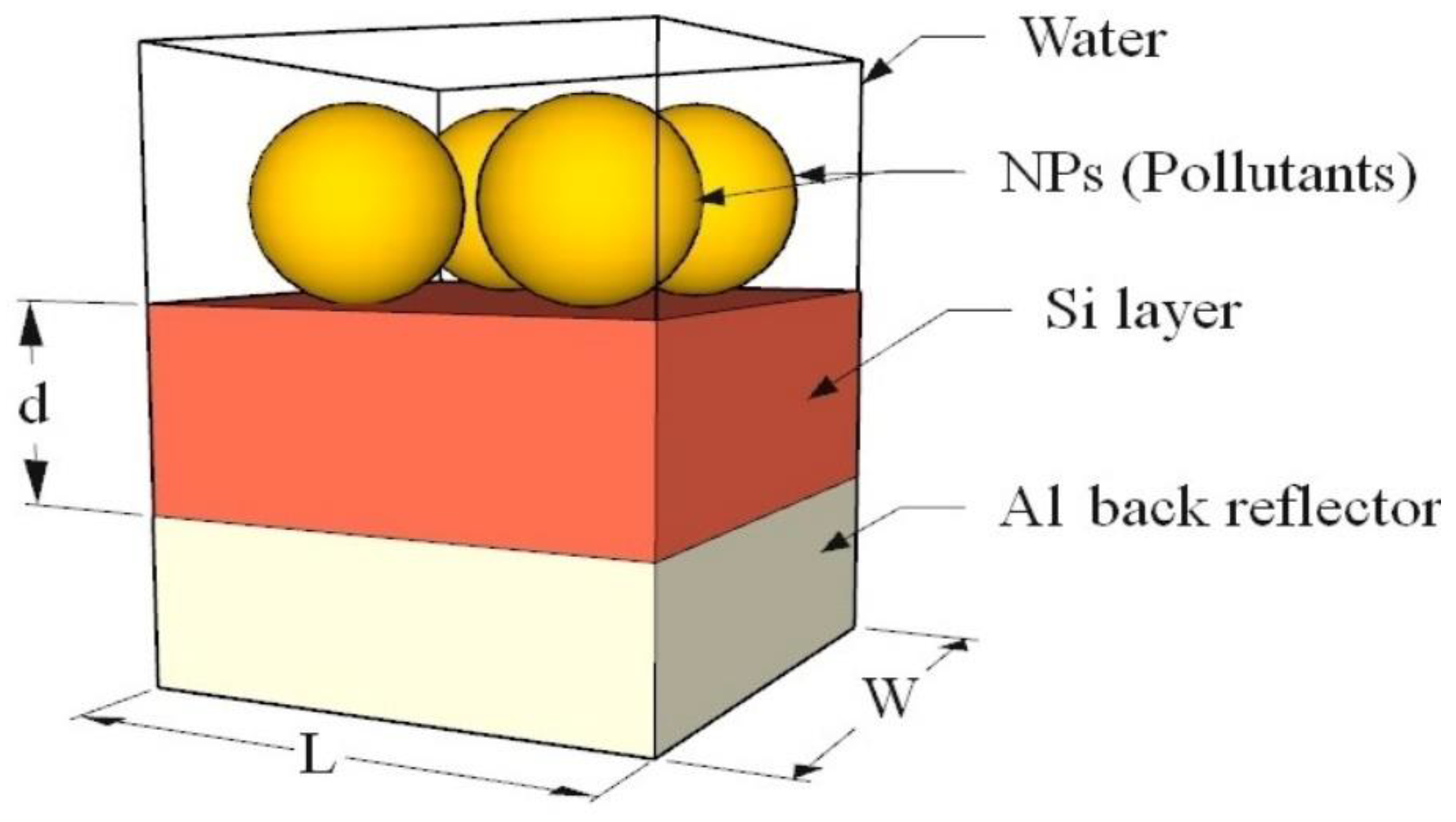
In this section, the optimization of the Si layer thickness is given, then the device’s performance in detecting various water pollutants is evaluated. Furthermore, the ability of the device to detect more than one water pollutant existing simultaneously is discussed.
3.1. Optimizing Si Layer Thickness
The device has been simulated for different thicknesses of the Si layer, , and with different Au NP sizes. The Si layer has been changed from 100 nm to 500 nm in a step of 100 nm, while the Au NP radius, r, takes the following values (50 nm, 100 nm, 200 nm, 300 nm, 400 nm, and 500 nm). In the simulation, it is assumed that the Au spherical NPs are touching each other with no separation between them. The absorption spectrum of the device is measured, and the peak wavelength (λmax), peak amplitude (I), and the FWHM of the absorption curve have been recorded for each case.
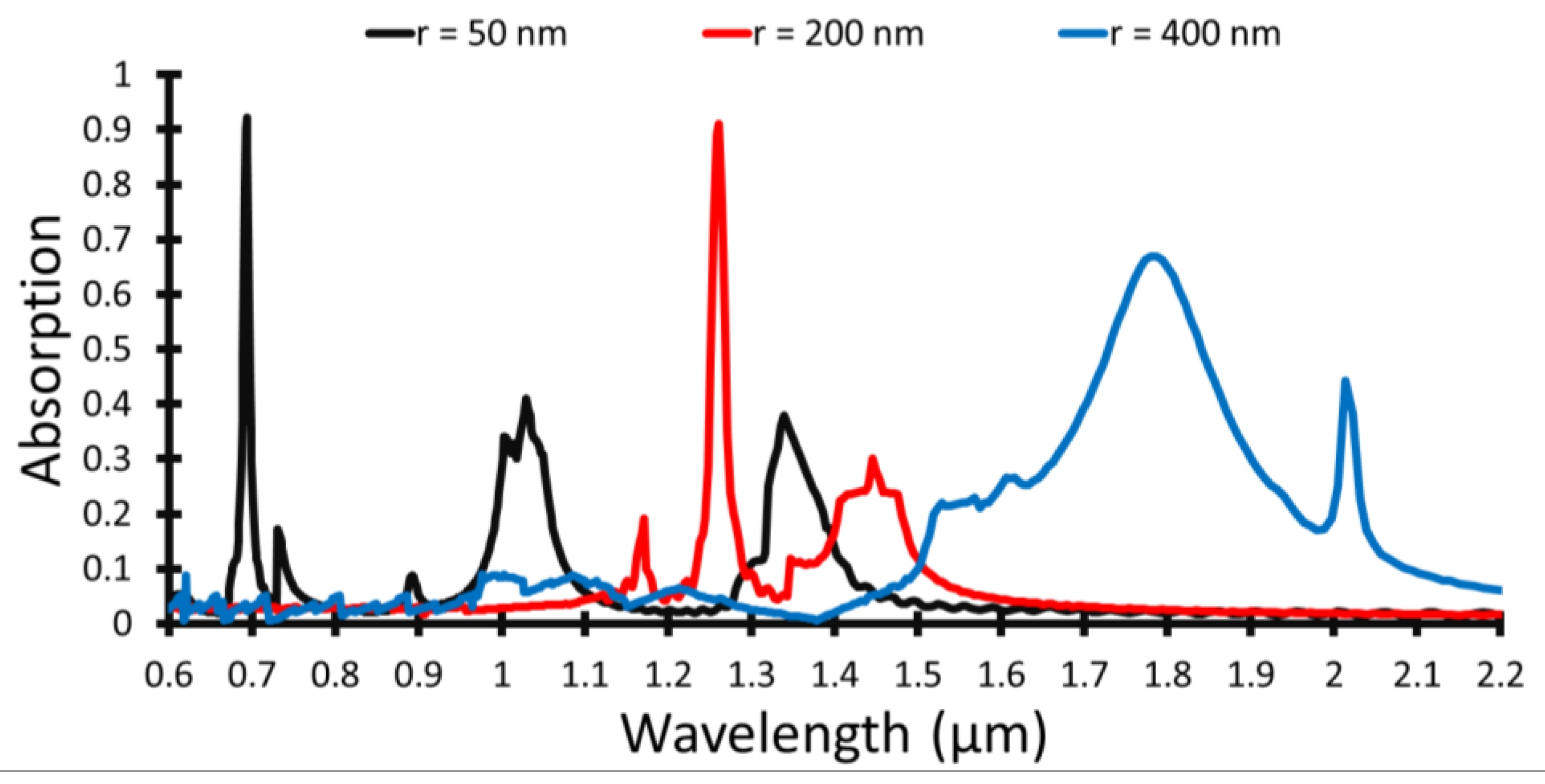
The absorbed light spectrum is shown in
Figure 2 with Au NP of radius 50 nm, 200 nm, and 400 nm when the Si layer is 300 nm thick. This is a sample of the measured spectrum where only three full-spectrum curves are selected to display the obtained absorption behavior. The absorption spectral shapes reveal that each NP size has size-dependent absorption characteristics, considering that the NP absorption cross-section depends on the Au NP size. Changing the NP size does not only change the peak wavelength but also changes the FWHM and relative intensity of the absorption spectrum.
Figure 3 shows a sample of the absorbed power distribution around the Au NP and in the Si layer for NPs of radius 500 nm at its maximum absorption wavelength (
λmax = 2.1 μm) and at a minimum absorption wavelength. It is clear from
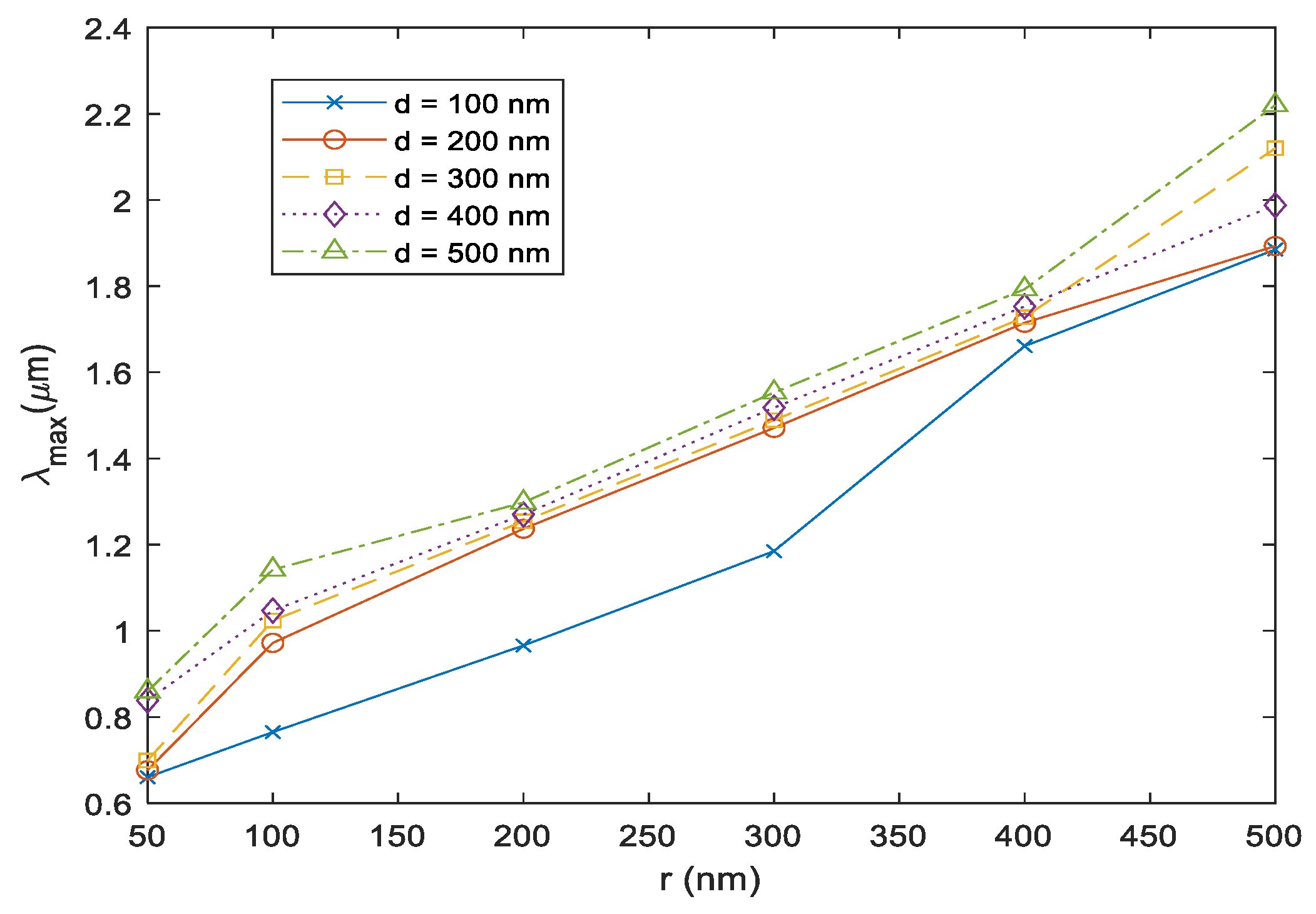
Figure 3a that the absorption power increases at the resonance wavelength of the Au NP, which increases the overall absorption of the sensor. The peak wavelength variation depending on the Au NP’s size (50 nm, 100 nm, 200 nm, 300 nm, 400 nm, and 500 nm) is shown in
Figure 4 for unoptimized Si layer thickness that varies from 100 to 500 nm. The observed absorption peak wavelength (
λmax) is redshifted as the size of the Au NP increases for all Si layer thickness values. This behavior is expected as the resonance wavelength of the Au plasmons is tunable depending on the particle shape, size, and number [
31]. When irradiated with electromagnetic waves, the metal NPs oscillate with size-dependent Eigen frequencies. This feature results from the confinement of the electron cloud at the metal-Si layer boundary. Using the Drude–Sommerfeld model of the dielectric function of the NP metal shows that plasmons resonance wavelength shifts to a longer wavelength as the size of the NP increases [
32,
33]. This NP behavior eventually leads to the size-dependent optical and electronic properties that could be optimized to suit devices applications by varying the nanoparticle size.
The dependence of the FWHM and absorption intensity on the size of the Au NPs and the thickness of the Si layer is shown in
Table 2. The data shows that the shape of the absorption curve varies depending on both the nanoparticle size and the thickness of the Si layer.
For all Si layer thickness values, FWHM and relative intensity seem to be distinct for each particle size, i.e., each particle size has a spectral shape that varies depending on the size. From the obtained data and encouraged by the systematic variation of the peak absorption wavelength, we elected the peak absorption wavelength as the parameter that will be used to optimize the Si layer thickness to maximize the shift in λmax as a function of the particle radius. This will increase the sensitivity of the sensor to NP size variations.
To optimize the Si layer thickness, an equation governing the peak absorption wavelength as a function of nanoparticle radius and Si layer thickness needs to be generated. Different interpolation techniques are adopted, including polynomials with various degrees and locally weighted smoothing quadratic regression (LOESS) to generate the required equation [
34]. These techniques have been utilized to find the best fitting models of the simulated data points shown in
Figure 4. The optimum model is selected based on the goodness of fit criterion considering the following statistics:
SSE measures the deviation in the fitted model as defined by Equation (6):
where
is the weights while
and the
are the actual data and the interpolated ones, respectively [
35]. A model with good fitting is when SSE is close to 0.
- 2.
R-Square
The metric indicates how well the fit describes the variability. It is defined as the proportion of the sum of squares of the regression (SSR) and the total sum of squares (SST). SSR is defined as:
SST is defined as the sum of squares that deviated from the mean and is defined as
where
SST =
SSR +
SSE. R-square is defined as:
R-Square can take values between 0 and 1, with a value close to 1 representing a good fitting model.
- 3.
Adjusted R-Square
The adjusted R-Square is defined by:
where
is defined as the number of response values and
is the residual degrees of freedom defined by the difference between
and the number of fitted coefficients estimated from the response values. The static can take any value between 0 and 1, with a value close to 1 representing a good fitting model.
- 4.
Root Mean Squared Error (RMSE)
It is defined as the square root of the mean square error (MSR). Again, a model with a value close to zero will represent a good fitting model.
Applying the previously mentioned interpolation techniques, the peak location of the maximum light absorption as a function of Si layer thickness
and the Au NP radius, r, is modeled by a polynomial of degree 5 as given by Equation (11):
where
is normalized by a mean of 300 nm and standard deviation (std) of 143.8 nm and
is normalized by a mean of 258.3 nm and std of 161.9 nm. The goodness of fit metrics is given in
Table 3. The recorded goodness of fit is the best result obtained when trying different interpolation techniques. The goodness of fit parameters is in the acceptable range, which indicates the high accuracy of the fitting equation.
The coefficients of the fitted polynomial using the adjusted R-square method with 95% confidence bounds are given in
Table 4. The coefficients shown are selected in the middle of the 95% confidence interval shown.
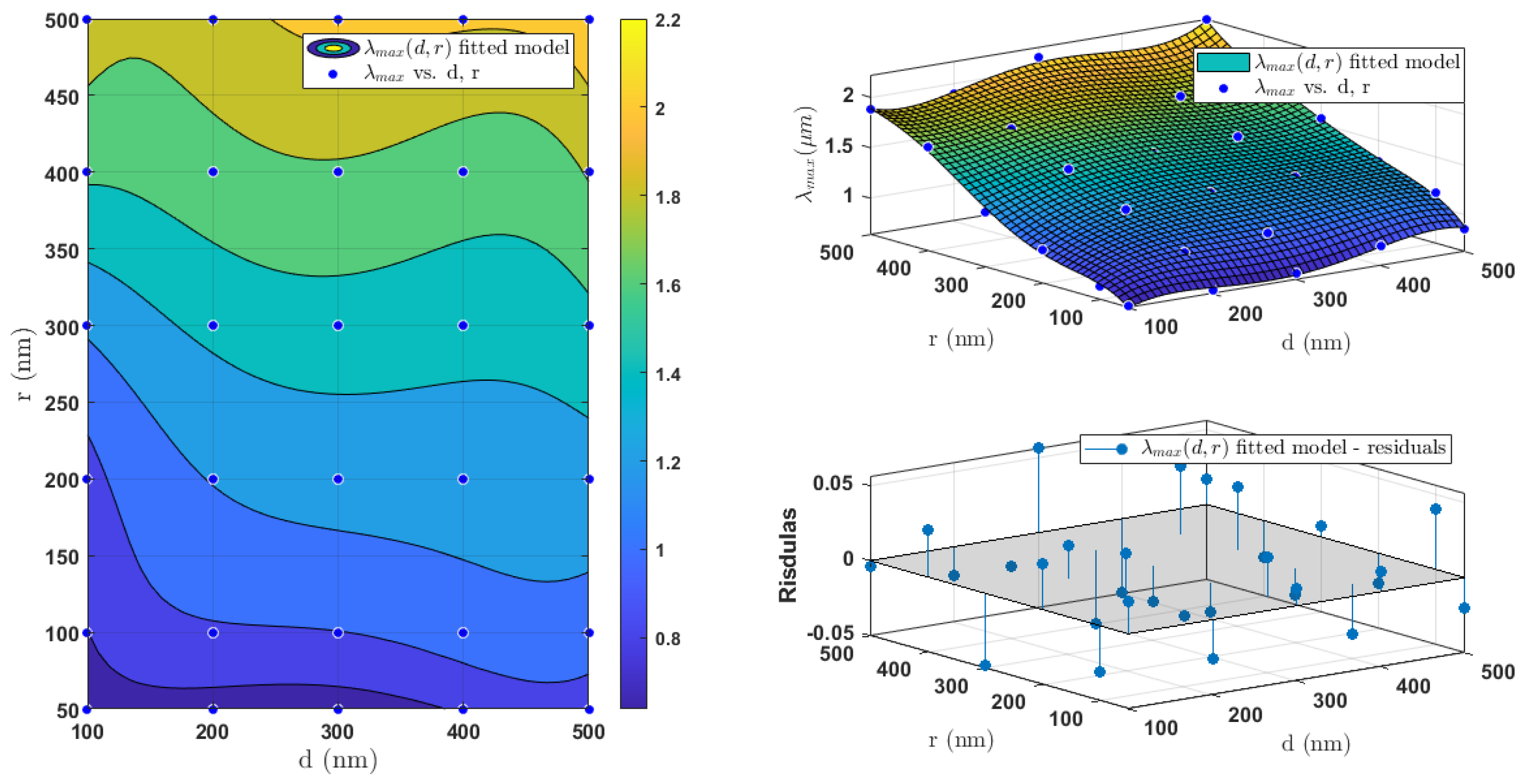
The fitted surface of
shown in
Figure 5 depicts that
is monotonically increasing as a function of both the radius and the Si layer thickness. The residuals plot in
Figure 5 indicates small interpolation errors between the actual data points and the fitted surface.
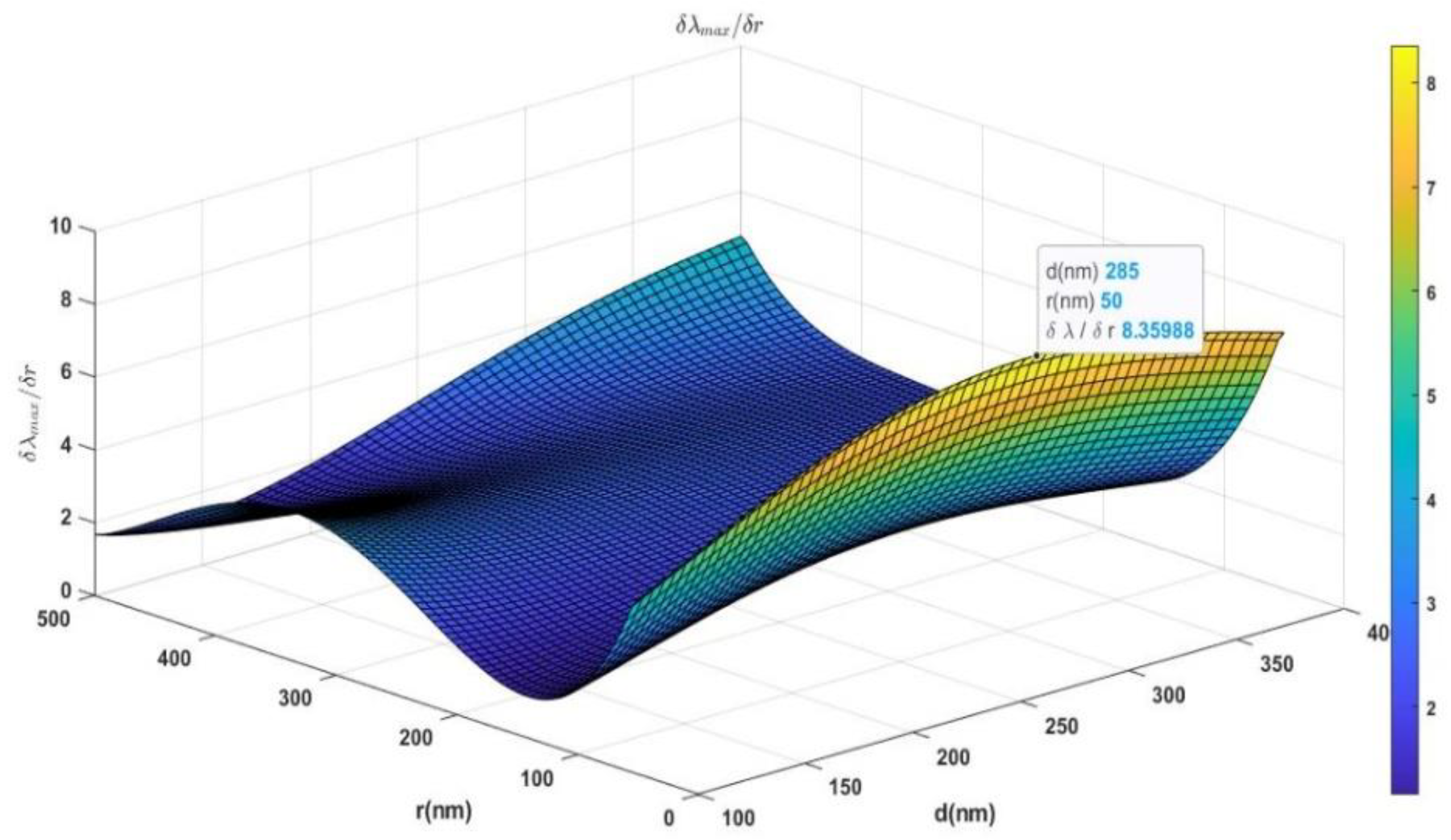
The gradient of the fitted surface,
is calculated and plotted in
Figure 6. The optimized thickness of the Si layer is determined from the maximum of the gradient. This optimum point is marked in
Figure 6, and it is found that the optimum thickness is 285 nm. This is the optimum thickness to detect the smallest used Au NP, r = 50 nm with size sensitivity
. So, the proposed structure Si layer thickness was set to 300 nm. By maximizing the size sensitivity of the device, the absorption peak wavelength could be used to detect the particle size with high sensitivity as there is no overlap between the different peaks.
The spectral peaks shown in
Figure 7 are obtained using the optimum Si layer thickness. These spectral peaks are truncated from the full absorption spectrum shown in
Figure 2 to illustrate the different features of the spectral peaks for all Au NPs sizes. There is a suitable wavelength span between each spectral peak as we maximized the size sensitivity of the device.
The effect of the concentration of Au NP on the peak wavelength is investigated, and it is observed that the peak wavelength redshifts as the number of Au NP increases. Additionally, the spectrum broadens as the concentration of the Au NP increases.
Figure 8 shows the dependence of the peak wavelength on the number of nanoparticles for the case where the Au NPs are of radius 200 nm and the Si layer thickness is 300 nm. The redshift of the spectrum with the increase of the number of nanoparticles is due to the coupling between different particles, which changes the electric field intensity on the surface. This causes a change in the oscillation frequency of the electrons, generating different cross-sections for the optical properties, including absorption and scattering [
36,
37,
38]. The increased broadening in the absorption spectrum is attributed to both the surface scattering of electrons as well as increased radiation damping [
33,
39,
40].
3.3. Detecting Water Pollutants Using the Designed Optimized Sensor
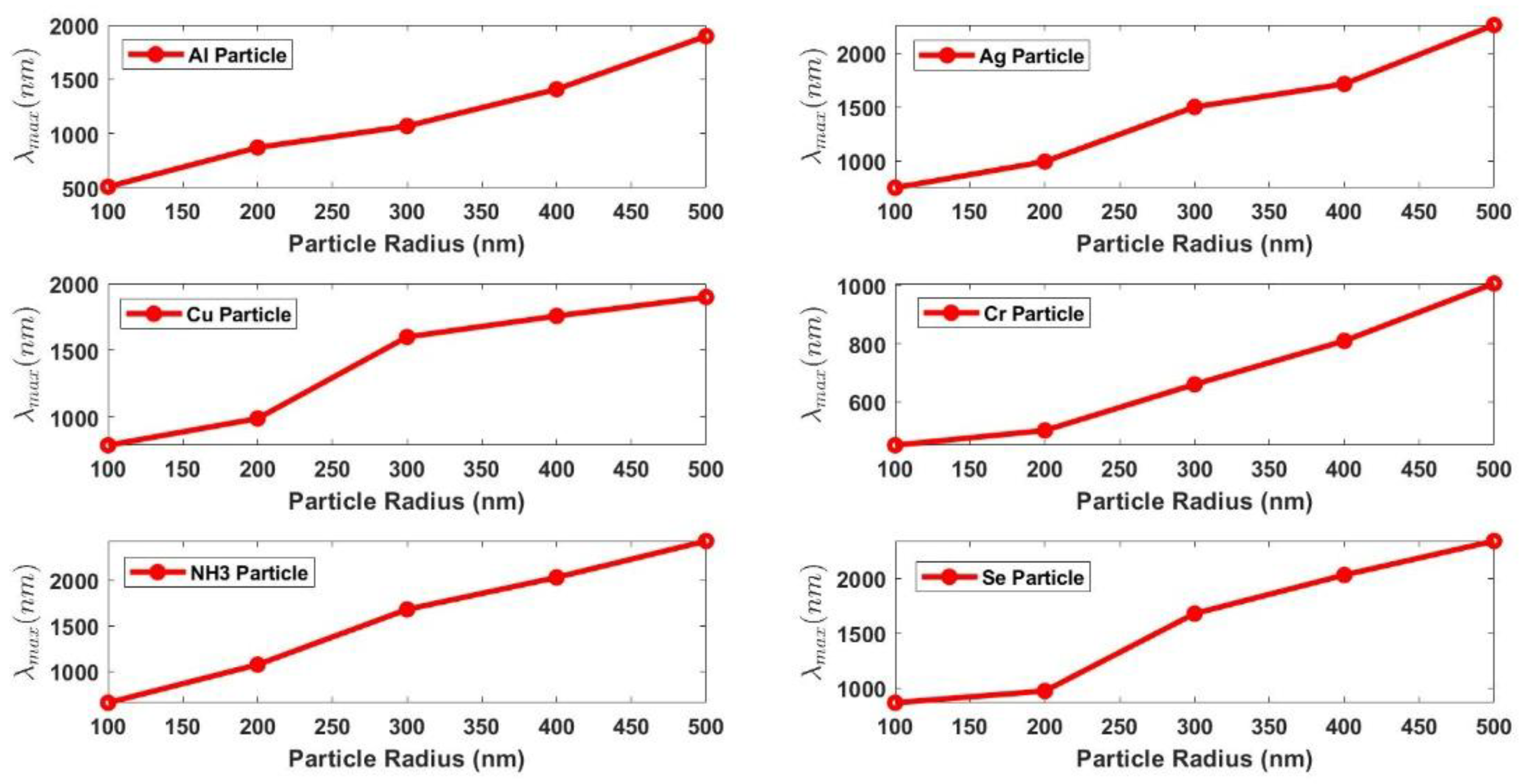
The designed Si-based sensor’s performance in detecting different sizes of water pollutants is investigated. The Au NPs on the top of the Si layer are replaced with six common water pollutants, Ag, Al, Cu, Cr, Se, and NH
3. As in the case of Au NPs, the absorption spectra are measured for different sizes ranging from 100 nm to 500 nm in a step of 100 nm. The spectrum curve’s peak wavelength, FWHM, and relative intensity are observed.
Figure 9 shows that the peak wavelength redshifts with the increase of the size of the pollutants. All the six pollutants’ absorption spectrum show the same behavior but with different size selectivity
, this is indicated by the different gradients of the six shown curves. The selectivity,
, takes the values from 2 to 7, which indicates the good selectivity of the sensor.
The FWHM and peak amplitude of the spectral absorption curves are shown in
Table 5a,b. The results for the six pollutants show that the absorption spectral shapes have a wide variation in FWHM values that are size dependent. These variations could be favorable as pollutants could be detected depending on the peak wavelength and the shape of the absorption spectrum, FWHM, and peak relative amplitude.
Figure 10 allocates all the simulated particles with different materials and different sizes. A good separation between the detected particles with different sizes is demonstrated, and the proposed structure can differentiate between these different cases. The zooming on the plotted graph shows that there is a potential that the particles can be detected efficiently. Additionally, we have normalized the features to be from 0 to 1 and plot the 3D feature space of the detected particles along with different sizes in
Figure 11. Again, it still shows a good separation between the different particles.
The sensor detection parameters are then calculated from Equations (1)–(3). The calculated values for the sensitivity, FOM, and Q-factor are presented in
Table 6. Our Sensitivity is comparable with the reported value for Ag, which is about 5000 nm/RIU, but our maximum sensitivity (11,300 nm/RIU) is much higher [
43]. Sharp spectral curves accompany this ultimate value of sensitivity in the case of Se that is indicated from the low values for FWHM in
Table 5 and large FOM values (750) in
Table 6.