1. Introduction
The most common grouse of any conscientious patient is loss of gingival levels that warrants treatment by a practitioner [
1]. Basic etiology of gingival recession has to be ascertained first; only after that can required surgical and non-surgical treatments be initiated to prevent its progression and avert its recurrence [
2]. The extent of gingival recession is higher in males than in females, and is higher in age groups above 30. The prevalence of recession was 37.8% in the younger age group (30 to 39 years), and conversely, the older age group had a prevalence of 90.4% [
3]. Gingival recession usually occurs without any symptoms, but it can cause sensitivity due to exposed root surfaces, predisposal to root caries and undermining of aesthetic profiles when involved in the anterior teeth [
4]. Patients’ aesthetic considerations are a primary requisite for root coverage procedures. Earlier, emphasis was mainly on achieving reduction of recession and increasing keratinized tissue. Recently, however, the goal has shifted towards attaining complete root coverage, owing to increasing aesthetic demands from patients. Multitudes of surgical techniques have been attempted for root coverage, with different clinical outcomes. Among them, the modified coronally advanced flap (MCAF) technique has shown good predictability, with 86.67% root coverage [
3,
4]. The variations included in the MCAF technique may increase success and predictability, which makes this method preferable. Connective tissue grafts, when used with the MCAF technique, enhance probability of complete root coverage. However, the need for a second surgical site could potentially create post-operative pain, bleeding and discomfort caused to both patients and clinicians [
5]. The MCAF technique has been used successfully to treat gingival recession. This approach is associated with a greater probability (87%) of obtaining complete root coverage (CRC) in treatment of localized recession compared to other techniques [
2]. Successful treatment for gingival recession continues to represent serious challenges in obtaining predictable results.
The entrance of LLLT into the evidence-based medical field, especially as treatment, was noted around 30 years ago. In 1971, Mester introduced LLLT and noticed improvement in wound healing via application of a low-energy (1 J/cm
2) ruby laser. The basic principle of LLLT is based on the bio-modulation or bio stimulation effect [
6]. The stimulatory effect of lasers induces changes in the structure, biochemical reactions and functional aspects of the cells. The basic energy of the living system is derived via ATP, and the laser system basically stimulates production of the pigments responsible for it. The biological mechanism behind the accelerated healing seen in laser biostimulation is said to be at a cellular level due to induction of intracellular metabolic alterations. These alterations lead to quicker cell division, proliferation rate, faster migration of fibroblastic cells and increased matrix production [
7]. Different studies have suggested that LLLT facilitates collagen synthesis, fibroblast and keratinocyte cell motility, angiogenesis and growth factor release. Photoactivation effects at the cellular level include early epithelialization, enhanced fibroblastic reactions, ingress of leucocytes and formation of new blood vessels in irradiated wounds [
8]. All of these cellular events hasten closure of wounds. Basic strength of a healing wound increases through maturation of underlying collagen fiber bundles, which improves resiliency of the wound [
9]. Reports of an analgesic effect of LLLT on nerves that supply the oral cavity demonstrate that LLLT decreases firing frequency of nociceptors, with a threshold effect seen in terms of irradiance required to exert maximal suppression [
10]. All types of laser treatment (red, infrared, pulsed and cw) were found to slow conduction in the nerve as well its velocity and also reduce compound action potential of irradiated nerves [
11].
When a diode laser was used to perform the laser-assisted laterally positioned flap technique, the procedure was shown to be more effective in the treatment of isolated Miller’s Class II recession defects than was the MCAF technique alone [
12]. Laser-assisted soft-tissue surgeries also shorten duration of treatment and provide very little bleeding at the operative site; the least trauma; improved surgical accuracy; and very minimal post-operative sequelae such as swelling, scarring and persisting pain.
Keeping all of these potential benefits in mind, a randomized, controlled split-mouth clinical trial was planned to compare the clinical outcome of the MCAF technique alone and that of the MCAF technique plus LLLT in order to assess effects of LLLT on clinical outcomes after MCAF operations in management of isolated gingival recession.
2. Materials and Methods
This study is a prospective randomized, controlled split-mouth and single-blinded clinical trial that compared the MCAF technique with a combination of the MCAF technique and LLLT after 6 months of surgery. Patients who visited the outpatient department and satisfied inclusion and exclusion criteria were selected for this study. Fifteen patients, each with one or two teeth displaying Miller’s Class I or Class II recession caused by traumatic tooth brushing on both sides of either the maxillary or mandibular arch, were included. Inclusion criteria were (1) age between 20–55 years; (2) Miller’s Class I or II recession in the maxillary or mandibular arch, with at least two buccal-adjacent recession defects; (3) gingival recession due to traumatic tooth brushing; (4) a full-mouth plaque score of ≤15%; (5) thick gingival phenotypes of ≥1.2 mm; and (6) willingness to participate in this study. Exclusion criteria were (1) having undergone any type of regenerative periodontal therapy six months prior to the preliminary examination, (2) being a smoker, (3) being systemically compromised, (4) being pregnant and/or a lactating woman, and (5) teeth with hopeless prognosis.
Each patient was treated with an initial phase of scaling and root planning, followed by individually tailored oral-hygiene instructions and brushing techniques for maintenance. Two weeks after completion of the initial phase of therapy, clinical parameters were recorded using a standardized acrylic occlusal stent with a UNC-15 probe. Gingival recession depth, gingival recession width, width of keratinized tissue, periodontal probing depth and wound healing index were assessed. A visual analogue scale was utilized to assess the outcome in patient-reported measure of pain. Patients were verbally informed, and the surgical procedure was explained in detail. Each and every patient was asked to sign written informed consent.
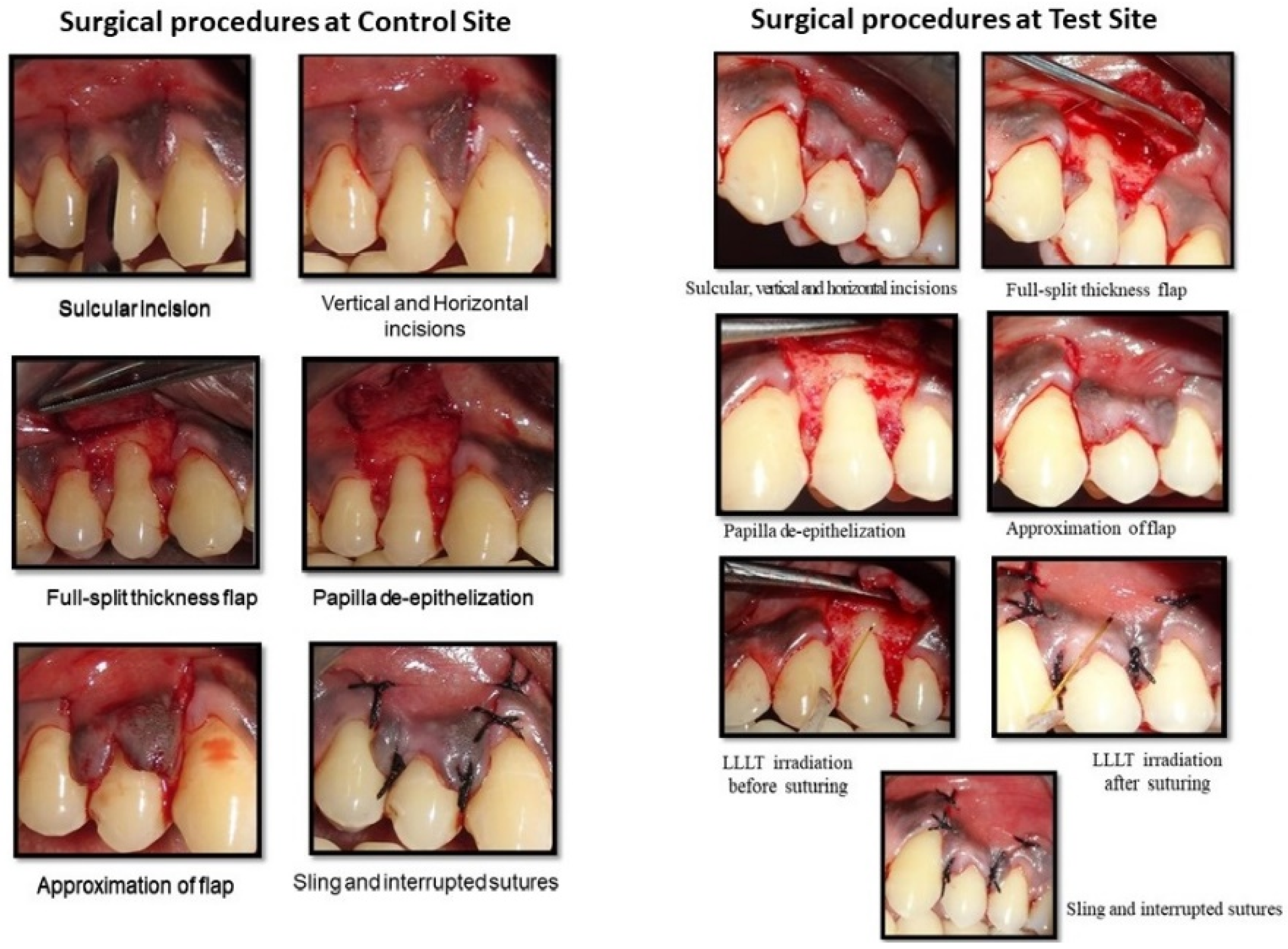
In the enrolled cases, randomization was carried out using a coin-toss method and assigned to test and control groups accordingly. In the control group, defects were treated with the modified coronally advanced flap procedure (MCAF), whereas the contralateral side (the test group) was treated with the modified coronally advanced flap procedure (MCAF) and low-level laser therapy (LLLT). The test and control sites each underwent an identical MCAF procedure for root coverage (
Figure 1).
The test group received 5 min of irradiation with a low-level laser to the adjacent surgical area and the inner surface of the mobilized MCAF before closure and immediately after flap closure. Irradiation was repeated on the test site for 5 min daily, for 5 days. Local anesthesia (2% lignocaine with 1:100,000 epinephrine) was administered. An intra-sulcular incision was made using a No.15 BP (Bard Parker) blade at the buccal aspect of the recession tooth and extended 3 mm horizontally in the mesial and distal interdental gingiva. Two divergent, oblique beveled incisions that extended beyond the mucogingival junction were made at the mesial and distal line angles of the most mesial and distal teeth with gingival recessions. The mucosa that lay beyond the root exposure, including even keratinized tissue, was reflected as a mucoperiosteal flap with aid of Molt’s periosteal elevator. This flap was extended about 3–4 mm apical to the bony dehiscence. This surgical step was taken to utilize periosteum and maximize thickness of soft tissues in the middle portion of the flap, eventually covering the avascular root surface. Hand curettes were used to gently debride exposed root surfaces. The most apical portion of the flap was a split-thickness flap to allow for coronal repositioning of the flap without tension.
It was essential that the flap be free of muscle insertions in order to coronally facilitate tension-free advancement. Therefore, with a number 15 blade, all muscle attachments were severed on the external mucosal surface. A sure way to check coronal advancement of a flap is to note whether it crosses the CEJ effortlessly. The flap should be stable in the final coronal position, even without sutures.
Vascularity of connective tissue could be improved through facial de-epithelialization of interdental papilla. To ensure optimum results, coronal displacement was 1–2 mm above the CEJ in both groups. A sling suture was placed to stabilize the flap in a coronal position, followed by interrupted sutures on vertical-releasing incisions in the apico-coronal direction, using Mersilk 4-0 suture material. For photo biomodulation, power was set at the minimal level, i.e., an 810 nm wavelength (diode laser; A.R.C. Laser FOX, Germany) and 120 mW power in a continuous mode for a total of 5 min. The tip was placed in contact mode and at 90 degrees to the operative area (
Table 1). Before suturing, irradiation was carried out on the exposed root surface, the adjacent surgical area and the inner surface of the mobilized flap. The flaps were then repositioned coronally and stabilized with sutures in both the test and control sites.
LLLT irradiation was repeated soon after closure of the flap in each test site for 5 min. Sutures were considered, as margins of each wound area were large. Laser irradiation was performed, with slight contact to tissue, from the margins toward the center of the wound in circular movements. A simulation of laser irradiation was carried out in the control site without actual activation of the device. Periodontal dressings were applied. Post-operatively, LLLT was repeated on the test sites for 5 min daily, for 5 days. The visual analog scale (VAS) was used to evaluate pain during the post-operative maintenance period. A 10 cm VAS, with verbal endpoints of ‘‘none’’ at the left end and ‘‘unbearable’’ at the right end, was prepared for each patient. Each of the subjects was asked to calibrate their pain level on this scale.
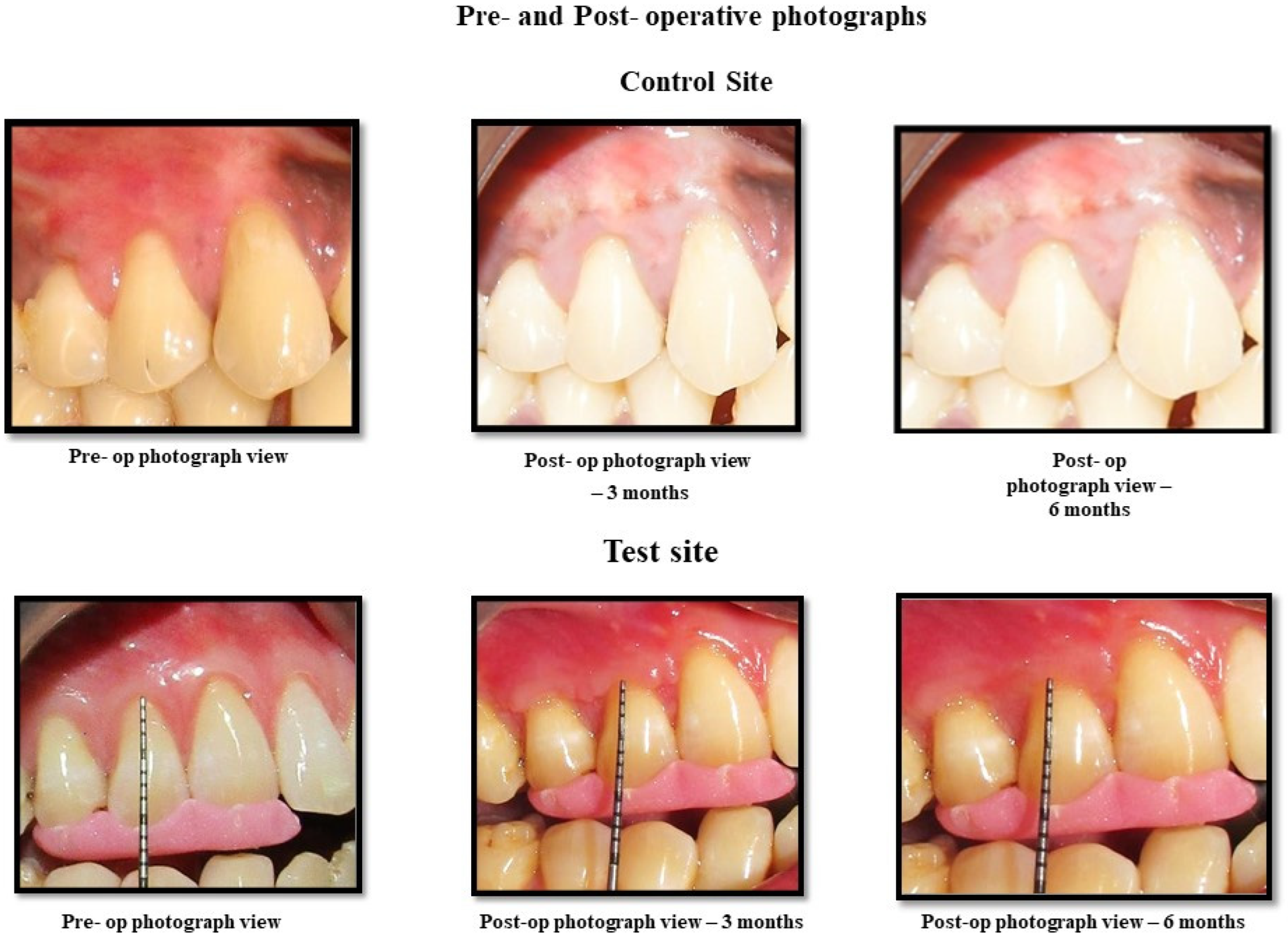
Post-operative maintenance/care consisting of 0.2% chlorhexidine mouth rinse 3 times daily for 3 weeks was recommended to patients. Analgesic ibuprofen, at 400 mg, was advised 3 times daily for 3 days. Patients were requested not to chew rigorously and to avoid brushing and flossing in the treated area for a period of 2 weeks. Patients were recalled for reinforcement of oral hygiene procedures, and clinical parameters were recorded at 6 weeks, 3 months and 6 months after surgical reconstruction. Pre-and post-operative photographs are shown in
Figure 2.
Data was entered in a Microsoft Excel worksheet and analyzed using SPSS (Statistical Package for Software and Social Science, version 23). Normality tests were performed to determine the nature of the data. A Kruskal–Wallis test and a Mann–Whitney test were performed to analyze those data. A p-value of less than 0.05 was considered statistically significant.
4. Discussion
The aim of this randomized, controlled split-mouth clinical trial was to assess the effect of LLLT on wound healing after a modified coronally advanced flap (MCAF) procedure for treatment of isolated recession-type defects. The total number of sites treated was 30, with 15 in the test group and 15 in the control group. Mean gingival recession depth and width showed significant improvement. Probing of pocket depth showed no major alterations. A moderate improvement was noted in clinical attachment levels. Overall, a gain in keratinized tissue width was reported, though the difference between the groups was negligible. The VAS score showed a significant difference between the control and test groups. The wound healing index showed no statistically significant difference.
Treatment of gingival recession using various modes, such as non-surgical or periodontal plastic procedures, has always been the norm of aesthetic therapeutic procedures. In spite of major evolutions, management of denuded roots still continues to be an unattainable goal outside of Miller’s Class I and Class II recession. The repertoire of treatment approaches comprises of soft-tissue pedicle grafts, free autogenous grafts, connective tissue grafts, guided tissue regeneration, the laterally sliding flap procedure, the double papilla flap procedure, the oblique rotated flap procedure, sub-epithelial connective tissue grafts, and any number of permutations and combinations of the same or more. When grafts that use native blood supply, namely pedicle grafts, are compared, the most convenient option is to coronally advance the flap. This technique circumvents added trauma of another surgical site and has the best possible color and contour match to adjacent tissues. Therefore, it is the simplest procedure that can heal uneventfully. The basic coronally advanced flap procedure can be combined with adjunctive treatment usage of platelet-rich fibrin, platelet-rich plasma, collagen membranes, enamel matrix derivatives, bone graft-substitutes, platelet concentrate grafts, collagen membranes seeded with autologous gingival fibroblasts, etc. After lasers were introduced, various types of laser therapy, such as photodynamic therapy, low-level laser therapy and laser photo therapy, have been attempted; among them, LLLT has elicited major interest due to its biostimulatory effects.
In vitro studies on effects of low-level laser irradiation on proliferation rate of human gingival fibroblasts showed that irradiated cells revealed considerably higher proliferation activity and suggested that these findings may be clinically relevant [
13]. Further, a recent systematic review revealed that LLLT with a diode laser stimulates human gingival fibroblasts; an increase in cell viability, proliferation, migration and protein synthesis was reported in irradiated cells. This accumulating evidence justified use of LLLT with intent to improve healing at the basic cellular level [
14]. LLLT has also been reported to promote gingival tissue repair and reduce post-operative symptoms of periodontal surgery [
15].
In opposition to the conventional coronally advanced procedure proposed by Allen and Miller in 1989 [
16], a modification with an additional vertical incision, proposed by Zuchelli et al. [
17], was inculcated in the present study. Placement of the gingival margin was 2 mm above the CEJ to counteract post-surgical gingival retraction in accordance with studies by Piniparato and Baldi et al. [
18,
19]. Most periodontal plastic surgical procedures are perceived as painful; studies carried out in 1993 and 1985 analyzed experience of pain after periodontal treatment and reported that 60% of patients experienced pain during the first week after surgery. Therefore, a visual analogue scale (0–10) that is highly subjective and dependent on individual experience was used in this study. Since the same patient served as test and control, a single blinding, wherein patients were unaware as to which side was laser treated, was essential. Overall the pain assessment scale revealed a significant difference between both sites, with the laser site eliciting less pain. Therefore, the parameters of LLLT application (mainly the wavelength of 810 nm) resulted in less pain intensity in the test site. This is in agreement with the study carried out by Ozelick et al. [
20], where pain reduction was found to be significantly higher in the laser group than in the control group two days after surgery. The findings of the present study suggest that laser application could be an effective tool in painful surgical procedures. This finding coincides with a study by Heidari et al. that reported on beneficial effects of LLLT on reduction of post-operative pain [
21].
Healing after mucogingival surgery depends on formation of a clot, maintenance of blood supply and formation of granulation tissue [
22]. Therefore, a thick gingival phenotype is probably more advantageous, as it is rich in vascularity. To optimize results, gingival thickness was taken into account during case selection. In general, lasers have been found to stimulate growth factors and to activate gingival fibroblasts and periodontal healing, which could contribute to improved wound stability. Therefore, in sites treated with the MCAF technique plus LLLT, healing was found to be superior compared to that in MCAF-technique-treated sites alone. Among the effects of LLLT, wound healing via increasing motility of human keratinocytes has been recorded; this healing promotes early epithelization, enhances fibroblast proliferation and better matrix synthesis and aids in neovascularization [
23].
In the present study, vertical incisions in almost all cases treated with the MCAF technique plus LLLT were observed to post-operatively merge with adjustment soft tissue. Conversely, in cases with the MCAF technique alone, merging of the vertical incision with the adjacent soft tissue was delayed in a few cases. Contradictory to the clinical trials by Damante et al. [
24], no added perks were reported in use of LLLT for healing. Edema was also reduced in the test sites, denoting better healing compared with the study by Amorium et al. [
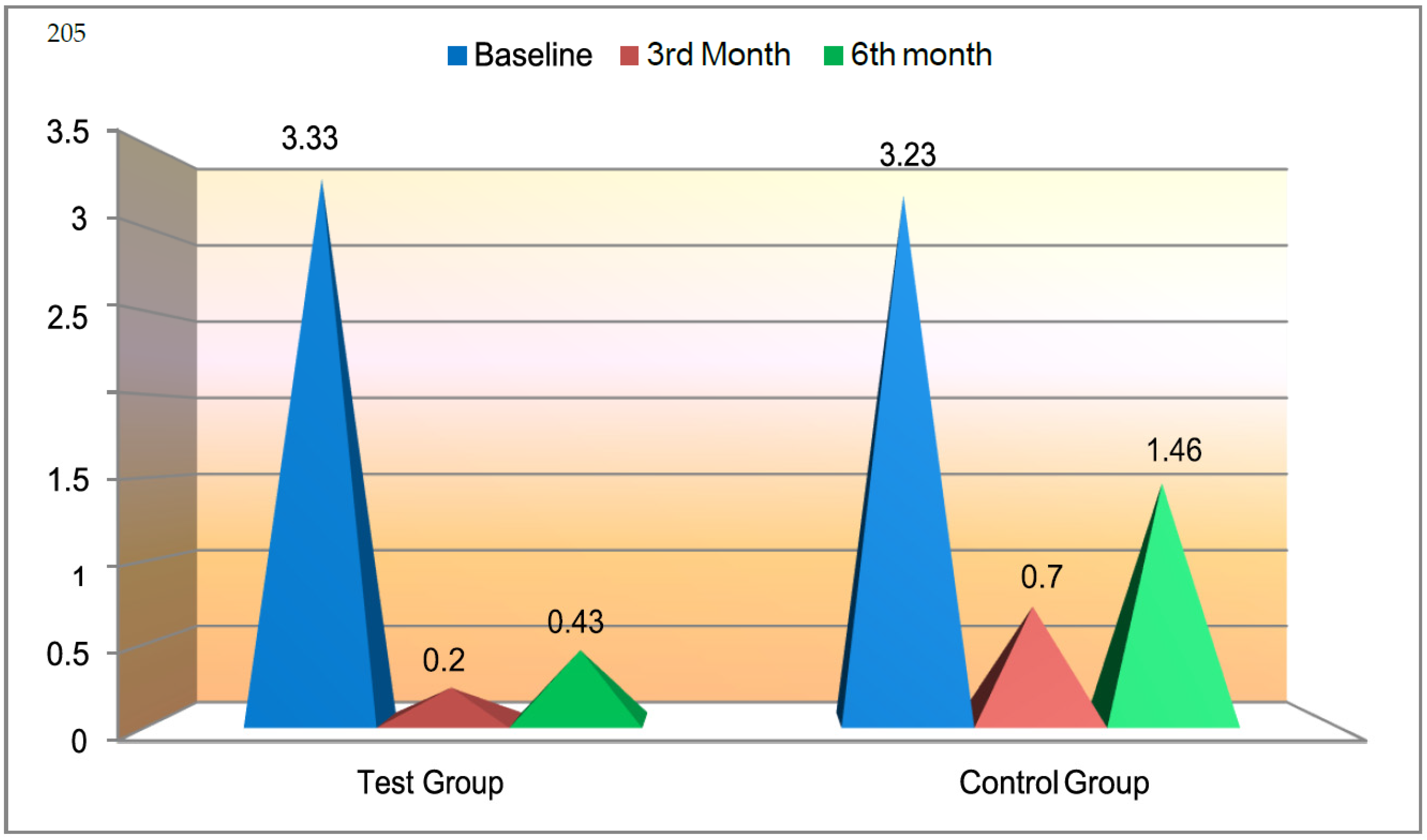
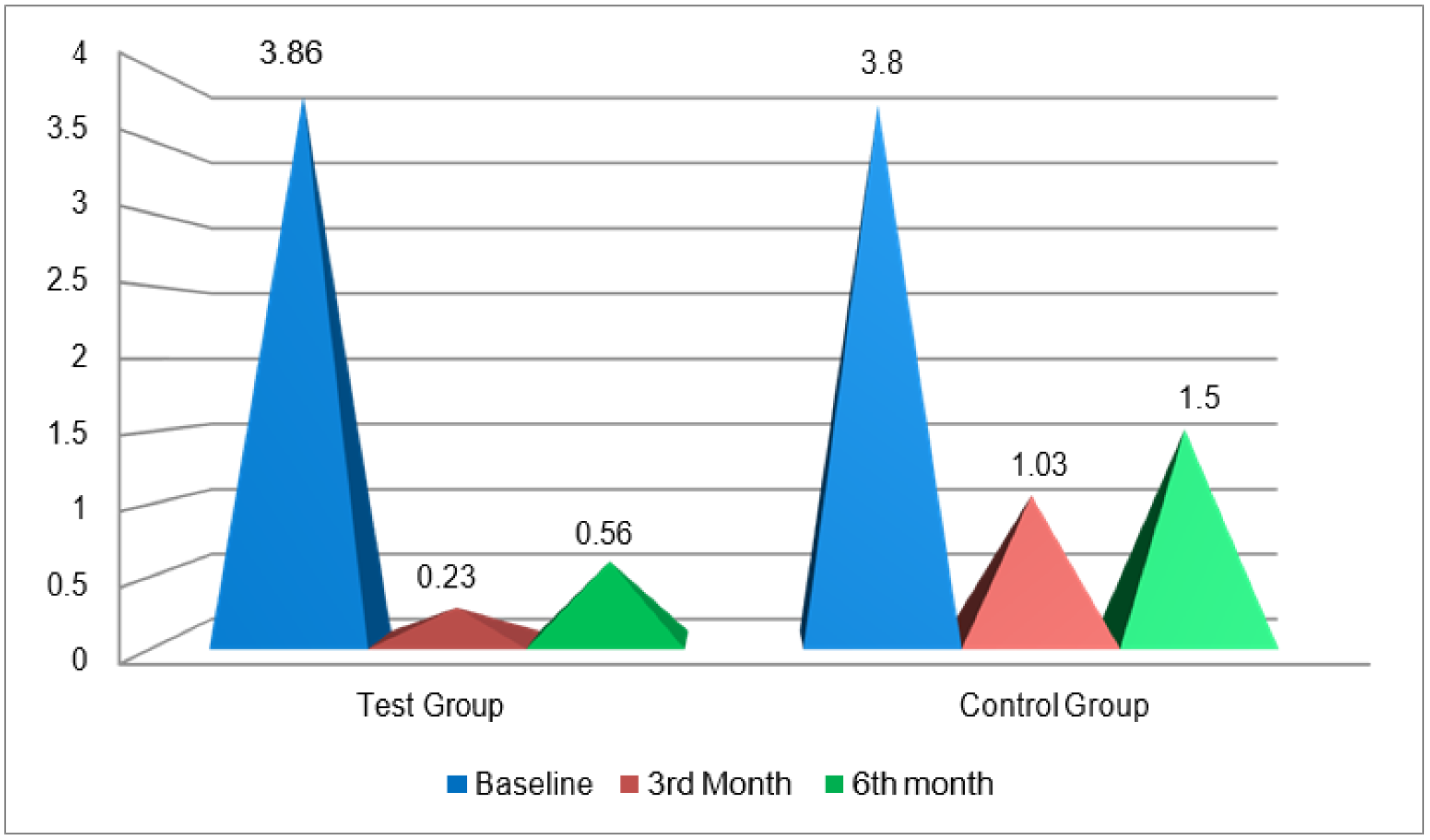
25]. Various measurements were included to quantify the amount of recession: gingival recession depth (GRD), gingival recession width (GRW), width of keratinized tissue (WKT), clinical attachment level (CAL) and pocket depth (PD). Among these, recession depth and width reduced drastically: more so in the test group, where GRD was recorded as 3.3 mm (baseline) to 0.4 mm (6 months), as opposed to 3.3 mm to 1.4 mm in the control groups. The difference was 1 mm. In the test group, GRW ranged from 3.8 mm (baseline) to 0.5 mm (6 months), whereas in the control group, GRW measured from 3.8 mm (baseline) to 1.5 mm (6 months); the difference was 1 mm. WKT increased from baseline to 3 and 6 months, and more gain of keratinized tissue was observed in test sites. The difference in gain among the groups, though statically significant, was not clinically relevant. This gain in KT was similar to that found in a study by Santana et al. [
26]. Basic periodontal clinical parameters, namely pocket depth (PD) and clinical attachment level (CAL), showed significant improvement, although the difference between test and control was negligible. Vital periodontal health indices were also recorded at baseline, 3 months and 6 months. As expected, after institution of periodontal treatment such as scaling, root planning and regular recall visits, reduction in scores of all indices was observed. When inter-group comparison of indices was carried out, no major differences were noted between the groups. Wound healing is best assessed when, along with clinical parameters, microbiological examination in the form of biopsy can be carried out. In the present study, biopsy could not be carried out due to ethical reasons. Several studies of lasers have shown favorable results when LLLT was used for multiple application times. In the present study, laser application was carried out for 5 min daily, for 5 days, which itself presented feasibility issues in terms of time spent on treatment and in finances. Therefore, patients could not be recalled at several time points.
In the current study, a split-mouth design was incorporated; evidence indicates systemic effects of the lasers that could have contributed to the results. Most likely, a bigger sample size could have elicited clearer results. Although the coin-toss method is widely practiced for randomization, it may be considered a limitation. At the outset of the present study, recession coverage in all sub-groups was found to be satisfactory. However, at the end of the study period (6 months), root coverage was found not to sustain in the control group as it had in the test group after surgical treatment. Significant improvement was also found in healing parameters in the laser-treated group. This accentuates fringe benefits of adjunctive use of low-level lasers.
Considering the outcomes above, usage of low-level therapy along with the modified coronally advanced flap procedure for coverage of denuded roots can be assumed to be a viable therapeutic option.