Waterproof Galvanometer Scanner-Based Handheld Photoacoustic Microscopy Probe for Wide-Field Vasculature Imaging In Vivo
Abstract
:1. Introduction
2. Materials and Methods
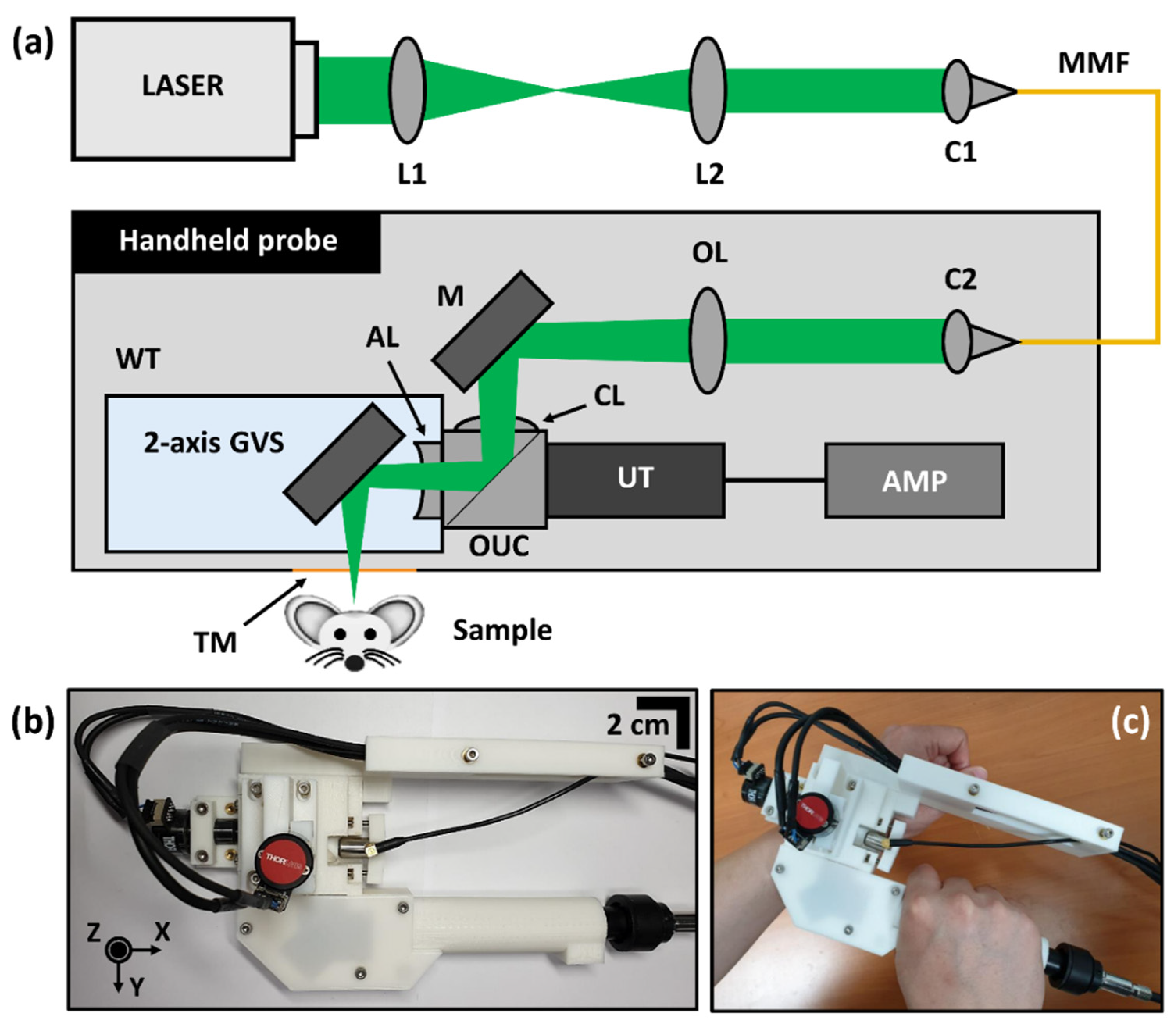
2.1. System Configuration of WP-GVS-HH-PAM
2.2. Animal Experiment Protocol
3. Results
3.1. Performance Evaluation of WP-GVS-HH-PAM
3.2. Phantom Images Using WP-GVS-HH-PAM
3.3. WP-GVS-HH-PAM for In Vivo Experiments with a Mouse
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Yao, J.; Wang, L.V. Photoacoustic tomography: Principles and advances. Electromagn. Waves (Camb. Mass) 2014, 147, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, C. Multimodal photoacoustic tomography. IEEE Trans. Multimed. 2013, 15, 975–982. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, J.; Kim, C. Multiplane spectroscopic whole-body photoacoustic imaging of small animals in vivo. Med. Biol. Eng. Comput. 2016, 54, 283–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeon, M.; Rich, L.J.; Hong, H.; Geng, J.; Zhang, Y.; Shi, S.; Barnhart, T.E.; Alexandridis, P.; Huizinga, J.D. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat. Nanotechnol. 2014, 9, 631–638. [Google Scholar] [CrossRef]
- Li, C.; Aguirre, A.; Gamelin, J.K.; Maurudis, A.; Zhu, Q.; Wang, L.V. Real-time photoacoustic tomography of cortical hemodynamics in small animals. J. Biomed. Opt. 2010, 15, 010509. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, L.V. Small-animal whole-body photoacoustic tomography: A review. IEEE Trans. Biomed. Eng. 2013, 61, 1380–1389. [Google Scholar] [CrossRef] [Green Version]
- Upputuri, P.K.; Pramanik, M. Recent advances toward preclinical and clinical translation of photoacoustic tomography: A review. J. Biomed. Opt. 2016, 22, 041006. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Performance characterization of low-cost, high-speed, portable pulsed laser diode photoacoustic tomography (PLD-PAT) system. Biomed. Opt. Express 2015, 6, 4118–4129. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wang, L.V. Photoacoustic microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, J.; Lee, D.; Baik, J.W.; Kim, C. Review on practical photoacoustic microscopy. Photoacoustics 2019, 15, 100141. [Google Scholar] [CrossRef]
- Kim, C.; Park, S.-J.; Kim, J.; Lee, S.; Lee, C.; Jeon, M.; Kim, J.; Oh, K.K. Objective-free optical-resolution photoacoustic microscopy. J. Biomed. Opt. 2012, 18, 010501. [Google Scholar] [CrossRef] [Green Version]
- Maslov, K.; Zhang, H.F.; Hu, S.; Wang, L.V. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett. 2008, 33, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.; Kim, J.; Kim, C. Acoustic resolution photoacoustic microscopy. Biomed. Eng. Lett. 2014, 4, 213–222. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.T.; Zhang, R.; Hai, P.; Zhang, C.; Pleitez, M.A.; Aft, R.L.; Novack, D.V.; Wang, L.V. Fast label-free multilayered histology-like imaging of human breast cancer by photoacoustic microscopy. Sci. Adv. 2017, 3, e1602168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Wong, T.T.; He, Y.; Li, L.; Zhang, R.; Yung, C.S.; Hwang, J.; Maslov, K.; Wang, L.V. High-resolution, high-contrast mid-infrared imaging of fresh biological samples with ultraviolet-localized photoacoustic microscopy. Nat. Photonics 2019, 13, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, J.; Ning, B.; Sun, N.; Wang, T.; Zuo, Z.; Hu, S. Functional and oxygen-metabolic photoacoustic microscopy of the awake mouse brain. Neuroimage 2017, 150, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, B.; Liu, W.; Wang, Y.-C.; Shi, J.; Li, Y.; Xu, S.; Sheng, H.; Zhou, Q.; Zou, J.; Hoffmann, U. High-speed widefield photoacoustic microscopy of small-animal hemodynamics. Biomed. Opt. Express 2018, 9, 4689–4701. [Google Scholar] [CrossRef]
- Park, K.; Kim, J.Y.; Lee, C.; Jeon, S.; Lim, G.; Kim, C. Handheld photoacoustic microscopy probe. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, P.; Xu, S.; Shi, J.; Li, L.; Yao, J.; Wang, L.; Zou, J.; Wang, L.V. Handheld optical-resolution photoacoustic microscopy. J. Biomed. Opt. 2016, 22, 041002. [Google Scholar] [CrossRef]
- Xu, S.; Huang, C.-H.; Zou, J. Microfabricated water-immersible scanning mirror with a small form factor for handheld ultrasound and photoacoustic microscopy. J. Micro/Nanolithography MEMS MOEMS 2015, 14, 035004. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, H.; Jin, T.; Qi, W.; Xie, H.; Xi, L. Ultracompact high-resolution photoacoustic microscopy. Opt. Lett. 2018, 43, 1615–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Song, C.; Xie, H.; Xi, L. Photoacoustic endomicroscopy based on a MEMS scanning mirror. Opt. Lett. 2017, 42, 4615–4618. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Chen, Q.; Guo, H.; Xie, H.; Xi, L. Miniaturized optical resolution photoacoustic microscope based on a microelectromechanical systems scanning mirror. Micromachines 2018, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Hajireza, P.; Shi, W.; Zemp, R.J. Real-time handheld optical-resolution photoacoustic microscopy. Opt. Express 2011, 19, 20097–20102. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, H.; Cheng, Z.; Wang, Z.; Zhang, L.; Yang, S. Miniaturized photoacoustic probe for in vivo imaging of subcutaneous microvessels within human skin. Quant. Imaging Med. Surg. 2019, 9, 807. [Google Scholar] [CrossRef]
- Jin, T.; Guo, H.; Jiang, H.; Ke, B.; Xi, L. Portable optical resolution photoacoustic microscopy (pORPAM) for human oral imaging. Opt. Lett. 2017, 42, 4434–4437. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Guo, H.; Yao, L.; Xie, H.; Jiang, H.; Xi, L. Portable optical-resolution photoacoustic microscopy for volumetric imaging of multiscale organisms. J. Biophotonics 2018, 11, e201700250. [Google Scholar] [CrossRef]
- Lee, J.; Han, S.; Seong, D.; Lee, J.; Park, S.; Wijesinghe, R.E.; Jeon, M.; Kim, J. Fully waterproof two-axis galvanometer scanner for enhanced wide-field optical-resolution photoacoustic microscopy. Opt. Lett. 2020, 45, 865–868. [Google Scholar] [CrossRef]
- Tsai, P.S.; Kaufhold, J.P.; Blinder, P.; Friedman, B.; Drew, P.J.; Karten, H.J.; Lyden, P.D.; Kleinfeld, D. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J. Neurosci. 2009, 29, 14553–14570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, A.; Guevara-Torres, A.; Schallek, J. Imaging single-cell blood flow in the smallest to largest vessels in the living retina. Elife 2019, 8, e45077. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Maslov, K.; Wang, L.V. Second-generation optical-resolution photoacoustic microscopy with improved sensitivity and speed. Opt. Lett. 2011, 36, 1134–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, D.; Han, S.; Lee, J.; Lee, E.; Kim, Y.; Lee, J.; Jeon, M.; Kim, J. Waterproof Galvanometer Scanner-Based Handheld Photoacoustic Microscopy Probe for Wide-Field Vasculature Imaging In Vivo. Photonics 2021, 8, 305. https://doi.org/10.3390/photonics8080305
Seong D, Han S, Lee J, Lee E, Kim Y, Lee J, Jeon M, Kim J. Waterproof Galvanometer Scanner-Based Handheld Photoacoustic Microscopy Probe for Wide-Field Vasculature Imaging In Vivo. Photonics. 2021; 8(8):305. https://doi.org/10.3390/photonics8080305
Chicago/Turabian StyleSeong, Daewoon, Sangyeob Han, Jaeyul Lee, Euimin Lee, Yoonseok Kim, Junsoo Lee, Mansik Jeon, and Jeehyun Kim. 2021. "Waterproof Galvanometer Scanner-Based Handheld Photoacoustic Microscopy Probe for Wide-Field Vasculature Imaging In Vivo" Photonics 8, no. 8: 305. https://doi.org/10.3390/photonics8080305
APA StyleSeong, D., Han, S., Lee, J., Lee, E., Kim, Y., Lee, J., Jeon, M., & Kim, J. (2021). Waterproof Galvanometer Scanner-Based Handheld Photoacoustic Microscopy Probe for Wide-Field Vasculature Imaging In Vivo. Photonics, 8(8), 305. https://doi.org/10.3390/photonics8080305






