Application of Adaptive Optics in Ophthalmology
Abstract
1. Introduction
2. Principles and Methods
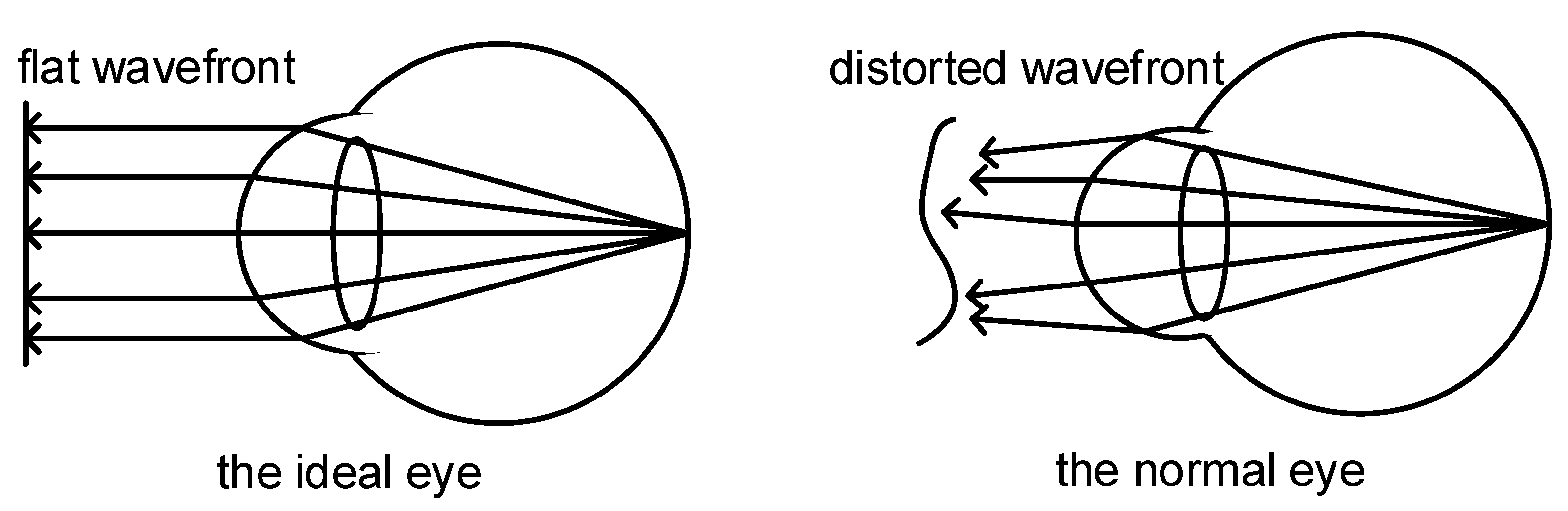
2.1. Wavefront Aberration in Human Eyes
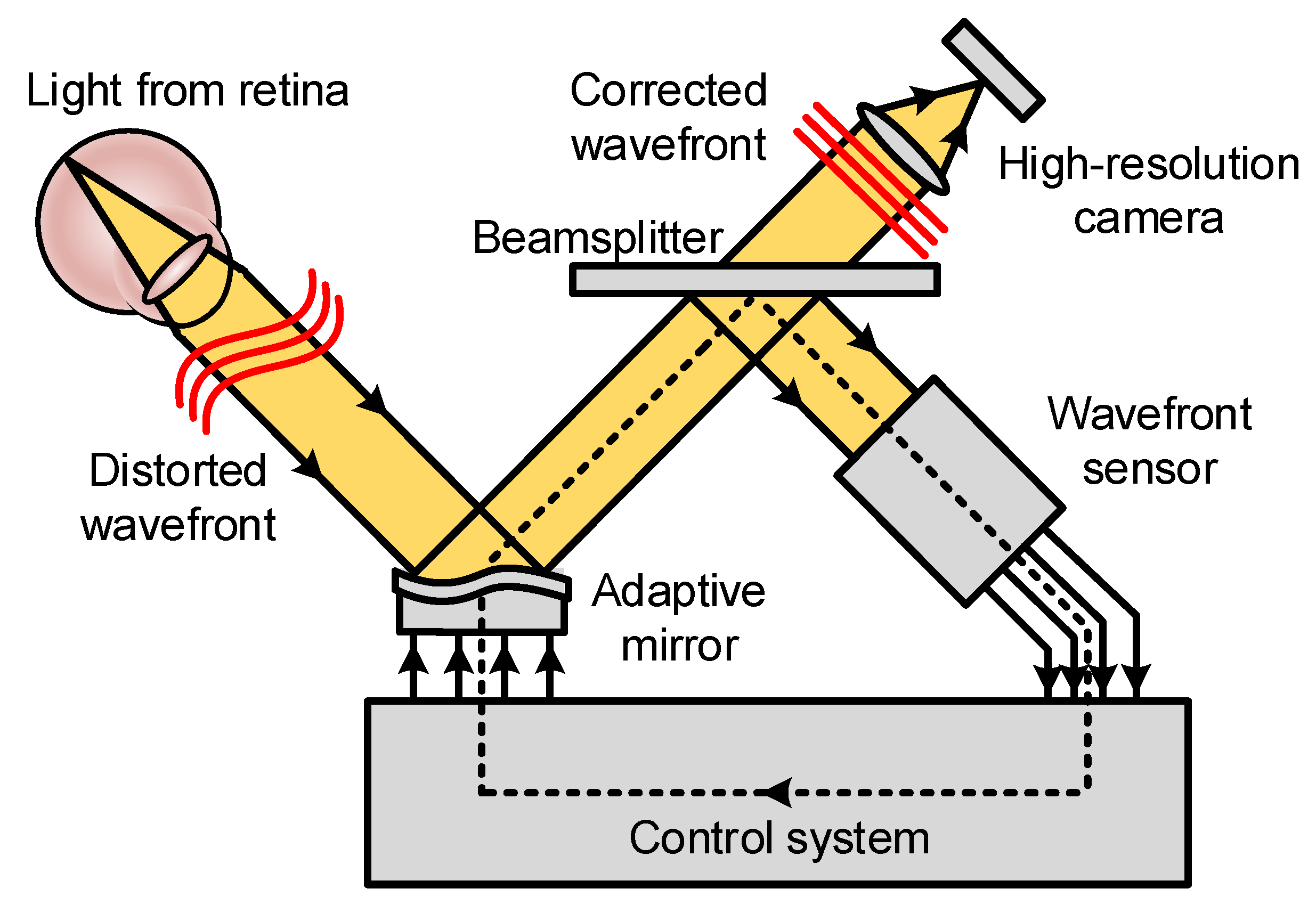
2.2. Basic Principles of Adaptive Optics
2.3. Sensorless AO and Computational AO
3. Application of Adaptive Optics in Ophthalmology
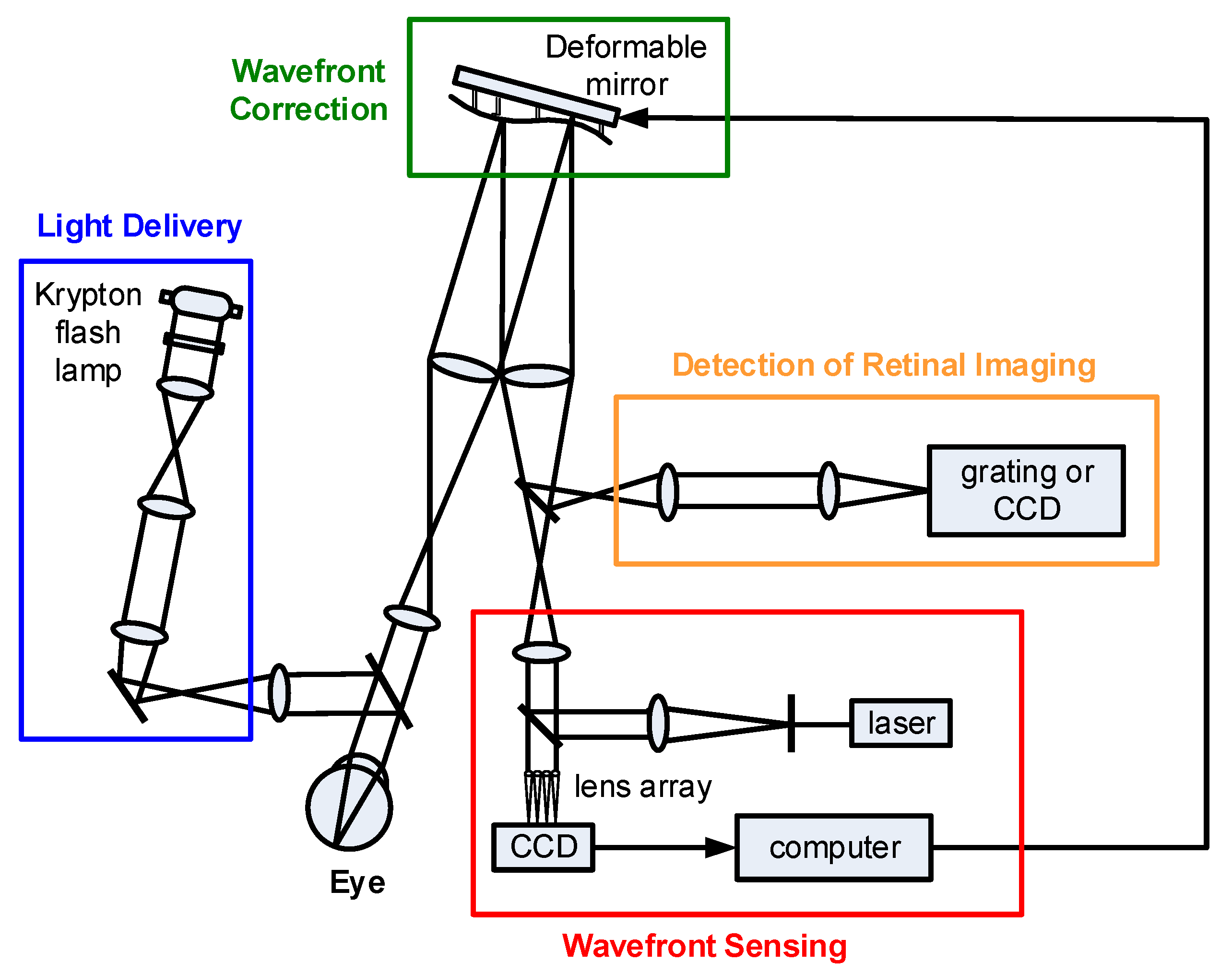
3.1. Adaptive Optics Fundus Camera (AO-FC)
3.2. Adaptive Optics Scanning Laser Ophthalmoscope (AO-SLO)
3.2.1. Basic Operation of AO-SLO
3.2.2. Eye Tracking Integration into AO-SLO
3.2.3. Split Detection Approaches of AO-SLO
3.2.4. Handheld Designs of AO-SLO
3.2.5. Algorithms and Applications of AO-SLO Retinal Imaging
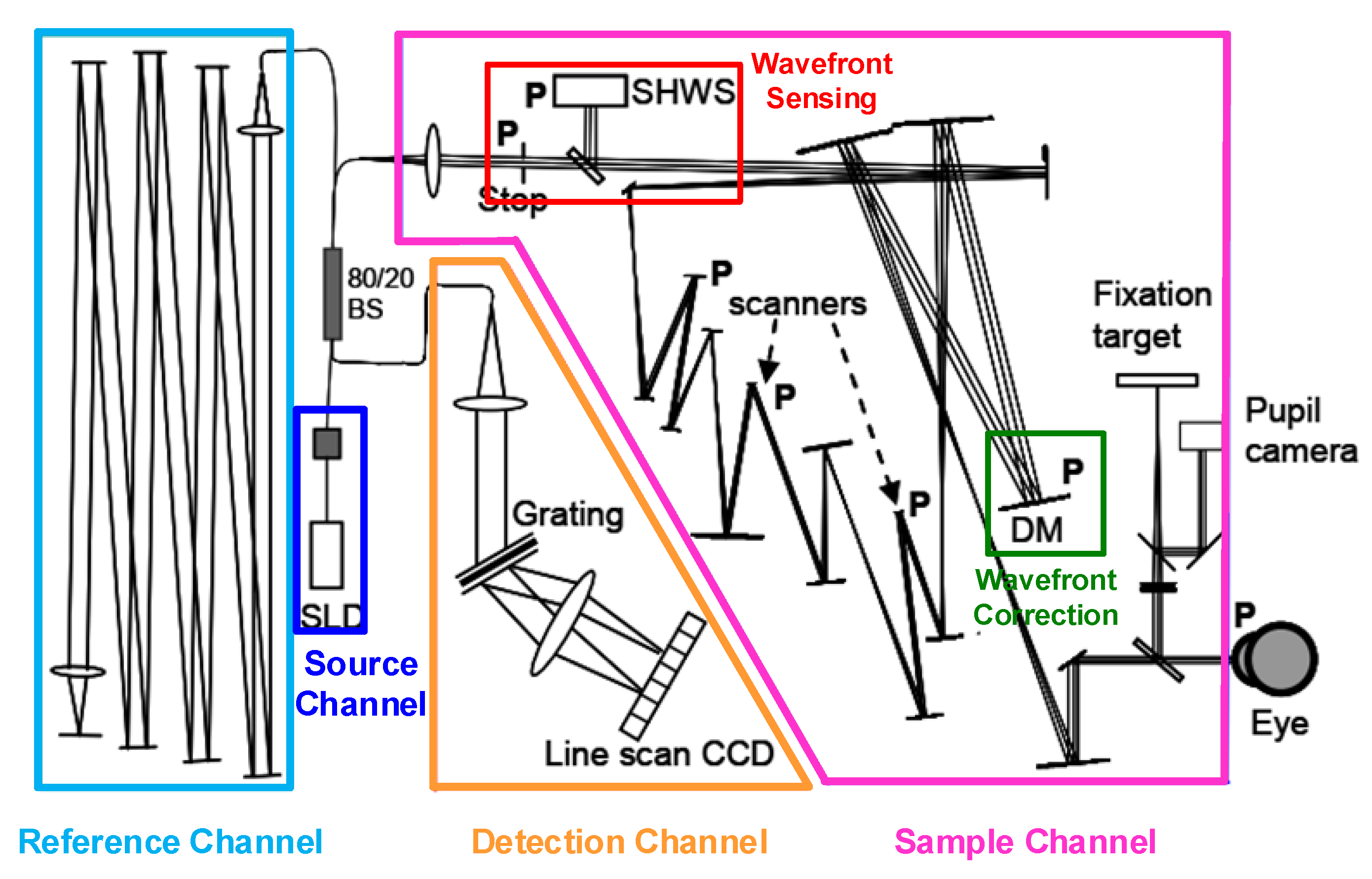
3.3. Adaptive Optics Optical Coherence Tomography (AO-OCT)
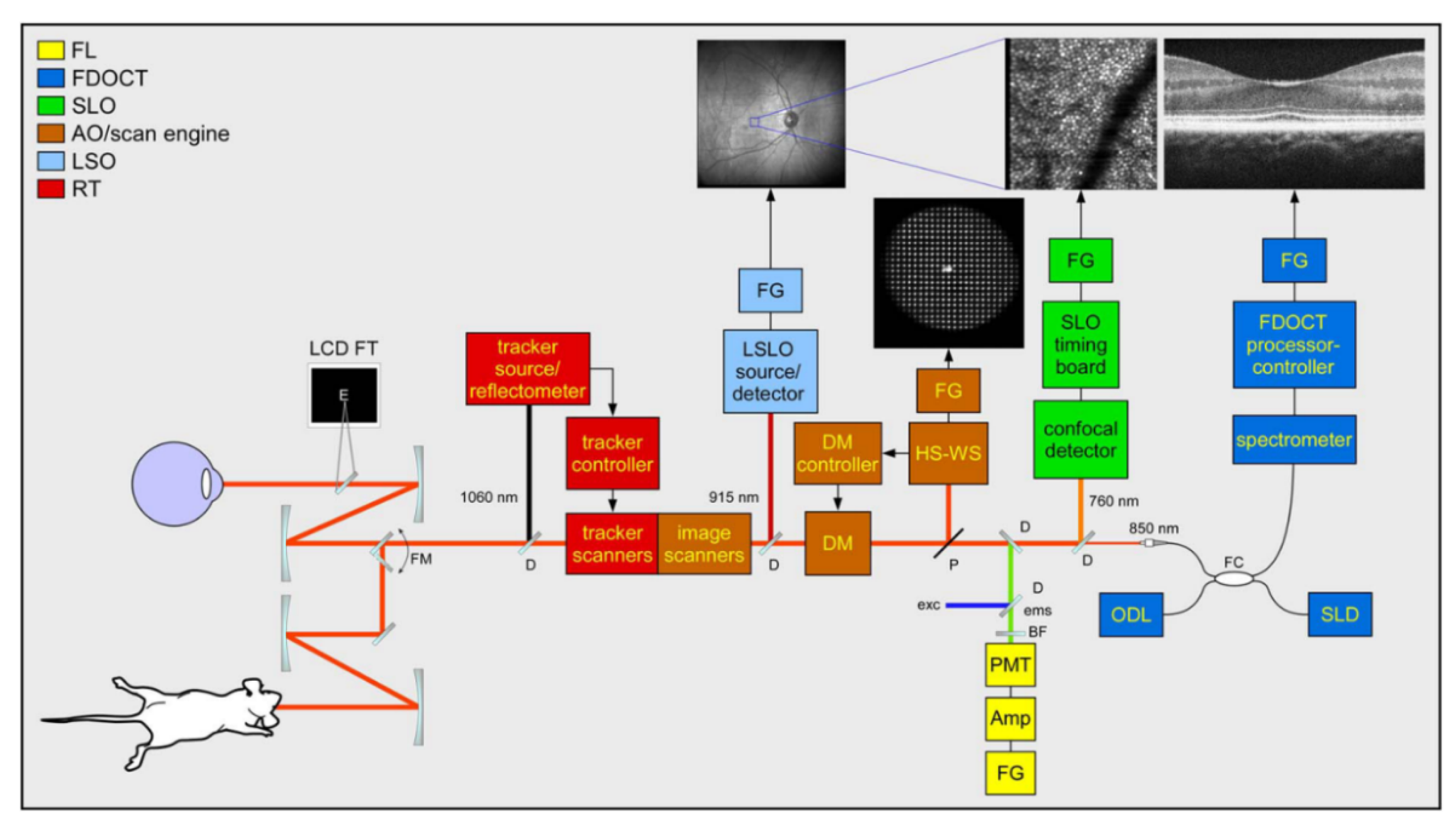
3.4. Multimodal Adaptive Optics Retinal Imaging Techniques
4. Summary and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, G.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Williams, D.R.; Morris, G.M.; Liang, J. Images of cone photoreceptors in the living human eye. Vis. Res. 1996, 36, 1067–1079. [Google Scholar] [CrossRef]
- Wade, A.; Fitzke, F. A fast, robust pattern recognition system for low light level image registration and its application to retinal imaging. Opt. Express 1998, 3, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Babcock, H.W. The possibility of compensating astronomical seeing. Publ. Astron. Soc. Pac. 1953, 65, 229–236. [Google Scholar] [CrossRef]
- Liang, J.; Grimm, B.; Goelz, S.; Bille, J.F. Objective measurement of wave aberrations of the human eye with the use of a Hartmann–Shack wave-front sensor. J. Opt. Soc. Am. A 1994, 11, 1949–1957. [Google Scholar] [CrossRef]
- Clarkson, D. Adaptive optics in ophthalmology-Emergence of diagnostic tools. Optician 2007, 234, 38–39. [Google Scholar]
- Godara, P.; Dubis, A.M.; Roorda, A.; Duncan, J.L.; Carroll, J. Adaptive optics retinal imaging: Emerging clinical applications. Optom. Vis. Sci. 2010, 87, 930–941. [Google Scholar] [CrossRef]
- Burns, S.A.; Elsner, A.E.; Sapoznik, K.A.; Warner, R.L.; Gast, T.J. Adaptive optics imaging of the human retina. Prog. Retin. Eye Res. 2019, 68, 1–30. [Google Scholar] [CrossRef]
- Gill, J.S.; Moosajee, M.; Dubis, A.M. Cellular imaging of inherited retinal diseases using adaptive optics. Eye 2019, 33, 1683–1698. [Google Scholar] [CrossRef]
- Bedggood, P.; Metha, A. Adaptive optics imaging of the retinal microvasculature. Clin. Exp. Optom. 2020, 103, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.; Guirao, A.; Cox, I.G.; Williams, D.R. Monochromatic aberrations of the human eye in a large population. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2001, 18, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Bille, J.F. High Resolution Imaging in Microscopy and Ophthalmology; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Akyol, E.; Hagag, A.M.; Sivaprasad, S.; Lotery, A.J. Adaptive optics: Principles and applications in ophthalmology. Eye 2021, 35, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Kozak, I. Retinal imaging using adaptive optics technology. Saudi J. Ophthalmol. 2014, 28, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Hampson, K.M.; Turcotte, R.; Miller, D.T.; Kurokawa, K.; Males, J.R.; Ji, N.; Booth, M.J. Adaptive optics for high-resolution imaging. Nat. Rev. Methods Primers 2021, 1, 68. [Google Scholar] [CrossRef]
- Roorda, A. Adaptive optics for studying visual function: A comprehensive review. J. Vis. 2011, 11, 6. [Google Scholar] [CrossRef]
- Hofer, H.; Sredar, N.; Queener, H.; Li, C.; Porter, J. Wavefront sensorless adaptive optics ophthalmoscopy in the human eye. Opt. Express 2011, 19, 14160–14171. [Google Scholar] [CrossRef]
- Wen, L.; Yang, P.; Wang, S.; Liu, W.; Chen, S.; Xu, B. A high speed model-based approach for wavefront sensorless adaptive optics systems. Opt. Laser Technol. 2018, 99, 124–132. [Google Scholar]
- Wong, K.S.K.; Jian, Y.F.; Cua, M.; Bonora, S.; Zawadzki, R.J.; Sarunic, M.V. In vivo imaging of human photoreceptor mosaic with wavefront sensorless adaptive optics optical coherence tomography. Biomed. Opt. Express 2015, 6, 580–590. [Google Scholar] [CrossRef]
- Polans, J.; Keller, B.; Carrasco-Zevallos, O.M.; LaRocca, F.; Cole, E.; Whitson, H.E.; Lad, E.M.; Farsiu, S.; Izatt, J.A. Wide-field retinal optical coherence tomography with wavefront sensorless adaptive optics for enhanced imaging of targeted regions. Biomed. Opt. Express 2017, 8, 16–37. [Google Scholar] [CrossRef]
- Zhou, X.L.; Bedggood, P.; Bui, B.; Nguyen, C.T.O.; He, Z.; Metha, A. Contrast-based sensorless adaptive optics for retinal imaging. Biomed. Opt. Express 2015, 6, 3577–3595. [Google Scholar] [CrossRef]
- Adie, S.G.; Graf, B.W.; Ahmad, A.; Carney, P.S.; Boppart, S.A. Computational adaptive optics for broadband optical interferometric tomography of biological tissue. Proc. Natl. Acad. Sci. USA 2012, 109, 7175–7180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Z.; Shemonski, N.D.; Adie, S.G.; Ahmad, A.; Bower, A.J.; Carney, P.S.; Boppart, S.A. Computed optical interferometric tomography for high-speed volumetric cellular imaging. Biomed. Opt. Express 2014, 5, 2988–3000. [Google Scholar] [CrossRef] [PubMed]
- South, F.A.; Liu, Y.-Z.; Bower, A.J.; Xu, Y.; Carney, P.S.; Boppart, S.A. Wavefront measurement using computational adaptive optics. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2018, 35, 466–473. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; South, F.A.; Xu, Y.; Carney, P.S.; Boppart, S.A. Computational optical coherence tomography [invited]. Biomed. Opt. Express 2017, 8, 1549–1574. [Google Scholar] [CrossRef] [PubMed]
- Booth, M.J. Adaptive optics in microscopy. Philos. Transact. A Math. Phys. Eng. Sci. 2007, 365, 2829–2843. [Google Scholar] [CrossRef] [PubMed]
- Zommer, S.; Ribak, E.N.; Lipson, S.G.; Adler, J. Simulated annealing in ocular adaptive optics. Opt. Lett. 2006, 31, 939–941. [Google Scholar] [CrossRef]
- Vorontsov, M.A. Decoupled stochastic parallel gradient descent optimization for adaptive optics: Integrated approach for wave-front sensor information fusion. J. Opt. Soc. Am. A 2002, 19, 356–368. [Google Scholar] [CrossRef]
- Huang, L.; Rao, C. Wavefront sensorless adaptive optics: A general model-based approach. Opt. Express 2011, 19, 371–379. [Google Scholar]
- Liu, Y.; Ma, J.; Li, B.; Chu, J. Hill-climbing algorithm based on Zernike modes for wavefront sensorless adaptive optics. Opt. Eng. 2013, 52, 016601. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Wang, M.; Jia, J. Wavefront sensorless adaptive optics based on the trust region method. Opt. Lett. 2015, 40, 1235–1237. [Google Scholar] [CrossRef]
- Jian, Y.; Lee, S.; Ju, M.; Heisler, M.; Ding, W.; Zawadzki, R.; Bonora, S.; Sarunic, M. Lens-based wavefront sensorless adaptive optics swept source OCT. Sci. Rep. 2015, 6, 27620. [Google Scholar] [CrossRef] [PubMed]
- Camino, A.; Ng, R.; Huang, J.; Guo, Y.; Ni, S.; Jia, Y.; Huang, D.; Jian, Y. Depth-resolved optimization of real-time sensorless adaptive optics optical coherence tomography. Opt. Lett. 2020, 45, 2612–2615. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, H.R.G.W.; Heisler, M.; Ju, M.J.; Wahl, D.; Bliek, L.; Kalkman, J.; Bonora, S.; Jian, Y.; Verhaegen, M.; Sarunic, M.V. Wavefront sensorless adaptive optics OCT with the DONE algorithm for in vivo human retinal imaging. Biomed. Opt. Express 2017, 8, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, P.; Hu, K.; Xu, B.; Li, H. Deep learning control model for adaptive optics systems. Appl. Opt. 2019, 58, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Xu, B.; Xu, Z.; Wen, L.; Yang, P.; Wang, S.; Dong, L. Self-learning control for wavefront sensorless adaptive optics system through deep reinforcement learning. Optik 2019, 178, 785–793. [Google Scholar]
- Durech, E.; Newberry, W.; Franke, J.; Sarunic, M. Wavefront sensor-less adaptive optics using deep reinforcement learning. Biomed. Opt. Express 2021, 12, 5423–5438. [Google Scholar] [CrossRef] [PubMed]
- Cunefare, D.; Langlo, C.S.; Patterson, E.J.; Blau, S.; Dubra, A.; Carroll, J.; Farsiu, S. Deep learning based detection of cone photoreceptors with multimodal adaptive optics scanning light ophthalmoscope images of achromatopsia. Biomed. Opt. Express 2018, 9, 3740–3756. [Google Scholar] [CrossRef]
- Cunefare, D.; Huckenpahler, A.L.; Patterson, E.J.; Dubra, A.; Farsiu, S. RAC-CNN: Multimodal deep learning based automatic detection and classification of rod and cone photoreceptors in adaptive optics scanning light ophthalmoscope images. Biomed. Opt. Express 2019, 10, 3815–3832. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, R.; Žurauskas, M.; Pande, P.; Bi, J.; Yuan, Q.; Wang, L.; Gao, Z.; Boppart, S.A. Automated fast computational adaptive optics for optical coherence tomography based on a stochastic parallel gradient descent algorithm. Opt. Express 2020, 28, 23306–23319. [Google Scholar] [CrossRef]
- Liang, J.; Williams, D.R.; Miller, D.T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A 1997, 14, 2884–2892. [Google Scholar] [CrossRef]
- Chew, A.L.; Sampson, D.M.; Kashani, I.; Chen, F.K. Agreement in cone density derived from gaze-directed single images versus wide-field montage using adaptive optics flood illumination ophthalmoscopy. Transl. Vis. Sci. Technol. 2017, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Gale, M.J.; Fay, J.D.; Faridi, A.; Titus, H.E.; Garg, A.K.; Michaels, K.V.; Erker, L.R.; Peters, D.; Smith, T.B.; et al. Assessment of different sampling methods for measuring and representing macular cone density using flood-illuminated adaptive optics. Investig. Ophth. Vis. Sci. 2015, 56, 5751–5763. [Google Scholar] [CrossRef] [PubMed]
- Rha, J.; Jonnal, R.S.; Thorn, K.E.; Qu, J.; Zhang, Y.; Miller, D.T. Adaptive optics flood-illumination camera for high speed retinal imaging. Opt. Express 2006, 14, 4552–4569. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nakazawa, N.; Bessho, K.; Kitaguchi, Y.; Maeda, N.; Fujikado, T.; Mihashi, T. Adaptive optics fundus camera using a liquid crystal phase modulator. Opt. Rev. 2008, 15, 173–180. [Google Scholar] [CrossRef]
- Lombardo, M.; Serrao, S.; Ducoli, P.; Lombardo, G. Variations in image optical quality of the eye and the sampling limit of resolution of the cone mosaic with axial length in young adults. J. Cataract. Refr. Surg. 2012, 38, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Nakamura, T.; Otsuka, M.; Miyakoshi, A.; Oiwake, T.; Ueda, T. Observation of microcystic changes in the inner retina with adaptive optics fundus camera. Investig. Ophth. Vis. Sci. 2014, 55, 2608. [Google Scholar]
- Gocho, K.; Akeo, K.; Kameya, S.; Yamaki, K.; Mizota, A.; Takahashi, H. The improvement of Spoke-Wheel pattern foveoschisis in a patient with X-linked retinoschisis treated with topical dorzolamide observed by high-resolution adaptive optics camera. Acta Ophthalmol. 2015, 93, ABS15-0537. [Google Scholar] [CrossRef]
- Soliman, M.K.; Sadiq, M.A.; Agarwal, A.; Sarwar, S.; Hassan, M.; Hanout, M.; Graf, F.; High, R.; Do, D.V.; Nguyen, Q.D.; et al. High-resolution imaging of parafoveal cones in different stages of diabetic retinopathy using adaptive optics fundus camera. PLoS ONE 2016, 11, e0152788. [Google Scholar] [CrossRef]
- Legras, R.; Gaudric, A.; Woog, K. Distribution of cone density, spacing and arrangement in adult healthy retinas with adaptive optics flood illumination. PLoS ONE 2018, 13, e0191141. [Google Scholar] [CrossRef] [PubMed]
- Markan, A.; Chawla, R.; Gupta, V.; Tripathi, M.; Sharma, A.; Kumar, A. Photoreceptor evaluation after successful macular hole closure: An adaptive optics study. Ther. Adv. Ophthalmol. 2019, 11, 251–258. [Google Scholar] [CrossRef]
- Nakamura, T.; Hayashi, A.; Oiwake, T. Long-term changes of retinal pigment epithelium in the eyes with Vogt-Koyanagi-Harada disease observed by adaptive optics imaging. Clin. Ophthalmol. 2019, 13, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Ueda-Consolvo, T.; Ozaki, H.; Nakamura, T.; Oiwake, T.; Hayashi, A. The association between cone density and visual function in the macula of patients with retinitis pigmentosa. Graef. Arch. Clin. Exp. 2019, 257, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Potic, J.; Bergin, C.; Giacuzzo, C.; Daruich, A.; Pournaras, J.A.; Kowalczuk, L.; Behar-Cohen, F.; Konstantinidis, L.; Wolfensberger, T.J. Changes in visual acuity and photoreceptor density using adaptive optics after retinal detachment repair. Retina 2020, 40, 376–386. [Google Scholar] [CrossRef]
- Ochinciuc, R.; Ochinciuc, U.; Stanca, H.T.; Barac, R.; Darabus, D.; Suta, M.; Balta, F.; Burcea, M. Photoreceptor assessment in focal laser-treated central serous chorioretinopathy using adaptive optics and fundus autofluorescence. Medicine 2020, 99, 195–236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Ciuffreda, K.J.; Jiang, H.; Zhou, K.; Lin, S.; Zheng, J.; Yu, X.; Vasudevan, B.; Liang, Y. Cone parameters in different vision levels from the adaptive optics imaging. Medicine 2021, 100, e25618. [Google Scholar] [CrossRef] [PubMed]
- Baltă, F.; Cristescu, I.-E.; Mirescu, A.-E.; Baltă, G.; Zemba, M.; Tofolean, I.T. Investigation of retinal microcirculation in diabetic patients using adaptive optics ophthalmoscopy and optical coherence angiography. J. Diabetes Res. 2022, 2022, 1516668. [Google Scholar] [CrossRef]
- Roorda, A.; Williams, D. The arrangement of the three cone classes in the living human eye. Nature 1999, 397, 520–522. [Google Scholar] [CrossRef]
- Wagner-Schuman, M.; Neitz, J.; Rha, J.; Williams, D.R.; Neitz, M.; Carroll, J. Color-deficient cone mosaics associated with Xq28 opsin mutations: A stop codon versus gene deletions. Vis. Res. 2010, 50, 2396–2402. [Google Scholar] [CrossRef]
- Koch, E.; Rosenbaum, D.; Brolly, A.; Sahel, J.A.; Chaumet-Riffaud, P.; Girerd, X.; Rossant, F.; Paques, M. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: Relationship with blood pressure and focal vascular changes. J. Hypertens. 2014, 32, 890–898. [Google Scholar] [CrossRef]
- Webb, R.H.; Hughes, G.W.; Pomerantzeff, O. Flying spot TV ophthalmoscope. Appl. Opt. 1980, 19, 2991–2997. [Google Scholar] [CrossRef]
- Webb, R.H.; Hughes, G.W. Scanning laser ophthalmoscope. IEEE Trans. Biomed. Eng. 1981, 28, 4884492. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.H.; Hughes, G.W.; Delori, F.C. Confocal scanning laser ophthalmoscope. Appl. Opt. 1987, 26, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Roorda, A. Applications of adaptive optics scanning laser ophthalmoscopy. Optom. Vis. Sci. 2010, 87, 260–268. [Google Scholar] [CrossRef]
- Dreher, A.W.; Bille, J.F.; Weinreb, R.N. Active optical depth resolution improvement of the laser tomographic scanner. Appl. Opt. 1989, 28, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Roorda, A.; Romero-Borja, F.; Donnelly, W., III; Queener, H.; Hebert, T.J.; Campbell, M.C.W. Adaptive optics scanning laser ophthalmoscopy. Opt. Express 2002, 10, 405–412. [Google Scholar] [CrossRef]
- Takayama, K.; Ooto, S.; Hangai, M.; Arakawa, N.; Oshima, S.; Shibata, N.; Hanebuchi, M.; Inoue, T.; Yoshimura, N. High-resolution imaging of the retinal nerve fiber layer in normal eyes using adaptive optics scanning laser ophthalmoscopy. PLoS ONE 2012, 7, e33158. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, K.; Roorda, A.; Romero-Borja, F. Theoretical modeling and evaluation of the axial resolution of the adaptive optics scanning laser ophthalmoscope. J. Biomed. Opt. 2004, 9, 132–138. [Google Scholar] [CrossRef]
- Gómez-Vieyra, A.; Dubra, A.; Malacara-Hernández, D.; Williams, D.R. First-order design of off-axis reflective ophthalmic adaptive optics systems using afocal telescopes. Opt. Express 2009, 17, 18906–18919. [Google Scholar] [CrossRef]
- Bedggood, P.; Daaboul, M.; Ashman, R.; Smith, G.; Metha, A. Characteristics of the human isoplanatic patch and implications for adaptive optics retinal imaging. J. Biomed. Opt. 2008, 3, 024008. [Google Scholar] [CrossRef]
- Ferguson, R.D.; Zhong, Z.; Hammer, D.X.; Mujat, M.; Patel, A.H.; Deng, C.; Zou, W.; Burns, S.A. Adaptive optics scanning laser ophthalmoscope with integrated wide-field retinal imaging and tracking. J. Opt. Soc. Am. A 2010, 27, 265–277. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Nozato, K.; Saito, K.; Williams, D.R.; Roorda, A.; Rossi, E.A. Closed-loop optical stabilization and digital image registration in adaptive optics scanning light ophthalmoscopy. Biomed. Opt. Express 2014, 5, 3174–3191. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Q.; Saito, K.; Nozato, K.; Williams, D.R.; Rossi, E.A. An adaptive optics imaging system designed for clinical use. Biomed. Opt. Express 2015, 6, 2120–2137. [Google Scholar] [CrossRef]
- Sheehy, C.K.; Tiruveedhula, P.; Sabesan, R.; Roorda, A. Active eye-tracking for an adaptive optics scanning laser ophthalmoscope. Biomed. Opt. Express 2015, 6, 2412–2423. [Google Scholar] [CrossRef]
- Chui, T.Y.P.; VanNasdale, D.A.; Burns, S.A. The use of forward scatter to improve retinal vascular imaging with an adaptive optics scanning laser ophthalmoscope. Biomed. Opt. Express 2012, 3, 2537–2549. [Google Scholar] [CrossRef]
- Scoles, D.; Sulai, Y.N.; Dubra, A. In vivo dark-field imaging of the retinal pigment epithelium cell mosaic. Biomed. Opt. Express 2013, 4, 1710–1723. [Google Scholar] [CrossRef]
- Scoles, D.; Sulai, Y.N.; Langlo, C.S.; Fishman, G.A.; Curcio, C.A.; Carroll, J.; Dubra, A. In vivo imaging of human cone photoreceptor inner segments. Investig. Ophth. Vis. Sci. 2014, 55, 4244–4251. [Google Scholar] [CrossRef]
- Rossi, E.A.; Granger, C.E.; Sharma, R.; Yang, Q.; Saito, K.; Schwarz, C.; Walters, S.; Nozato, K.; Zhang, J.; Kawakami, T.; et al. Imaging individual neurons in the retinal ganglion cell layer of the living eye. Proc. Natl. Acad. Sci. USA 2017, 114, 586–591. [Google Scholar] [CrossRef]
- Sapoznik, K.A.; Luo, T.; Castro, A.; Sawides, L.; Warner, R.L.; Burns, S.A. Enhanced retinal vasculature imaging with a rapidly configurable aperture. Biomed. Opt. Express 2018, 9, 1323–1333. [Google Scholar] [CrossRef]
- Litts, K.M.; Cooper, R.F.; Duncan, J.L.; Carroll, J. Photoreceptor-based biomarkers in AOSLO retinal imaging. Investig. Ophth. Vis. Sci. 2017, 58, BIO255–BIO267. [Google Scholar] [CrossRef]
- Sun, L.W.; Johnson, R.D.; Langlo, C.S.; Cooper, R.F.; Razeen, M.M.; Russillo, M.C.; Dubra, A.; Connor, T.B., Jr.; Han, D.P.; Pennesi, M.E.; et al. Assessing photoreceptor structure in retinitis pigmentosa and Usher syndrome. Investig. Ophth. Vis. Sci. 2016, 57, 2428–2442. [Google Scholar] [CrossRef]
- Sun, L.W.; Carroll, J.; Lujan, B.J. Photoreceptor disruption and vision loss associated with central serous retinopathy. Am. J. Ophthalmol. Case Rep. 2017, 8, 74–77. [Google Scholar] [CrossRef]
- Sajdak, B.S.; Salmon, A.E.; Cava, J.A.; Allen, K.P.; Freling, S.; Ramamirtham, R.; Norton, T.T.; Roorda, A.; Carroll, J. Noninvasive imaging of the tree shrew eye: Wavefront analysis and retinal imaging with correlative histology. Exp. Eye Res. 2019, 185, 107683. [Google Scholar] [CrossRef]
- Sredar, N.; Razeen, M.; Kowalski, B.; Carroll, J.; Dubra, A. Comparison of confocal and non-confocal split-detection cone photoreceptor imaging. Biomed. Opt. Express 2021, 12, 737–755. [Google Scholar] [CrossRef]
- DuBose, T.; Nankivil, D.; LaRocca, F.; Waterman, G.; Hagan, K.; Polans, J.; Keller, B.; Tran-Viet, D.; Vajzovic, L.; Kuo, A.N.; et al. Handheld adaptive optics scanning laser ophthalmoscope. Optica 2018, 5, 1027–1036. [Google Scholar] [CrossRef]
- Hagan, K.; DuBose, T.; Cunefare, D.; Waterman, G.; Park, J.; Simmerer, C.; Kuo, A.N.; McNabb, R.P.; Izatt, J.A.; Farsiu, S. Multimodal handheld adaptive optics scanning laser ophthalmoscope. Opt. Lett. 2020, 45, 4940–4943. [Google Scholar] [CrossRef]
- Li, H.; Lu, J.; Shi, G.; Zhang, Y. Tracking features in retinal images of adaptive optics confocal scanning laser ophthalmoscope using KLT-SIFT algorithm. Biomed. Opt. Express 2010, 1, 10–15. [Google Scholar] [CrossRef]
- Chen, G.; Huo, B.; Tong, F.; Zhu, B. AOSLO Video Image Stabilization Algorithm Based on Harris-sift. J. Simul. 2015, 3, 105–107. [Google Scholar]
- Salmon, A.E.; Cooper, R.F.; Langlo, C.S.; Baghaie, A.; Dubra, A.; Carroll, J. An automated reference frame selection (ARFS) algorithm for cone imaging with adaptive optics scanning light ophthalmoscopy. Transl. Vis. Sci. Technol. 2017, 6, 9. [Google Scholar] [CrossRef]
- Dubra, A.; Harvey, Z. Registration of 2D Images from Fast Scanning Ophthalmic Instruments. Biomed. Image Regist. 2010, 6024, 60–71. [Google Scholar]
- Chen, H.; He, Y.; Wei, L.; Li, X.; Zhang, Y. Automatic dewarping of retina images in adaptive optics confocal scanning laser ophthalmoscope. IEEE Access 2019, 7, 59585–59599. [Google Scholar] [CrossRef]
- Chen, M.; Cooper, R.F.; Han, G.K.; Gee, J.; Brainard, D.H.; Morgan, J.I.W. Multi-modal automatic montaging of adaptive optics retinal images. Biomed. Opt. Express 2016, 7, 4899–4918. [Google Scholar] [CrossRef]
- Cunefare, D.; Cooper, R.F.; Higgins, B.; Katz, D.F.; Dubra, A.; Carroll, J.; Farsiu, S. Automatic detection of cone photoreceptors in split detector adaptive optics scanning light ophthalmoscope images. Biomed. Opt. Express 2016, 7, 2036–2050. [Google Scholar] [CrossRef]
- Davidson, B.; Kalitzeos, A.; Carroll, J.; Dubra, A.; Ourselin, S.; Michaelides, M.; Bergeles, C. Automatic cone photoreceptor localisation in healthy and stargardt afflicted retinas using deep learning. Sci. Rep. 2018, 8, 7911. [Google Scholar] [CrossRef]
- Young, L.K.; Smithson, H.E. Emulated retinal image capture (ERICA) to test, train and validate processing of retinal images. Sci. Rep. 2021, 11, 11225. [Google Scholar]
- Dubra, A.; Sulai, Y.; Norris, J.L.; Cooper, R.F.; Dubis, A.M.; Williams, D.R.; Carroll, J. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed. Opt. Express 2011, 2, 1864–1876. [Google Scholar] [CrossRef]
- Duncan, J.L.; Zhang, Y.; Gandhi, J.; Nakanishi, C.; Othman, M.; Branham, K.E.H.; Swaroop, A.; Roorda, A. High-resolution imaging with adaptive optics in patients with inherited retinal degeneration. Investig. Ophth Vis. Sci. 2007, 48, 3283–3291. [Google Scholar] [CrossRef]
- Merino, D.; Duncan, J.L.; Tiruveedhula, P.; Roorda, A. Observation of cone and rod photoreceptors in normal subjects and patients using a new generation adaptive optics scanning laser ophthalmoscope. Biomed. Opt. Express 2011, 2, 2189–2201. [Google Scholar]
- Morgan, J.I.W.; Hunter, J.J.; Masella, B.; Wolfe, R.; Gray, D.C.; Merigan, W.H.; Delori, F.C.; Williams, D.R. Light-induced retinal changes observed with high-resolution autofluorescence imaging of the retinal pigment epithelium. Investig. Ophth. Vis. Sci. 2008, 49, 3715–3729. [Google Scholar] [CrossRef]
- Tam, J.; Roorda, A. Speed quantification and tracking of moving objects in adaptive optics scanning laser ophthalmoscopy. J. Biomed. Opt. 2011, 16, 036002. [Google Scholar] [CrossRef]
- Arthur, E.; Elsner, A.E.; Sapoznik, K.A.; Papay, J.A.; Muller, M.S.; Burns, S.A. Distances from capillaries to arterioles or venules measured using OCTA and AOSLO. Investig. Ophth. Vis. Sci. 2019, 60, 1833–1844. [Google Scholar] [CrossRef]
- Gofas-Salas, E.; Rui, Y.; Mecê, P.; Zhang, M.; Snyder, V.C.; Vienola, K.V.; Lee, D.M.W.; Sahel, J.A.; Grieve, K.; Rossi, E.A. Design of a radial multi-offset detection pattern for in vivo phase contrast imaging of the inner retina in humans. Biomed. Opt. Express 2021, 13, 117–132. [Google Scholar] [CrossRef]
- Geng, Y.; Dubra, A.; Yin, L. Adaptive optics retinal imaging in the living mouse eye. Biomed. Opt. Express 2012, 3, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Rivero, E.B.; Clark, M.E.; Witherspoon, C.D.; Spaide, R.F.; Girkin, C.A.; Owsley, C.; Curcio, C.A. Photoreceptor perturbation around subretinal drusenoid deposits revealed by adaptive optics scanning laser ophthalmoscopy. Am. J. Ophthalmol. 2014, 158, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Meadway, A.; Wang, X.; Curcio, C.A.; Zhang, Y. Microstructure of subretinal drusenoid deposits revealed by adaptive optics imaging. Biomed. Opt. Express 2014, 5, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Chui, T.Y.P.; Dubow, M.; Pinhas, A.; Shah, N.; Gan, A.; Weitz, R.; Sulai, Y.N.; Dubra, A.; Rosen, R.B. Comparison of adaptive optics scanning light ophthalmoscopic fluorescein angiography and offset pinhole imaging. Biomed. Opt. Express 2014, 5, 1173–1189. [Google Scholar] [CrossRef] [PubMed]
- Makiyama, Y.; Ooto, S.; Hangai, M.; Takayama, K.; Uji, A.; Oishi, A.; Ogino, K.; Nakagawa, S.; Yoshimura, N. Macular cone abnormalities in retinitis pigmentosa with preserved central vision using adaptive optics scanning laser ophthalmoscopy. PLoS ONE 2013, 8, e79447. [Google Scholar] [CrossRef]
- Nakatake, S.; Murakami, Y.; Funatsu, J.; Koyanagi, Y.; Akiyama, M.; Momozawa, Y.; Ishibashi, T.; Sonoda, K.H.; Ikeda, Y. Early detection of cone photoreceptor cell loss in retinitis pigmentosa using adaptive optics scanning laser ophthalmoscopy. Graef. Arch. Clin. Exp. Ophthalmol. 2019, 257, 1169–1181. [Google Scholar] [CrossRef]
- Vilupuru, A.S.; Rangaswamy, N.V.; Frishman, L.J.; Smith, E.L., III; Harwerth, R.S.; Roorda, A. Adaptive optics scanning laser ophthalmoscopy for in vivo imaging of lamina cribrosa. J. Opt. Soc. Am. A 2007, 24, 1417–1425. [Google Scholar] [CrossRef]
- Akagi, T.; Hangai, M.; Takayama, K.; Nonaka, A.; Ooto, S.; Yoshimura, N. In vivo imaging of lamina cribrosa pores by adaptive optics scanning laser ophthalmoscopy. Investig. Ophth. Vis. Sci. 2012, 53, 4111–4119. [Google Scholar] [CrossRef]
- Ooto, S.; Hangai, M.; Takayama, K.; Arakawa, N.; Tsujikawa, A.; Koizumi, H.; Oshima, S.; Yoshimura, N. High-resolution photoreceptor imaging in idiopathic macular telangiectasia type 2 using adaptive optics scanning laser ophthalmoscopy. Investig. Ophth. Vis. Sci. 2011, 52, 5541–5550. [Google Scholar] [CrossRef]
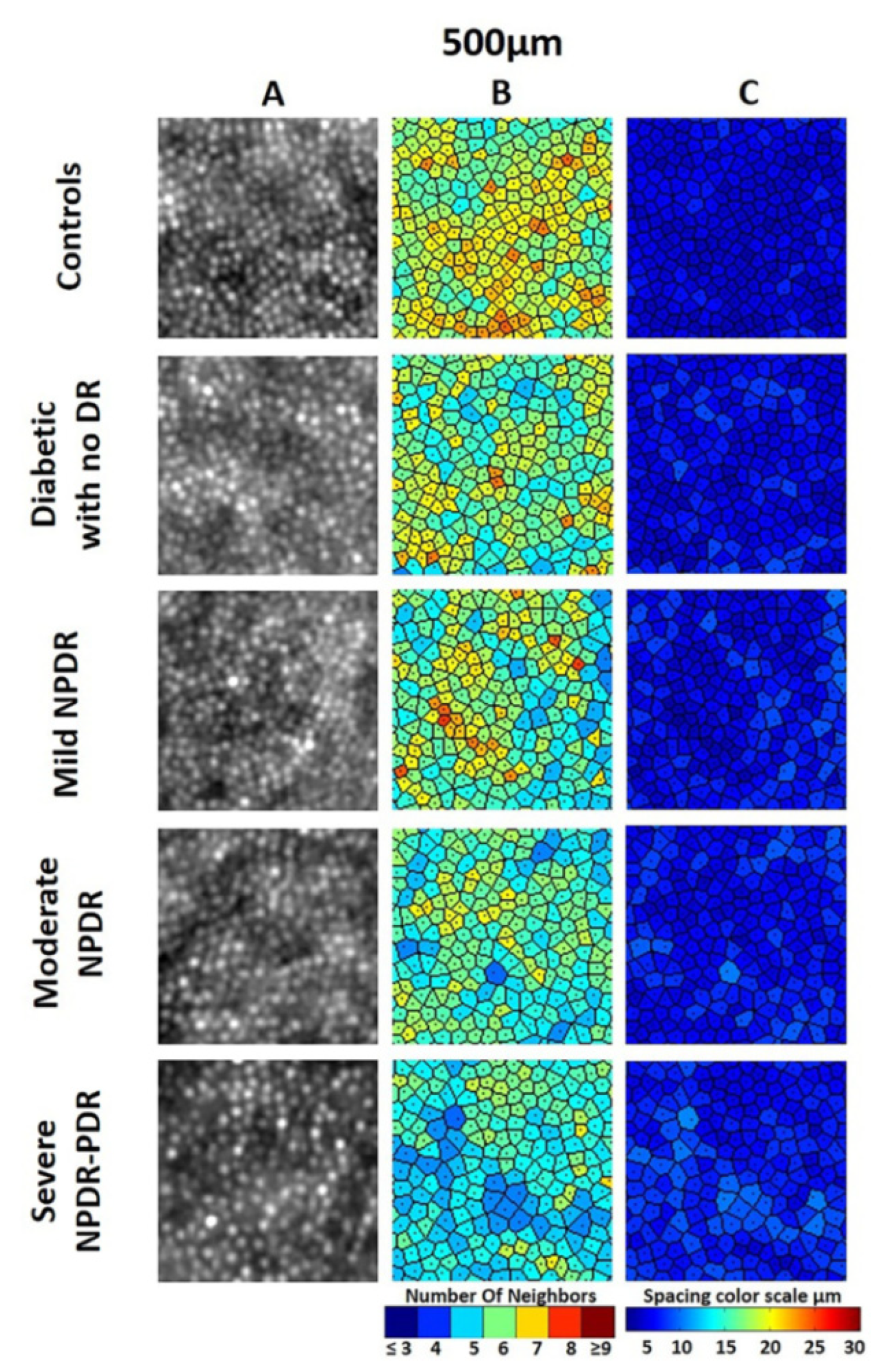
- Arichika, S.; Uji, A.; Murakami, T.; Unoki, N.; Yoshitake, S.; Dodo, Y.; Ooto, S.; Miyamoto, K.; Yoshimura, N. Retinal hemorheologic characterization of early-stage diabetic retinopathy using adaptive optics scanning laser ophthalmoscopy. Investig. Ophth. Vis. Sci. 2014, 55, 8513–8522. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.A.; Elsner, A.E.; Chui, T.Y.; VanNasdale, D.A.; Christopher, A.C.; Gast, T.J.; Malinovsky, V.E.; Phan, A.D.T. In vivo adaptive optics microvascular imaging in diabetic patients without clinically severe diabetic retinopathy. Biomed. Opt. Express 2014, 5, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Arichika, S.; Uji, A.; Ooto, S.; Muraoka, Y.; Yoshimura, N. Effects of age and blood pressure on the retinal arterial wall, analyzed using adaptive optics scanning laser ophthalmoscopy. Sci. Rep. 2015, 5, 12283. [Google Scholar] [CrossRef] [PubMed]
- Mkrtchyan, M.; Duncan, J.L.; Lujan, B.J.; Roorda, A.; Merino, D.; Thirkill, C.E. Outer retinal structure in patients with acute zonal occult outer retinopathy. Int. J. Ophthalmol. 2012, 153, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Williams, Z.R.; Rossi, E.A.; DiLoreto, D.A. In vivo adaptive optics ophthalmoscopy correlated with histopathologic results in cancer-associated retinopathy. Ophthalmol. Retin. 2018, 2, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowski, M.; Leitgeb, R.; Kowalczyk, A.; Bajraszewski, T.; Fercher, A.F. In vivo human retinal imaging by Fourier domain optical coherence tomography. J. Biomed. Opt. 2002, 7, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Duguid, G. Optical coherence tomography--a review of the principles and contemporary uses in retinal investigation. Eye 2004, 18, 561–570. [Google Scholar] [CrossRef]
- Nassif, N.A.; Cense, B.; Park, B.H.; Pierce, M.C.; Yun, S.H.; Bouma, B.E.; Tearney, G.J.; Chen, T.C.; de Boer, J.F. In vivo high-resolution video-rate spectral-domain optical coherence tomography of the human retina and optic nerve. Opt. Express 2004, 12, 367–376. [Google Scholar] [CrossRef]
- Miller, D.T.; Kurokawa, K. Cellular-scale imaging of transparent retina l structures and processes using adaptive optics optical coherence tomography. Annu. Rev. Vis. Sci. 2020, 6, 115–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Rha, J.; Jonnal, R.S.; Miller, D.T. Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina. Opt. Express 2005, 13, 4792–4811. [Google Scholar] [CrossRef]
- Zawadzki, R.J.; Jones, S.M.; Olivier, S.S.; Zhao, M.; Bower, B.A.; Izatt, J.A.; Choi, S.; Laut, S.; Werner, J.S. Adaptive optics optical coherence tomography for high-resolution and high-speed 3-D retinal in vivo imaging. Opt. Express 2005, 13, 8532–8546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cense, B.; Rha, J.; Jonnal, R.S.; Gao, W.; Zawadzki, R.J.; Werner, J.S.; Jones, S.; Olivier, S.; Miller, D.T. High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography. Opt. Express 2006, 14, 4380–4394. [Google Scholar] [CrossRef] [PubMed]
- Polans, J.; Cunefare, D.; Cole, E.; Keller, B.; Mettu, P.S.; Cousins, S.W.; Allingham, M.J.; Izatt, J.A.; Farsiu, S. Enhanced visualization of peripheral retinal vasculature with wavefront sensorless adaptive optics optical coherence tomography angiography in diabetic patients. Opt. Lett. 2017, 42, 17–20. [Google Scholar] [CrossRef]
- Reddikumar, M.; Tanabe, A.; Hashimoto, N.; Cense, B. Optical coherence tomography with a 2.8-mm beam diameter and sensorless defocus and astigmatism correction. J. Biomed. Opt. 2017, 22, 026005. [Google Scholar] [CrossRef][Green Version]
- Camino, A.; Zang, P.; Athwal, A.; Ni, S.; Jia, Y.; Huang, D.; Jian, Y. Sensorless adaptive-optics optical coherence tomographic angiography. Opt. Express 2020, 11, 3952–3967. [Google Scholar] [CrossRef] [PubMed]
- Shemonski, N.D.; South, F.A.; Liu, Y.-Z.; Adie, S.G.; Carney, P.S.; Boppart, S.A. Computational high resolution optical imaging of the living human retina. Nat. Photonics 2015, 9, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, D.; Spahr, H.; Hain, C.; Sudkamp, H.; Franke, G.; Pfäffle, C.; Winter, C.; Hüttmann, G. Aberration-free volumetric high-speed imaging of in vivo retina. Sci. Rep. 2016, 6, 35209. [Google Scholar] [CrossRef]
- Ginner, L.; Kumar, A.; Fechtig, D.; Wurster, L.M.; Salas, M.; Pircher, M.; Leitgeb, R.A. Noniterative digital aberration correction for cellular resolution retinal optical coherence tomography in vivo. Optica 2017, 4, 924–931. [Google Scholar] [CrossRef]
- South, F.A.; Kurokawa, K.; Liu, Z.; Liu, Y.-Z.; Miller, D.T.; Boppart, S.A. Combined hardware and computational optical wavefront correction. Biomed. Opt. Express 2018, 9, 2562–2574. [Google Scholar] [CrossRef]
- Zhang, F.; Kurokawa, K.; Lassoued, A.; Crowell, J.A.; Miller, D.T. Cone photoreceptor classification in the living human eye from photostimulation-induced phase dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 7951–7956. [Google Scholar] [CrossRef]
- Liu, Z.; Kocaoglu, O.P.; Miller, D.T. 3D Imaging of retinal pigment epithelial cells in the living human retina. Investig. Ophth. Vis. Sci. 2016, 57, OCT533–OCT543. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kurokawa, K.; Hammer, D.X.; Miller, D.T. In vivo measurement of organelle motility in human retinal pigment epithelial cells. Biomed. Opt. Express 2019, 10, 4142–4158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kurokawa, K.; Zhang, F.; Lee, J.J.; Miller, D.T. Imaging and quantifying ganglion cells and other transparent neurons in the living human retina. Proc. Natl. Acad. Sci. USA 2017, 114, 12803–12808. [Google Scholar] [CrossRef] [PubMed]
- Hammer, D.X.; Agrawal, A.; Villanueva, R.; Saeedi, O.; Liu, Z. Label-free adaptive optics imaging of human retinal macrophage distribution and dynamics. Proc. Natl. Acad. Sci. USA 2020, 117, 30661–30669. [Google Scholar] [CrossRef] [PubMed]
- Nadler, Z.; Wang, B.; Wollstein, G.; Nevins, J.E.; Ishikawa, H.; Bilonick, R.; Kagemann, L.; Sigal, I.A.; Ferguson, R.D.; Patel, A.; et al. Repeatability of in vivo 3D lamina cribrosa microarchitecture using adaptive optics spectral domain optical coherence tomography. Biomed. Opt. Express 2014, 5, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Nadler, Z.; Wang, B.; Schuman, J.S.; Ferguson, R.D.; Patel, A.; Hammer, D.X.; Bilonick, R.A.; Ishikawa, H.; Kagemann, L.; Sigal, I.A. In vivo three-dimensional characterization of the healthy human lamina cribrosa with adaptive optics spectral-domain optical coherence tomography. Investig. Ophth. Vis. Sci. 2014, 55, 6459–6466. [Google Scholar] [CrossRef] [PubMed]
- Cense, B.; Gao, W.; Brown, J.M.; Jones, S.M.; Jonnal, R.S.; Mujat, M.; Park, B.H.; Boer, J.F.; Miller, D.T. Retinal imaging with polarization-sensitive optical coherence tomography and adaptive optics. Opt. Express 2009, 17, 21634–21651. [Google Scholar] [CrossRef]
- Pandiyan, V.P.; Jiang, X.; Maloney-Bertelli, A.; Kuchenbecker, J.A.; Sharma, U.; Sabesan, R. High-speed adaptive optics line-scan OCT for cellular-resolution optoretinography. Biomed. Opt. Express 2020, 11, 5274–5296. [Google Scholar] [CrossRef]
- Pandiyan, V.P.; Jiang, X.; Kuchenbecker, J.A.; Sabesan, R. Reflective mirror-based line-scan adaptive optics OCT for imaging retinal structure and function. Biomed. Opt. Express 2021, 12, 5865–5880. [Google Scholar] [CrossRef]
- Zawadzki, R.J.; Jones, S.M.; Pilli, S.; Balderas-Mata, S.; Kim, D.Y.; Olivier, S.S.; Werner, J.S. Integrated adaptive optics optical coherence tomography and adaptive optics scanning laser ophthalmoscope system for simultaneous cellular resolution in vivo retinal imaging. Biomed. Opt. express 2011, 2, 1674–1686. [Google Scholar] [CrossRef]
- Mujat, M.; Ferguson, R.D.; Patel, A.H.; Iftimia, N.; Lue, N.; Hammer, D.X. High resolution multimodal clinical ophthalmic imaging system. Opt. Express 2010, 18, 11607–11621. [Google Scholar] [CrossRef] [PubMed]
- Hammer, D.X.; Ferguson, R.D.; Mujat, M.; Patel, A.; Plumb, E.; Iftimia, N.; Chui, T.Y.P.; Akula, J.D.; Fulton, A.B. Multimodal adaptive optics retinal imager: Design and performance. J. Opt. Soc. Am. 2012, 29, 2598–2607. [Google Scholar] [CrossRef] [PubMed]
- Meadway, A.; Girkin, C.A.; Zhang, Y. A dual-modal retinal imaging system with adaptive optics. Opt. Express 2013, 21, 29792–29807. [Google Scholar] [CrossRef] [PubMed]
- Felberer, F.; Kroisamer, J.-S.; Baumann, B.; Zotter, S.; Schmidt-Erfurth, U.; Hitzenberger, C.K.; Pircher, M. Adaptive optics SLO/OCT for 3D imaging of human photoreceptors in vivo. Biomed. Opt. Express 2014, 5, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.; Drexler, W.; Levecq, X.; Lamory, B.; Ritter, M.; Prager, S.; Hafner, J.; Schmidt-Erfurth, U.; Pircher, M. Multi-modal adaptive optics system including fundus photography and optical coherence tomography for the clinical setting. Biomed. Opt. Express 2016, 7, 1783–1796. [Google Scholar] [CrossRef]
- Hafner, J.; Salas, M.; Scholda, C.; Vogl, W.D.; Drexler, W.; Schmidt-Erfurth, U.; Pircher, M.; Karst, S. Dynamic changes of retinal microaneurysms in diabetes imaged with in vivo adaptive optics optical coherence tomography. Investig. Ophth. Vis. Sci. 2018, 59, 5932–5940. [Google Scholar] [CrossRef]
- Karst, S.G.; Salas, M.; Hafner, J.; Scholda, C.; Vogl, W.D.; Drexler, W.; Pircher, M.; Schmidt-Erfurth, U. Three-dimensional analysis of retinal microaneurysms with adaptive optics optical coherence tomography. Retina 2019, 39, 465–472. [Google Scholar] [CrossRef]
- Liu, Z.; Tam, J.; Saeedi, O.; Hammer, D.X. Trans-retinal cellular imaging with multimodal adaptive optics. Biomed. Opt. Express 2018, 9, 4246–4262. [Google Scholar] [CrossRef]
- Wahl, D.J.; Ng, R.; Ju, M.J.; Jian, Y.; Sarunic, M.V. Sensorless adaptive optics multimodal en-face small animal retinal imaging. Biomed. Opt. Express 2019, 10, 252–267. [Google Scholar] [CrossRef]
- Bower, A.J.; Liu, T.; Aguilera, N.; Li, J.; Liu, J.; Lu, R.; Giannini, J.P.; Huryn, L.A.; Dubra, A.; Liu, Z.; et al. Integrating adaptive optics-SLO and OCT for multimodal visualization of the human retinal pigment epithelial mosaic. Biomed. Opt. Express 2021, 12, 1449–1466. [Google Scholar] [CrossRef]

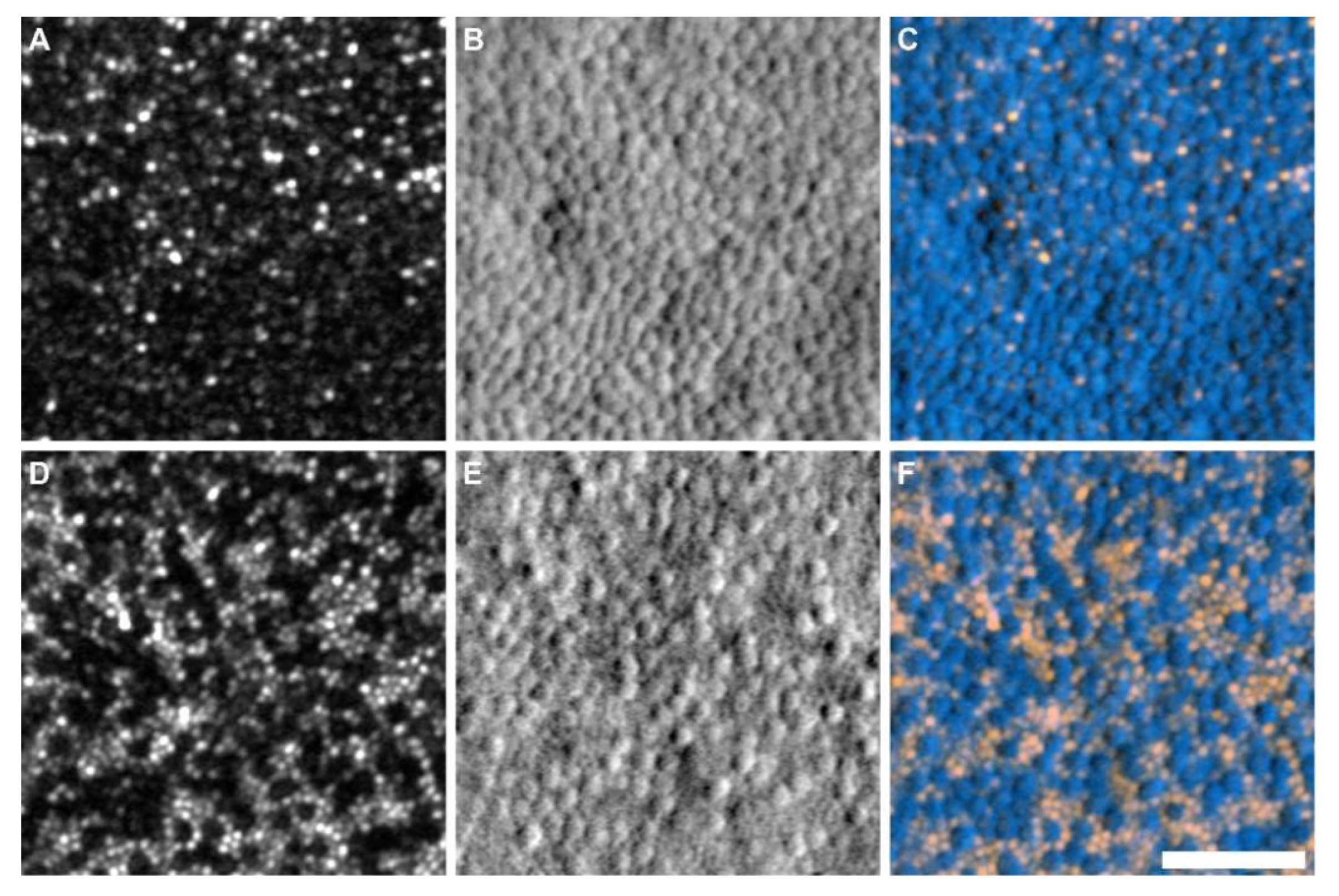

| Year/Authors | Algorithm | Description | Advantages |
|---|---|---|---|
| 2015/Chen et al. | Harris-SIFT [88] | Harris-SIFT algorithm matches feature vectors; random sample consensus (RANSAC) algorithm verifies the matching accuracy to obtain the motion vector estimation; the fixed frame compensation method is used to realize the motion compensation. | The algorithm could effectively eliminate jitter and enhance the contrast of video image. |
| 2017/Salmon et al. | Automated reference frame selection (ARFS) [89] | ARFS comprises two main modules: distortion detection to select the least distorted frame(s) from an image sequence and motion tracking to allow selection of multiple reference frames from distinct spatial locations. | ARFS outperformed expert observers in selecting minimally distorted reference frames in AOSLO image sequences. |
| 2018/Davidson et al. | MultiDimensional recurrent neural network (MDRNN) [94] | A powerful deep learning framework is used for automatic localization of cone photoreceptors in AO-SLO split-detection images. | The approach was demonstrated to be the most robust, most accurate, and appreciably faster algorithm for automatic cone localization in healthy and Stargardt afflicted retinas. |
| 2018/Cunefare et al. | Late fusion dual mode convolutional neural networks (LF-DM-CNN) [38] | A new deep learning-based approach combines information from the confocal and non-confocal AO-SLO models to detect cones in subjects with achromatopsia. | The method outperformed the state-of-the-art automated techniques and is on a par with human grading. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wu, Z.; Qi, M.; Li, Y.; Zhang, M.; Liao, D.; Gao, P. Application of Adaptive Optics in Ophthalmology. Photonics 2022, 9, 288. https://doi.org/10.3390/photonics9050288
Liu L, Wu Z, Qi M, Li Y, Zhang M, Liao D, Gao P. Application of Adaptive Optics in Ophthalmology. Photonics. 2022; 9(5):288. https://doi.org/10.3390/photonics9050288
Chicago/Turabian StyleLiu, Lixin, Zhaoqing Wu, Meijie Qi, Yanru Li, Meiling Zhang, Dingying Liao, and Peng Gao. 2022. "Application of Adaptive Optics in Ophthalmology" Photonics 9, no. 5: 288. https://doi.org/10.3390/photonics9050288
APA StyleLiu, L., Wu, Z., Qi, M., Li, Y., Zhang, M., Liao, D., & Gao, P. (2022). Application of Adaptive Optics in Ophthalmology. Photonics, 9(5), 288. https://doi.org/10.3390/photonics9050288






