Establishment of Personalized Finite Element Model of Crystalline Lens Based on Sweep-Source Optical Coherence Tomography
Abstract
1. Introduction
2. Methods
2.1. Subjects
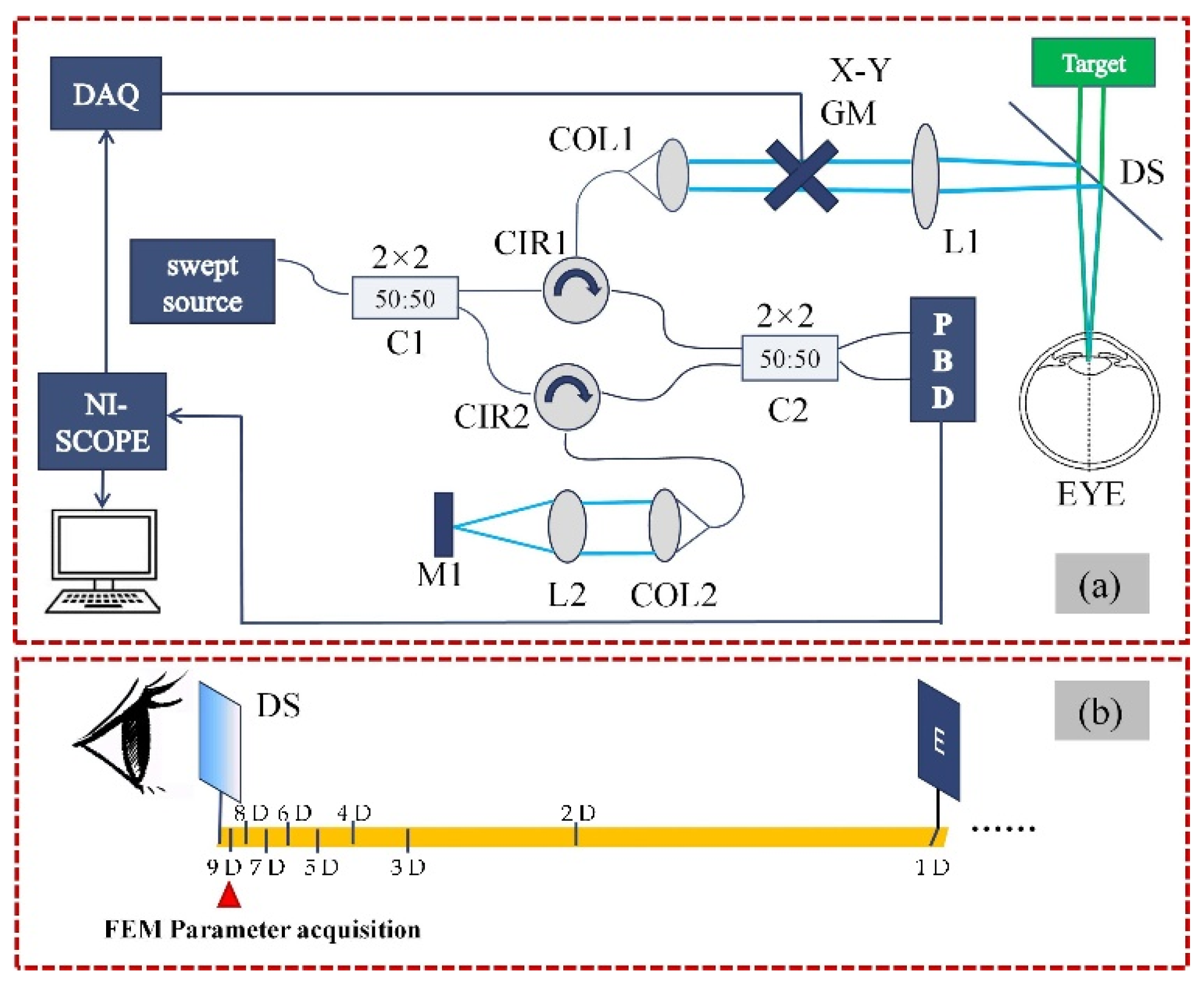
2.2. Measurement System and Data Acquisition
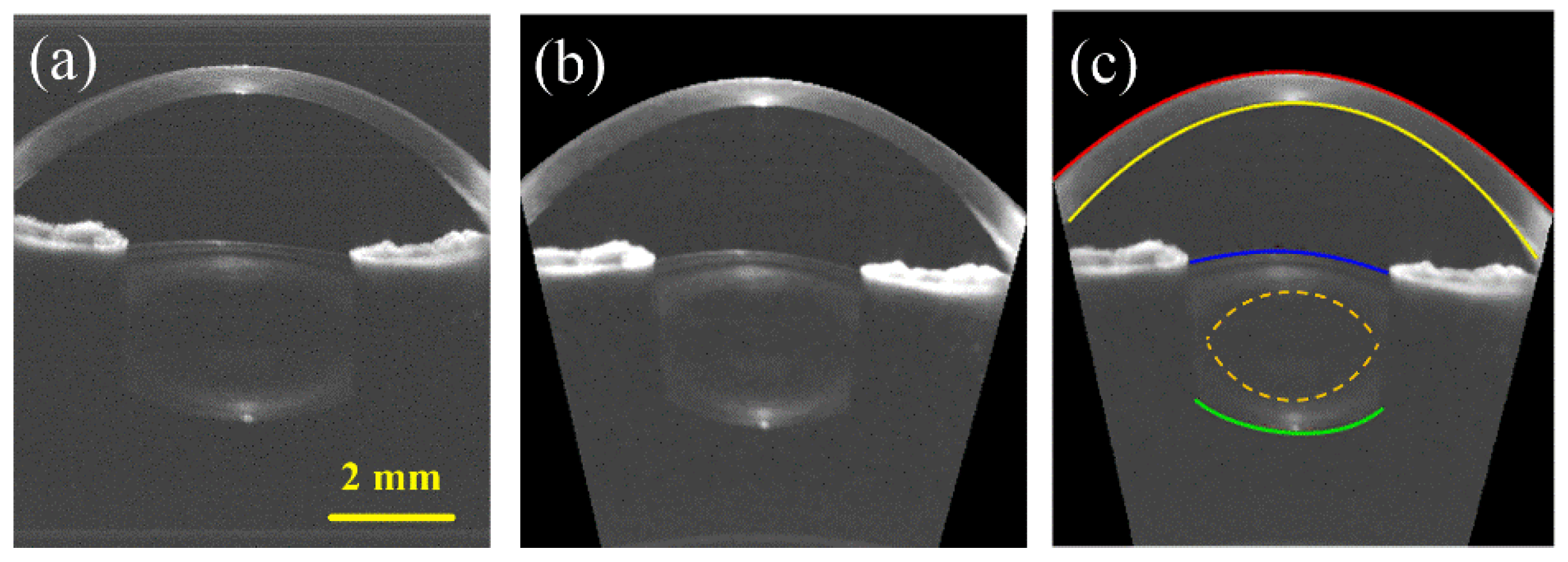
2.3. Image Processing
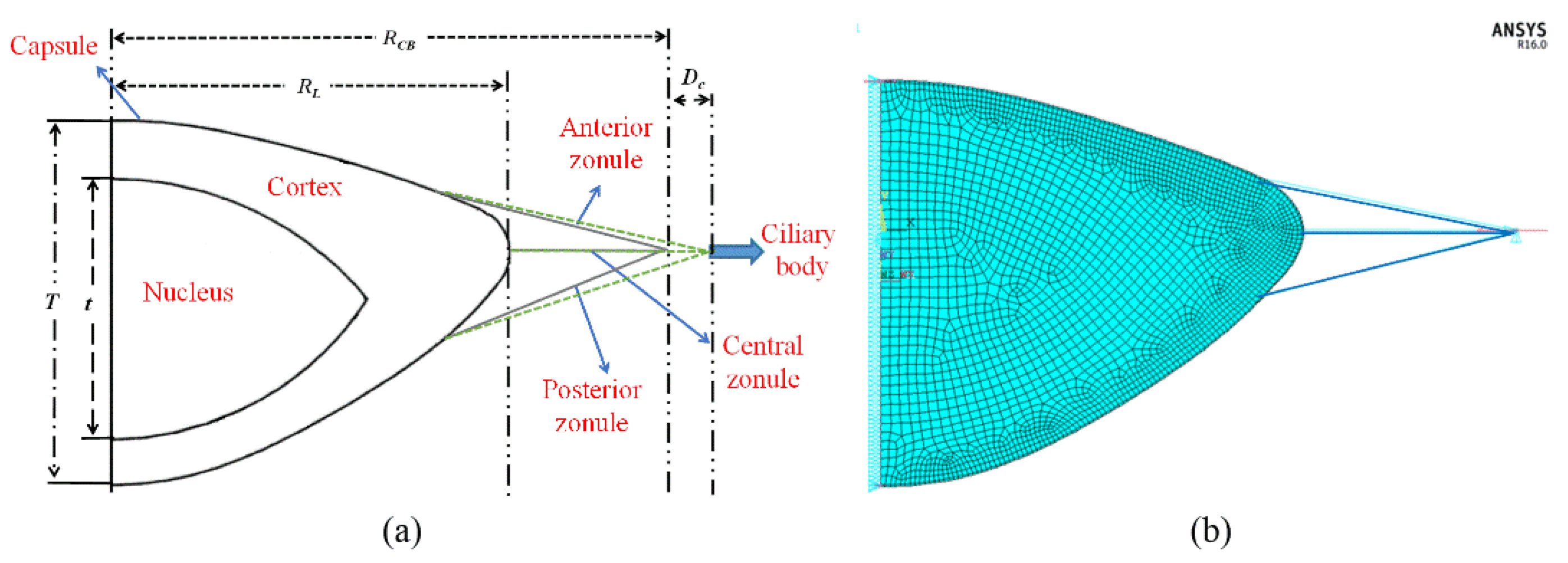
2.4. Finite Element Modeling
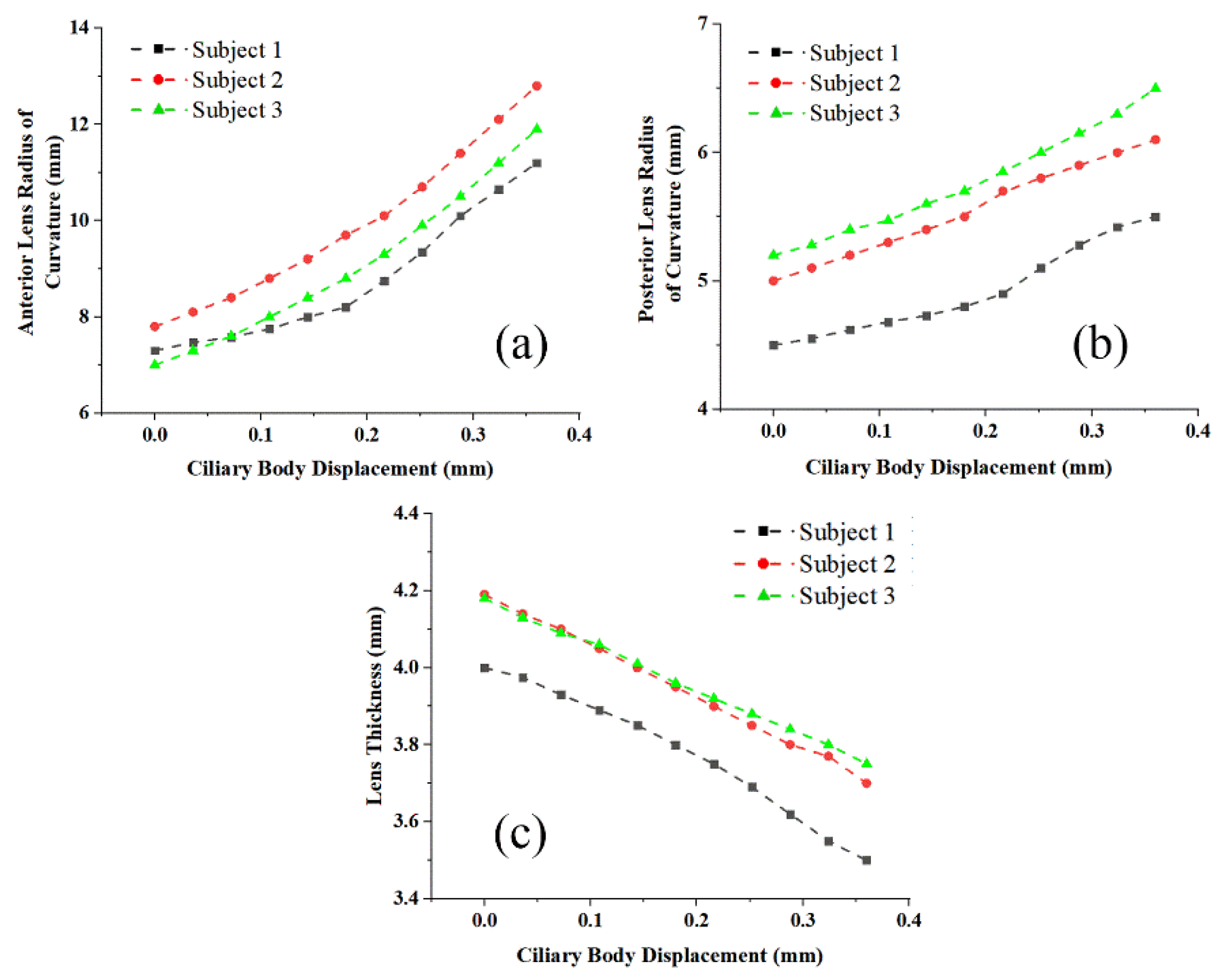
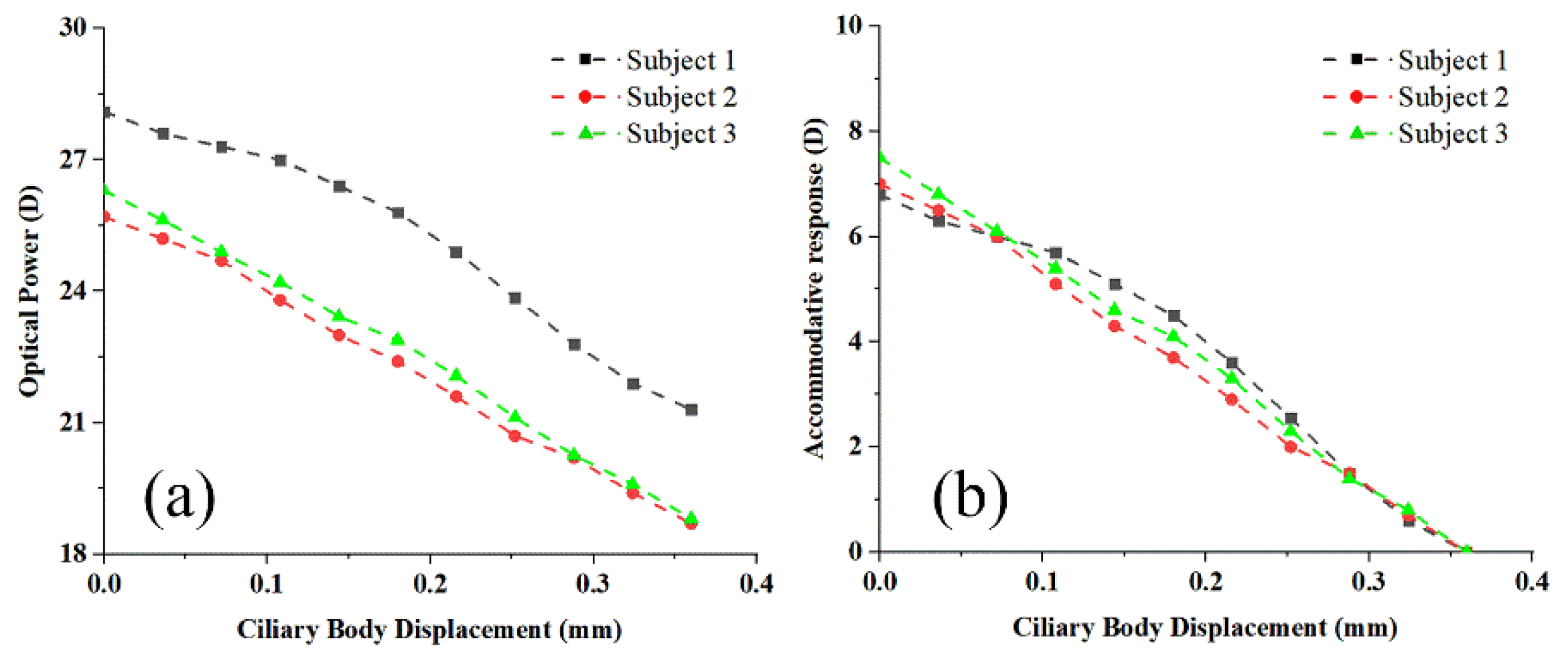
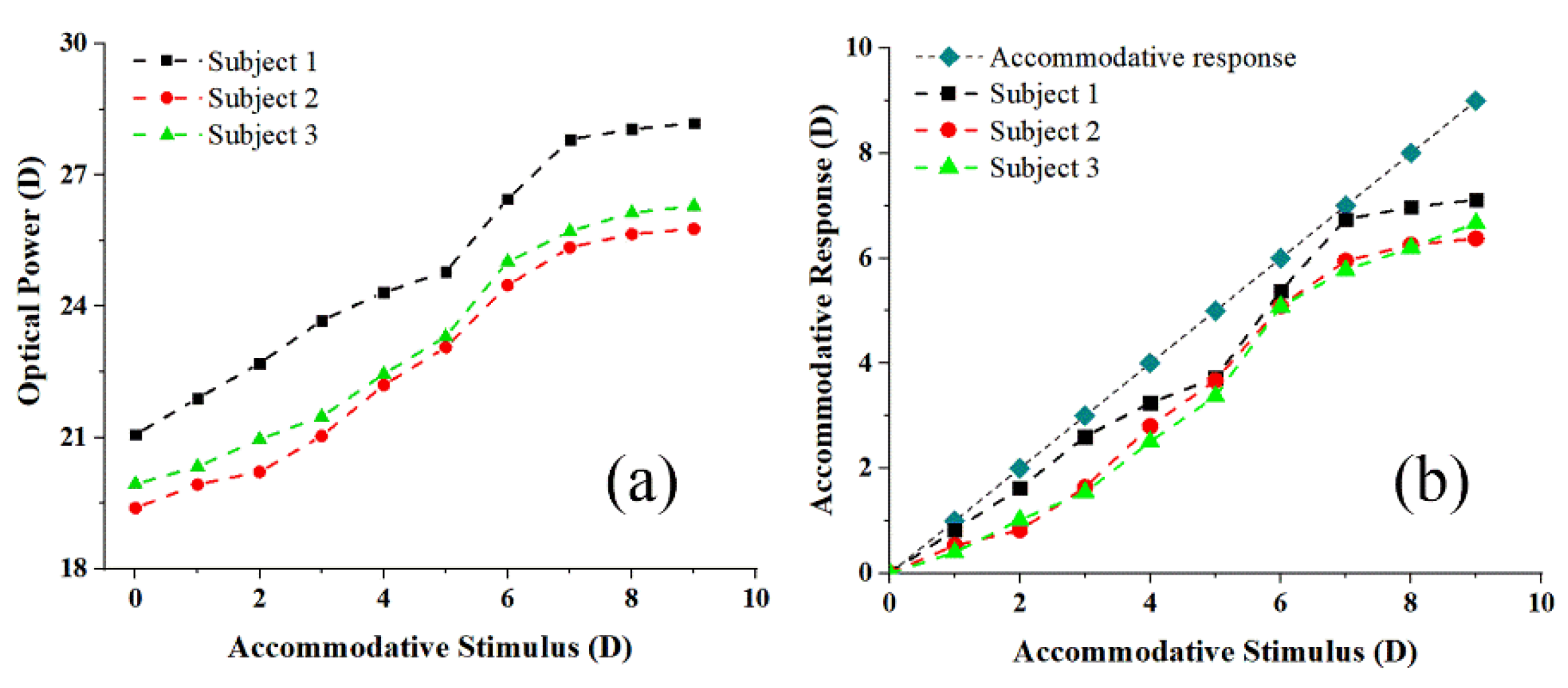
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Truffer, O.; Abler, D.; Pajic, B.; Grabner, G.; Kraker, H.; Büchler, P. Optimization of surgical parameters based on patient-specific models: Application to arcuate keratotomy. J. Cataract. Refract. Surg. 2018, 45, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Zein, M. Vision Restoration: Cataract Surgery and Surgical Correction of Myopia, Hyperopia, and Presbyopia. Med. Clin. North Am. 2021, 105, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Enriquez, E.; Pérez-Merino, P.; Durán-Poveda, S.; Jiménez-Alfaro, I.; Marcos, S. Estimation of intraocular lens position from full crystalline lens geometry: Towards a new generation of intraocular lens power calculation formulas. Sci. Rep. 2018, 8, 2045–2322. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Nandyala, S.; Martinez-Enriquez, E.; Heilman, B.M.; Augusteyn, R.C.; de Castro, A.; Ruggeri, M.; Parel, J.A.; Marcos, S.; Manns, F. Isolated human crystalline lens three-dimensional shape: A comparison between Indian and European populations. Exp. Eye Res. 2021, 205, 108481. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.; Langenbucher, A. Relationship between effective lens position and axial position of a thick intraocular lens. PLoS ONE 2018, 13, e0198824. [Google Scholar] [CrossRef]
- Martinez-Enriquez, E.; de Castro, A.; Mohamed, A.; Sravani, N.G.; Ruggeri, M.; Manns, F.; Marcos, S. Age-Related Changes to the Three-Dimensional Full Shape of the Isolated Human Crystalline Lens. Investig. Ophthalmol. Vis. Sci. 2020, 61, 11. [Google Scholar] [CrossRef]
- Canovas, C.; Artal, P. Customized eye models for determining optimized intraocular lenses power. Biomed. Opt. Express 2011, 2, 1649–1662. [Google Scholar] [CrossRef]
- Küchle, M.; Nguyen, N.X.; Langenbucher, A.; Gusek-Schneider, G.C.; Seitz, B.; Hanna, K.D. Implantation of a new accommodative posterior chamber intraocular lens. J. Refract. Surg. 2002, 18, 208–216. [Google Scholar] [CrossRef]
- Burd, H.J.; Judge, S.J.; Cross, J.A. Numerical modelling of the accommodating lens. Vis. Res. 2002, 42, 2235–2251. [Google Scholar] [CrossRef]
- Shung, V.W. An Analysis of a Crystalline Lens Subjected to Equatorial Periodic Pulls. Ph.D. Thesis, The University of Texas at Arlington, Arlington, TX, USA, 2002. [Google Scholar]
- Kasprzak, H.T. New approximation for the whole profile of the human crystalline lens. Ophthalmic Physiol. Opt. 2000, 20, 31–43. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, X.; Wang, X.; Liu, A. Modeling of Human Crystalline Lens. In Proceedings of the International Conference on Bioinformatics & Biomedical Engineering, Shanghai, China, 16–18 May 2008; IEEE: Piscataway, NJ, USA, 2008. [Google Scholar]
- Weale, R.A. Why we need reading-glasses before a zimmer-frame. Vis. Res. 2000, 40, 2233–2240. [Google Scholar] [CrossRef][Green Version]
- Wyatt, H.J. Application of a simple mechanical model of accommodation to the aging eye. Vis. Res. 1993, 33, 731–738. [Google Scholar] [CrossRef]
- Martin, H.; Guthoff, R.; Terwee, T.; Schmitz, K.-P. Comparison of the accommodation theories of Coleman and of Helmholtz by finite element simulations. Vis. Res. 2005, 45, 2910–2915. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.; Wang, B. Modeling and Visualization of Human Lens Based on OpenGL. In Proceedings of the 12th National Academic Conference on Image Graphics, Singapore, 23–25 February 2019; Association for Computing Machinery: New York, NY, USA, 2005; pp. 684–688. [Google Scholar]
- Welch-Phillips, A.; Gibbons, D.; Ahern, D.P.; Butler, J.S. What Is Finite Element Analysis? Clin. Spine Surg. 2020, 33, 323–324. [Google Scholar] [CrossRef]
- Sánchez, P.; Moutsouris, K.; Pandolfi, A. Biomechanical and optical behavior of human corneas before and after photorefractive keratectomy. J. Cataract. Refract. Surg. 2014, 40, 905–917. [Google Scholar] [CrossRef]
- Amini, R.; Jouzdani, S.; Barocas, V.H. Increased iris–lens contact following spontaneous blinking: Mathematical modeling. J. Biomech. 2012, 45, 2293–2296. [Google Scholar] [CrossRef][Green Version]
- Tse, K.M.; Lee, H.P.; Shabana, N.; Loon, S.C.; Watson, P.G.; Thean, S.Y.L.H. Do shapes and dimensions of scleral flap and sclerostomy influence aqueous outflow in trabeculectomy? A finite element simulation approach. Br. J. Ophthalmol. 2012, 96, 432–437. [Google Scholar] [CrossRef]
- Wang, Z.; Pokki, J.; Ergeneman, O.; Nelson, B.J.; Hirai, S. Viscoelastic interaction between intraocular microrobots and vitreous humor: A finite element approach. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 4937–4940. [Google Scholar]
- Norman, R.E.; Flanagan, J.G.; Sigal, I.A.; Rausch, S.M.K.; Tertinegg, I.; Ethier, C.R. Finite element modeling of the human sclera: Influence on optic nerve head biomechanics and connections with glaucoma. Exp. Eye Res. 2011, 93, 4–12. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; Wang, B.; Chen, Y.; Yang, H. Parametric 3D Visualization of Human Crystalline Lens based on OpenGL. In Proceedings of the International Conference on Medical Imaging and Tele-Medicine (MIT2005), Wuyi Mountain, China, 16–19 August 2005. [Google Scholar]
- Lanchares, E.; Navarro, R.; Calvo, B. Hyperelastic modelling of the crystalline lens: Accommodation and presbyopia. J. Optom. 2012, 5, 110–120. [Google Scholar] [CrossRef]
- Eppig, T.; Gillner, M.; Zoric, K.; Jäger, J.; Löffler, A.; Langenbucher, A. Biomechanical eye model and measurement setup for investigating accommodating intraocular lenses. Z Med. Phys. 2013, 23, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Manns, F.; Pham, T.; Parel, J.M. Predicting the performance of accommodating intraocular lenses using ray tracing. J. Cataract. Refract. Surg. 2006, 32, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Wang, Z.Q.; Liu, Y.J.; Wang, Y. A novel approach to design intraocular lenses with extended depth of focus in a pseudophakic eye model. Optik 2011, 122, 991–995. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Yan, W.; Tong, Z. Intraocular lens design for treating high myopia based on individual eye model. Optik 2007, 118, 88–93. [Google Scholar]
- Rosales, P.; Marcos, S. Customized computer models of eyes with intraocular lenses. Opt. Express 2007, 15, 2204–2218. [Google Scholar] [CrossRef]
- Zhang, W. Personalized hemodynamic modeling and numerical study of pulmonary hypertension. Med. Biomech. 2021, 36, 64. [Google Scholar]
- Wang, N.; Chen, G.; Li, J. Personalized modeling method of liver pipeline based on improved GA. J. Minzu Univ. China (Nat. Sci. Ed.) 2016, 25, 75–79. [Google Scholar]
- Deng, X.; Song, J.; Lin, Z.; Xia, C. Personalized digital modeling-assisted selection of external locking plates for distal tibial fractures. Chin. J. Mod. Surg. 2019, 23, 345–348. [Google Scholar]
- Timoney, P.J.; Breathnach, C.S. Allvar Gullstrand and the slit lamp 1911. Ir. J. Med. Sci. 2013, 182, 301–305. [Google Scholar] [CrossRef]
- Rosales, P.; Dubbelman, M.; Marcos, S.; van der Heijde, R. Crystalline lens radii of curvature from Purkinje and Scheimpflug imaging. J. Vis. 2006, 6, 1057–1067. [Google Scholar] [CrossRef]
- Domínguez-Vicent, A.; Monsálvez-Romín, D.; del Águila-Carrasco, A.J.; Ferrer-Blasco, T.; Montés-Micó, R. Changes in the anterior chamber during accommodation assessed with a Scheimpflug system. J. Cataract. Refract. Surg. 2014, 40, 1790–1797. [Google Scholar] [CrossRef]
- Hermans, E.A.; Pouwels, P.J.; Dubbelman, M.; Kuijer, J.P.; van der Heijde, R.G.; Heethaar, R.M. Constant volume of the human lens and decrease in surface area of the capsular bag during accommodation: An MRI and Scheimpflug study. Investig. Ophthalmol. Vis. Sci. 2009, 50, 281–289. [Google Scholar] [CrossRef]
- Kasthurirangan, S.; Markwell, E.L.; Atchison, D.A.; Pope, J.M. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J. Vis. 2011, 11, 19. [Google Scholar] [CrossRef]
- Ramasubramanian, V.; Glasser, A. Objective measurement of accommodative biometric changes using ultrasound biomicroscopy. J. Cataract. Refract. Surg. 2015, 41, 511–526. [Google Scholar] [CrossRef]
- Koretz, J.E.; Strenk, S.A.; Strenk, L.M.; Semmlow, J.L. Scheimpflug and high-resolution magnetic resonance imaging of the anterior segment: A comparative study. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2004, 21, 346–354. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Hee, M.R.; Izatt, J.A.; Swanson, E.A.; Huang, D.; Schuman, J.S.; Lin, C.P.; Puliafito, C.A.; Fujimoto, J.G. Optical coherence tomography of the human retina. Arch. Ophthalmol. 1995, 113, 325–332. [Google Scholar] [CrossRef]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical coherence tomography today: Speed, contrast, and multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef]
- Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Lin, C.P.; Huang, D.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch. Ophthalmol. 1994, 112, 1584–1589. [Google Scholar] [CrossRef]
- Ehlers, J.P. Intraoperative optical coherence tomography: Past, present, and future. Eye 2016, 30, 193–201. [Google Scholar] [CrossRef]
- de Boer, J.F.; Leitgeb, R.; Wojtkowski, M. Twenty-five years of optical coherence tomography: The paradigm shift in sensitivity and speed provided by Fourier domain OCT [Invited]. Biomed. Opt. Express 2017, 8, 3248–3280. [Google Scholar] [CrossRef]
- Rodríguez-Aramendía, A.; Díaz-Doutón, F.; Fernández-Trullàs, J.; Falgueras, P.; González, L.; Pujol, J.; Grulkowski, I.; Güell, J.L. Whole anterior segment and retinal swept source OCT for comprehensive ocular screening. Biomed. Opt. Express 2021, 12, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Zevallos, O.M.; Keller, B.; Viehland, C.; Shen, L.; Waterman, G.; Todorich, B.; Shieh, C.; Hahn, P.; Farsiu, S.; Kuo, A.N.; et al. Live volumetric (4D) visualization and guidance of in vivo human ophthalmic surgery with intraoperative optical coherence tomography. Sci. Rep. 2016, 6, 31689. [Google Scholar] [CrossRef] [PubMed]
- Adhi, M.; Liu, J.J.; Qavi, A.H.; Grulkowski, I.; Lu, C.D.; Mohler, K.J.; Ferrara, D.; Kraus, M.F.; Baumal, C.R.; Witkin, A.J.; et al. Choroidal analysis in healthy eyes using swept-source optical coherence tomography compared to spectral domain optical coherence tomography. Am. J. Ophthalmol. 2014, 157, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Grulkowski, I.; Liu, J.J.; Potsaid, B.; Jayaraman, V.; Jiang, J.; Fujimoto, J.G.; Cable, A.E. High-precision, high-accuracy ultralong-range swept-source optical coherence tomography using vertical cavity surface emitting laser light source. Opt. Lett. 2013, 38, 673–675. [Google Scholar] [CrossRef]
- Grulkowski, I.; Liu, J.J.; Potsaid, B.; Jayaraman, V.; Lu, C.D.; Jiang, J.; Cable, A.E.; Duker, J.S.; Fujimoto, J.G. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers. Biomed. Opt. Express 2012, 3, 2733–2751. [Google Scholar] [CrossRef]
- Dhalla, A.H.; Nankivil, D.; Bustamante, T.; Kuo, A.; Izatt, J.A. Simultaneous swept source optical coherence tomography of the anterior segment and retina using coherence revival. Opt. Lett. 2012, 37, 1883–1885. [Google Scholar] [CrossRef]
- Fernández-Vigo, J.I.; Shi, H.; Kudsieh, B.; Arriola-Villalobos, P.; Gómez-de-Liaño, L.D.; García-Feijóo, J.; Fernández-Vigo, J.Á. Ciliary muscle dimensions by swept-source optical coherence tomography and correlation study in a large population. Acta Ophthalmol. 2020, 98, e487–e494. [Google Scholar] [CrossRef]
- Kong, M.; Gao, Z.; Chen, L.; Li, X. Effect of crystalline lens surfaces and refractive index on image quality by model simulation analysis. Chin. Opt. Lett. 2008, 6, 377–380. [Google Scholar] [CrossRef]
- Zhu, D.; Shao, Y.; Leng, L.; Xu, Z.; Wang, J.; Lu, F.; Shen, M. Automatic biometry of the anterior segment during accommodation imaged by optical coherence tomography. Eye Contact Lens 2014, 40, 232–238. [Google Scholar] [CrossRef]
- Wolfson, S.J. Canny Scanning. Keats-Shelley Rev. 2022, 36, 21. [Google Scholar] [CrossRef]
- Zhihong, N. Curve fitting method for the first and second derivatives of known type value points—Quintic polynomial method. Mech. Des. Res. 1994, 3, 14–15. [Google Scholar]
- Dubbelman, M.; van der Heijde, G.L.; Weeber, H.A. Change in shape of the aging human crystalline lens with accommodation. Vis. Res. 2005, 45, 117–132. [Google Scholar] [CrossRef]
- Martin, H.; Stachs, O.; Guthoff, R.; Grabow, N. Finite-Elemente-Modellierung des Auges zur Untersuchung der Akkommodation [Finite Element Modelling of the Eye for the Investigation of Accommodation]. Klin. Monbl. Augenheilkd. 2016, 233, 1357–1361. [Google Scholar]
- Strenk, S.A.; Semmlow, J.L.; Strenk, L.M.; Munoz, P.; Gronlund-Jacob, J.; DeMarco, J.K. Age-related changes in human ciliary muscle and lens: A magnetic resonance imaging study. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1162–1169. [Google Scholar]
- Krag, S.; Olsen, T.; Andreassen, T.T. Biomechanical characteristics of the human anterior lens capsule in relation to age. Investig. Ophthalmol. Vis. Sci. 1997, 38, 357–363. [Google Scholar]
- Vaghefi, E.; Malcolm, D.T.; Jacobs, M.D.; Donaldson, P.J. Development of a 3D finite element model of lens microcirculation. Biomed. Eng. Online 2012, 11, 69. [Google Scholar] [CrossRef]
- Pour, H.M.; Kanapathipillai, S.; Zarrabi, K.; Manns, F.; Ho, A. Stretch-dependent changes in surface profiles of the human crystalline lens during accommodation: A finite element study. Clin. Exp. Optom. 2015, 98, 126–137. [Google Scholar] [CrossRef]
- Pérez-Merino, P.; Velasco-Ocana, M.; Martinez-Enriquez, E.; Marcos, S. OCT-based crystalline lens topography in accommodating eyes. Biomed. Opt. Express 2015, 6, 5039–5054. [Google Scholar] [CrossRef]
- Doyle, L.; Little, J.A.; Saunders, K.J. Repeatability of OCT lens thickness measures with age and accommodation. Optom. Vis. Sci. 2013, 90, 1396–1405. [Google Scholar] [CrossRef]
- Martinez-Enriquez, E.; Pérez-Merino, P.; Velasco-Ocana, M.; Marcos, S. OCT-based full crystalline lens shape change during accommodation in vivo. Biomed. Opt. Express 2017, 8, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.F. The elastic constants of the human lens. J. Physiol. 1971, 212, 147–180. [Google Scholar] [CrossRef] [PubMed]
- Wilde, G.S.; Burd, H.J.; Judge, S.J. Shear modulus data for the human lens determined from a spinning lens test. Exp. Eye Res. 2012, 97, 36–48. [Google Scholar] [CrossRef] [PubMed]




| Parameter | a | b | c | d | e |
|---|---|---|---|---|---|
| ALC (Subject 1) | −0.001 | 0.0106 | −0.0299 | −0.0686 | 2.2 |
| PLC (Subject 1) | −0.0001 | 0.0007 | −0.0065 | 0.1111 | −2 |
| ALC (Subject 2) | −0.001 | 0.0087 | −0.0202 | −0.0637 | 2.2 |
| PLC (Subject 2) | 0.0047 | −0.0368 | 0.0826 | 0.1034 | −1.99 |
| ALC (Subject 3) | 0.0005 | −0.0046 | 0.0155 | −0.0715 | 2.09 |
| PLC (Subject 3) | 0.0037 | −0.0307 | 0.0715 | 0.096 | −2.1 |
| Parameter | Subject 1 | Subject 2 | Subject 3 |
|---|---|---|---|
| Ciliary body radius, Rcb [mm] | 6.465 | 6.492 | 6.51 |
| Lens radius, RL | 4.32 | 4.3 | 4.28 |
| Nucleus thickness, t [mm] | 2.97 | 2.6 | 2.69 |
| Capsule Young’s modulus [N/mm2] | 1.3 | 1.2 | 1.15 |
| Nuclear Young’s modulus [N/mm2] | 0.00055 | 0.00055 | 0.00055 |
| Cortex Young’s modulus [N/mm2] | 0.00342 | 0.00342 | 0.00342 |
| Anterior zonule stiffness [N/mm] | 0.066 | 0.066 | 0.066 |
| Central zonule stiffness [N/mm] | 0.011 | 0.011 | 0.011 |
| Posterior zonule stiffness [N/mm] | 0.033 | 0.033 | 0.033 |
| Capsule Poisson’s ratio | 0.47 | 0.47 | 0.47 |
| Nuclear and cortex ratio | 0.49 | 0.49 | 0.49 |
| Subject | ALC | PLC | TH | RP |
|---|---|---|---|---|
| Subject 1 | 0.38 (4.5%) | 0.10 (2.0%) | 0.067 (1.8%) | 0.45 (1.8%) |
| Subject 2 | 0.31 (3.3%) | 0.07 (1.3%) | 0.058 (1.5%) | 0.36 (1.6%) |
| Subject 3 | 0.25 (2.8%) | 0.12 (2.2%) | 0.09 (2.3%) | 0.59 (2.5%) |
| Average | 0.313 (3.53%) | 0.096 (1.83%) | 0.071 (1.86%) | 0.466 (1.96%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Li, A.; Liu, J.; Zhao, Y.; Zhu, K.; Li, Z.; Lin, Y.; Yan, S.; Lv, H.; Wang, S.; et al. Establishment of Personalized Finite Element Model of Crystalline Lens Based on Sweep-Source Optical Coherence Tomography. Photonics 2022, 9, 803. https://doi.org/10.3390/photonics9110803
Liu G, Li A, Liu J, Zhao Y, Zhu K, Li Z, Lin Y, Yan S, Lv H, Wang S, et al. Establishment of Personalized Finite Element Model of Crystalline Lens Based on Sweep-Source Optical Coherence Tomography. Photonics. 2022; 9(11):803. https://doi.org/10.3390/photonics9110803
Chicago/Turabian StyleLiu, Guangheng, Ang Li, Jian Liu, Yuqian Zhao, Keliang Zhu, Zhen Li, Yang Lin, Shixin Yan, Hongyu Lv, Shuanglian Wang, and et al. 2022. "Establishment of Personalized Finite Element Model of Crystalline Lens Based on Sweep-Source Optical Coherence Tomography" Photonics 9, no. 11: 803. https://doi.org/10.3390/photonics9110803
APA StyleLiu, G., Li, A., Liu, J., Zhao, Y., Zhu, K., Li, Z., Lin, Y., Yan, S., Lv, H., Wang, S., Yu, Y., Wang, Y., Luan, J., & Ma, Z. (2022). Establishment of Personalized Finite Element Model of Crystalline Lens Based on Sweep-Source Optical Coherence Tomography. Photonics, 9(11), 803. https://doi.org/10.3390/photonics9110803






