Abstract
Photobiomodulation (PBM) has demonstrated positive effects on the muscle repair process. The aim of the study was to evaluate the effects of infrared PBM using different light sources—low-level laser (LLL) at 780 nm (40 or 70 mW, 10 J/cm2, 0.4 J) or LED at 850 nm (40 or 70 mW, 0.13 J/cm2, 0.4 J)—and dosimetric parameters on the proliferation and migration of muscle cells. The results showed that LLL 40 mW and 70 mW, with the same radiation exposure, led to an increase in proliferation after 24 h, but no differences at 48 and 72 h. Cells irradiated with LED 70 mW exhibited an increase in proliferation in comparison to the control group and 40mW after 24 and 48 h, but not at 72 h. Moreover, cell migration was greater in comparison to the control after 6 and 24 h, and no differences were found at 12 h when LLL was used with an output power of 70 mW. Furthermore, no differences were found at 6 and 12 h with the 70 mW output power-LED, but an increase was observed in the cell migration after 24 h. In conclusion, PBM using different light sources and dosimetric parameters was able to modulate the proliferation of C2C12 myoblasts, but only PBM at 70 mW was able to modulate the migration of these cells.
1. Introduction
Skeletal muscle corresponds to approximately 40% of the mass of the human body. This tissue plays fundamental roles, such as the generation of force, control of skeletal movements, and physical protection of many other organs [1]. Due to its functions, skeletal muscle is one of the most frequently injured tissues, and its regenerative capacity is of fundamental importance to the adequate reestablishment of its functions [1,2]. In some situations, however, muscle regeneration is slow and may lead to incomplete functional recovery as a result of chronic injury or the formation of fibrous tissue which can directly interfere with its functioning [3].
The regeneration of skeletal muscle after an injury is possible due to the presence of precursor cells, known as satellite cells (SCs) [4,5]. SCs remain in a quiescent state between the basal lamina and sarcolemma in mature muscle fibers and are activated after an injury, initiating a proliferation process in which these cells are now denominated myoblasts. Myoblasts subsequently undergo an initial differentiation and fusion phase, uniting for the formation of multinucleated myotubes, which then fuse with the damaged muscle fibers or form new muscle fibers to promote regeneration [3,6,7,8,9]. The C2C12 myoblast line is derived from mouse satellite cells and provides a good cellular model for studying the phases of myogenesis due to its capacity to proliferate and differentiate in a process similar to what occurs in vivo [8,10,11,12].
Photobiomodulation (PBM) has been widely used to enhance the muscle repair process. This noninvasive treatment modality consists of the application of light with a power output lower than 500 mW and a wavelength ranging from 400 to 1100 nm. The effects of this technique include the stimulation of tissue repair, analgesia, and a reduction in the inflammatory process, promoting the healing of injuries and preventing necrosis and tissue damage [7,8,10,13,14,15]. In addition, da Silva Neto Trajano et al. (2016) [7,10] used low-level laser (LLL) with different radiant exposure (10, 35, and 70 J/cm2) on C2C12 myoblasts, which are the same dosimetric parameters used in clinical protocols for treatment of muscle injury exposure and showed at low or high radiant exposure a stimulation in the elimination of non-viable cells by inflammatory machinery or the recovery of viable cells.
In vivo and in vitro studies report positive effects of PBM in the treatment of muscle tissue. In vivo studies have demonstrated a reduction in the production of inflammatory cytokines [16], a reduction in myonecrosis, an increase in the number of blood vessels, better organization of collagen fibers, and a reduction in the acute inflammatory process [13,17]. In vitro studies analyzing the effects of PBM using on C2C12 myoblasts have demonstrated increase in the potential of the mitochondrial membrane [18], increase in cell viability and creatine kinase activity interrelated with differentiate phase [19], and the activation of the mitochondrial respiratory chain [20]. In a recent published study [11], it was described that PBM is capable to increase the viability, proliferation, and differentiation of irradiated C2C12 myoblasts cultivated with products of proinflammatory macrophages. However, questions remain regarding the use of this treatment modality due mainly to the variety of combinations of the dosimetric parameters employed.
Thus, the aim of the present study was to perform a comparative analysis of the effects of PBM using different light sources (LLL and LED) and different dosimetric parameters on the proliferation and migration of myoblasts to broaden knowledge on cell responses to this treatment modality than can assist in the establishment of novel protocols for use in clinical trials.
2. Materials and Methods
2.1. Cell Culture
The C2C12 myogenic precursor cell line (mouse satellite cells) (ATCC® CRL-1772™) was used in this study. The cells were cultured in proliferation medium composed of Dulbecco’s modified Eagle medium (DMEM, Vitrocell, Campinas, SP, Brazil) supplemented with 10% fetal bovine serum (FBS, Vitrocell, Campinas, SP, Brazil) and 1% antibiotic–antimycotic solution incubated (HEPA class 3110, Thermo Electron Corporation, OH, USA) at 37 °C in a humidified atmosphere with 5% CO2. The cells used for the experiments had viability higher than 95%.
Experimental Groups
C2C12 cells were divided into the following groups: (a) control (not irradiated); (b) LLL I (40 mW, 0.4 J of total energy); (c) LLL II (70 mW, 0.4 J of total energy); (d) LED I (40 mW, 0.4 J of total energy); and (e) LED II (70 mW, 0.4 J of total energy).
2.2. Irradiation Procedure
2.2.1. PBM Using LLL
The cells in all groups submitted to PBM with LLL and the respective control group were centrifuged (1200 rpm, 5 min, 4 °C) for the formation of the cellular precipitate at the bottom of conical 50-mL Falcon tubes (KASVI®, São José dos Pinhais, PR, Brazil). The LLL groups were irradiated with the bottom of the tube positioned in contact and perpendicular to the laser device to reach the cells directly and equally, due to the fact of the smaller beam area dimension could not reach the entire culture dish (0.34 cm2) and to reduce the interference of the culture medium [21].
LLL was performed with an aluminum–gallium–arsenide (AlGaAs) diode laser (Twin Laser, MM Optics, São Carlos, SP, Brazil) using the parameters listed in Table 1. The output power was verified using a laser-check power meter (Coherent, Portland, OR, USA) prior to use, and the experiments were conducted in an environment with partial darkness.

Table 1.
PBM parameters (LLL).
The cells in the groups irradiated with LLL and the respective control group were re-suspended and plated (4 × 104/well for myonuclei counting and 1.4 × 105 for cell migration) in six-well plates (KASVI®).
2.2.2. PBM Using LED
The cells in all groups were plated (4 × 104/well for myonuclei counting and 1.4 × 105 for cell migration) in 35-mm dishes (Corning Cell Culture Dish, Corning, NY, USA) and incubated for three hours to enable cell adhesion. The culture medium was then removed to avoid its interference in the absorption of the photons [21]. LED was applied using the parameters described in Table 2. Due to the higher beam area of the LED, the irradiation was performed on the upper part of the dishes so that the beam could reach the entire culture dish (35 mm; KASVI®), as displayed in Supplementary Figure S1. After the application of LED, the culture medium was returned to the dishes.

Table 2.
PBM parameters (LED).
The output power was verified using the laser-check power meter prior to the use of the LED device. For such, the culture dishes were positioned at a standardized distance of 3.1 cm. All experiments were conducted in an environment with partial darkness.
2.3. May–Grunwald and Giemsa Staining for Quantifying Cell Proliferation
After the irradiation procedure, the analysis of cell proliferation was performed using May–Grunwald and Giemsa staining in all experimental groups (LLL and LED) at 24, 48, and 72 h to enable a clear visualization of cells morphology and count myonuclei [11,22,23,24,25]. The cells were washed with cold PBS 1X and fixed with the addition of methanol (Synth, Diadema, SP, Brazil) for 5 min, followed by the addition of May–Grunwald stain diluted (1:3) in sodium phosphate buffer solution (NaH2PO4, 1 mM; Na2HPO4, 1 mM; pH 5.8) for 5 min. The wells were washed twice with distilled water, followed by the addition of Giemsa stain (Renylab, Barcelona, MG, Brazil) diluted (1:20) in sodium phosphate buffer for 10 min. The wells were then washed with PBS 1X and the plates were kept at room temperature to dry.
Next, five images per plate were obtained from random regions of two wells in three independent assays of all the experimental groups using an inverted phase microscope (Eclipse TE 2000U, Nikon, Melville, NY, USA) with 20× magnification and 1.5× correction. The images were analyzed by a previously trained blinded evaluator with the aid of the Image Processing and Analysis in Java (Image J) software (National Institute of Health). Counts were performed of the total quantity of myonuclei in each image.
2.4. Cell Migration
After the irradiation procedures, the cells were plated (35 mm Corning Cell Culture Dish, NY, USA) until reaching 80–90% confluence, and the analysis of cell migration was performed using the scratch assay in all experimental groups (LLL and LED). Scratch wounds were mechanically created in the monolayer of cells by a 200 µL plastic tip and cells debris were removed with PBS and a new proliferation medium was added to each well. Images of the wound area were obtained immediately after the scratch was induced as well as after 6, 12, and 24 h. These periods were determined as described by Ferreira et al. (2018) [8].
Pictures were taken immediately after the scratch with an inverted phase microscope (Eclipse TE 2000U, Nikon, Melville, NY, USA) with 10× magnification and processed using the Image J software. Next, three measures at fixed points were traced in the scratch region to determine the width (in pixels) of each one (Figure 1), followed by the calculation of the mean number of pixels per image. The experiments were performed with duplicate samples in three independent assays, and three measures at fixed points, resulting in eighteen measures traced in the scratch region per experimental group.
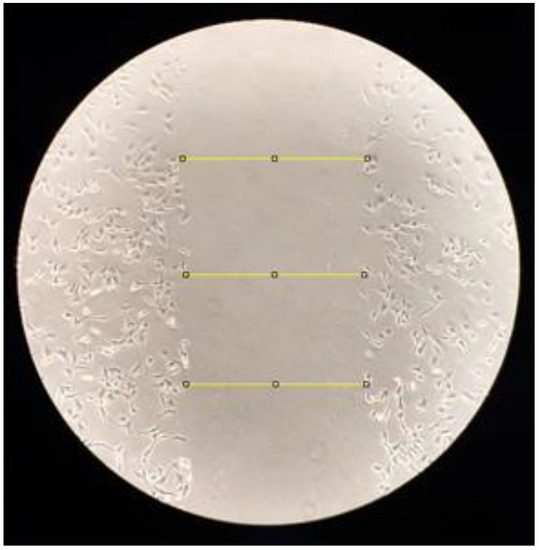
Figure 1.
Measures to determine the width after scratch.
2.5. Statistical Analysis
Data analysis was performed using GraphPad Prism 5.01® (GraphPad Software, San Diego, CA, USA). All experiments were performed with duplicate samples in three independent assays. The Kolmogorov–Smirnov test was used to determine the normality of the data. The data were expressed as mean and standard error of the mean. Means were compared between groups using one-way analysis of variance (ANOVA) followed by Tukey’s test.
3. Results
3.1. Cell Proliferation
The images of the cells with May–Grunwald–Giemsa stain in the LLL groups at the different evaluation times are displayed in Supplementary Figure S2. Twenty-four hours after the application of LLL (Figure 2A), the number of myonuclei was higher in the 40 mW (p < 0.001) and 70 mW (p < 0.001) groups compared to the control group. At 48 h (Figure 2B) and 72 h (Figure 2C), the proliferation indices were similar in all groups, with no significant difference.
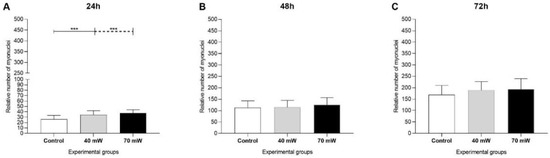
Figure 2.
Proliferation of C2C12 myoblasts submitted to LLL after (A) 24 h, (B) 48 h, and (C) 72 h (ANOVA/Tukey); *** p < 0.001.
The images of the cells with May–Grunwald–Giemsa stain in the LED groups at the different evaluation times are displayed in Supplementary Figure S3. After 24 h and 48 h of culturing (Figure 3A,B), the number of myonuclei was significantly higher in the 70 mW group compared to the control group (p < 0.001) and 40 mW group (p < 0.001). Seventy-two hours after the application of LED (Figure 3C), no significant differences among groups were found regarding the number of myonuclei.
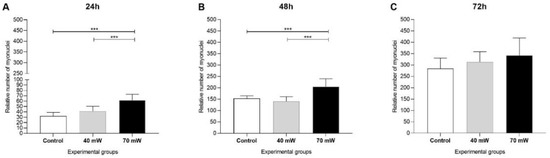
Figure 3.
Proliferation of C2C12 myoblasts submitted to LED after (A) 24 h, (B) 48 h, and (C) 72 h (ANOVA/Tukey); *** p < 0.001.
3.2. Cell Migration
The images of scratch assay using the cells submitted to the LLL are displayed in Figure 4. Evaluations were performed at 0 h (Figure 4A–C), 6 h (Figure 4D–F), 12 h (Figure 4G–I), and 24 h (Figure 4J–L).

Figure 4.
Closure of wound after 0 h: (A) Control, (B) LLL I 40 mW, (C) LLL II 70 mW; after 6 h: (D) Control, (E) LLL I 40 mW, (F) LLL II 70 mW; after 12 h: (G) Control, (H) LLL I 40 mW, (I) LLL II 70 mW; after 24 h: (J) Control, (K) LLL I 40 mW, (L) LLL II 70 mW.
Figure 5 displays the wound area of the LLL groups at the different evaluation times. After six hours (Figure 5A), migration was significantly greater in the LLL 70 mW group (p < 0.05) compared to the control group. No significant differences were found among the groups at 12 h. After 24 h of incubation (Figure 5C), the 70 mW group once again demonstrated significant results (p < 0.01) in comparison to the control group, with closure at several points of the wound.
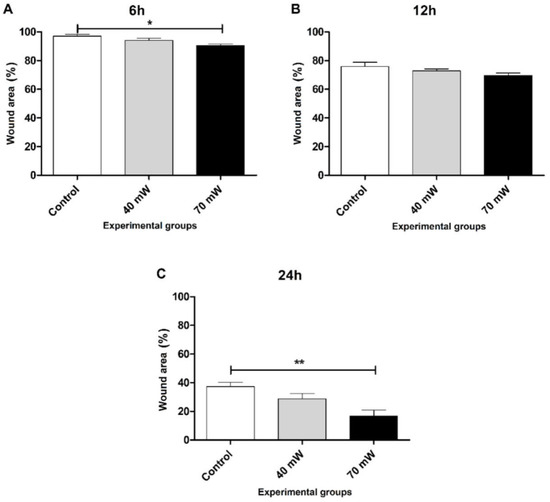
Figure 5.
Wound area closure (A) 6 h, (B) 12 h, and (C) 24 h after to perform scratch wounds and the irradiation with LLL(ANOVA/Tukey); * p < 0.05 vs. control ** p < 0.01 vs. control.
The images of the scratch assay using the cells submitted to the LED are displayed in Figure 6. Evaluations were performed at 0 h (Figure 6A–C), 6 h (Figure 6D–F), 12 h (Figure 6G–I), and 24 h (Figure 6J–L), as described previously.

Figure 6.
Closure of wound after 0 h: (A) Control, (B) LED I 40 mW, (C) LED II 70 mW; after 6 h: (D) Control, (E) LED I 40 mW, (F) LED II 70 mW; after 12 h: (G) Control, (H) LED I 40 mW, (I) LED II 70 mW; after 24 h: (J) Control, (K) LED I 40 mW, (L) LED II 70 mW.
Figure 7 displays the wound area of the LED groups at the different evaluation times. No statistically significant differences were found among the groups at 6 and 12 h. After 24 h of incubation, migration was significantly greater in the 70 mW group (p < 0.05) compared to the control group.
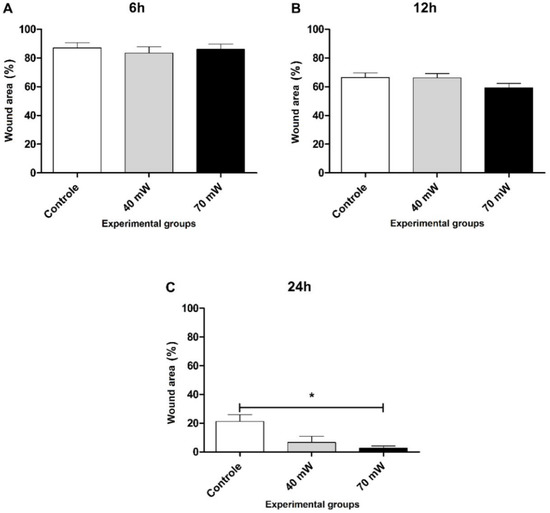
Figure 7.
Wound area closure (A) 6 h, (B) 12 h, and (C) 24 h after to perform the scratch wounds and the irradiation with LED (ANOVA/Tukey); * p < 0.05 vs. control.
4. Discussion
In the present study, we evaluated the proliferation and migration of C2C12 myoblasts irradiated with different light sources and different dosimetric parameters, with the aim of checking how the PBM using different light resources interfere with myogenesis and the success of muscle repair as well as assisting future studies in the optimization of PBM parameters used for muscle repair.
Hamblin (2017) [26] emphasizes the understanding of PBM mechanisms in the tertiary effects, at molecular and cellular levels, providing additional explanations for its use for multiple injuries conditions. Studies indicate that PBM affects the proliferation and differentiation of muscle cells. Baskov et al. (2022) [27] cited that the influence of near-infrared light on ATP activation and cell proliferation occurs through the interaction of photons with intracellular water layers. However, the wide range of parameters used in terms of output power, wavelength, type of emission, radiant exposure, irradiation time, dosage, total energy delivered, application method, and interval between sessions has led to different results among the studies found in the literature [28]. In the present study, C2C12 cells were irradiated with two different infrared light sources (LLL or LED) and two different output powers (40 and 70 mW) with each light source to analyze the effects on the proliferation and migration of these cells. The present findings are of extreme relevance to muscle regeneration, as this process directly depends on the proliferation and migration of satellite cells activated after an injury and the differentiation (fusion) of muscle fibers for the reestablishment of the injured muscle area. Based on the present findings, the use of a higher output power (70 mW) with either LLL or LED may be the best choice for enhancing the muscle regeneration process.
The fusion is a differentiation process of C2C12 myoblasts into myotubes [29]. During this phase, mononucleated cells fuse together to form multinucleated myotubes, and this sequence leads to the terminal differentiation to regenerate injured muscle area or form a new muscle fiber [2,4].
Regarding the proliferation, LED at output powers of 70 mW achieved better results in comparison to the control group and 40 mW 24 and 48 h after irradiation. The results differed when LLL was used, where differences in 40 mW and 70 mW in comparison to the control group were found only at 24 h. According with these results, Santos et al. (2020) [11] found that LLL therapy (780 nm, 70 mW, 26.25 J/cm2) caused an increase in proliferation at 24 and 48 h on undifferentiated and differentiation induced C2C12 cells cultivated in a conditioned media of proinflammatory macrophages also irradiated with the same parameters. PBM was capable of inducing an increase in myonuclei in the same evaluated periods in the present study.
Ferreira et al. (2009) [30] and Mesquita-Ferrari et al. (2011) [31] used LLL with different wavelength and parameters (including 780 nm; 15, 40 or 70 mW; 3.8, 10 and 17.5 J/cm2) on C2C12 myoblasts cultivated in proliferation medium and under nutritional conditions, showing no differences on cell viability and proliferation in the groups treated after 24, 48, and 72 h under the conditions evaluated. Some of parameters of the study were similar to those used in the present investigation, which had a positive effect on increasing the proliferation of C2C12 myoblasts.
The results of the scratch assay demonstrate that both LLL and LED were effective at improving cell migration only, with an output power of 70 mW. Considering LLL, an increase in cell migration was observed in comparison to the control group at 6 and 24 h after the induction of the wound. Similar results were found in the groups irradiated with LED, as no significant difference was found when an output power of 40 mW was used, but 70 mW led to a significant increase in cell migration at 24 h compared to the control group.
Ferreira et al. (2018) [8] found that LLL did not affect cell viability, but reduced the migration (scratch assay) and proliferation of C2C12 cells. This divergence in relation to the cell proliferation found in the present study may be explained by the difference in the dosimetric parameters and the wavelength used by the authors cited (wavelength of 660 nm and output power of 20 mW). There are several limitations of in vitro scratch assay method, comprising the longer time before performing the analysis and a large number of seeded cells; however, this is an approachable method that does not require any specialized equipment [32].
When incorrect PBM parameters are used, treatment will likely be ineffective. There is a biphasic response curve with regards to very low and very high doses of radiant exposure (J/cm2) and irradiance (mW/cm2), which can have undesirable, inhibitory effects [26,33]. This biphasic response to the dose signifies that there is an ideal dose for each tissue and the dose must be increased until encountering this ideal level. When this level is surpassed, however, the response in the target tissue may be diminished or completely eliminated, and it is even possible to encounter negative or inhibitory effects [33]. Analyzing the results, the biphasic response to PBM may partially explain the findings, as the inhibition of cell migration was found at a low output power of 20 mW in the study by Ferreira et al. (2018) [8], the maintenance of cell migration was found at 40 mW and an increase was found when 70 mW was used in the present investigation.
In a previous study [31] evaluating the effects of LLL with different output powers (15, 25, 40, and 70 mW) on the proliferation of C2C12 myoblasts based on a mitochondrial analysis, no significant improvements in comparison to the control group were found, even when 70 mW was used. This divergence from the present findings may be explained by the fact that the authors used a radiant exposure nearly two-fold higher (17.5 J/cm2) than that employed in the present investigation.
The LED group had a higher cell proliferation in comparison to the LLL group in the first 24 and 48 h, and the results were only similar at the 72-h evaluation. However, a comparison of the different light sources used in this study is not possible due to differences in the wavelength range of LED and LLL, beam area (0.04 cm2 for LLL and 3 cm2 for LED), wavelength, radiant exposure, and irradiance of the devices, even though the calculated final energy was 0.4 J in both cases.
In this study, PBM with LLL led to an increase in the proliferation after 24 h (40 and 70 mW) and cell migration after 6 and 24 h (70 mW). Using LED, an increase in the proliferation was found after 24 and 48 h (70 mW) and an increase in cell migration was found after 24 h (70 mW).
Although the study design was useful, the energy delivered by PBM in all groups was the same (0.4 J). Therefore, differences in output power (40 vs. 70 mW) must have led to the different results found. Moreover, it should be stressed that the present data obtained from cell cultures cannot be directly transposed to the clinical condition, as other cells and stimuli (hormones) are present under in vivo conditions and exert an influence on the entire repair process and response to PBM.
In the present study, the LLL or LED experimental groups were not compared to each other due to the fact that some dosimetric parameters of irradiation were different, generated by the higher beam area of the LED device in comparison to LLL, consequently modifying the radiant exposure and irradiance. Furthermore, it is important to consider that the two light sources used in the present study present different features, such as the coherence, which could be implicated with the different results obtained [34,35].
5. Conclusions
In conclusion, the PBM using LLL induced an increase in cell proliferation regardless of the output power used, but only stimulated cell migration when the output power of 70 mW was used. Furthermore, LED treatment only induced an increase in cell proliferation and migration when an output power of 70 mW was used.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/photonics9070469/s1, Figure S1: Laser (A) and LED (B) devices positioned for irradiation; Figure S2: Stained C2C12 myoblasts submitted to LLL; Figure S3: Stained C2C12 myoblasts submitted to LED.
Author Contributions
Conceptualization, R.L., T.C.d.S.M. and R.A.M.-F.; methodology, R.L. and T.C.d.S.M.; software, R.L. and L.A.; validation, M.F.S.D.R., K.P.S.F. and R.A.M.-F.; formal analysis, R.L. and L.A.; investigation, R.L. and T.C.d.S.M.; resources, R.A.M.-F.; data curation, M.F.S.D.R. and K.P.S.F.; writing—original draft preparation, R.L. and T.C.d.S.M.; writing—review and editing, R.A.M.-F.; visualization, K.P.S.F. and S.K.B.; supervision R.A.M.-F.; project administration, S.K.B. and R.A.M.-F.; funding acquisition, R.A.M.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation (FAPESP), grant number 2020/13976-0, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), grant number 88887.481244/2020-00 and UNINOVE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (R.A.M.F.*) upon reasonable request.
Acknowledgments
Nove de Julho University for administrative and technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, W.; Kim, J.; Park, H.S.; Jeon, J.S. Development of Microfluidic Stretch System for Studying Recovery of Damaged Skeletal Muscle Cells. Micromachines 2018, 9, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uezumi, A.; Ikemoto-Uezumi, M.; Tsuchida, K. Roles of Nonmyogenic Mesenchymal Progenitors in Pathogenesis and Regeneration of Skeletal Muscle. Front. Physiol. 2014, 5, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippin, L.I.; Moreira, A.J.; Marroni, N.P.; Xavier, R.M. Nitric Oxide and Repair of Skeletal Muscle Injury. Nitric Oxide 2009, 21, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Regulation of Muscle Growth and Regeneration by the Immune System. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Gherardi, A.P.R.K.; Chazaud, B. Inflammatory Monocytes Recruited after Skeletal Muscle Injury Switch into Anti-inflammatory Macrophages to Support Myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Ranzato, E.; Balbo, V.; Boccafoschi, F.; Mazzucco, L.; Burlando, B. Scratch Wound Closure of C2C12 Mouse Myoblasts Is Enhanced by Human Platelet Lysate. Cell. Biol. Int. 2009, 33, 911–917. [Google Scholar] [CrossRef]
- da Silva Neto Trajano, L.A.; Stumbo, A.C.; da Silva, C.L.; Mencalha, A.L.; Fonseca, A.S. Low-Level Infrared Laser Modulates Muscle Repair and Chromosome Stabilization Genes in Myoblasts. Lasers. Med. Sci. 2016, 31, 1161–1167. [Google Scholar] [CrossRef]
- Ferreira, J.H.; Cury, S.S.; Vechetti-júnior, I.J.; Fernandez, G.J.; Moraes, L.N.; Alves, C.A.B.; Freire, P.P.; Freitas, C.E.A.; Dal-pai-silva, M.; Carvalho, R.F. Low-Level Laser Irradiation Induces a Transcriptional Myotube-like Profile in C2C12 Myoblasts. Lasers Med. Sci. 2018, 8, 1673–1683. [Google Scholar] [CrossRef] [Green Version]
- Kitakaze, T.; Oshimo, M.; Kobayashi, Y.; Ryu, M.; Suzuki, Y.A.; Inui, H.; Harada, N.; Yamaji, R. Lactoferrin Promotes Murine C2C12 Myoblast Proliferation and Differentiation and Myotube Hypertrophy. Mol. Med. Rep. 2018, 17, 5912–5920. [Google Scholar] [CrossRef] [Green Version]
- da Silva Neto Trajano, L.A.; da Silva, C.L.; de Carvalho, S.N.; Cortez, E.; Mencalha, A.L.; de Souza da Fonseca, A.; Stumbo, A.C. Cell Viability, Reactive Oxygen Species, Apoptosis, and Necrosis in Myoblast Cultures Exposed to Low-Level Infrared Laser. Lasers Med. Sci. 2016, 31, 841–848. [Google Scholar] [CrossRef]
- Santos, T.C.; de Brito Sousa, K.; Andreo, L.; Martinelli, A.; Rodrigues, M.F.S.D.; Bussadori, S.K.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A. Effect of Photobiomodulation on C2C12 Myoblasts Cultivated in M1 Macrophage-Conditioned Media. Photoch. Photob. 2020, 4, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Bolus, D.J.; Shanmugam, G.; Narasimhan, M.; Rajasekaran, N.S. Recurrent Heat Shock Impairs the Proliferation and Differentiation of C2C12 Myoblasts. Cell Stress Chaper. 2018, 23, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Andreo, L.; Mesquita-ferrari, R.A.; Ribeiro, Ã.B.G.; Benitte, A. Effects of Myogenic Precursor Cells (C2C12) Transplantation and Low-Level Laser Therapy on Muscle Repair. Lasers Surg. Med. 2018, 50, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.M.D.; Malamo, A.G.; Larkin-Kaiser, K.A.; Borsa, P.A.; Adhihetty, P.J. Effect of Near-Infrared Light Exposure on Mitochondrial Signaling in C2C12 Muscle Cells. Mitoch. Resear. Soc. 2014, 14, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Serrage, H.J.; Joanisse, S.; Cooper, P.R.; Palin, W.; Hadis, M.; Darch, O.; Philp, A.; Milward, M.R. Differential Responses of Myoblasts and Myotubes to Photobiomodulation Are Associated with Mitochondrial Number. J. Biophot. 2019, 12, e201800411. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.N.; Ribeiro, B.G.; Fernandes, K.P.S.; Souza, N.H.C.; Rocha, L.A.; Nunes, F.D.; Bussadori, S.K.; Mesquita-Ferrari, R.A. Comparative Effects of Low-Level Laser Therapy Pre- and Post-Injury on MRNA Expression of MyoD, Myogenin, and IL-6 during the Skeletal Muscle Repair. Lasers Med. Sci. 2016, 31, 679–685. [Google Scholar] [CrossRef]
- Souza, N.H.C.; Mesquita-Ferrari, R.A.; Rodrigues, M.F.S.D.; da Silva, D.F.T.; Ribeiro, B.G.; Alves, A.N.; Garcia, M.P.; Nunes, F.D.; da Silva Junior, E.M.; França, C.M.; et al. Photobiomodulation and Different Macrophages Phenotypes during Muscle Tissue Repair. J. Cell. Mol. Med. 2018, 22, 4922–4934. [Google Scholar] [CrossRef]
- Ferraresi, C.; Kaippert, B.; Avci, P.; Huang, Y.; Victor, M.; De Sousa, P.; Bagnato, V.S.; Parizotto, N.A. Low-Level Laser (Light) Therapy Increases Mitochondrial Membrane Potential and ATP Synthesis in C2C12 Myotubes with a Peak Response at 3–6 Hours. Photochem. Photobiol. 2016, 91, 411–416. [Google Scholar] [CrossRef]
- Mesquita-Ferrari, R.A.; Alves, A.N.; de Oliveira Cardoso, V.; Artilheiro, P.P.; Bussadori, S.K.; Rocha, L.A.; Nunes, F.D.; Fernandes, K.P.S. Low-Level Laser Irradiation Modulates Cell Viability and Creatine Kinase Activity in C2C12 Muscle Cells during the Differentiation Process. Lasers Med. Sci. 2015, 30, 2209–2213. [Google Scholar] [CrossRef]
- Silveira, P.C.L.; Ferreira, G.K.; Zaccaron, R.P.; Glaser, V.; Remor, A.P.; Mendes, C.; Pinho, R.A.; Latini, A. Effects of Photobiomodulation on Mitochondria of Brain, Muscle, and C6 Astroglioma Cells. Med. Eng. Phys. 2019, 71, 108–113. [Google Scholar] [CrossRef]
- Silva, D.F.T.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S.; Raele, M.P.; Wetter, N.U.; Deana, A.M. Effective Transmission of Light for Media Culture, Plates and Tubes. Photoch. Photob. 2012, 88, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Sakamoto, K. Lipopolysaccharide Inhibits Myogenic Differentiation of C2C12 Myoblasts through the Toll-like Receptor 4-Nuclear Factor-ΚB Signaling Pathway and Myoblast-Derived Tumor Necrosis Factor-α. PLoS ONE 2017, 12, e0182040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veliça, P.; Bunce, C.M. A Quick, Simple and Unbiased Method to Quantify C2C12 Myogenic Differentiation. Muscle Nerve 2011, 44, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Pansters, N.A.M.; van der Velden, J.L.J.; Kelders, M.C.J.M.; Laeremans, H.; Schols, A.M.W.J.; Langen, R.C.J. Segregation of Myoblast Fusion and Muscle-Specific Gene Expression by Distinct Ligand-Dependent Inactivation of GSK-3b. Cell. Mol. Life Sci. 2011, 68, 523–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizunoya, W.; Tashima, A.; Sato, Y.; Tatsumi, R.; Ikeuchi, Y. The Growth-Promoting Activity of Egg White Proteins in the C2C12 Myoblast Cell Line. Anim. Sci. J. 2015, 86, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and Applications of the Anti-Inflammatory Effects of Photobiomodulation. AIMS Bioph. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Baskov, A.; Borshchenko, I.A.; Baskov, V.; Shekhter, A.; Sobol, E. Laser Reconstruction of Spinal Discs Experiments and Clinic. Appl. Sci. 2022, 12, 675. [Google Scholar] [CrossRef]
- Zein, R.; Selting, W.; Hamblin, M.R. Review of Light Parameters and Photobiomodulation Efficacy: Dive into Complexity. J. Biomed. Opt. 2018, 23, 120901. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, P.; Reddy, B.; Millet, L.; Wei, C.; Zorlutuna, P.; Bao, G.; Bashir, R. Patterning the Differentiation of C2C12 Skeletal Myoblasts. Integr. Biol. 2011, 3, 897–909. [Google Scholar] [CrossRef]
- Ferreira, M.P.P.; Ferrari, R.A.M.; Gravalos, E.D.; Martins, M.D.; Bussadori, S.K.; Gonzalez, D.A.B.; Fernandes, K.P.S. Effect of Low-Energy Gallium-Aluminum-Arsenide and Aluminium Gallium Indium Phosphide Laser Irradiation on the Viability of C2C12 Myoblasts in a Muscle Injury Model. Photomed. Laser Surg. 2009, 27, 901–906. [Google Scholar] [CrossRef]
- Mesquita-Ferrari, R.A.; Ribeiro, R.; Souza, N.H.C.; Silva, C.A.A.; Martins, M.D.; Bussadori, S.K.; Fernandes, K.P.S. No Effect of Low-Level Lasers on in Vitro Myoblast Culture. Indian J. Exp. Biol. 2011, 49, 423–428. [Google Scholar] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naderi, M.S.; Razzaghi, M.; Djavid, G.E.; Hajebrahimi, Z. A Comparative Study of 660 nm Low-Level Laser and Light Emitted Diode in Proliferative Effects of Fibroblast Cells. Lasers Med. Sci. 2017, 8, S46–S50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photoch. Photob. 2018, 17, 1003–1017. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).