Synthesis, Structures, and Photoluminescence of Two Novel Zinc(II) Compounds Containing 2-Acetylpyridine-aminoguanidine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Physicochemical Properties of the Complexes
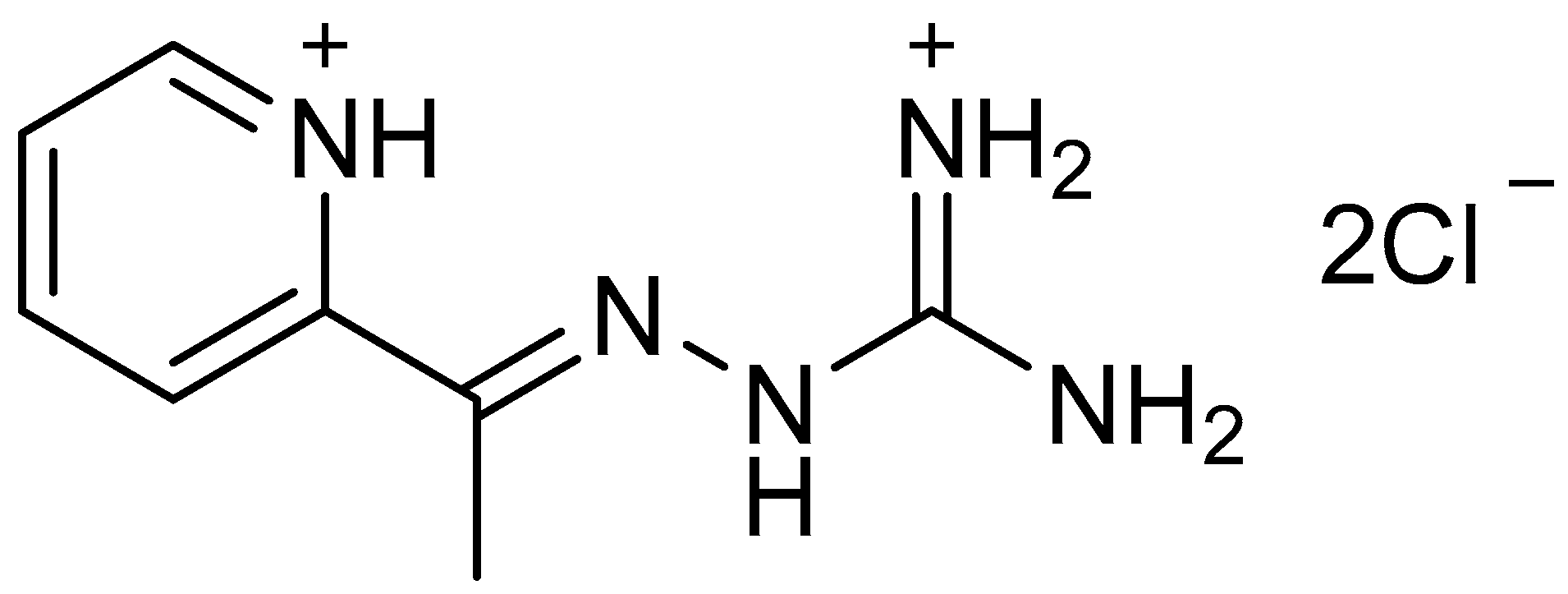
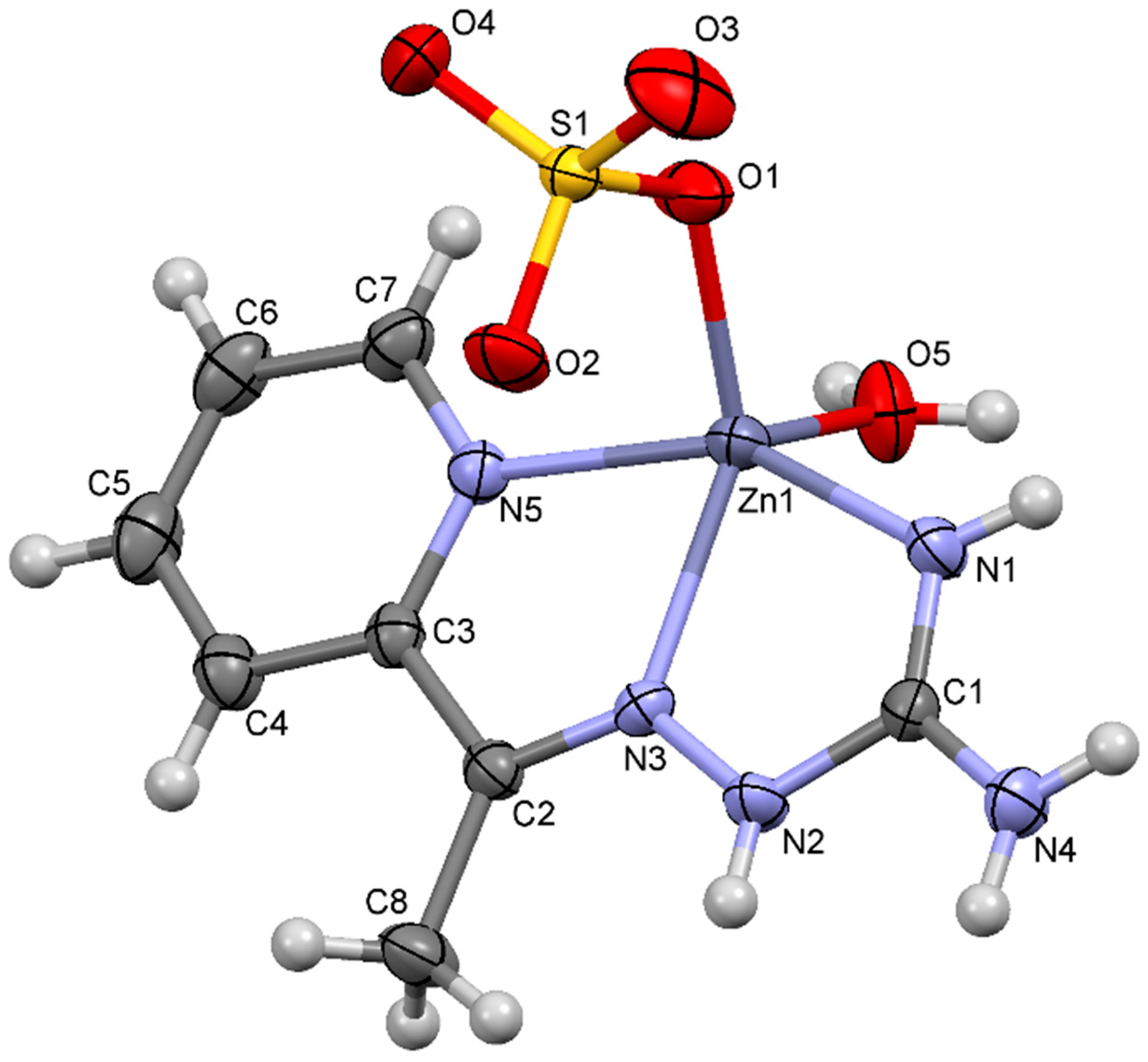
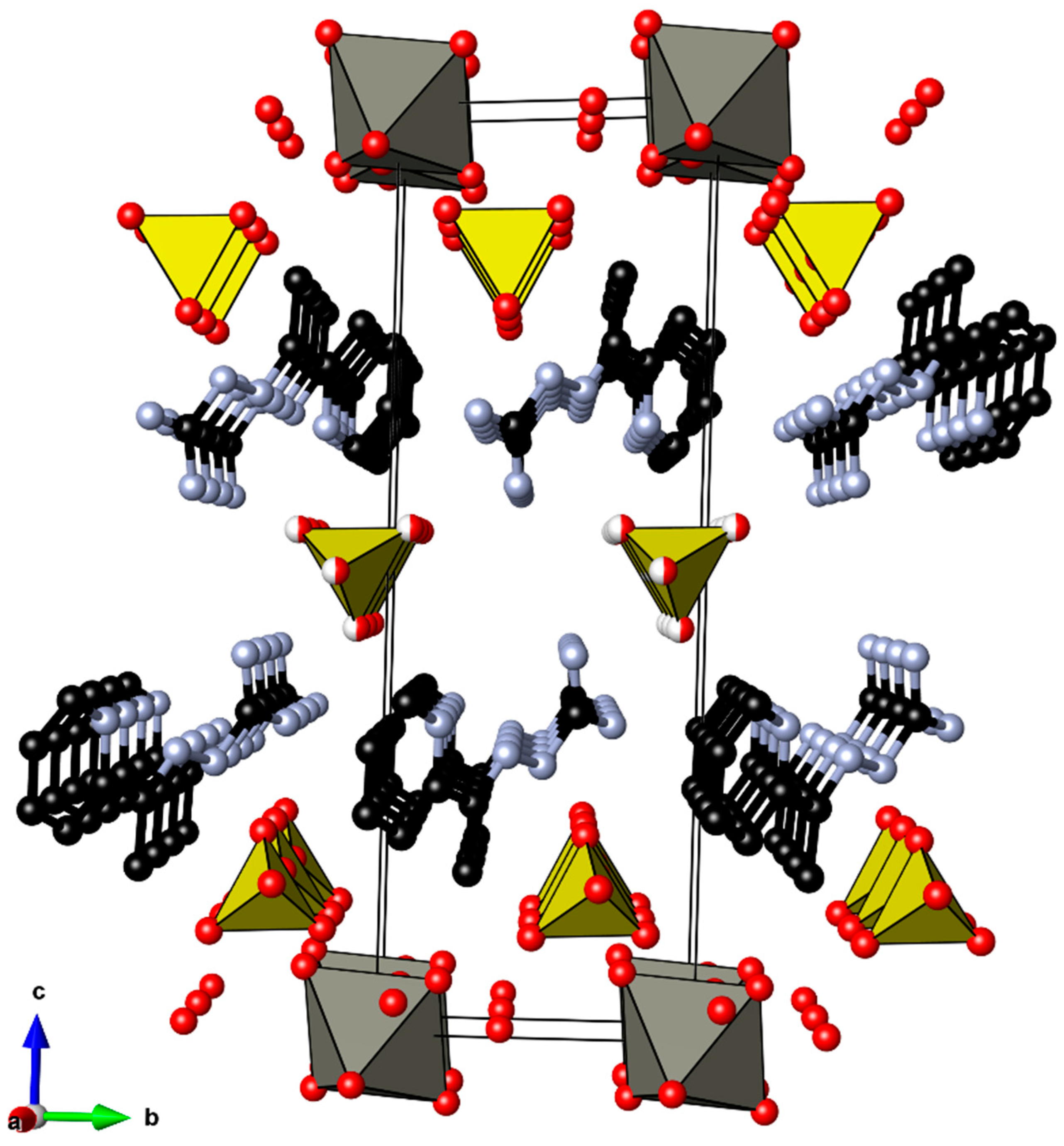
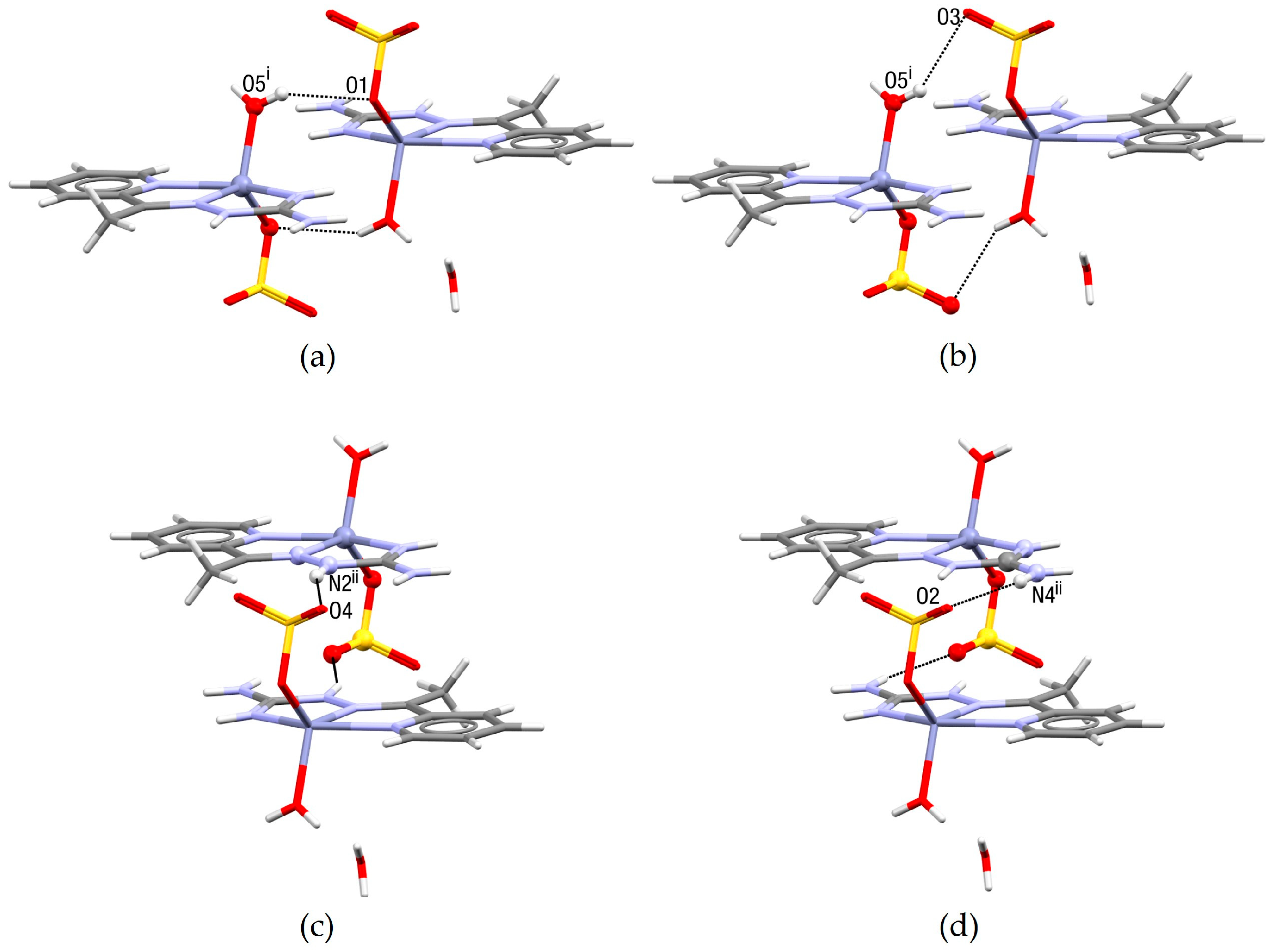
2.2. Crystal Structure
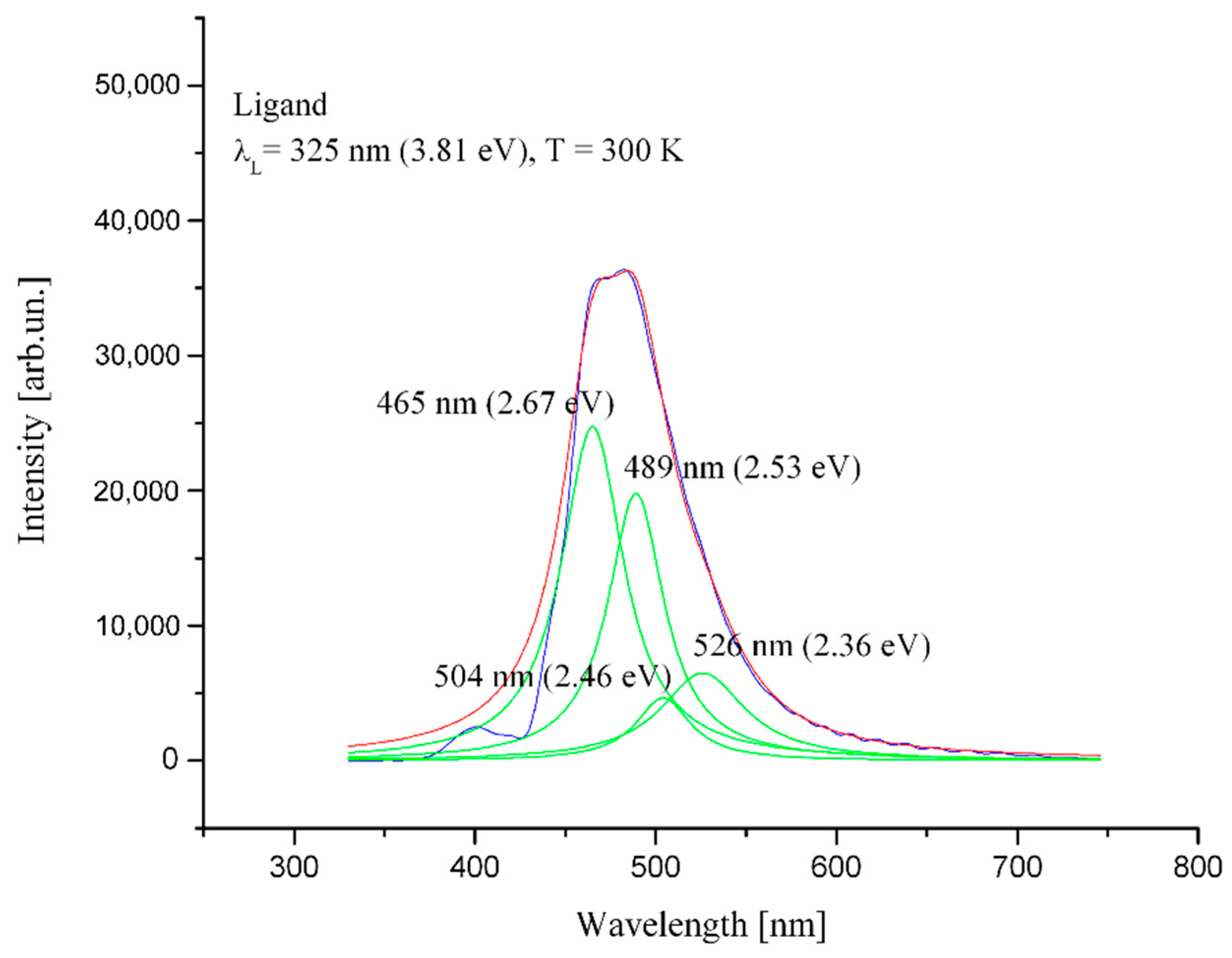
2.3. Photoluminescence Measurement
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Synthesis of Complexes
3.3. Single Crystal X-ray Diffraction
3.4. Photoluminescence Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández-Molina, R.; Mederos, A. Acyclic and Macrocyclic Schiff Base Ligands. Compr. Coord. Chem. II 2003, 1, 411–446. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sutar, A.K. Catalytic Activities of Schiff Base Transition Metal Complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Wong, W.Y. Energy Materials Based on Metal Schiff Base Complexes. Coord. Chem. Rev. 2018, 355, 180–198. [Google Scholar] [CrossRef]
- Di Bella, S.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D. Zinc(II) as a Versatile Template for Efficient Dipolar and Octupolar Second-Order Nonlinear Optical Molecular Materials. Inorganics 2018, 6, 133. [Google Scholar] [CrossRef]
- Matozzo, P.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D.; Biagini, P.; Fantacci, S.; Marinotto, D. Communication A Chiral Bis(Salicylaldiminato)Zinc(II) Complex with Second-Order Nonlinear Optical and Luminescent Properties in Solution. Inorganics 2020, 8, 25. [Google Scholar] [CrossRef]
- Hossain, A.M.S.; Méndez-Arriaga, J.M.; Xia, C.; Xie, J.; Gómez-Ruiz, S. Metal Complexes with ONS Donor Schiff Bases. A Review. Polyhedron 2022, 217, 115692. [Google Scholar] [CrossRef]
- Gao, X.S.; Ni, C.C.; Ren, X.M. Syntheses, Crystal Structures, Photoluminescent and Magnetic Properties of Complexes of Zinc(II) and Copper(II) with Schiff-Base Ligands Derived from 2,6-Diacetylpyridine. Polyhedron 2017, 138, 225–231. [Google Scholar] [CrossRef]
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable Metal Complexes for Organic Light-Emitting Diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- Ji, Y.F.; Wang, R.; Ding, S.; Du, C.F.; Liu, Z.L. Synthesis, Crystal Structures and Fluorescence Studies of Three New Zn(II) Complexes with Multidentate Schiff Base Ligands. Inorg. Chem. Commun. 2012, 16, 47–50. [Google Scholar] [CrossRef]
- Basak, S.; Sen, S.; Banerjee, S.; Mitra, S.; Rosair, G.; Rodriguez, M.T.G. Three New Pseudohalide Bridged Dinuclear Zn(II) Schiff Base Complexes: Synthesis, Crystal Structures and Fluorescence Studies. Polyhedron 2007, 26, 5104–5112. [Google Scholar] [CrossRef]
- Araškov, J.B.; Višnjevac, A.; Popović, J.; Blagojević, V.; Fernandes, H.S.; Sousa, S.F.; Novaković, I.; Padrón, J.M.; Holló, B.B.; Monge, M.; et al. Zn(Ii) Complexes with Thiazolyl-Hydrazones: Structure, Intermolecular Interactions, Photophysical Properties, Computational Study and Anticancer Activity. CrystEngComm 2022, 24, 5194–5214. [Google Scholar] [CrossRef]
- Ejarque, D.; Calvet, T.; Font-Bardia, M.; Pons, J. Steric Crowding of a Series of Pyridine Based Ligands Influencing the Photophysical Properties of Zn(II) Complexes. CrystEngComm 2021, 23, 6199–6213. [Google Scholar] [CrossRef]
- Taguchi, T.; Sugiura, M.; Hamada, Y.; Miwa, I. In Vivo Formation of a Schiff Base of Aminoguanidine with Pyridoxal Phosphate. Biochem. Pharm. 1998, 55, 1667–1671. [Google Scholar] [CrossRef]
- Vojinović-Ješić, L.S.; Radanović, M.M. Koordinaciona Hemija Aminogvanidina i Njegovih Šifovih Baza; Faculty of Sciences, University of Novi Sad: Novi Sad, Serbia, 2017; ISBN 9788670314542. [Google Scholar]
- Garcia, C.V.; Parrilha, G.L.; Rodrigues, B.L.; Barbeira, P.J.S.; Clarke, R.M.; Storr, T.; Beraldo, H. Cobalt(III) Complexes with 2-Acetylpyridine-Derived Schiff Bases: Studies Investigating Ligand Release upon Reduction. Polyhedron 2017, 124, 86–95. [Google Scholar] [CrossRef]
- Jelić, M.G.; Boukos, N.; Lalović, M.M.; Romčević, N.Ž.; Leovac, V.M.; Hadžić, B.B.; Baloš, S.S.; Jovanović, L.S.; Slankamenac, M.P.; Živanov, M.B.; et al. Synthesis, Structure and Photoluminescence Properties of Copper(II) and Cobalt(III) Complexes with Pyridoxalaminoguanidine. Opt. Mater. 2013, 35, 2728–2735. [Google Scholar] [CrossRef]
- Radanović, M.M.; Jelić, M.G.; Romčević, N.; Boukos, N.; Vojinović-Ješić, L.S.; Leovac, V.M.; Hadžić, B.B.; Bajac, B.M.; Nad, L.F.; Chandrinou, C.; et al. Synthesis, Structure and Photoluminescence of (PLAGH)2[ZnCl4] and Comparative Analysis of Photoluminescence Properties with Tris(2,2′-Bipyridine)Ruthenium(II). Mater. Res. Bull. 2015, 70, 951–957. [Google Scholar] [CrossRef]
- Jelić, M.G.; Georgiadou, D.G.; Radanović, M.M.; Romčević, N.Z.; Giannakopoulos, K.P.; Leovac, V.M.; Nađ, L.F.; Vojinović-ješić, L.S.; Edited by Jelena Radovanovic, G.; Stepić, M.; et al. Efficient Electron Injecting Layer for PLEDs Based on (PLAGH)2[ZnCl4]. Opt. Quant. Electron. 2016, 48, 276. [Google Scholar] [CrossRef]
- Jelić, M.G.; Romčević, N.Z.; Hadžić, B.B.; Lalović, M.M.; Slankamenac, M.P.; Živanov, M.B. Photoluminescence Study of Cobalt (III) and Copper (II) Complexes with the Schiff Base of Pyridoxal and Aminoguanidine. Phys. Scr. Top. Issues 2014, T162, 014010. [Google Scholar] [CrossRef]
- Radanović, M.M.; Rodić, M.V.; Vojinović-Ješić, L.S.; Armaković, S.; Armaković, S.J.; Leovac, V.M. Complexes of Zn(II) and Cd(II) with 2-Acetylpyridine -Aminoguanidine—Syntheses, Structures and DFT Calculations. Inorg. Chim. Acta 2018, 473, 160–168. [Google Scholar] [CrossRef]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterisation of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Vojinović-Ješić, L.S.; Radanović, M.M.; Rodić, M.V.; Živković-Radovanović, V.; Jovanović, L.S.; Leovac, V.M. Syntheses and Characterization of 2-Acetylpyridine-Aminoguanidine and Its Copper(II) Complexes: Crystallographic and Antimicrobial Study. Polyhedron 2016, 117, 526–534. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 978-0-471-74493-1. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Radanović, M.M.; Novaković, S.B.; Vojinović-ješić, L.S.; Rodić, M.V.; Leovac, V.M. 2-Acetylpyrydine-Aminoguanidine Schiff Base-Novel Ligand Salt and Zinc(II) Complex Containing Thiocyanate. J. Serb. Chem. Soc. 2018, 83, 157–166. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System; Rigaku Corporation: Oxford, UK, 2015. [Google Scholar]
- Sheldrick, G.M. Foundations and Advances SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Spek, A.L. Biological Crystallography Structure Validation in Chemical Crystallography. Acta Crystallogr. Sect. D 2009, D65, 148–155. [Google Scholar] [CrossRef] [Green Version]



| Bonds | Distance (Å) | Bonds | Angle (°) | ||
|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | ||
| M–O1 | 2.1644 (14) | 1.9913 (15) | N3–N2–C1 | 117.28 (17) | 115.11 (17) |
| M–O1 i | 2.1644 (14) | – | N1–C1–N4 | 122.1 (2) | 127.2 (2) |
| M–O2 | 2.0466 (15) | – | N2–C1–N1 | 120.24 (19) | 118.20 (18) |
| M–O2 i | 2.0466 (15) | – | C3–N5–C7 | 122.6 (3) | 118.98 (19) |
| M–O3 | 2.0603 (15) | – | |||
| M–O3 i | 2.0603 (15) | – | |||
| M–O5 | – | 2.0135 (18) | |||
| M–N1 | – | 2.0400 (19) | |||
| M–N3 | – | 2.1115 (17) | |||
| M–N5 | – | 2.1712 (17) | |||
| C1–N1 | 1.316 (3) | 1.286 (3) | |||
| C1–N2 | 1.350 (3) | 1.376 (3) | |||
| C1–N4 | 1.309 (3) | 1.337 (3) | |||
| N2–N3 | 1.372 (2) | 1.349 (2) | |||
| C2–N3 | 1.281 (3) | 1.278 (3) |
| Bond | Distances (Å) | Angles (°) | |
|---|---|---|---|
| D—H···A | H···A | D···A | D–H···A |
| O1—H1B···S1 | 3.02 (3) | 3.7856 (16) | 156 (2) |
| O1—H1B···O5 | 1.93 (3) | 2.756 (2) | 175 (3) |
| O1—H1A···S1 i | 2.89 (3) | 3.5686 (15) | 152 (3) |
| O1—H1A···O8 i | 2.01 (3) | 2.755 (2) | 172 (3) |
| O2—H2A···S1 ii | 2.89 (3) | 3.6713 (18) | 155 (2) |
| O2—H2A···O8 ii | 1.83 (3) | 2.674 (2) | 178 (3) |
| O2—H2B···O4 iii | 2.01 (3) | 2.727 (2) | 175 (3) |
| O3—H3B···O7 iv | 1.91 (3) | 2.713 (2) | 172 (3) |
| O3—H3A···O4 ii | 1.90 (3) | 2.707 (2) | 173 (3) |
| N3—H3···S1 | 2.95 (2) | 3.7332 (18) | 151 (2) |
| N3—H3···O5 | 2.07 (3) | 2.913 (2) | 164 (2) |
| N4—H4A···S2 v | 2.83 | 3.635 (2) | 158 |
| N4—H4A···O11 v | 1.97 | 2.825 (4) | 172 |
| N4—H4A···O12 vi | 1.95 | 2.757 (4) | 155 |
| N4—H4B···S1 | 2.86 | 3.6809 (19) | 161 |
| N4—H4B···O6 | 1.94 | 2.760 (2) | 158 |
| N5—H5A···S2 v | 2.95 | 3.730 (2) | 152 |
| N5—H5A···O10 v | 1.88 | 2.715 (4) | 163 |
| N5—H5A···O12 vi | 2.48 | 3.144 (4) | 135 |
| N5—H5B···O9 vii | 2.33 | 3.160 (4) | 164 |
| N5—H5B···O13 | 2.00 | 2.814 (4) | 157 |
| N1—H1···S2 vii | 2.86 | 3.458 (2) | 128 |
| N1—H1···O9 vii | 1.73 | 2.506 (4) | 149 |
| N1—H1···O13 | 2.20 | 3.019 (5) | 159 |
| O4—H4C···S1 ii | 2.82 (3) | 3.5505 (19) | 154 (3) |
| O4—H4C···O7 ii | 2.02 (3) | 2.807 (2) | 176 (3) |
| O4—H4D···O1 viii | 2.05 (3) | 2.831 (2) | 171 (3) |
| Distances (Å) | Angles (°) | ||
|---|---|---|---|
| D–H∙∙∙A | D–A | H∙∙∙A | D–H∙∙∙A |
| O5–H5A∙∙∙O6 | 1.840 (18) | 2.652 (3) | 175 (3) |
| O5–H5B∙∙∙O1 i | 2.31 (2) | 3.026 (3) | 150 (3) |
| O5–H5B∙∙∙O3 i | 2.34 (2) | 3.025 (3) | 146 (3) |
| N2–H2∙∙∙O2 ii | 1.976 (18) | 2.737 (2) | 154 (2) |
| N4–H4A∙∙∙O2 iii | 2.66 (2) | 3.295 (3) | 134 (2) |
| N4–H4A∙∙∙O4 iii | 2.145 (18) | 2.974 (3) | 168 (2) |
| N4–H4A∙∙∙O2 ii | 2.16 (2) | 2.914 (3) | 147 (2) |
| 1 | 2 | |
|---|---|---|
| Chemical formula | C16H44N10O21S3Zn | C8H15N5O6SZn |
| Formula weight | 874.16 | 374.68 |
| Temperature, K | 293 (2) | 294 (2) |
| Wavelength, Å | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Monoclinic |
| Space group | 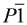 | P21/n |
| a/Å | 6.3640 (2) | 9.7385 (7) |
| b/Å | 7.2609 (3) | 7.8010 (7) |
| c/Å | 19.9173 (12) | 17.6698 (15) |
| α/° | 87.989 (4) | 90 |
| β/° | 88.451 (5) | 93.153 (7) |
| γ/° | 68.731 (4) | 90 |
| V/Å3 | 857.03 (7) | 1340.34 (19) |
| Z | 1 | 4 |
| Dc/g cm−3 | 1.694 | 1.857 |
| µ/mm−1 | 0.997 | 2.025 |
| Absorption correction | Analytical | Analytical |
| Tmax, Tmin | 0.885, 0.732 | 0.764, 0.432 |
| Crystal size, mm | 0.46 × 0.38 × 0.16 | 0.11 × 0.25 × 0.65 |
| θ range, ° | 3.01–29.1 | 2.9–29.0 |
| Reflections collected | 8472 | 8954 |
| Independent reflections | 3818 | 3112 |
| Rint | 0.023 | 0.022 |
| Restraints, parameters | 0. 296 | 8, 215 |
| Goodness of fit on F2 | 1.086 | 1.050 |
| R1 (I > 2σ (I)) | 0.032 | 0.029 |
| R1 (all data) | 0.036 | 0.035 |
| wR2 (I > 2σ (I)) | 0.078 | 0.068 |
| wR2 (all data) | 0.081 | 0.072 |
| Δρmax, Δρmin/e A−3 | 0.38, −0.41 | 0.49, −0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radanović, M.M.; Vojinović-Ješić, L.S.; Jelić, M.G.; Sakellis, E.; Barta Holló, B.; Leovac, V.M.; Rodić, M.V. Synthesis, Structures, and Photoluminescence of Two Novel Zinc(II) Compounds Containing 2-Acetylpyridine-aminoguanidine. Inorganics 2022, 10, 147. https://doi.org/10.3390/inorganics10100147
Radanović MM, Vojinović-Ješić LS, Jelić MG, Sakellis E, Barta Holló B, Leovac VM, Rodić MV. Synthesis, Structures, and Photoluminescence of Two Novel Zinc(II) Compounds Containing 2-Acetylpyridine-aminoguanidine. Inorganics. 2022; 10(10):147. https://doi.org/10.3390/inorganics10100147
Chicago/Turabian StyleRadanović, Mirjana M., Ljiljana S. Vojinović-Ješić, Miodrag G. Jelić, Elias Sakellis, Berta Barta Holló, Vukadin M. Leovac, and Marko V. Rodić. 2022. "Synthesis, Structures, and Photoluminescence of Two Novel Zinc(II) Compounds Containing 2-Acetylpyridine-aminoguanidine" Inorganics 10, no. 10: 147. https://doi.org/10.3390/inorganics10100147
APA StyleRadanović, M. M., Vojinović-Ješić, L. S., Jelić, M. G., Sakellis, E., Barta Holló, B., Leovac, V. M., & Rodić, M. V. (2022). Synthesis, Structures, and Photoluminescence of Two Novel Zinc(II) Compounds Containing 2-Acetylpyridine-aminoguanidine. Inorganics, 10(10), 147. https://doi.org/10.3390/inorganics10100147









