Intravital Imaging with Two-Photon Microscopy: A Look into the Kidney
Abstract
1. Introduction
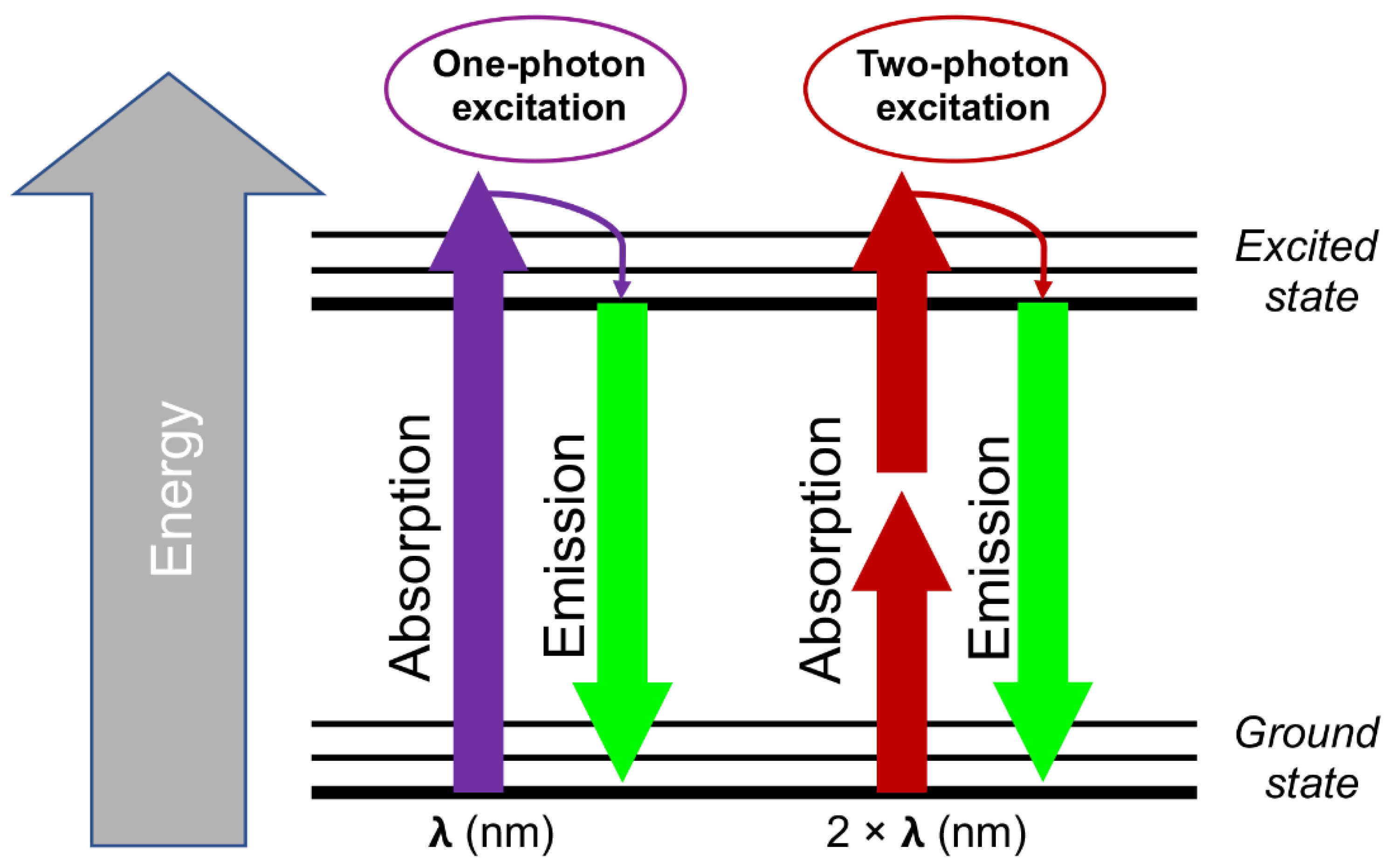
2. Principles of Two-Photon Microscopy
3. Main Applications of Two-Photon Microscopy
3.1. Renal Autofluorescence to Study Metabolic Functions
3.2. Second Harmonic Generation (SHG) to Study Renal Fibrosis
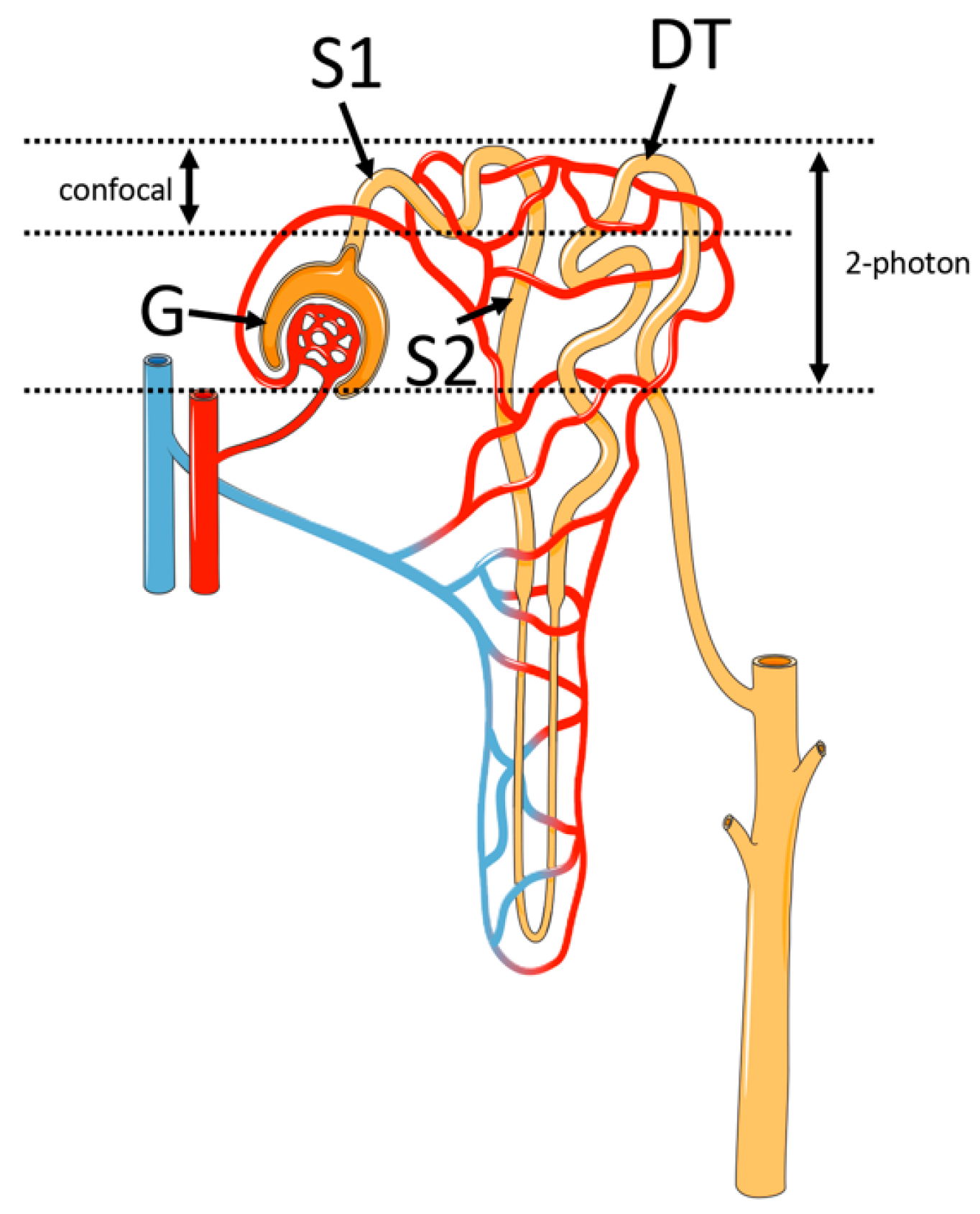
3.3. Single-Nephron Glomerular Filtration Rate (SNGFR) Assessment
3.4. Organic Cations Transport Evaluation
3.5. Renal Tissue Regeneration
4. Imaging Data Processing and Machine Learning
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parslow, A.C.; Clayton, A.H.A.; Lock, P.; Scott, A.M. Confocal Microscopy Reveals Cell Surface Receptor Aggregation Through Image Correlation Spectroscopy. J. Vis. Exp. 2018, 138, e57164. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, J.D.; Prajna, N.V.; Palepu, S.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Lalitha, P.; Macleod, D.; Burton, M.J. Cellular morphological changes detected by laser scanning in vivo confocal microscopy associated with clinical outcome in fungal keratitis. Sci. Rep. 2019, 9, 8334. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Caterino, M.; Cevenini, A.; Jung, V.; Chhuon, C.; Lipecka, J.; Fedele, R.; Guerrera, I.C.; Ruoppolo, M. Proteomics Reveals that Methylmalonyl-CoA Mutase Modulates Cell Architecture and Increases Susceptibility to Stress. Int. J. Mol. Sci. 2020, 21, 4998. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Fiocchetti, M.; Ascenzi, P.; Marino, M.; Caterino, M.; Ruoppolo, M. Proteomic and Bioinformatic Investigation of Altered Pathways in Neuroglobin-Deficient Breast Cancer Cells. Molecules 2021, 26, 2397. [Google Scholar] [CrossRef]
- Costanzo, M.; Caterino, M.; Cevenini, A.; Jung, V.; Chhuon, C.; Lipecka, J.; Fedele, R.; Guerrera, I.C.; Ruoppolo, M. Dataset of a comparative proteomics experiment in a methylmalonyl-CoA mutase knockout HEK 293 cell model. Data Br. 2020, 33, 106453. [Google Scholar] [CrossRef]
- Gonzalez Melo, M.; Remacle, N.; Cudré-Cung, H.-P.; Roux, C.; Poms, M.; Cudalbu, C.; Barroso, M.; Gersting, S.W.; Feichtinger, R.G.; Mayr, J.A.; et al. The first knock-in rat model for glutaric aciduria type I allows further insights into pathophysiology in brain and periphery. Mol. Genet. Metab. 2021, 133, 157–181. [Google Scholar] [CrossRef]
- Pygall, S.R.; Whetstone, J.; Timmins, P.; Melia, C.D. Pharmaceutical applications of confocal laser scanning microscopy: The physical characterisation of pharmaceutical systems. Adv. Drug Deliv. Rev. 2007, 59, 1434–1452. [Google Scholar] [CrossRef]
- Prosperi, F.; Suzumoto, Y.; Marzuillo, P.; Costanzo, V.; Jelen, S.; Iervolino, A.; Guarino, S.; La Manna, A.; Miraglia Del Giudice, E.; Perna, A.F.; et al. Characterization of five novel vasopressin V2 receptor mutants causing nephrogenic diabetes insipidus reveals a role of tolvaptan for M272R-V2R mutation. Sci. Rep. 2020, 10, 16383. [Google Scholar] [CrossRef]
- Rigby, P.J.; Goldie, R.G. Confocal microscopy in biomedical research. Croat. Med. J. 1999, 40, 346–352. [Google Scholar]
- Kitamura, A. Pinhole Closure Improves Spatial Resolution in Confocal Scanning Microscopy. In Live Cell Imaging; Humana: New York, NY, USA, 2021; pp. 385–389. [Google Scholar]
- Ghosh, S.; Nandi, S.; Ghosh, C.; Bhattacharyya, K. Fluorescence Dynamics in the Endoplasmic Reticulum of a Live Cell: Time-Resolved Confocal Microscopy. ChemPhysChem 2016, 17, 2818–2823. [Google Scholar] [CrossRef]
- Ilyin, S.E.; Flynn, M.C.; Plata-Salamán, C.R. Fiber-optic monitoring coupled with confocal microscopy for imaging gene expression in vitro and in vivo. J. Neurosci. Methods 2001, 108, 91–96. [Google Scholar] [CrossRef]
- Pike, J.A.; Styles, I.B.; Rappoport, J.Z.; Heath, J.K. Quantifying receptor trafficking and colocalization with confocal microscopy. Methods 2017, 115, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Peti-Peterdi, J.; Toma, I.; Sipos, A.; Vargas, S.L. Multiphoton Imaging of Renal Regulatory Mechanisms. Physiology 2009, 24, 88–96. [Google Scholar] [CrossRef]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Hall, A.M.; Molitoris, B.A. Dynamic Multiphoton Microscopy: Focusing Light on Acute Kidney Injury. Physiology 2014, 29, 334–342. [Google Scholar] [CrossRef]
- Benninger, R.K.P.; Piston, D.W. Two-Photon Excitation Microscopy for the Study of Living Cells and Tissues. Curr. Protoc. Cell Biol. 2013, 59, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Göppert-Mayer, M. Über Elementarakte mit zwei Quantensprüngen. Ann. Phys. 1931, 401, 273–294. [Google Scholar] [CrossRef]
- Kaiser, W.; Garrett, C.G.B. Two-Photon Excitation in Two-Photon Excitation in CaF2: Eu2+. Phys. Rev. Lett. 1961, 7, 229–231. [Google Scholar] [CrossRef]
- Sezgin, E. Super-resolution optical microscopy for studying membrane structure and dynamics. J. Phys. Condens. Matter 2017, 29, 273001. [Google Scholar] [CrossRef]
- Gu, M.; Sheppard, C.J.R. Comparison of three-dimensional imaging properties between two-photon and single-photon fluorescence microscopy. J. Microsc. 1995, 177, 128–137. [Google Scholar] [CrossRef]
- Wilson, T. Resolution and optical sectioning in the confocal microscope. J. Microsc. 2011, 244, 113–121. [Google Scholar] [CrossRef]
- Sankaran, J.; Balasubramanian, H.; Tang, W.H.; Ng, X.W.; Röllin, A.; Wohland, T. Simultaneous spatiotemporal super-resolution and multi-parametric fluorescence microscopy. Nat. Commun. 2021, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.P. Temporal resolution in fluorescence imaging. Front. Mol. Biosci. 2014, 1, 11. [Google Scholar] [CrossRef]
- Yang, Z.; Samanta, S.; Yan, W.; Yu, B.; Qu, J. Super-resolution Microscopy for Biological Imaging. In Optical Imaging in Human Disease and Biological Research; Springer: Cham, Switzerland, 2021; pp. 23–43. [Google Scholar]
- Fahrbach, F.O.; Gurchenkov, V.; Alessandri, K.; Nassoy, P.; Rohrbach, A. Light-sheet microscopy in thick media using scanned Bessel beams and two-photon fluorescence excitation. Opt. Express 2013, 21, 13824. [Google Scholar] [CrossRef]
- Schermelleh, L.; Ferrand, A.; Huser, T.; Eggeling, C.; Sauer, M.; Biehlmaier, O.; Drummen, G.P.C. Super-resolution microscopy demystified. Nat. Cell Biol. 2019, 21, 72–84. [Google Scholar] [CrossRef]
- Urban, B.E.; Yi, J.; Chen, S.; Dong, B.; Zhu, Y.; DeVries, S.H.; Backman, V.; Zhang, H.F. Super-resolution two-photon microscopy via scanning patterned illumination. Phys. Rev. E 2015, 91, 042703. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Liang, Y.; Sarsfield, S.; Jiang, W.; Lu, R.; Dudman, J.T.; Aponte, Y.; Ji, N. High-throughput synapse-resolving two-photon fluorescence microendoscopy for deep-brain volumetric imaging in vivo. Elife 2019, 8, e40805. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Medina, F.A.; Hassan, A.; Perillo, E.P.; Hagan, K.; Shams Kazmi, S.M.; Dunn, A.K. In vivo multiphoton microscopy beyond 1 mm in the brain. In Optics in the Life Sciences Congress; OSA: Washington, DC, USA, 2017; p. BrM4B.5. [Google Scholar]
- Molitoris, B.A.; Sandoval, R.M. Intravital multiphoton microscopy of dynamic renal processes. Am. J. Physiol. Physiol. 2005, 288, F1084–F1089. [Google Scholar] [CrossRef]
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef]
- Svoboda, K.; Yasuda, R. Principles of Two-Photon Excitation Microscopy and Its Applications to Neuroscience. Neuron 2006, 50, 823–839. [Google Scholar] [CrossRef]
- Caterino, M.; Ruoppolo, M.; Costanzo, M.; Albano, L.; Crisci, D.; Sotgiu, G.; Saderi, L.; Montella, A.; Franconi, F.; Campesi, I. Sex Affects Human Premature Neonates’ Blood Metabolome According to Gestational Age, Parenteral Nutrition, and Caffeine Treatment. Metabolites 2021, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Caterino, M.; Ruoppolo, M.; Villani, G.R.D.; Marchese, E.; Costanzo, M.; Sotgiu, G.; Dore, S.; Franconi, F.; Campesi, I. Influence of Sex on Urinary Organic Acids: A Cross-Sectional Study in Children. Int. J. Mol. Sci. 2020, 21, 582. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Caterino, M.; Fedele, R.; Cevenini, A.; Pontillo, M.; Barra, L.; Ruoppolo, M. COVIDomics: The Proteomic and Metabolomic Signatures of COVID-19. Int. J. Mol. Sci. 2022, 23, 2414. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Unwin, R.J.; Parker, N.; Duchen, M.R. Multiphoton Imaging Reveals Differences in Mitochondrial Function between Nephron Segments. J. Am. Soc. Nephrol. 2009, 20, 1293–1302. [Google Scholar] [CrossRef]
- Sandoval, R.M.; Molitoris, B.A. Intravital multiphoton microscopy as a tool for studying renal physiology and pathophysiology. Methods 2017, 128, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Bugarski, M.; Martins, J.R.; Haenni, D.; Hall, A.M. Multiphoton imaging reveals axial differences in metabolic autofluorescence signals along the kidney proximal tubule. Am. J. Physiol. Physiol. 2018, 315, F1613–F1625. [Google Scholar] [CrossRef]
- Caterino, M.; Costanzo, M.; Fedele, R.; Cevenini, A.; Gelzo, M.; Di Minno, A.; Andolfo, I.; Capasso, M.; Russo, R.; Annunziata, A.; et al. The Serum Metabolome of Moderate and Severe COVID-19 Patients Reflects Possible Liver Alterations Involving Carbon and Nitrogen Metabolism. Int. J. Mol. Sci. 2021, 22, 9548. [Google Scholar] [CrossRef]
- Shanley, P.F.; Brezis, M.; Spokes, K.; Silva, P.; Epstein, F.H.; Rosen, S. Differential Responsiveness of Proximal Tubule Segments to Metabolic Inhibitors in the Isolated Perfused Rat Kidney. Am. J. Kidney Dis. 1986, 7, 76–83. [Google Scholar] [CrossRef]
- Hall, A.M.; Rhodes, G.J.; Sandoval, R.M.; Corridon, P.R.; Molitoris, B.A. In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int. 2013, 83, 72–83. [Google Scholar] [CrossRef]
- Manganelli, V.; Salvatori, I.; Costanzo, M.; Capozzi, A.; Caissutti, D.; Caterino, M.; Valle, C.; Ferri, A.; Sorice, M.; Ruoppolo, M.; et al. Overexpression of Neuroglobin Promotes Energy Metabolism and Autophagy Induction in Human Neuroblastoma SH-SY5Y Cells. Cells 2021, 10, 3394. [Google Scholar] [CrossRef]
- Hato, T.; Winfree, S.; Dagher, P.C. Intravital imaging of the kidney. Methods 2017, 128, 33–39. [Google Scholar] [CrossRef]
- Zipfel, W.R.; Williams, R.M.; Christie, R.; Nikitin, A.Y.; Hyman, B.T.; Webb, W.W. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA 2003, 100, 7075–7080. [Google Scholar] [CrossRef]
- Reeve, J.E.; Anderson, H.L.; Clays, K. Dyes for biological second harmonic generation imaging. Phys. Chem. Chem. Phys. 2010, 12, 13484. [Google Scholar] [CrossRef]
- Small, D.M.; Sanchez, W.Y.; Gobe, G.C. Intravital Multiphoton Imaging of the Kidney: Tubular Structure and Metabolism. In Kidney Research; Humana Press: New York, NY, USA, 2016; pp. 155–172. [Google Scholar]
- Strupler, M.; Hernest, M.; Fligny, C.; Martin, J.-L.; Tharaux, P.-L.; Schanne-Klein, M.-C. Second harmonic microscopy to quantify renal interstitial fibrosis and arterial remodeling. J. Biomed. Opt. 2008, 13, 054041. [Google Scholar] [CrossRef]
- Nucciotti, V.; Stringari, C.; Sacconi, L.; Vanzi, F.; Fusi, L.; Linari, M.; Piazzesi, G.; Lombardi, V.; Pavone, F.S. Probing myosin structural conformation in vivo by second-harmonic generation microscopy. Proc. Natl. Acad. Sci. USA 2010, 107, 7763–7768. [Google Scholar] [CrossRef]
- Yu, C.-H.; Langowitz, N.; Wu, H.-Y.; Farhadifar, R.; Brugues, J.; Yoo, T.Y.; Needleman, D. Measuring Microtubule Polarity in Spindles with Second-Harmonic Generation. Biophys. J. 2014, 106, 1578–1587. [Google Scholar] [CrossRef]
- Petrillo, F.; Iervolino, A.; Angrisano, T.; Jelen, S.; Costanzo, V.; D’Acierno, M.; Cheng, L.; Wu, Q.; Guerriero, I.; Mazzarella, M.C.; et al. Dysregulation of Principal Cell miRNAs Facilitates Epigenetic Regulation of AQP2 and Results in Nephrogenic Diabetes Insipidus. J. Am. Soc. Nephrol. 2021, 32, 1339–1354. [Google Scholar] [CrossRef]
- Ranjit, S.; Dobrinskikh, E.; Montford, J.; Dvornikov, A.; Lehman, A.; Orlicky, D.J.; Nemenoff, R.; Gratton, E.; Levi, M.; Furgeson, S. Label-free fluorescence lifetime and second harmonic generation imaging microscopy improves quantification of experimental renal fibrosis. Kidney Int. 2016, 90, 1123–1128. [Google Scholar] [CrossRef]
- Perry, S.W.; Burke, R.M.; Brown, E.B. Two-Photon and Second Harmonic Microscopy in Clinical and Translational Cancer Research. Ann. Biomed. Eng. 2012, 40, 277–291. [Google Scholar] [CrossRef]
- Caterino, M.; Zacchia, M.; Costanzo, M.; Bruno, G.; Arcaniolo, D.; Trepiccione, F.; Siciliano, R.A.; Mazzeo, M.F.; Ruoppolo, M.; Capasso, G. Urine Proteomics Revealed a Significant Correlation Between Urine-Fibronectin Abundance and Estimated-GFR Decline in Patients with Bardet-Biedl Syndrome. Kidney Blood Press. Res. 2018, 43, 389–405. [Google Scholar] [CrossRef]
- Gonzalez Melo, M.; Fontana, A.; Viertl, D.; Allenbach, G.; Prior, J.O.; Rotman, S.; Feichtinger, R.G.; Mayr, J.A.; Costanzo, M.; Caterino, M.; et al. A knock-in rat model unravels acute and chronic renal toxicity in glutaric aciduria type I. Mol. Genet. Metab. 2021, 134, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Mathew, J.; Lerman, L.O.; Lieske, J.C.; Larson, J.J.; Alexander, M.P.; Poggio, E.; Glassock, R.J.; Rule, A.D. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N. Engl. J. Med. 2017, 376, 2349–2357. [Google Scholar] [CrossRef]
- Vallon, V. Micropuncturing the nephron. Pflügers Arch. Eur. J. Physiol. 2009, 458, 189–201. [Google Scholar] [CrossRef]
- Kang, J.J.; Toma, I.; Sipos, A.; McCulloch, F.; Peti-Peterdi, J. Quantitative imaging of basic functions in renal (patho)physiology. Am. J. Physiol. Physiol. 2006, 291, F495–F502. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, V.; D’Apolito, L.; Sardella, D.; Iervolino, A.; La Manna, G.; Capasso, G.; Frische, S.; Trepiccione, F. Single nephron glomerular filtration rate measured by linescan multiphoton microscopy compared to conventional micropuncture. Pflügers Arch. Eur. J. Physiol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Ferrell, N.; Sandoval, R.M.; Bian, A.; Campos-Bilderback, S.B.; Molitoris, B.A.; Fissell, W.H. Shear stress is normalized in glomerular capillaries following ⅚ nephrectomy. Am. J. Physiol. Physiol. 2015, 308, F588–F593. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Schlatter, E. Regulation of organic cation transport. Pflügers Arch. Eur. J. Physiol. 2005, 449, 423–441. [Google Scholar] [CrossRef]
- Motohashi, H.; Inui, K. Organic Cation Transporter OCTs (SLC22) and MATEs (SLC47) in the Human Kidney. AAPS J. 2013, 15, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Glorieux, G.; Thornalley, P.; Schepers, E.; Meert, N.; Jankowski, J.; Jankowski, V.; Argiles, A.; Anderstam, B.; Brunet, P.; et al. Review on uraemic toxins III: Recommendations for handling uraemic retention solutes in vitro towards a standardized approach for research on uraemia. Nephrol. Dial. Transplant. 2007, 22, 3381–3390. [Google Scholar] [CrossRef] [PubMed]
- Hörbelt, M.; Wotzlaw, C.; Sutton, T.A.; Molitoris, B.A.; Philipp, T.; Kribben, A.; Fandrey, J.; Pietruck, F. Organic cation transport in the rat kidney in vivo visualized by time-resolved two-photon microscopy. Kidney Int. 2007, 72, 422–429. [Google Scholar] [CrossRef]
- Engbjerg, J.S.; Costanzo, V.; Sardella, D.; Bordoni, L.; Jakobsen, S.; D’Apolito, L.; Frøkiær, J.; Trepiccione, F.; Capasso, G.; Frische, S. The Probe for Renal Organic Cation Secretion (4-Dimethylaminostyryl)-N-Methylpyridinium (ASP+)) Shows Amplified Fluorescence by Binding to Albumin and Is Accumulated In Vivo. Mol. Imaging 2022, 2022, 7908357. [Google Scholar] [CrossRef]
- Meyer-Schwesinger, C. The Role of Renal Progenitors in Renal Regeneration. Nephron 2016, 132, 101–109. [Google Scholar] [CrossRef]
- Schiessl, I.M.; Grill, A.; Fremter, K.; Steppan, D.; Hellmuth, M.-K.; Castrop, H. Renal Interstitial Platelet-Derived Growth Factor Receptor- β Cells Support Proximal Tubular Regeneration. J. Am. Soc. Nephrol. 2018, 29, 1383–1396. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, S.; Sun, H.; Wang, L.; Li, H.; Zhao, J.; Zhang, C.; Li, N.; Guo, Z.; Han, Z.; et al. In vivo two-photon microscopy reveals the contribution of Sox9+ cell to kidney regeneration in a mouse model with extracellular vesicle treatment. J. Biol. Chem. 2020, 295, 12203–12213. [Google Scholar] [CrossRef]
- Rhodes, G.J. Surgical preparation of rats and mice for intravital microscopic imaging of abdominal organs. Methods 2017, 128, 129–138. [Google Scholar] [CrossRef]
- Dunn, K.W.; Sutton, T.A.; Sandoval, R.M. Live-Animal Imaging of Renal Function by Multiphoton Microscopy. Curr. Protoc. Cytom. 2018, 83, 12–19. [Google Scholar] [CrossRef]
- Soulet, D.; Lamontagne-Proulx, J.; Aubé, B.; Davalos, D. Multiphoton intravital microscopy in small animals: Motion artefact challenges and technical solutions. J. Microsc. 2020, 278, 3–17. [Google Scholar] [CrossRef]
- Flotho, P.; Nomura, S.; Kuhn, B.; Strauss, D.J. Software for Non-Parametric Image Registration of 2-Photon Imaging Data. J. Biophotonics 2022, e202100330, online ahead of print. [Google Scholar] [CrossRef]
- Ritsma, L.; Steller, E.J.A.; Ellenbroek, S.I.J.; Kranenburg, O.; Borel Rinkes, I.H.M.; van Rheenen, J. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat. Protoc. 2013, 8, 583–594. [Google Scholar] [CrossRef]
- Bölke, T.; Krapf, L.; Orzekowsky-Schroeder, R.; Vossmeyer, T.; Dimitrijevic, J.; Weller, H.; Schüth, A.; Klinger, A.; Hüttmann, G.; Gebert, A. Data-adaptive image-denoising for detecting and quantifying nanoparticle entry in mucosal tissues through intravital 2-photon microscopy. Beilstein J. Nanotechnol. 2014, 5, 2016–2025. [Google Scholar] [CrossRef]
- Xiao, S.; Mertz, J. Contrast improvement in two-photon microscopy with instantaneous differential aberration imaging. Biomed. Opt. Express 2019, 10, 2467. [Google Scholar] [CrossRef] [PubMed]
- Kan, A. Machine learning applications in cell image analysis. Immunol. Cell Biol. 2017, 95, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Mougeot, G.; Dubos, T.; Chausse, F.; Péry, E.; Graumann, K.; Tatout, C.; Evans, D.E.; Desset, S. Deep learning—Promises for 3D nuclear imaging: A guide for biologists. J. Cell Sci. 2022, 135, jcs258986. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Qin, X.; Nair, S.; Huang, X.; Liu, Y. Label-free detection of rare circulating tumor cells by image analysis and machine learning. Sci. Rep. 2020, 10, 12226. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schlingmann, S.; Jonke, Ž.; Avci, P.; Padrón-Laso, V.; Neumeier, F.; Koveshazi, I.; Ikeliani, I.U.; Patzer, K.; Kunrad, E.; et al. Machine Learning Based Prediction of Squamous Cell Carcinoma in Ex Vivo Confocal Laser Scanning Microscopy. Cancers 2021, 13, 5522. [Google Scholar] [CrossRef] [PubMed]
- Kromp, F.; Bozsaky, E.; Rifatbegovic, F.; Fischer, L.; Ambros, M.; Berneder, M.; Weiss, T.; Lazic, D.; Dörr, W.; Hanbury, A.; et al. An annotated fluorescence image dataset for training nuclear segmentation methods. Sci. Data 2020, 7, 262. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.; Haenni, D.; Bugarski, M.; Polesel, M.; Schuh, C.; Hall, A.M. Intravital kidney microscopy: Entering a new era. Kidney Int. 2021, 100, 527–535. [Google Scholar] [CrossRef]
- Dunn, K.W.; Sandoval, R.M.; Kelly, K.J.; Dagher, P.C.; Tanner, G.A.; Atkinson, S.J.; Bacallao, R.L.; Molitoris, B.A. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am. J. Physiol. Physiol. 2002, 283, C905–C916. [Google Scholar] [CrossRef]
- Hackl, M.J.; Burford, J.L.; Villanueva, K.; Lam, L.; Suszták, K.; Schermer, B.; Benzing, T.; Peti-Peterdi, J. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat. Med. 2013, 19, 1661–1666. [Google Scholar] [CrossRef]
- Rovira-Halbach, G.; Alt, J.M.; Brunkhorst, R.; Frei, U.; Kühn, K.; Stolte, H. Single nephron hyperfiltration and proteinuria in a newly selected rat strain with superficial glomeruli. Ren. Physiol. 1986, 9, 317–325. [Google Scholar] [CrossRef]
- Saritas, T.; Puelles, V.G.; Su, X.-T.; Ellison, D.H.; Kramann, R. Optical Clearing and Imaging of Immunolabeled Kidney Tissue. J. Vis. Exp. 2019, 149, e60002. [Google Scholar] [CrossRef]
- Lin, M.-H.; Chen, J.-C.; Tian, X.; Lee, C.-M.; Yu, I.-S.; Lo, Y.-F.; Uchida, S.; Huang, C.-L.; Chen, B.-C.; Cheng, C.-J. Impairment in renal medulla development underlies salt wasting in Clc-k2 channel deficiency. JCI Insight 2021, 6, e151039. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanzo, V.; Costanzo, M. Intravital Imaging with Two-Photon Microscopy: A Look into the Kidney. Photonics 2022, 9, 294. https://doi.org/10.3390/photonics9050294
Costanzo V, Costanzo M. Intravital Imaging with Two-Photon Microscopy: A Look into the Kidney. Photonics. 2022; 9(5):294. https://doi.org/10.3390/photonics9050294
Chicago/Turabian StyleCostanzo, Vincenzo, and Michele Costanzo. 2022. "Intravital Imaging with Two-Photon Microscopy: A Look into the Kidney" Photonics 9, no. 5: 294. https://doi.org/10.3390/photonics9050294
APA StyleCostanzo, V., & Costanzo, M. (2022). Intravital Imaging with Two-Photon Microscopy: A Look into the Kidney. Photonics, 9(5), 294. https://doi.org/10.3390/photonics9050294





