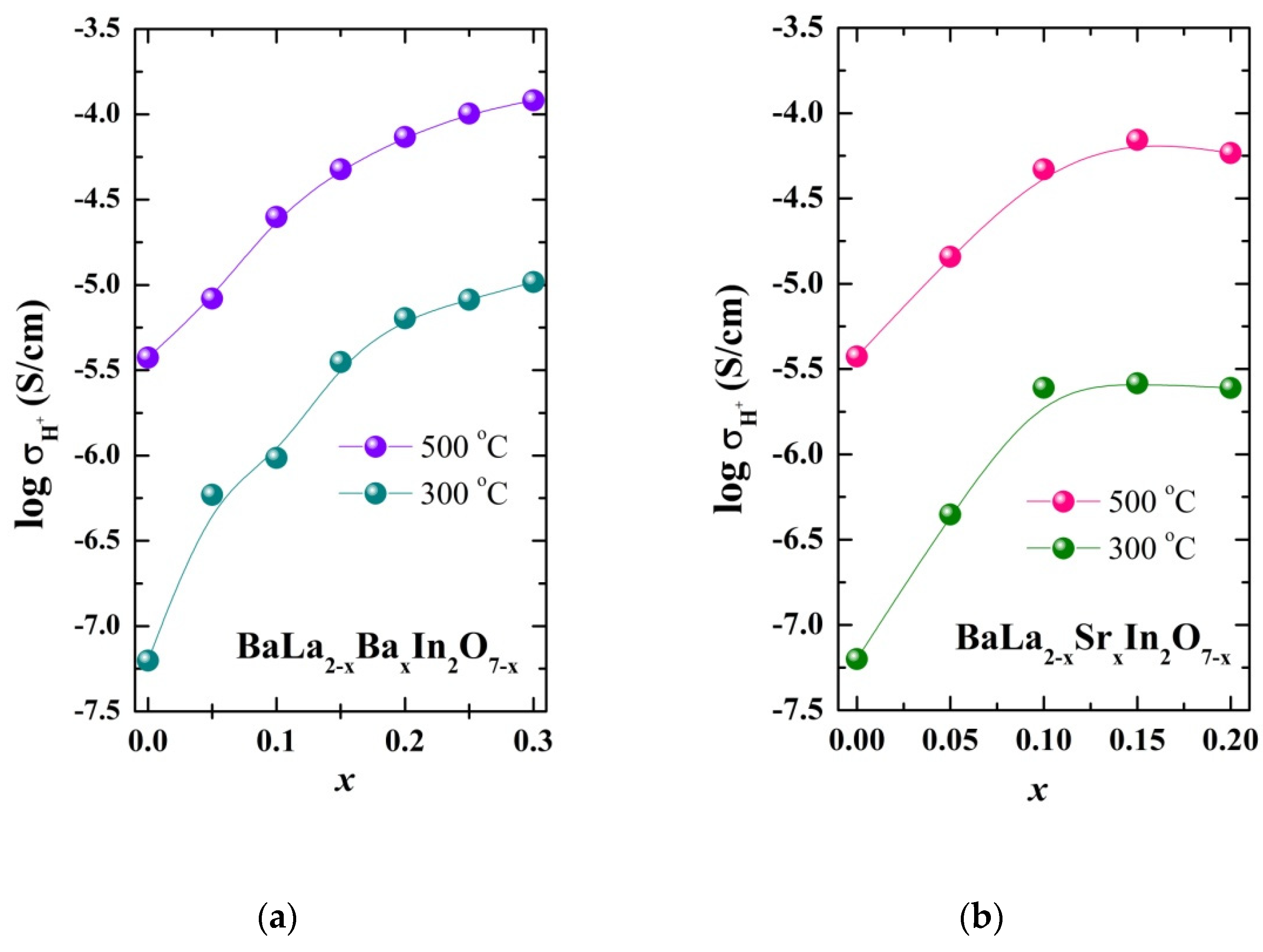
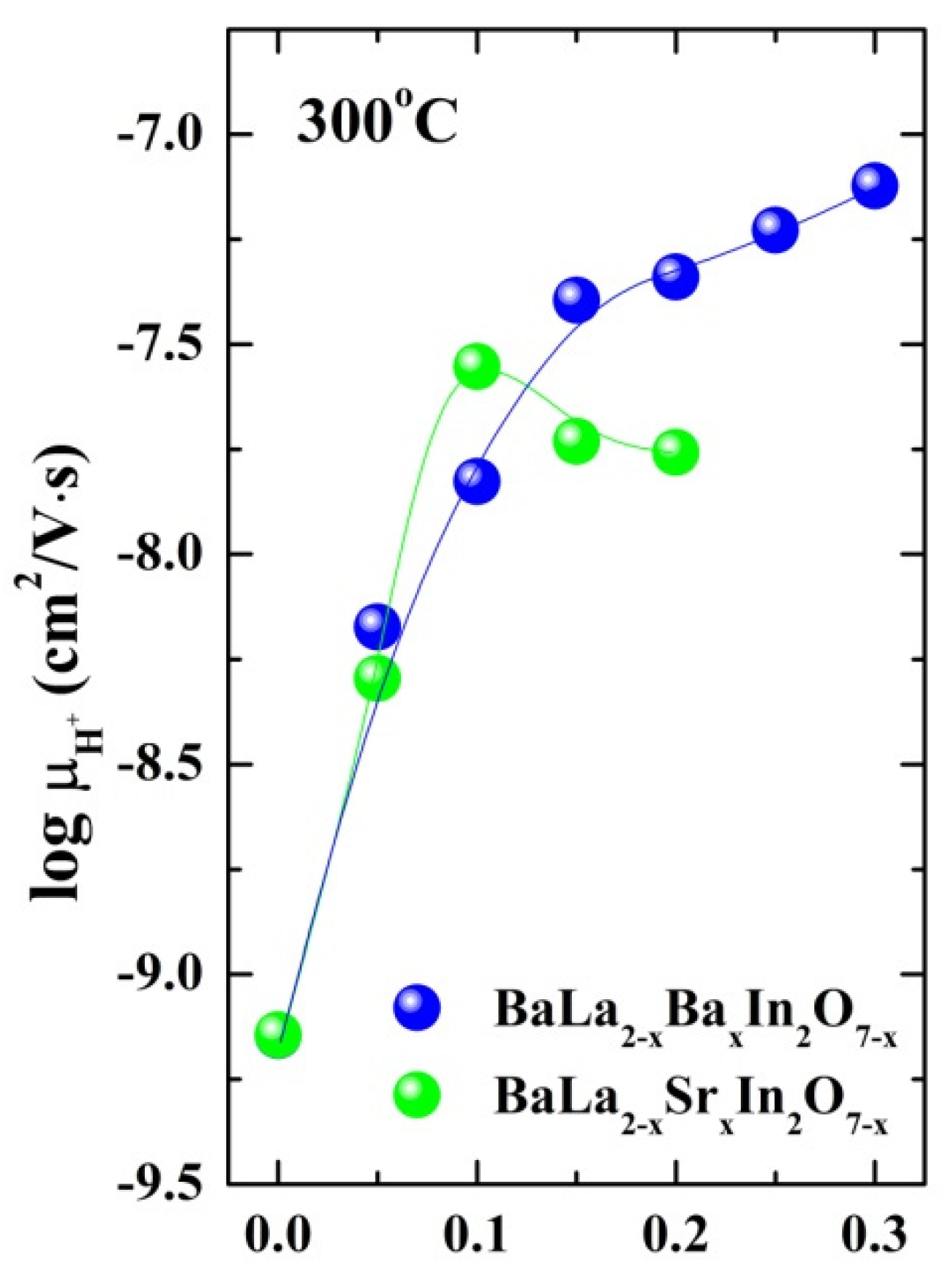
2.2. Electrical Conductivity Investigations
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruddlesden, S.N.; Popper, P. New compounds of the K2NiF4 type. Acta Cryst. 1957, 10, 538–539. [Google Scholar] [CrossRef]
- Ruddlesden, S.N.; Popper, P. The compound Sr3Ti2O7 and its structure. Acta Cryst. 1958, 11, 54–55. [Google Scholar] [CrossRef] [Green Version]
- Aurivillius, B. Mixed bismuth oxides with layer lattices: I. Structure type of CaBi2B2O9. Arkiv Kem 1949, 1, 463–480. [Google Scholar]
- Dion, M.; Ganne, M.; Tournoux, M. Nouvelles familles de phases MIMII2Nb3O10 a feuillets «perovskites». Mater. Res. Bull. 1981, 16, 1429–1435. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Lewandowski, J.T.; Johnson, J.W. Ion exchange of the layered perovskite KCa2Nb3O10 by protons. J. Less-Common Met. 1986, 116, 137–145. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Johnson, J.W.; Lewandowski, J.T. Interlayer chemistry between thick transition-metal oxide layers: Synthesis and intercalation reactions of K[Ca2Nan−3NbnO3n+1]. Inorg. Chem. 1985, 24, 3727–3729. [Google Scholar] [CrossRef]
- Domen, K.; Yoshimura, J.; Sekine, T.; Tanaka, A.; Onishi, T. A novel series of photocatalysts with an ion-exchangeable layered structure of niobate. Catal Lett. 1990, 4, 339–343. [Google Scholar] [CrossRef]
- Machida, M.; Yabunaka, J.; Kijima, T. Efficient photocatalytic decomposition of water with the novel layered tantalate RbNdTa2O7. Chem. Commun. 1999, 1939–1940. [Google Scholar] [CrossRef]
- Machida, M.; Miyazaki, K.; Matsushima, S.; Arai, M. Photocatalytic properties of layered perovskite tantalates, MLnTa2O7 (M = Cs, Rb, Na, and H.; Ln = La, Pr, Nd, and Sm). J. Mater. Chem. 2003, 13, 1433–1437. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Zvereva, I.A. Photocatalytic activity of layered perovskite-like oxides in practically valuable chemical reactions. Russ. Chem. Rev. 2016, 85, 248–279. [Google Scholar] [CrossRef]
- Krasheninnikova, O.V.; Syrov, E.V.; Smirnov, S.M.; Suleimanov, E.V.; Fukina, D.G.; Knyazev, A.V.; Titaev, D.N. Synthesis, crystal structure and photocatalytic activity of new Dion-Jacobson type titanoniobates. J. Solid State Chem. 2022, 315, 123445. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Ekthammathat, N.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T. Hydrothermal synthesis, structure, and optical properties of pure and silver-doped Bi2MoO6 nanoplates. Russ. J. Phys. Chem. 2015, 89, 2443–2448. [Google Scholar] [CrossRef]
- Chawla, H.; Chandra, A.; Ingole, P.P.; Garg, S. Recent advancements in enhancement of photocatalytic activity using bismuth-based metal oxides Bi2MO6 (M = W, Mo, Cr) for environmental remediation and clean energy production. Ind. Eng. Chem. Res. 2021, 95, 1–15. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Recent progress in structural development and band engineering of perovskites materials for photocatalytic solar hydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 19078–19111. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Deng, Y.; Qian, Y.; Jia, X.; Ma, M.; Yang, C.; Liu, K.; Wang, Z.; Qu, S.; et al. The application of perovskite materials in solar water splitting. J. Semicond. 2020, 41, 011701. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, G.; Wang, L.; Irvine, J.T.S. Inorganic perovskite photocatalysts for solar energy utilization. Chem. Soc. Rev. 2016, 45, 5951–5984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Zhang, J.; Gong, J. Tantalum-based semiconductors for solar water splitting. Chem. Soc. Rev. 2014, 43, 4395–4422. [Google Scholar] [CrossRef]
- Benedek, N.A.; Rondinelli, J.M.; Djani, H.; Ghosez, P.; Lightfoot, P. Understanding ferroelectricity in layered perovskites: New ideas and insights from theory and experiments. Dalton Trans. 2015, 44, 10543–10558. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, W.C.; Rodrigues, G.L.C.; Araújo, B.S.; de Aguiar, F.A.A.; de Abreu Silva, A.N.A.; Fechine, P.B.A.; de Araujo Paschoal, C.W.; Ayala, A.P. Pressure-induced structural phase transitions in the multiferroic four-layer Aurivillius ceramic Bi5FeTi3O15. Ceram. Int. 2020, 46, 18056–180621. [Google Scholar] [CrossRef]
- Zulhadjri; Wendari, T.P.; Ikhram, M.; Putri, Y.E.; Septiani, U.; Imelda. Enhanced dielectric and ferroelectric responses in La3+/Ti4+ co-substituted SrBi2Ta2O9 Aurivillius phase. Ceram. Int. 2022, 48, 10328–103321. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, S.; Wang, F.; Liu, J.; Shi, J.; Xing, J.; Chen, Q.; Zhu, J.; Wang, Q. Bismuth titanate based piezoceramics: Structural evolutions and electrical behaviors at different sintering temperatures. J. Alloys Compd. 2021, 882, 160637. [Google Scholar] [CrossRef]
- Chen, C.; Ning, H.; Lepadatu, S.; Cain, M.; Yan, H.; Reece, M.J. Ferroelectricity in Dion-Jacobson ABiNb2O7 (A = Rb, Cs) compounds. J. Mater. Chem. C 2015, 3, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Kudo, A.; Kaneko, E. Photoluminescence of layered perovskite oxides with triple-octahedra slabs containing titanium and niobium. J. Mater. Sci. Lett. 1997, 16, 224–226. [Google Scholar] [CrossRef]
- Pavani, K.; Graça, M.P.F.; Kumar, J.S.; Neves, A.J. Photoluminescence varied by selective excitation in BiGdWO6:Eu3+ phosphor. Opt. Mater. 2017, 74, 120–127. [Google Scholar] [CrossRef]
- Mamidi, S.; Gundeboina, R.; Kurra, S.; Velchuri, R.; Muga, V. Aurivillius family of layered perovskites, BiREWO6 (RE = La, Pr, Gd, and Dy): Synthesis, characterization, and photocatalytic studies. Comptes Rendus Chim. 2018, 21, 547–552. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, X.; Zhao, J.; Molokeev, M.; Lin, Z.; Liu, Q.; Xia, Z. Two-dimensional-layered perovskite ALaTa2O7:Bi3+ (A = K and Na) phosphors with versatile structures and tunable photoluminescence. ACS Appl. Mater. Interfaces 2018, 10, 24648–24655. [Google Scholar] [CrossRef]
- Panda, D.P.; Singh, A.K.; Kundu, T.K.; Sundaresan, A. Visible-light excited polar Dion-Jacobson Rb(Bi1−xEux)2Ti2NbO10 perovskites: Photoluminescence properties and in vitro bioimaging. J. Mater. Chem. B 2022, 10, 935–944. [Google Scholar] [CrossRef]
- Yadav, D.; Nirala, G.; Kumar, U.; Upadhyay, S. Investigation on structural and optical properties of system Sr2Ce1−xNaxO4 (0.0 ≤ x ≤ 0.10). J. Mater. Sci. Mater. Electron. 2021, 32, 8064–8080. [Google Scholar] [CrossRef]
- Schaak, E.R.; Mallouk, T.E. KLnTiO4 (Ln = La, Nd, Sm, Eu, Gd, Dy): A new series of Ruddlesden-Popper phases synthesized by ion-exchange of HLnTiO4. J. Solid State Chem. 2001, 161, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Nishimoto, S.; Matsuda, M.; Miyake, M. Novel protonated and hydrated n = 1 Ruddlesden-Popper phases, HxNa1−xLaTiO4−yH2O, formed by ion-exchange/intercalation reaction. J. Solid State Chem. 2005, 178, 811–818. [Google Scholar] [CrossRef]
- Nishimoto, S.; Matsuda, M.; Harjo, S.; Hoshikawa, A.; Kamiyama, T.; Ishigaki, T.; Miyake, M. Structural change in a series of protonated layered perovskite compounds, HLnTiO4 (Ln = La, Nd and Y). J. Solid State Chem. 2006, 179, 1892–1897. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Silyukov, O.I.; Mazur, A.S.; Zvereva, I.A. Synthesis and thermal stability of new inorganic-organic perovskite-like hybrids based on layered titanates HLnTiO4 (Ln = La, Nd). Ceram. Int. 2020, 46, 5058–5068. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, Y.; Fan, L.; Li, Y.; Wei, Y.; Lin, J.; Wu, J. Synthesis and photochemical properties of La-doped HCa2Nb3O10. Int. J. Hydrogen Energy 2008, 33, 6432–6438. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, R.; Waterhouse, G.I.N.; Zhang, T. Recent advances in niobium-based semiconductors for solar hydrogen production. Coord. Chem. Rev. 2020, 419, 213399. [Google Scholar] [CrossRef]
- Fujii, K.; Esaki, Y.; Omoto, K.; Yashima, M.; Hoshikawa, A.; Ishigaki, T.; Hester, J.R. New perovskite-related structure family of oxide-ion conducting materials NdBaInO4. Chem. Mater. 2014, 26, 2488–2491. [Google Scholar] [CrossRef]
- Fujii, K.; Shiraiwa, M.; Esaki, Y.; Yashima, M.; Kim, S.J.; Lee, S. Improved oxide-ion conductivity of NdBaInO4 by Sr doping. J. Mater. Chem. A 2015, 3, 11985. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, T.; Yan, Y.; Sakai, T.; Ida, S. Oxide ion conductivity in doped NdBaInO4. Solid State Ion. 2016, 288, 262–265. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Lu, F.; Xu, J.; Kuang, X. Acceptor doping and oxygen vacancy migration in layered perovskite NdBa1−nO4-based mixed conductors. J. Phys. Chem. C 2016, 120, 6416–6426. [Google Scholar] [CrossRef]
- Fijii, K.; Yashima, M. Discovery and development of BaNdInO4 -A brief review. J. Ceram. Soc. Jpn. 2018, 126, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Shiraiwa, M.; Nagao, M.; Fujii, K.; Tanaka, I.; Yashima, M.; Baque, L.; Basbus, J.F.; Mogni, L.V.; Skinner, S.J. Protonic conduction in the BaNdInO4 structure achieved by acceptor doping. Chem. Mater. 2021, 33, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, M.; Kido, T.; Fujii, K.; Yashima, M. High-temperature proton conductors based on the (110) layered perovskite BaNdScO4. J. Mat. Chem. A 2021, 9, 8607. [Google Scholar] [CrossRef]
- Kato, S.; Ogasawara, M.; Sugai, M.; Nakata, S. Synthesis and oxide ion conductivity of new layered perovskite La1−xSr1+xInO4−d. Solid State Ion. 2002, 149, 53–57. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Aguadero, A. Low activation energies for interstitial oxygen conduction in the layered perovskites La1+xSr1−xInO4+d. J. Mater. Chem. A 2015, 3, 17797–17803. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Fernández-Díaz, M.T.; Aguadero, A. Introduction of interstitial oxygen atoms in the layered perovskite LaSrIn1−xBxO4+δ system (B=Zr, Ti). Solid State Ion. 2015, 282, 82–87. [Google Scholar] [CrossRef]
- Troncoso, L.; Mariño, C.; Arce, M.D.; Alonso, J.A. Dual oxygen defects in layered La1.2Sr0.8−xBaxInO4+d (x = 0.2, 0.3) oxide-ion conductors: A neutron diffraction study. Materials 2019, 12, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troncoso, L.; Arce, M.D.; Fernández-Díaz, M.T.; Mogni, L.V.; Alonso, J.A. Water insertion and combined interstitial-vacancy oxygen conduction in the layered perovskites La1.2Sr0.8−xBaxInO4+δ. New J. Chem. 2019, 43, 6087–6094. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Electrical properties of new protonic conductors Ba1+xLa1−xInO4−0.5x with Ruddlesden-Popper structure. J. Solid State Electrochem. 2020, 24, 1497–1508. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I. Improvement of oxygen-ionic and protonic conductivity of BaLaInO4 through Ti doping. Ionics 2020, 26, 5075–5088. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I. Ba2+/Ti4+- co-doped layered perovskite BaLaInO4: The structure and ionic (O2−, H+) conductivity. Int. J. Hydrogen Energy 2021, 46, 16868–16877. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Effect of acceptor and donor doping on the state of protons in block-layered structures based on BaLaInO4. Solid State Commun. 2021, 323, 14093. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Anokhina, I.; Gilev, A.; Cheremisina, P. Novel mid-temperature Y3+ → In3+ doped proton conductors based on the layered perovskite BaLaInO4. Ceram. Int. 2022, 48, 15677–15685. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A.; Korona, D.; Davletbaev, K. Novel proton-conducting layered perovskite based on BaLaInO4 with two different cations in B-sublattice: Synthesis, hydration, ionic (O2-, H+) conductivity. Int. J. Hydrogen Energy 2022, 47, 18972–18982. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Materials AIILnInO4 with Ruddlesden-Popper structure for electrochemical applications: Relationship between ion (oxygen-ion, proton) conductivity, water uptake and structural changes. Materials 2022, 15, 114. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Korona, D.; Kreimesh, H.; Fedorova, I. Protonic Transport in layered perovskites BaLanInnO3n+1 (n = 1, 2) with Ruddlesden-Popper Structur. Appl. Sci. 2022, 12, 4082. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Belova, K.; Egorova, A.; Abakumova, E.; Medvedev, D. Layered perovskites BaM2In2O7 (M = La, Nd): From the structure to the ionic (O2−, H+) conductivity. Materials 2022, 15, 3488. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Korona, D.; Abakumova, E.; Medvedev, D. Novel mixed oxygen-electronic conductors based on BaLa2In2O7 with two-layer Ruddlesden-Popper structure. Ceram 2022, 48, 35376–35385. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]





| Sample | a, b (Å) | c (Å) | Vcell (Å3) | Water Uptake (mol) |
|---|---|---|---|---|
| 0 | 5.914(9) | 20.846(5) | 729.3365 | 0.17 |
| 0.05 | 5.915(2) | 20.869(0) | 730.1977 | 0.15 |
| 0.10 | 5.916(3) | 20.870(4) | 730.5183 | 0.18 |
| 0.15 | 5.916(4) | 20.871(3) | 730.5745 | 0.18 |
| 0.20 | 5.917(2) | 20.872(1) | 730.8001 | 0.19 |
| Sample | a, b (Å) | c (Å) | Vcell (Å3) | Water Uptake (mol) |
|---|---|---|---|---|
| 0 | 5.914(9) | 20.846(5) | 729.3365 | 0.17 |
| 0.05 | 5.915(1) | 20.859(0) | 729.8232 | 0.16 |
| 0.10 | 5.916(3) | 20.870(4) | 730.5183 | 0.17 |
| 0.15 | 5.920(4) | 20.899(3) | 732.5442 | 0.19 |
| 0.20 | 5.927(5) | 20.940(1) | 735.7357 | 0.20 |
| 0.25 | 5.941(5) | 20.949(1) | 739.5330 | 0.21 |
| 0.30 | 5.956(6) | 20.954(9) | 743.5025 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasova, N.; Bedarkova, A.; Animitsa, I.; Belova, K.; Abakumova, E.; Cheremisina, P.; Medvedev, D. Oxygen Ion and Proton Transport in Alkali-Earth Doped Layered Perovskites Based on BaLa2In2O7. Inorganics 2022, 10, 161. https://doi.org/10.3390/inorganics10100161
Tarasova N, Bedarkova A, Animitsa I, Belova K, Abakumova E, Cheremisina P, Medvedev D. Oxygen Ion and Proton Transport in Alkali-Earth Doped Layered Perovskites Based on BaLa2In2O7. Inorganics. 2022; 10(10):161. https://doi.org/10.3390/inorganics10100161
Chicago/Turabian StyleTarasova, Nataliia, Anzhelika Bedarkova, Irina Animitsa, Ksenia Belova, Ekaterina Abakumova, Polina Cheremisina, and Dmitry Medvedev. 2022. "Oxygen Ion and Proton Transport in Alkali-Earth Doped Layered Perovskites Based on BaLa2In2O7" Inorganics 10, no. 10: 161. https://doi.org/10.3390/inorganics10100161
APA StyleTarasova, N., Bedarkova, A., Animitsa, I., Belova, K., Abakumova, E., Cheremisina, P., & Medvedev, D. (2022). Oxygen Ion and Proton Transport in Alkali-Earth Doped Layered Perovskites Based on BaLa2In2O7. Inorganics, 10(10), 161. https://doi.org/10.3390/inorganics10100161








