Capture Mechanism of Cadmium in Agricultural Soil Via Iron-Modified Graphene
Abstract
:1. Introduction
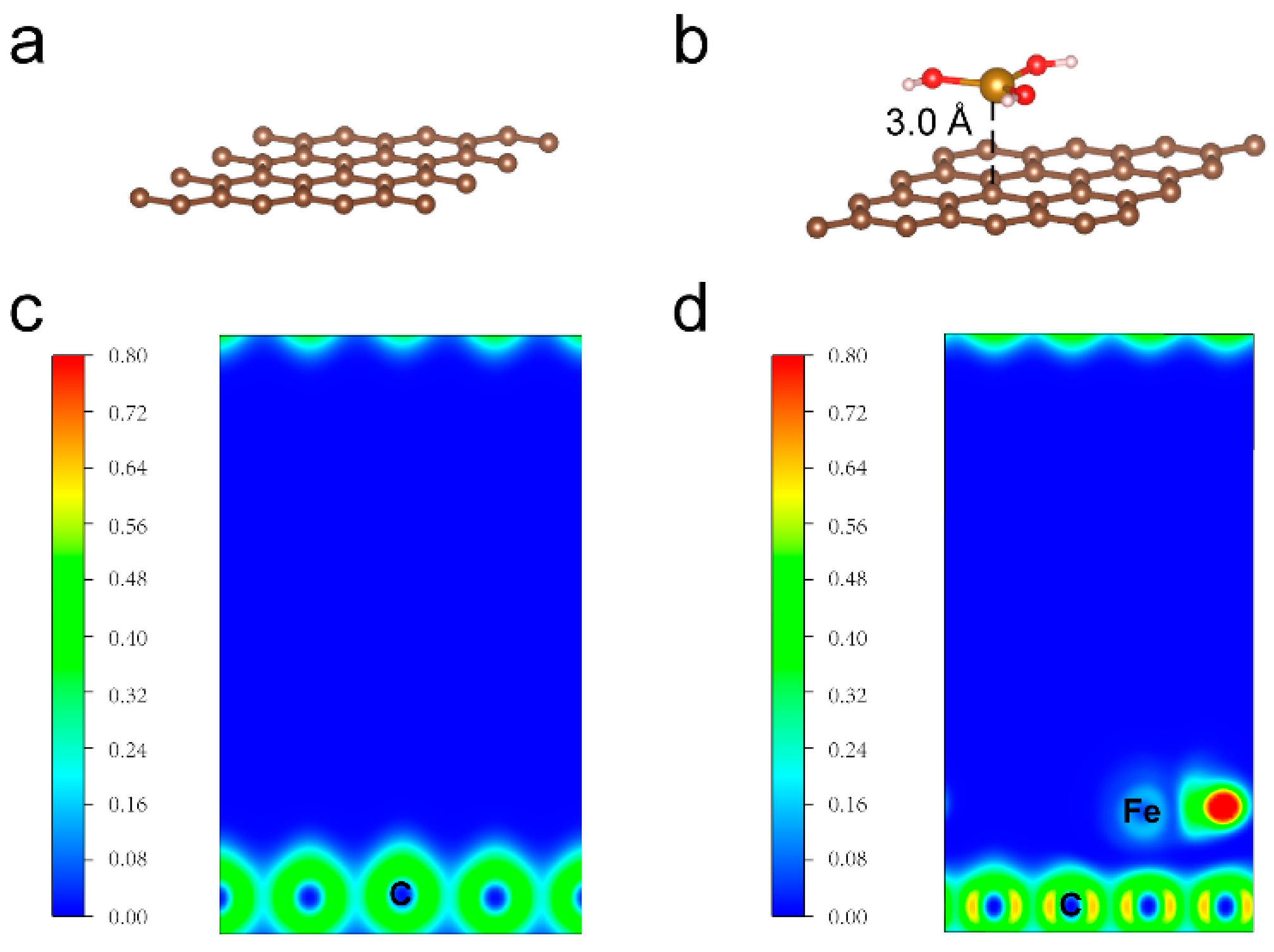
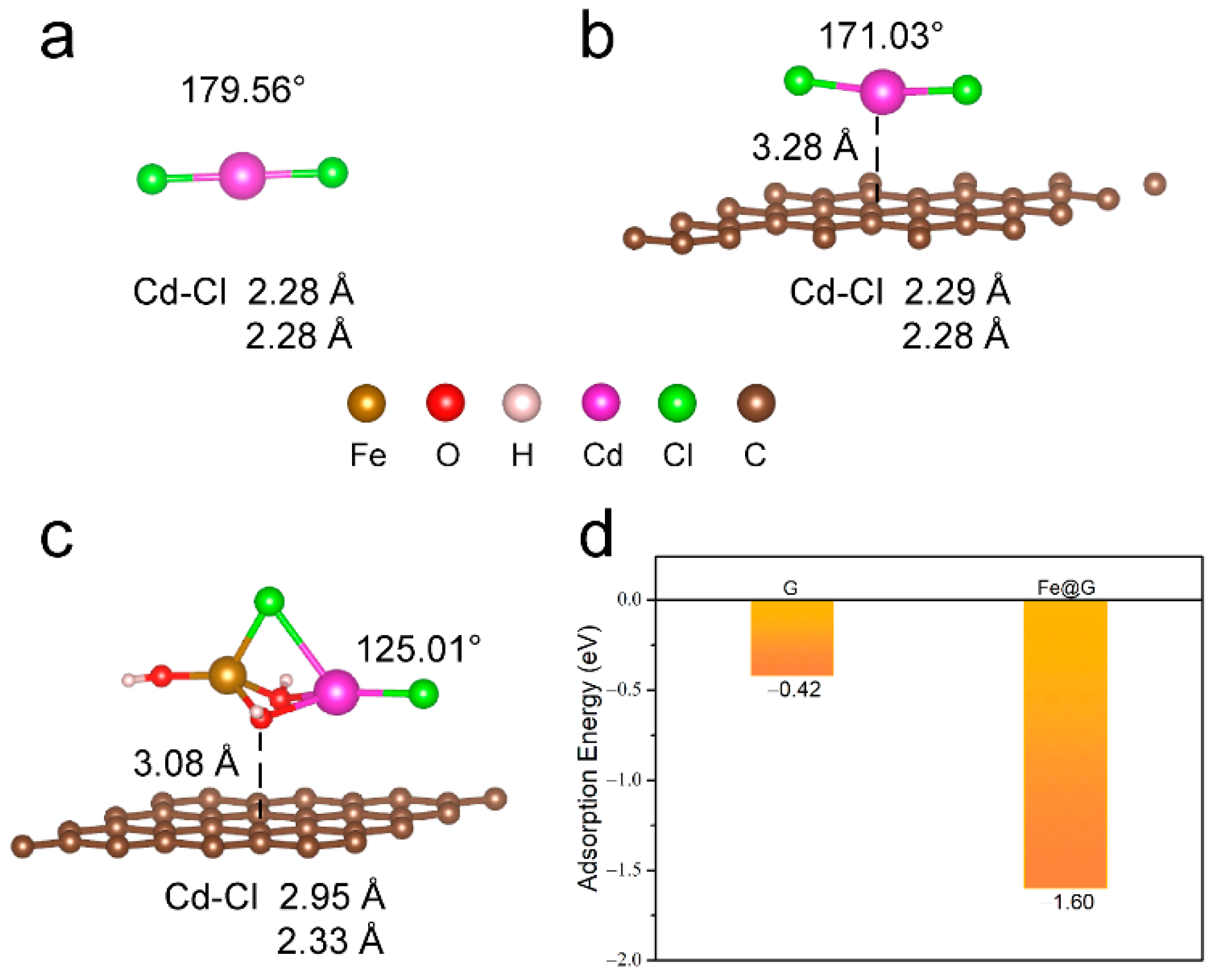
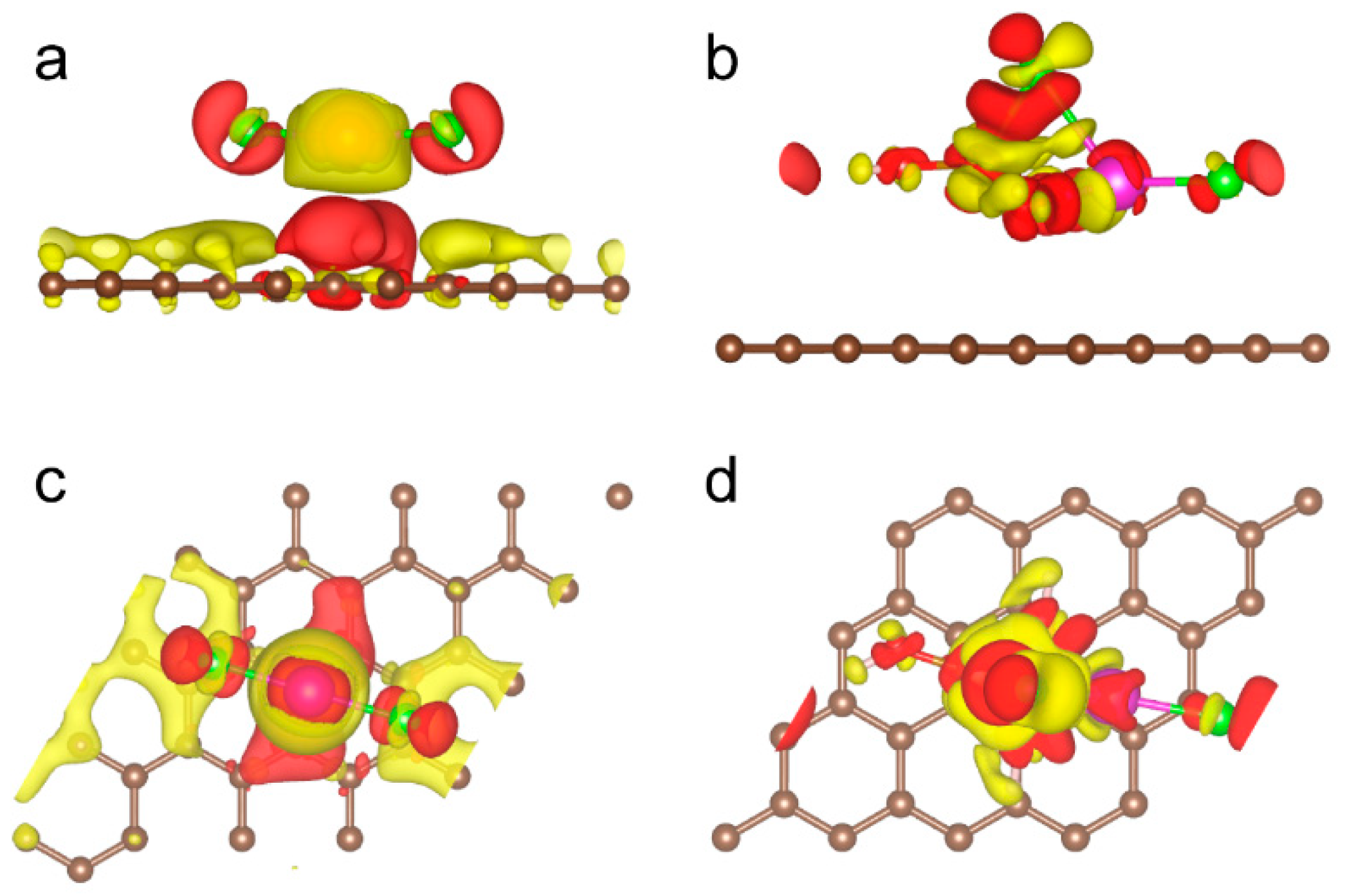
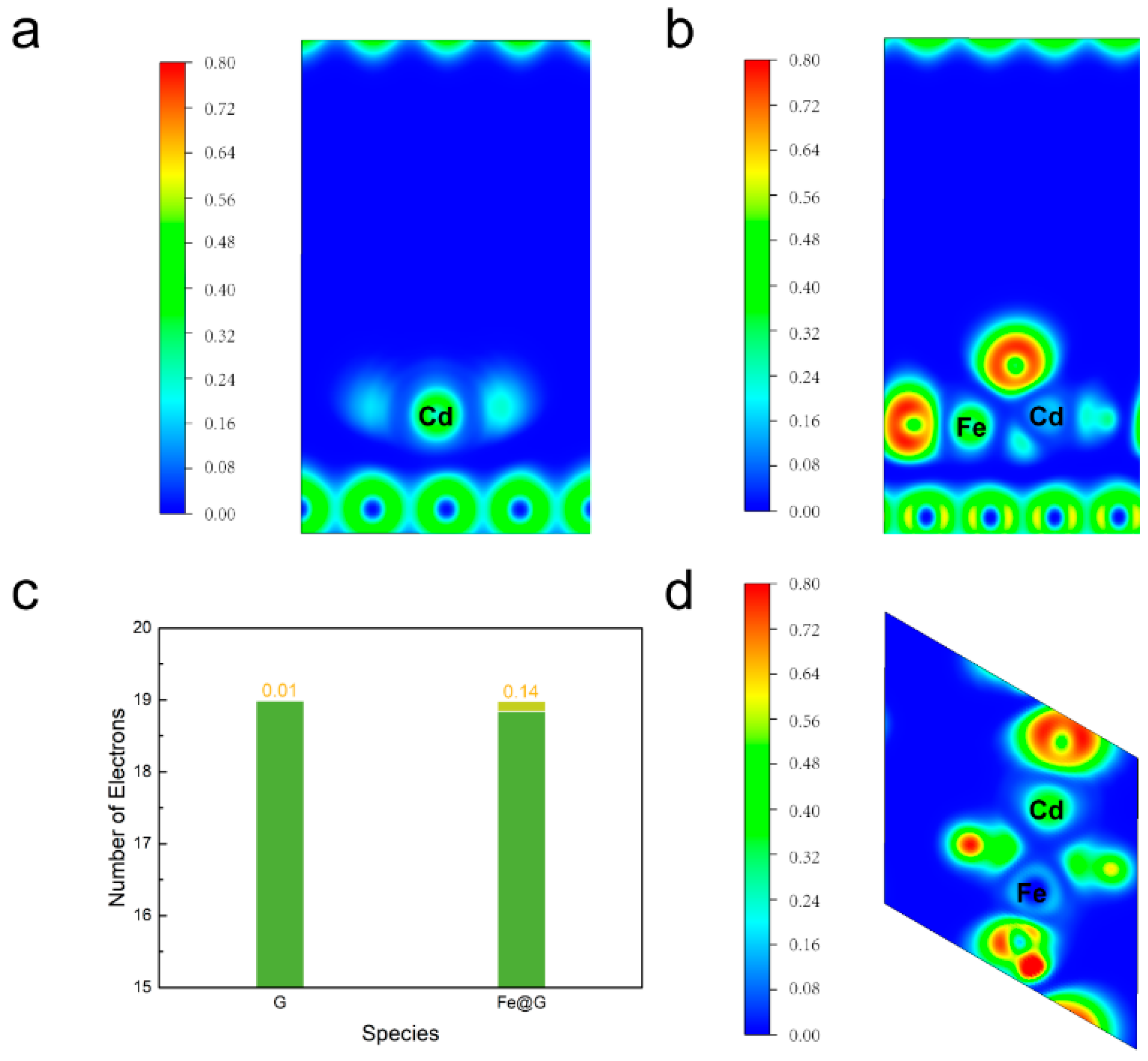
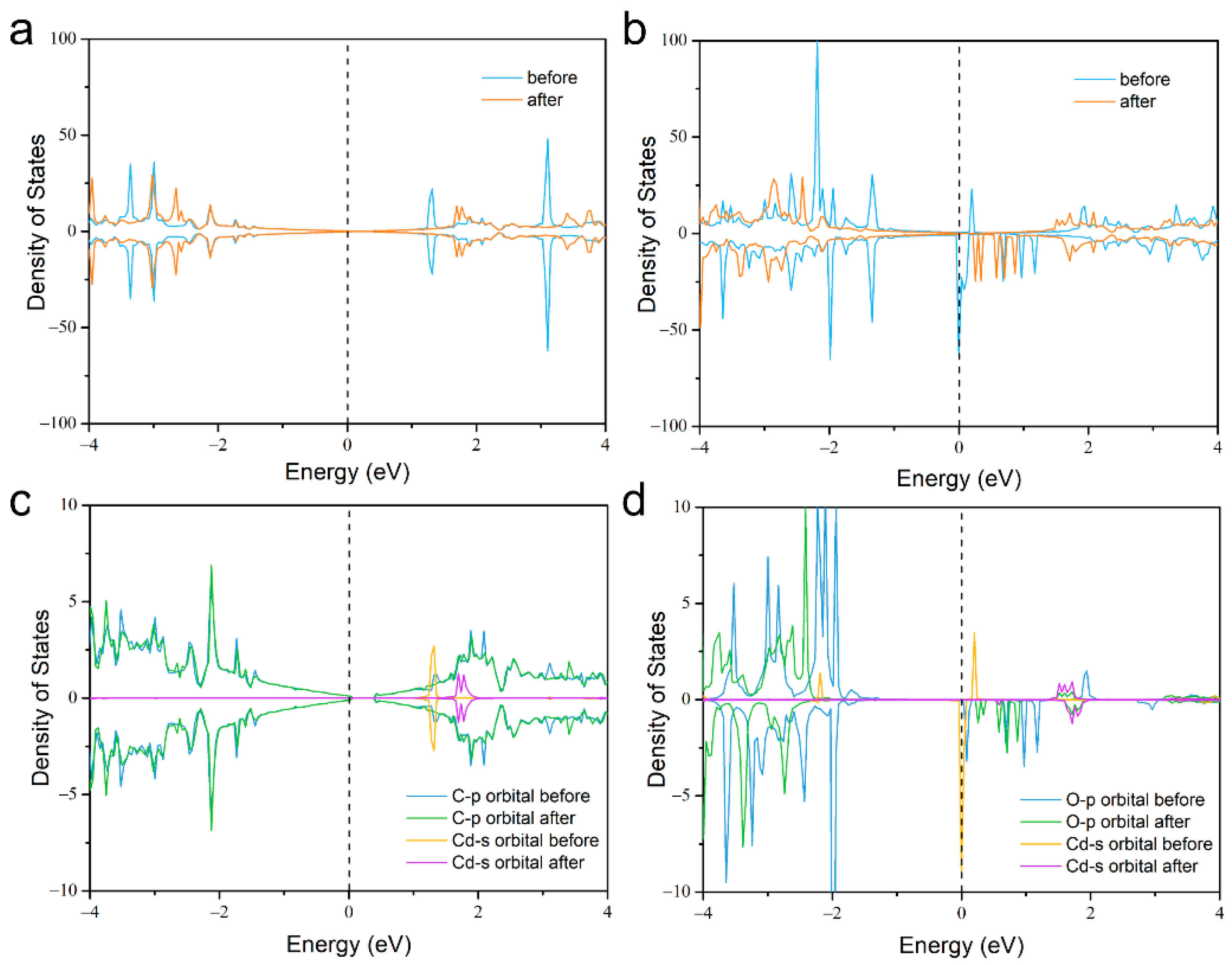
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Kotaki, K.; Gunji, F.; Kubo, N.; Kobayashi, S.; Ito, T.; Itabashi, H. Suppression of cadmium uptake in rice using fermented bark as a soil amendment. Chemosphere 2016, 148, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q. Integrated amendment and ecological restoration of polluted soils by heavy metals. Proc. Strategy Soil Environ. Prot. New Century China 1998, 26–29. [Google Scholar]
- Du, Z.-Y.; Liu, Y.-J.; Tian, L.-X.; Wang, J.-T.; Wang, Y.; Liang, G.-Y. Effect of dietary lipid level on growth, feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2005, 11, 139–146. [Google Scholar] [CrossRef]
- Zong, Y.; Xiao, Q.; Lu, S. Biochar derived from cadmium-contaminated rice straw at various pyrolysis temperatures: Cadmium immobilization mechanisms and environmental implication. Bioresour. Technol. 2021, 321, 124459. [Google Scholar] [CrossRef]
- Sun, L.; Wang, R.; Tang, W.; Chen, Y.; Zhou, J.; Ma, H.; Li, S.; Deng, H.; Han, L.; Chen, Y.; et al. Robust identification of low-Cd rice varieties by boosting the genotypic effect of grain Cd accumulation in combination with marker-assisted selection. J. Hazard Mater. 2022, 424, 127703. [Google Scholar] [CrossRef]
- Satarug, S.; Moore, M. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Perspect 2004, 112, 1099–1103. [Google Scholar] [CrossRef]
- Haider, F.; Liqun, C.; Coulter, J.; Cheema, S.A.; Wu, J.; Zhang, R.; Ma, W.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotox Environ. Safe 2021, 211, 111887. [Google Scholar] [CrossRef]
- Shen, Z.; Fan, X.; Hou, D.; Jin, F.; O’Connor, D.; Tsang, D.C.W.; Ok, Y.S.; Alessi, D.S. Risk evaluation of biochars produced from Cd-contaminated rice straw and optimization of its production for Cd removal. Chemosphere 2019, 233, 149–156. [Google Scholar] [CrossRef]
- Yang, W.-T.; Gu, J.-F.; Zou, J.-L.; Zhou, H.; Zeng, Q.-R.; Liao, B.-H. Impacts of rapeseed dregs on Cd availability in contaminated acid soil and Cd translocation and accumulation in rice plants. Environ. Sci. Pollut Res. 2016, 23, 20853–20861. [Google Scholar] [CrossRef] [PubMed]
- Chaney, R. How does contamination of rice soils with Cd and Zn cause high incidence of human Cd disease in subsistence rice farmers. Curr. Pollut. Rep. 2015, 1, 13–22. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, K. Itai-itai disease: Renal tubular osteomalacia induced by environmental exposure to cadmium—historical review and perspectives. Soil Sci. Plant Nutr. 2016, 62, 319–326. [Google Scholar] [CrossRef]
- Zanuzzi, A.; Faz, A.; Acosta, J. Chemical stabilization of metals in the environment: A feasible alternative for remediation of mine soils. Environ. Earth Sci. 2013, 70, 2623–2632. [Google Scholar] [CrossRef]
- Rees, F.; Simonnot, M.; Morel, J. Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.-X.; Ye, X.-Z.; Xiao, X.-D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotox Environ. Safe 2016, 132, 94–100. [Google Scholar] [CrossRef]
- Rajendran, M.; Shi, L.; Wu, C.; Li, W.; An, W.; Liu, Z.; Xue, S. Effect of sulfur and sulfur-iron modified biochar on cadmium availability and transfer in the soil-rice system. Chemosphere 2019, 222, 314–322. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.; Thies, J.; Masiello, C.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota–a review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Baolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lin, H.; He, Y.; Zhang, Y. The influence of corncob-based biochar on remediation of arsenic and cadmium in yellow soil and cinnamon soil. Sci. Total Environ. 2020, 717, 137014. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Altschul, E.; Hedin, R.; Nakles, D.; Dzombak, D.A. Sequestration enhancement of metals in soils by addition of iron oxides recovered from coal mine drainage sites. Soil Sediment Contam. 2014, 23, 374–388. [Google Scholar] [CrossRef]
- Chen, S.; Chen, W.; Shih, C. Heavy metal removal from wastewater using zero-valent iron nanoparticles. Water Sci. Technol. 2008, 58, 1947–1954. [Google Scholar] [CrossRef]
- Rehman, M.; Rizwan, M.; Khalid, H.; Ali, S.; Naeem, A.; Yousaf, B.; Liu, G.; Sabir, M.; Farooq, M. Farmyard manure alone and combined with immobilizing amendments reduced cadmium accumulation in wheat and rice grains grown in field irrigated with raw effluents. Chemosphere 2018, 199, 468–476. [Google Scholar] [CrossRef]
- Guo, F.; Ding, C.; Zhou, Z.; Huang, G.; Wang, X. Effects of combined amendments on crop yield and cadmium uptake in two cadmium contaminated soils under rice-wheat rotation. Ecotox Environ. Safe 2018, 148, 303–310. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, T.; Wang, X.; Li, F.; Lv, Y.; Cui, J.; Zeng, X.; Yuan, Y.; Liu, C. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef]
- Yin, D.; Wang, X.; Peng, B.; Tan, C.; Ma, L.Q. Effect of biochar and Fe-biochar on Cd and As mobility and transfer in soil-rice system. Chemosphere 2017, 186, 928–937. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, S.; Wang, T.; Yang, S.Z.; Johannessen, B.; Chen, H.; Liu, C.; Ye, Y.; Wu, Y.; Peng, Y.; et al. Theoretical calculation guided design of single-atom catalysts towards fast kinetic and long-life Li-S batteries. Nano Lett. 2019, 20, 1252–1261. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.; Liu, S.; Tan, Y.; Cheng, Z.; Shen, Z. FeN3-embedded carbon as an efficient sorbent for mercury adsorption: A theoretical study. Chem. Eng. J. 2019, 374, 1337–1343. [Google Scholar] [CrossRef]
- Hou, T.; Chen, X.; Peng, H.-J.; Huang, J.; Li, B.-Q.; Zhang, Q.; Li, B. Design Principles for Heteroatom-Doped Nanocarbon to Achieve Strong Anchoring of Polysulfides for Lithium-Sulfur Batteries. Small 2016, 12, 3283–3291. [Google Scholar] [CrossRef]
- Dai, J.; Yuan, J. Modulating the electronic and magnetic structures of P-doped graphene by molecule doping. J. Phys. Condens. Matter 2010, 22, 225501. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, X.; Xiong, P.; Zhang, J.; Song, J.; Yan, K.; Gao, X.; Liu, H.; Wang, G. Interface Engineering of MXene Composite Separator for High-Performance Li-Se and Na-Se Batteries. Adv. Energy Mater. 2020, 10, 2000446. [Google Scholar] [CrossRef]
- Froyen, S. Brillouin-zone integration by Fourier quadrature: Special points for superlattice and supercell calculations. Phys. Rev. B 1989, 39, 3168. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, T.; Chang, H.; Wang, F.; Wen, J.; Sun, H.; Hossain, M.; Xie, Q.; Zhao, Y.; Wu, Y. Computational Design of Single Mo Atom Anchored Defective Boron Phosphide Monolayer as a High-performance Electrocatalyst for the Nitrogen Reduction Reaction. Energy Environ. Mater. 2021, 4, 255–262. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Q. Magnetism of phthalocyanine-based organometallic single porous sheet. J. Am. Chem. Soc. 2011, 133, 15113–15119. [Google Scholar] [CrossRef]
- Ozugurlu, E. Cd-doped ZnO nanoparticles: An experimental and first-principles DFT studies. J. Alloy Compd. 2021, 861, 158620. [Google Scholar] [CrossRef]
- Luo, J.; Sun, M.; Ritt, C.; Liu, X.; Pei, Y.; Crittenden, J.C.; Elimelech, M. Tuning Pb(II) Adsorption from Aqueous Solutions on Ultrathin Iron Oxychloride (FeOCl) Nanosheets. Environ. Sci. Technol. 2019, 53, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Hu, J.; Zhou, W.; Long, P.; Ma, X.; Zhang, F.; Wu, Y.; Wu, X.; Dai, J.; Fu, Z. Capture Mechanism of Cadmium in Agricultural Soil Via Iron-Modified Graphene. Inorganics 2022, 10, 150. https://doi.org/10.3390/inorganics10100150
Wang H, Hu J, Zhou W, Long P, Ma X, Zhang F, Wu Y, Wu X, Dai J, Fu Z. Capture Mechanism of Cadmium in Agricultural Soil Via Iron-Modified Graphene. Inorganics. 2022; 10(10):150. https://doi.org/10.3390/inorganics10100150
Chicago/Turabian StyleWang, Hongrui, Junping Hu, Wentao Zhou, Pan Long, Xin Ma, Feng Zhang, Yuping Wu, Xiongwei Wu, Jiayu Dai, and Zhiqiang Fu. 2022. "Capture Mechanism of Cadmium in Agricultural Soil Via Iron-Modified Graphene" Inorganics 10, no. 10: 150. https://doi.org/10.3390/inorganics10100150
APA StyleWang, H., Hu, J., Zhou, W., Long, P., Ma, X., Zhang, F., Wu, Y., Wu, X., Dai, J., & Fu, Z. (2022). Capture Mechanism of Cadmium in Agricultural Soil Via Iron-Modified Graphene. Inorganics, 10(10), 150. https://doi.org/10.3390/inorganics10100150






