Abstract
Is it possible to obtain good results in myopia of 2 or fewer diopters (D) with transepithelial photorefractive keratectomy (TransPRK) changing the optical zone and epithelium thickness? We retrospectively analyzed two groups of 296 eyes with a minimum follow-up of 4 months. Group A had 2 or less D, treated with an optical zone (OZ) 0.2 mm bigger than recommended, and a central epithelium thickness of 60 microns, and group B had 2 D to 5 D, with the recommended optical zone, and a 55-micron epithelium ablation at the center. The outcomes were not different between the two myopic ranges; the postop uncorrected distance visual acuity was 20/20 ± 4 in both groups (p = 0.2), which was −0.3 ± 0.8 lines worse than the preoperative corrected distance visual acuity in both groups (p = 0.5). The safety of the treatments resulted in a change of 0.0 ± 0.7 lines in the low myopia group, versus a gain of +0.1 ± 0.8 lines in the moderate myopia group (p = 0.1). The deviation from the intended target was −0.04 ± 0.33 D in the low myopia group and +0.07 ± 0.32 D in the moderate myopia group (p < 0.0001); the postoperative spherical equivalent was 0.00 ± 0.33 D in the low myopia group and +0.10 ± 0.31 D in the moderate myopia group (p < 0.0001). The postop refractive astigmatism was 0.32 ± 0.16 D in both groups (p = 0.5). In conclusion, the refractive and visual outcomes after TransPRK are comparable in low myopia changing the optical zone and epithelium thickness versus moderate myopia with standard optical zone and epithelium thickness.
1. Introduction
Transepithelial photorefractive keratectomy (TransPRK) using the AMARIS laser platform (Schwind eye-tech-solutions, Kleinostheim, Germany) is a one-step surface ablation consisting of a reverse aspheric photorefractive keratectomy (PRK) followed by a phototherapeutic keratectomy (PTK) to complete the stromal ablation [1,2]. The PTK of the epithelium is standardized by the software of the laser system, with an aspherical profile of 55 µm ablation at the center and 65 µm at the 4 mm periphery [3].
One of the first papers describing this technique was a theoretical work published by Arba and Awwad [4]. In that theoretical work [4], the authors described potential refractive deviations and the outcomes if the epithelium is thinner or thicker than that given by the default algorithm of the software, which was 55 µm at the center. In cases where the epithelium is thicker than the applied epithelial ablation profile, the resulting effective optical zone (OZ) will be reduced. In cases where the epithelium is thinner, there is some waste of tissue, as there is more ablation on the stroma than precalculated. For higher refractive corrections or larger OZs, not only is the maximum ablation deeper but also, the slope of the ablation is steeper (and maximum at the periphery of the OZ). Thus, in the cases that the actual epithelium was thicker than what is assumed in the model, the effects in the reduction in the achieved OZ (respect to the planned OZ) are smaller for higher refractive corrections or larger OZs. The larger the planned refractive correction, the less of a difference between the planned OZ and achieved OZ, and as with higher corrections or larger OZs, we get deeper ablation depths.
Based on this theoretical calculation, they recommend not performing TransPRK treatments (with the default epithelial profile settings) for myopia below −1.00 diopter (D). At the time of this study, it was not possible in the clinical situation to measure epithelium thickness with a diagnostic device in a reliable way. For that reason, we conducted a retrospective study for low myopia changing the epithelium thickness to 60 microns at the center and 70 microns at 4 mm at the periphery and enlarging the recommended OZ 0.2 mm and compared that with a group of moderate myopia treated with TransPRK treated in the standard manner of the software, not changing the epithelium thickness or the recommended OZ. We have not changed the increase in epithelium thickness from the center to the periphery to not change the shape of the ablation profile.
2. Materials and Methods
2.1. The Patients
The patients were treated between January 2017 and December 2019. We retrospectively selected two groups of eyes. The first group of consecutive eyes was treated with TransPRK with spherical equivalent (SEQ) of 2 diopters or less with a minimum follow up of 4 months (Group A) changing the epithelium thickness and optical zone with 296 eyes from a group, and then, a consecutive group with the same number of eyes was treated with the same technique without changing the OZ and epithelium thickness with myopia between 2 and 5 diopters with a minimum follow up of 4 months (Group B). Proper informed consent was obtained from each patient, and the data were de-identified for clinical data calculation and for publication. Patients were enrolled in the study if they had a preoperative corrected distance visual acuity (CDVA)p of 20/32, or better, using the standardized Snellen Charts with international standardization organization (ISO), stable refraction for 1 year before the study, and discontinued contact lens use for at least 2 weeks before the preoperative evaluation.
The exclusion criteria were systemic rheumatic or immunological illness, a preoperative central corneal thickness less than 440 microns systemic, a calculated postoperative corneal bed thickness less than 350 microns after ablation, previous ocular surgery, asymmetric topography of 1 D or more, or keratometry higher than 49 D.
2.2. Clinical Evaluation
Preoperatively, all patients underwent complete ophthalmological examination, with the determination of refractive defect under manifest and cycloplegic conditions measuring uncorrected distance visual acuity (UDVA) and best-corrected distance visual acuity (CDVA), pupillometry, corneal topography and Scheimpflug corneal pachymetry with a SIRIUS topo-tomographer (Costruzione Strumenti Oftalmici, Florence, Italy), aberrometry with the Peramis (SCHWIND eye-tech-solutions, Kleinostheim, Germany), and a fundus evaluation with a dilated pupil. Postoperative control was at 1 week, after 1 month, and after 4 months. One day postoperatively, the UDVA was measured, and the patient had a slit-lamp examination of the anterior segment. The same examinations as those performed preoperatively (except dilated fundoscopy unless warranted) were performed at 1 week, 1 month, and 4 months.
2.3. Treatment Plan
We used the surface ablation TransPRK with an aberration-free ablation pattern [5,6], planning the treatment with the ORK-CAM planning module [7,8]. The profile includes treatment in the one-step ablation of the epithelium and transepithelial PRK of a stromal correction. The profile is aspheric, compensating for the energy loss at the periphery [9,10]. The aspheric ablation of the epithelium is defined on a parabolic epithelial thickness profile (with default values of 55 microns at the center and 65 microns at the 4 mm periphery). We used Smart Pulse Technology (SPT) in all cases; the SPT algorithm is a piece of laser pulse technology software aimed at reducing the surface irregularity of the residual stromal bed at the end of treatment [11]. The laser spot has a truncated super-Gaussian spot shape; this spot profile is combined with a truncation at 14% of the peak intensity.
2.4. Surgical Technique
This retrospective cohort study was based on two consecutive case series of patients treated by a single surgeon (DdO), and TransPRK was used to correct myopic astigmatism, at Aurelios Augenlaserzentrum, Recklinghausen, Germany.
The sphere and cylinder values were entered into the laser based on the manifest refraction with nomogram adjustments based on the data of previously operated eyes using the Datagraph-med software (Wendelstein, Germany). Data for simulated keratometry at 3 mm were also used to compensate for the geometry of the eye [9].
The surgical technique has been described previously [12], and we used the AMARIS laser platform (SCHWIND eye-tech-solutions, Kleinostheim, Germany) with a 1050 Hz infrared eye tracker with simultaneous limbus, pupil, and torsion tracking [13,14], centering the ablation on the corneal vertex [15,16]. The laser uses a randomized flying-spot ablation pattern to minimize the thermal load of the treatment [17,18].
After the surgery, a soft bandage contact lens (Air Optix Night & Day, base curve 8.4) was applied for 4 days. The patients took Isoptomax eye drops (Novartis Pharma Gmbh, Basel, Switzerland) four times a day for 2 weeks, Fluormetholon eye drops (Fluoropos Ursapharm GmbH, Saarbrücken, Germany) three times per day for another 6 weeks, and preservative-free lubricants for 2 months as needed, and beyond, if necessary.
In the standard ablation used in group B of myopic eyes with 2 to 5 D of myopia, a defined epithelial thickness profile (55 microns in the center and 65 microns at 4 mm radial distance) was ablated. The epithelial profile was based on the normal epithelium, as well as on the refractive neutrality of an epithelial ablation [19,20,21,22,23,24,25,26,27,28,29,30,31,32].
In group A, with 2 D or less, the epithelial profile ablation was changed to 60 microns at the center and 70 microns at 4 mm. At this point, we did not measure the epithelium thickness. As the amount of correction ≤2 D was small, we wanted to obtain a minimum OZ of 6.5 mm, knowing that, theoretically, if the epithelium is thicker than 55 microns at the center, we obtain a smaller OZ or, in the worst case, a very small correction, perhaps without clinical correction. Therefore, we decided to use an OZ 0.2 mm bigger and plan a thicker epithelial profile so that we could assume that the treatment was deep enough to penetrate the epithelium. The only disadvantage of this choice was the waste of some tissue, as we went deeper than necessary, but this potential waste may not be clinically relevant for small corrections.
The epithelium thickness for the low myopia group has been changed to 60//70 representing merely 5 µm everywhere from the default setting (55//65). This way the ablation adds a 5 µm buffer at all locations (resembling a 5 µm flat PTK), but the profile (i.e., the refractive correction) remains the same.
The OZ has been enlarged by 0.2 mm (in diameter), representing merely a ~2µm increase in ablation depth. This way the ablation adds a 2 µm buffer at all locations and increases the ablation zone by 0.2 mm (resembling a 2 µm flat PTK with a tapered TZ), but the profile (i.e., the refractive correction) remains the same.
The ablation rate is higher in epithelium than in stroma, and it increases with the stromal depth. This ablation rate is also compensated by the laser system [33], and if we change the epithelium thickness to 5 microns deeper, it will, theoretically, ablate stroma where it should ablate epithelium. As this occurs on the entire surface, the final curvature of the surface will be the same.
2.5. Excimer Laser
The laser ablation algorithm used a flying-spot delivery system that operates at 1050 Hz with a super-Gaussian beam profile of 0.54 mm full width-half maximum [3]. It works with an inverted sequentialization of the epithelium profile and aspherical correction without breaks. Depending on the planned refractive correction, approximately 80% of the corneal ablation was performed with a high fluence level (~440 mJ/cm2) and 20%, using a low fluence level (~300 mJ/cm2) for fine correction and smoothing the ablated area [11]. The Spot placement was randomized to prevent heat buildup between laser pulses [17,18,34]. An aspiration system, with laminar flow dynamics, was incorporated to reduce debris and heat build-up.
2.6. Data Analysis
Refractive and visual outcomes were analyzed using the Excel software (Microsoft Corp. Redmond, Washington). The logMAR visual acuities were converted to Snellen acuities for data reporting, using the Visual Acuity Conversion Chart of the Journal of Cataract & Refractive Surgery. A p value less than 0.05 was considered statistically significant. Data for up to 4 months postoperatively are reported here. The normality of the samples was assessed using the back-of-the-envelope and the quantil-quantil methods. The intergroups comparisons were assessed using unpaired Student’s t-tests, whereas preoperative to postoperative changes were assessed using paired Student’s t-tests.
3. Results
3.1. Demographics
Table 1 summarizes the values used for both groups. Age, astigmatism magnitude, and CDVA were (as expected) not normally distributed in either group; whereas TZ and ablation depth were also not normally distributed in the low myopia group.

Table 1.
Demographic data of the two groups.
3.2. Efficacy
The efficacy of the treatments was not different between the two myopic groups (Figure 1). The preop CDVA was 20/19 ± 4 in the low myopia group and 20/18 ± 3 in the moderate myopia group (p = 0.1); the postop UDVA was 20/20 ± 4 in both groups (p = 0.2). The postop UDVA was −0.3 ± 0.8 lines worse than the preop CDVA in both groups (p = 0.5) (Figure 2). This corresponds to 94% ± 13% efficacy index for the low myopia group and 95% ± 15% efficacy index for the moderate myopia group (p = 0.3).
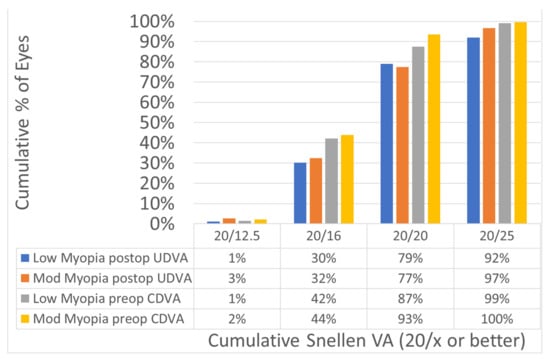
Figure 1.
Efficacy comparing the preoperatively corrected distance visual acuity (CDVA) with the postoperatively uncorrected distance visual acuity (UDVA). The efficacy of the treatments was not different between the two myopic groups: 30% of the eyes in the low myopia and 32% of the eyes in the moderate myopia achieved UDVA of 20/16 or better postoperatively.
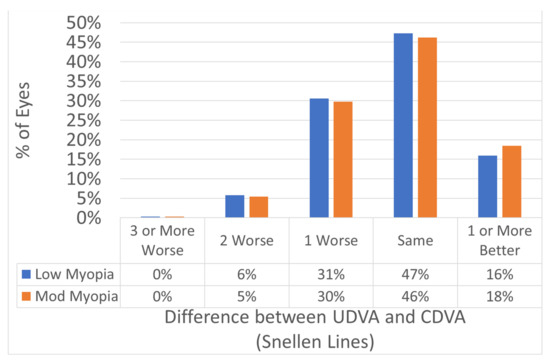
Figure 2.
Efficacy expressed in Snellen lines. The postop UDVA was −0.3 ± SD 0.8 lines worse than the preop CDVA in both groups (p = 0.5). In 94% of the eyes the postoperative UDVA was within 1 line of the preoperative CDVA.
3.3. Safety
The safety of the treatments was not different between the two myopic ranges (Figure 3). The change in CDVA from preop to postop was 0.0 ± 0.7 lines in the low myopia group, and +0.1 ± 0.8 lines gain in the moderate myopia group (p = 0.1). This corresponds to 101% ± 18% safety index for the low myopia group and 103% ± 22% safety index for the moderate myopia group (p = 0.1).
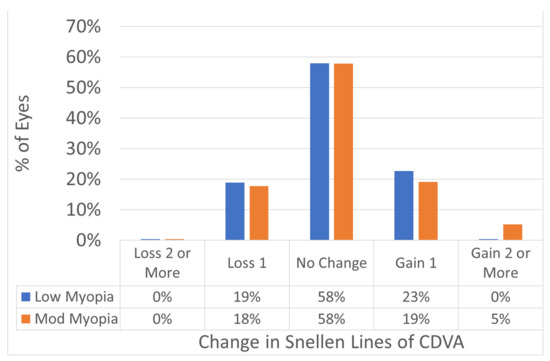
Figure 3.
Safety change for CDVA preoperatively versus postoperatively in Snellen lines. The change in CDVA from preop to postop was 0.0 ± SD 0.7 lines in the low myopia group, and +0.1 ± SD 0.8 lines gain in the moderate myopia group (p = 0.1). No eye lost 2 or more lines of CDVA in either group.
3.4. Predictability
The predictability of the treatments was not different between the two myopic ranges (Figure 4, Figure 5 and Figure 6). The scattergram of achieved vs. attempted correction was very similar for both groups (Figure 4). The deviation from the intended target was −0.04 ± 0.33 D in the low myopia group, and +0.07 ± 0.32 D in the moderate myopia group (p < 0.0001); the postop SEQ was 0.00 ± 0.33 D in the low myopia group, and +0.10 ± 0.31 D in the moderate myopia group (p < 0.0001) (Figure 5). Postop refractive astigmatism was 0.32 ± 0.16 D in both groups (p = 0.5) (Figure 6). The SEQ did not change in a relevant manner between 1M and 4M in either group (Figure 7).
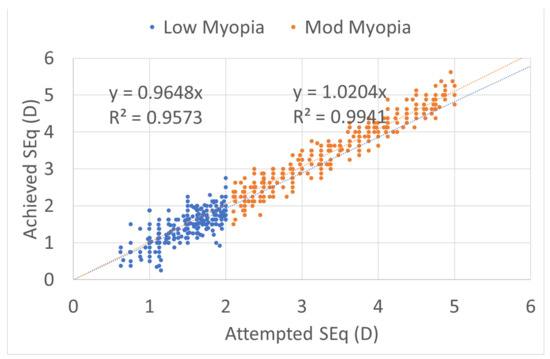
Figure 4.
Predictability achieved versus attempted spherical equivalent (SEQ) for both myopic groups, low myopia with a small undercorrection, and moderate myopia with a small overcorrection, regression analysis and R2 coefficient of determination in both groups near to 1. The predictability of the treatments was not different between the two myopic ranges. The scattergram of achieved vs. attempted correction was very similar for both groups.
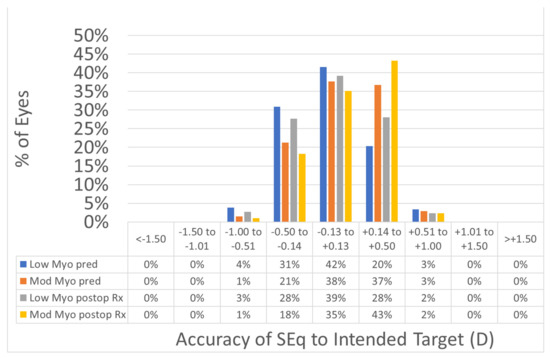
Figure 5.
Accuracy expressed in SEQ deviation from the intended versus achieved. The predictability of the treatments was not different between the two myopic ranges. The deviation from the intended target was −0.04 ± 0.33 D in the low myopia group, and +0.07 ± 0.32 D in the moderate myopia group (p < 0.0001); the postop SEQ was 0.00 ± 0.33 D in the low myopia group, and +0.10 ± 0.31 D in the moderate myopia group (p < 0.0001). In 94% of the eyes in the low myopia group and 96% of the eyes in the moderate myopia group, the SEQ was within 0.5D from the intended target.
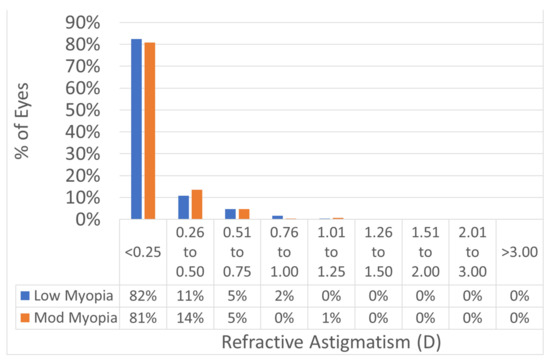
Figure 6.
Postoperative refractive astigmatism. The predictability of the treatments was not different between the two myopic ranges. Postop refractive astigmatism was 0.32 ± 0.16 D in both groups (p = 0.5). In 93% of the eyes in the low myopia group and 94% of the eyes in the moderate myopia group, the postoperative refractive astigmatism was within 0.5D from the intended target.
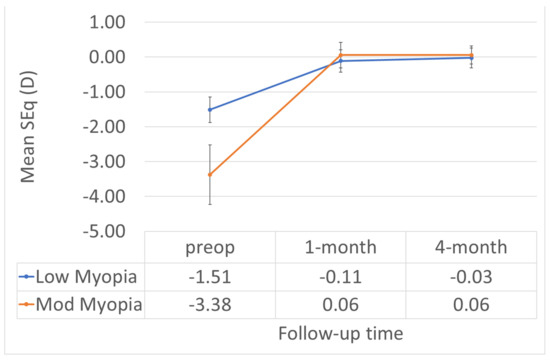
Figure 7.
The SEQ did not change in a relevant manner between 1M and 4M in either group.
3.5. Adverse Events
Neither adverse events nor complications were observed intra- or postoperatively.
4. Discussion
Many groups have reported the efficacy and safety of TransPRK procedures [1,35,36,37,38,39,40]. The manufacturer does not recommend treating very small amounts or refractive errors (below 0.75 D) with TransPRK, as, theoretically, the results could be largely affected by the patient’s epithelial profile. As we wanted to treat small amounts of myopia with TransPRK, we changed the standard, as we see many advantages with the TransPRK method, and performed some changes in the treatment. We always used an OZ 0.2 mm bigger than what we would have selected for, e.g., a PRK treatment and used a theoretically thicker epithelium. This change is like performing an aspherical phototherapeutic change in the cornea, which can waste more tissue than necessary, as we might go deeper than needed but still obtain the desired OZ. If we change the epithelium thickness to 5 microns deeper, it will, theoretically, ablate stroma when it should ablate epithelium. As this occurs on the entire surface, the final curvature of the surface will be the same. Therefore, it does not theoretically change the refractive result.
The epithelium thickness recommended by the software is based on published studies. Diverse studies have found that, centrally, the epithelium is between 52 and 54 microns, with a standard deviation of 7 microns, which has been proved by confocal microscopy [41], optical coherence tomography [42], and high-frequency ultrasound [20].
The AMARIS standard epithelial ablation algorithm may cause, in some cases, more stroma to be ablated than necessary if the epithelium is thinner than the precalculated value, and, in cases where the epithelium is thicker, a smaller optical zone [4]. This effect will be appreciated in small amounts of correction, which is why we decided to make some changes in the low myopia group. We planned a thicker epithelium for the aspheric PTK component and used a larger OZ. It seems that this change has no detrimental effect on the outcomes, as our results show. In any case, there is wasted tissue when the actual corneal epithelial profile is thinner than the applied epithelial ablation profile. However, we avoid the achieved optical zone being reduced when the actual corneal epithelial profile is thicker than the applied epithelial ablation profile, and additional refractive errors are potentially induced when the actual difference center-to-periphery in the corneal epithelial profile is different than that in the applied epithelial ablation profile. Jun et al. investigated the outcomes of mechanical PRK and TransPRK for myopia of 2 D or lower, treating with a mean OZ of 7.1 mm; there were no significant differences in the postoperative visual acuity and refractive error [43]. As the authors also mention, the application of a large OZ in cornea refractive surgery provides several advantages, such as reduced postoperative night vision disturbances and halos, fewer optical aberrations, and fewer regressions.
With the TransPRK method, the diameter of epithelial removal is calculated to match the ablation zone of the refractive part, thus decreasing the wound surface and speeding up the healing process in comparison to PRK or LASEK (laser epithelial keratomileusis), resulting in a faster visual recovery. Lee et al. [44] reported that the epithelium regrowth was the fastest in the TransPRK-treated group, with a mean time of 2.5 days, with 3 days in the PRK group, and 3.5 days in the LASEK group. With the SPT, a smooth corneal surface after ablation may lead to faster and better vision recovery [11,45].
Transepithelial approaches are aimed at modifying the cornea across the epithelium, allowing, theoretically, higher correspondence between the anterior corneal surface and corneal topography and the ablation profile compared to other refractive surgery techniques performed on deeper stromal layers of the cornea [39]. Therefore, the likelihood of error reduces to the difference in the photoablation rate between the stroma and epithelial tissue. This difference (approximately 20% higher in epithelium) is negligible when a small amount of tissue is involved. Jun et al. have recently performed a study regarding transepithelial PRK and the inter-individual epithelial thickness profile variability and the associated refractive effect if we used the standard epithelial thickness for all the cases; they found that the results were effective for thin and thick epithelium corneas, but the astigmatism was less corrected in eyes with thicker epithelium corneas [46].
In terms of astigmatic corrections, the mean asymmetry reported for the epithelial thickness of the normal population (if not considered in the profile) corresponds to 0.24 D of residual astigmatism. In corneas with important toricity (leading to corneal astigmatism), the epithelial layer may also exhibit a different toricity than the underlying Bowman’s membrane. Clinically significant reduction in the amount of anterior astigmatism has been reported when moving from the anterior epithelial (air/tear film) interface to the Bowman’s layer surface, in an in vivo study using slit scanning topography. This partial compensation demonstrates that there are azimuthal differences in the epithelial thickness distribution. As the epithelial thickness profile along the steepest meridian may differ from the thickness profile along the flattest meridian. This would depend on the epithelial ablation profile and the underlying epithelial toricty. The application of a rotationally symmetrical transepithelial profile on a toric corneal surface may also induce lenticules of wasted tissue (or variations in the achieved OZ) with an oval perimeter. All these uncertainties may reduce the precision of astigmatic correction and limit any benefit provided by TransPRK ablations.
While this is not a big problem for the spherical component marginal amounts of coma (decentered epithelium thinning compared to visual axis) and astigmatism (different epithelial profile in all four cardinal directions) may be induced (with a mean value of ∼0.25 D worse astigmatism correction with TransPRK for normal populations, but peaks of up to 0.63 D).
In the future, the advent of high-resolution OCT technique could allow proper representation of the epithelial layer, leading to customized epithelial ablations.
Using radial symmetric epithelial ablation profiles the risk of extra ∼0.25 D of residual astigmatism (up to ±0.5 D) shall be considered.
We retrospectively analyzed and compare the outcomes of almost 600 eyes after TransPRK, divided into 296 consecutively treated eyes with low myopia (up to 2D of myopia) and 296 consecutive eyes with moderate myopia (from 2D to 5D of myopia). As a general rule, the two eyes of normal subjects are very similar and using both eyes of subjects to increase the statistical power of the analysis is a controversial methodology. We included 296 consecutive eyes irrespective of whether two eyes of the same subject were included. For (two) 296 eyes cohorts (meaning at least 148 patients at each cohort) the effect is likely negligible. Further, this “virtual increase in statistical power” would actually emphasize (potentially bias) type I errors (false positive by rejection of a true null hypothesis). Our series show no differences among groups (despite the fact that both eyes of the patients were included).
In the current study, the deviation from the intended target was −0.04 ± 0.33 D, in the low myopia group, and +0.07 ± 0.32 D in the moderate myopia group (p < 0.0001); the postop SEQ was 0.00 ± 0.33 D, in the low myopia group, and +0.10 ± 0.31 D in the moderate myopia group (p < 0.0001). These results may have actually been affected by the changes in epithelium thickness profile and enlarging the OZ on the final refractive outcomes. This can only be done by comparing to a 3rd and 4th cohorts of low and moderate myopia treated with PRK instead of TransPRK. We have not followed this approach, since TransPRK is the preferred method in the clinic. We have, however, referred to previous works [43,46] which deal with the same aspects from a different perspective. Jun et al. [43] comparing PRK and TransPRK in low myopia, and other work [46] comparing TransPRK using the standard epithelium setting of 55//65 µm on eyes with actual central epithelial thickness <50 µm vs. >60 µm.
Among the major drawbacks of surface ablation are immediate postoperative discomfort and slower visual acuity recovery; however, the results appear to be comparable by 1 month after surgery [47,48].
The results for patient’s satisfaction score, glare, or halo would have provided a more comprehensive view on the comparison. We did not administer any patient reported outcomes questionnaire.
The efficacy and safety were evaluated only based on VA. The proper analysis is based on Figure 2 and Figure 3, where the preoperative CDVA as baseline is subtracted. Both revealed no differences between groups. Figure 1 suffers from the fact that preop CDVA may have been different between the groups.
Recent reports using the same laser platform [35], as well as our own experience [49] using surface ablation with this laser and this cohort, found refractive stability after surface ablation from 6 weeks to 3 months postoperatively.
There are new possibilities to measure the epithelium thickness preoperatively with new optical coherence devices so that we can provide the exact amount of the epithelium thickness to be ablated. Future studies will determine if we obtain clinically, and significantly, better results.
5. Conclusions
TransPRK with small amounts of myopia using a larger optical zone and a thicker epithelial profile is as safe and effective as TransPRK for moderate amounts of myopia using standard optical zone and epithelial thickness.
Author Contributions
Conceptualization, D.d.O.; methodology, D.d.O. and S.A.-M.; software, D.v.R. and S.A.-M.; validation, S.A.-M. and D.d.O.; formal analysis, D.d.O. and D.v.R.; investigation, D.d.O. and D.v.R.; resources, D.d.O. and D.v.R.; data curation, D.d.O. and D.v.R.; writing—original draft preparation, D.d.O.; writing—review and editing, D.d.O. and S.A.-M.; visualization, D.d.O. and S.A.-M.; supervision, D.d.O. and S.A.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. As the study was retrospective, the Institutional Board decided that we did not need an ethical approval number.
Informed Consent Statement
Informed consent was completed for all patients.
Data Availability Statement
All data were fully anonymized and are available upon request.
Conflicts of Interest
D.d.O. is a Consultant for Schwind eye-tech-solutions and S.A.-M. is an employee of Schwind eye-tech-solutions. D.v.R. has no conflict of interest.
References
- Aslanides, I.M.; Padroni, S.; Arba Mosquera, S.; Ioannides, A.; Mukherjee, A. Comparison of Single-Step Reverse Transepithelial All-Surface Laser Ablation (ASLA) to Alcohol-Assisted Photorefractive Keratectomy. Clin. Ophthalmol. 2012, 6, 973–980. [Google Scholar] [CrossRef]
- Lin, D.T.C.; Holland, S.P.; Verma, S.; Hogden, J.; Arba-Mosquera, S. Postoperative Corneal Asphericity in Low, Moderate, and High Myopic Eyes After Transepithelial PRK Using a New Pulse Allocation. J. Refract. Surg. 2017, 33, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Arba-Mosquera, S.; Hollerbach, T. Ablation Resolution in Laser Corneal Refractive Surgery: The Dual Fluence Concept of the AMARIS Platform. Adv. Opt. Technol. 2010, 2010, 1–13. [Google Scholar] [CrossRef]
- Arba Mosquera, S.; Awwad, S.T. Theoretical Analyses of the Refractive Implications of Transepithelial PRK Ablations. Br. J. Ophthalmol. 2013, 97, 905–911. [Google Scholar] [CrossRef]
- de Ortueta, D.; Arba Mosquera, S. Mathematical Properties of Asphericity: A Method to Calculate with Asphericities. J. Refract. Surg. 2008, 24, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Arba-Mosquera, S.; Merayo-Lloves, J.; de Ortueta, D. Asphericity Analysis Using Corneal Wavefront and Topographic Meridional Fits. J. Biomed. Opt. 2010, 15, 028003. [Google Scholar] [CrossRef][Green Version]
- Arba-Mosquera, S.; de Ortueta, D. Analysis of Optimized Profiles for “aberration-Free” Refractive Surgery. Ophthalmic Physiol. Opt. 2009, 29, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Arbelaez, M.C.; Vidal, C.; Jabri, B.A.; Arba Mosquera, S. LASIK for Myopia with Aspheric “Aberration Neutral” Ablations Using the ESIRIS Laser System. J. Refract. Surg. 2009, 25, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Arba-Mosquera, S.; de Ortueta, D. Geometrical Analysis of the Loss of Ablation Efficiency at Non-Normal Incidence. Opt. Express 2021, 16, 3877–3895. [Google Scholar] [CrossRef]
- de Ortueta, D.; Mosquera, S.A.; Haecker, C. Theoretical Considerations on the Hyperopic Shift Effect Observed When Treating Negative Cylinder in Laser Refractive Surgery. J. Emmetropia 2010, 1, 23–28. [Google Scholar]
- Vinciguerra, P.; Camesasca, F.I.; Vinciguerra, R.; Arba-Mosquera, S.; Torres, I.; Morenghi, E.; Randleman, J.B. Advanced Surface Ablation With a New Software for the Reduction of Ablation Irregularities. J. Refract. Surg. 2017, 33, 89–95. [Google Scholar] [CrossRef] [PubMed]
- de Ortueta, D. Transepithelial Photorefractive Keratektomy after a Clear Lens Exchange. Vision 2021, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Arba-Mosquera, S.; Merayo-Lloves, J.; de Ortueta, D. Clinical Effects of Pure Cyclotorsional Errors during Refractive Surgery. Invest. Ophthalmol. Vis. Sci. 2008, 49, 4828–4836. [Google Scholar] [CrossRef] [PubMed]
- Adib-Moghaddam, S.; Soleyman-Jahi, S.; Tofighi, S.; Tefagh, G.; Arba-Mosquera, S.; Kontadakis, G.; Kymionis, G.D. Factors Associated With Ocular Cyclotorsion Detected by High-Speed Dual-Detection Eye Tracker During Single-Step Transepithelial Photorefractive Keratectomy. J. Refract. Surg. 2018, 34, 736–744. [Google Scholar] [CrossRef]
- de Ortueta, D.; Schreyger, F.D. Centration on the Cornea Vertex Normal during Hyperopic Refractive Photoablation Using Videokeratoscopy. J. Refract. Surg. 2007, 23, 198–200. [Google Scholar] [CrossRef]
- Arbelaez, M.C.; Vidal, C.; Arba-Mosquera, S. Clinical Outcomes of Corneal Vertex versus Central Pupil References with Aberration-Free Ablation Strategies and LASIK. Invest. Ophthalmol. Vis. Sci. 2008, 49, 5287–5294. [Google Scholar] [CrossRef]
- Brunsmann, U.; Sauer, U.; Dressler, K.; Triefenbach, N.; Mosquera, S.A. Minimisation of the Thermal Load of the Ablation in High-Speed Laser Corneal Refractive Surgery: The ‘Intelligent Thermal Effect Control’ of the AMARIS Platform. J. Refract. Surg. 2010, 57, 466–479. [Google Scholar] [CrossRef]
- de Ortueta, D.; Magnago, T.; Triefenbach, N.; Arba Mosquera, S.; Sauer, U.; Brunsmann, U. In Vivo Measurements of Thermal Load during Ablation in High-Speed Laser Corneal Refractive Surgery. J. Refract. Surg. 2012, 28, 53–58. [Google Scholar] [CrossRef]
- Simon, G.; Legeais, J.M.; Parel, J.M. [Optical power of the corneal epithelium]. J. Fr. Ophtalmol. 1993, 16, 41–47. [Google Scholar]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M.; Silverman, R.H.; Coleman, D.J. Epithelial Thickness in the Normal Cornea: Three-Dimensional Display with Artemis Very High-Frequency Digital Ultrasound. J. Refract. Surg. 2008, 24, 571–581. [Google Scholar] [CrossRef]
- Feng, Y.; Simpson, T.L. Comparison of Human Central Cornea and Limbus in Vivo Using Optical Coherence Tomography. Optom. Vis. Sci. 2005, 82, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Simpson, T.L. Corneal, Limbal, and Conjunctival Epithelial Thickness from Optical Coherence Tomography. Optom. Vis. Sci. 2008, 85, E880–E883. [Google Scholar] [CrossRef]
- Gatinel, D.; Racine, L.; Hoang-Xuan, T. Contribution of the Corneal Epithelium to Anterior Corneal Topography in Patients Having Myopic Photorefractive Keratectomy. J. Cataract Refract. Surg. 2007, 33, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Jones, L.; Simpson, T. Thickness Mapping of the Cornea and Epithelium Using Optical Coherence Tomography. Optom. Vis. Sci. 2008, 85, E963–E976. [Google Scholar] [CrossRef]
- Salah-Mabed, I.; Saad, A.; Gatinel, D. Topography of the Corneal Epithelium and Bowman Layer in Low to Moderately Myopic Eyes. J. Cataract Refract. Surg. 2016, 42, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tao, A.; Jiang, H.; Xu, Z.; Perez, V.; Wang, J. Vertical and Horizontal Corneal Epithelial Thickness Profile Using Ultra-High Resolution and Long Scan Depth Optical Coherence Tomography. PLoS ONE 2014, 9, e97962. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Gobbe, M.; Archer, T.J.; Silverman, R.H.; Coleman, D.J. Epithelial, Stromal, and Total Corneal Thickness in Keratoconus: Three-Dimensional Display with Artemis Very-High Frequency Digital Ultrasound. J. Refract. Surg. 2010, 26, 259–271. [Google Scholar] [CrossRef]
- Patel, S.V.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. Confocal Microscopy in Vivo in Corneas of Long-Term Contact Lens Wearers. Invest. Ophthalmol. Vis. Sci. 2002, 43, 995–1003. [Google Scholar]
- Ivarsen, A.; Fledelius, W.; Hjortdal, J.Ø. Three-Year Changes in Epithelial and Stromal Thickness after PRK or LASIK for High Myopia. Invest. Ophthalmol. Vis. Sci. 2009, 50, 2061. [Google Scholar] [CrossRef]
- Vega-Estrada, A.; Mimouni, M.; Espla, E.; Alió del Barrio, J.; Alio, J.L. Corneal Epithelial Thickness Intrasubject Repeatability and Its Relation With Visual Limitation in Keratoconus. Am. J. Ophthalmol. 2019, 200, 255–262. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Srivannaboon, S.; Gobbe, M.; Archer, T.; Silverman, R.H.; Sutton, H.; Coleman, D.J. Epithelial Thickness Profile Changes Induced by Myopic LASIK as Measured by Artemis Very High-Frequency Digital Ultrasound. J. Refract. Surg. 2009, 25, 444–450. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M. Corneal Epithelial Thickness Profile in the Diagnosis of Keratoconus. J. Refract. Surg. 2009, 25, 604–610. [Google Scholar] [CrossRef]
- Seiler, T.; Kriegerowski, M.; Schnoy, N.; Bende, T. Ablation Rate of Human Corneal Epithelium and Bowman’s Layer with the Excimer Laser (193 Nm). Refract. Corneal Surg. 1990, 6, 99–102. [Google Scholar] [CrossRef]
- Arba-Mosquera, S.; Shraiki, M. Analysis of the PMMA and Cornea Temperature Rise during Excimer Laser Ablation. J. Mod. Opt. 2010, 57, 400–407. [Google Scholar] [CrossRef]
- Kaluzny, B.J.; Cieslinska, I.; Mosquera, S.A.; Verma, S. Single-Step Transepithelial PRK vs Alcohol-Assisted PRK in Myopia and Compound Myopic Astigmatism Correction. Medicine 2016, 95, e1993. [Google Scholar] [CrossRef]
- Antonios, R.; Abdul Fattah, M.; Arba Mosquera, S.; Abiad, B.H.; Sleiman, K.; Awwad, S.T. Single-Step Transepithelial versus Alcohol-Assisted Photorefractive Keratectomy in the Treatment of High Myopia: A Comparative Evaluation over 12 Months. Br. J. Ophthalmol. 2017, 101, 1106–1112. [Google Scholar] [CrossRef]
- Camellin, M.; Arba Mosquera, S. Simultaneous Aspheric Wavefront-Guided Transepithelial Photorefractive Keratectomy and Phototherapeutic Keratectomy to Correct Aberrations and Refractive Errors after Corneal Surgery. J. Cataract Refract. Surg. 2010, 36, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Adib-Moghaddam, S.; Soleyman-Jahi, S.; Adili-Aghdam, F.; Arba Mosquera, S.; Hoorshad, N.; Tofighi, S. Single-Step Transepithelial Photorefractive Keratectomy in High Myopia: Qualitative and Quantitative Visual Functions. Int. J. Ophthalmol. 2017, 10, 445–452. [Google Scholar] [CrossRef] [PubMed]
- de Ortueta, D.; von Rüden, D.; Verma, S.; Magnago, T.; Arba-Mosquera, S. Transepithelial Photorefractive Keratectomy in Moderate to High Astigmatism With a Non-Wavefront–Guided Aberration-Neutral Ablation Profile. J. Refract. Surg. 2018, 34, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, A.; Fahed, D.; Khalil, K.; Dunia, I.; Menassa, J.; El Rami, H.; Chlela, E.; Fahed, S. Transepithelial Photorefractive Keratectomy: Clinical Results. J. Cataract Refract. Surg. 2011, 37, 1852–1857. [Google Scholar] [CrossRef]
- Eckard, A.; Stave, J.; Guthoff, R.F. In Vivo Investigations of the Corneal Epithelium with the Confocal Rostock Laser Scanning Microscope (RLSM). Cornea 2006, 25, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Sin, S.; Simpson, T.L. The Repeatability of Corneal and Corneal Epithelial Thickness Measurements Using Optical Coherence Tomography. Optom. Vis. Sci. 2006, 83, 360–365. [Google Scholar] [CrossRef]
- Jun, I.; Yong Kang, D.S.; Arba-Mosquera, S.; Jean, S.K.; Kim, E.K.; Seo, K.Y.; Kim, T.-I. Clinical Outcomes of Mechanical and Transepithelial Photorefractive Keratectomy in Low Myopia with a Large Ablation Zone. J. Cataract Refract. Surg. 2019, 45, 977–984. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, K.S.; Kim, J.K.; Kim, H.C.; Seo, K.R.; Kim, E.K. Epithelial Healing and Clinical Outcomes in Excimer Laser Photorefractive Surgery Following Three Epithelial Removal Techniques: Mechanical, Alcohol, and Excimer Laser. Am. J. Ophthalmol. 2005, 139, 56–63. [Google Scholar] [CrossRef]
- Lin, D.T.C.; Holland, S.P.; Verma, S.; Hogden, J.; Arba-Mosquera, S. Immediate and Short Term Visual Recovery after SmartSurf(ACE) Photorefractive Keratectomy. J. Optom. 2019, 12, 240–247. [Google Scholar] [CrossRef]
- Jun, I.; Kang, D.S.Y.; Arba-Mosquera, S.; Kim, E.K.; Seo, K.Y.; Kim, T.-I. Clinical Outcomes of Transepithelial Photorefractive Keratectomy According to Epithelial Thickness. J. Refract. Surg. 2018, 34, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-J.; Yan, H.-T.; Nakahori, Y. Evaluation of the Effectiveness of Laser in Situ Keratomileusis and Photorefractive Keratectomy for Myopia: A Meta-Analysis. J. Med. Investig. 2003, 50, 180–186. [Google Scholar] [PubMed]
- Luger, M.H.A.; Ewering, T.; Arba-Mosquera, S. Myopia Correction with Transepithelial Photorefractive Keratectomy versus Femtosecond-Assisted Laser in Situ Keratomileusis: One-Year Case-Matched Analysis. J. Cataract Refract. Surg. 2016, 42, 1579–1587. [Google Scholar] [CrossRef]
- de Ortueta, D.; von Rüden, D. Transepithelial photorefractive keratectomy: Results and clinical experiences. Ophthalmologe 2018, 116, 534–541. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).