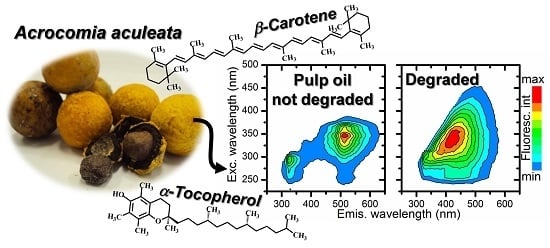
Optical Analysis of the Oils Obtained from Acrocomia aculeata (Jacq.) Lodd: Mapping Absorption-Emission Profiles in an Induced Oxidation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Collection, Oil Extraction and Degradation
2.2. Fatty Acid Composition
2.3. Physicochemical Characterization
2.4. Total Polyphenol and Carotenoids Content
2.5. Antioxidant Activity
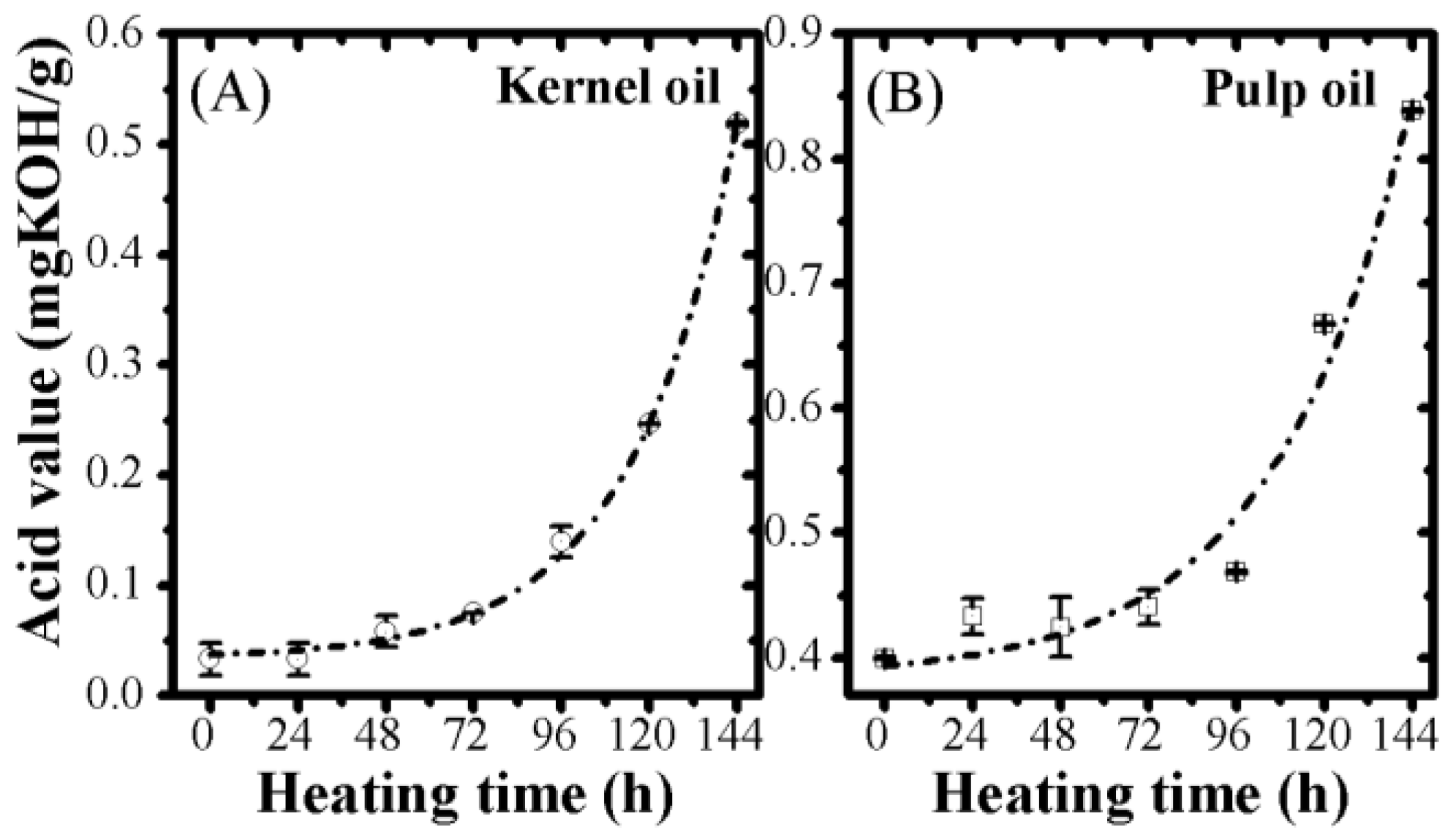
2.6. Acid Value
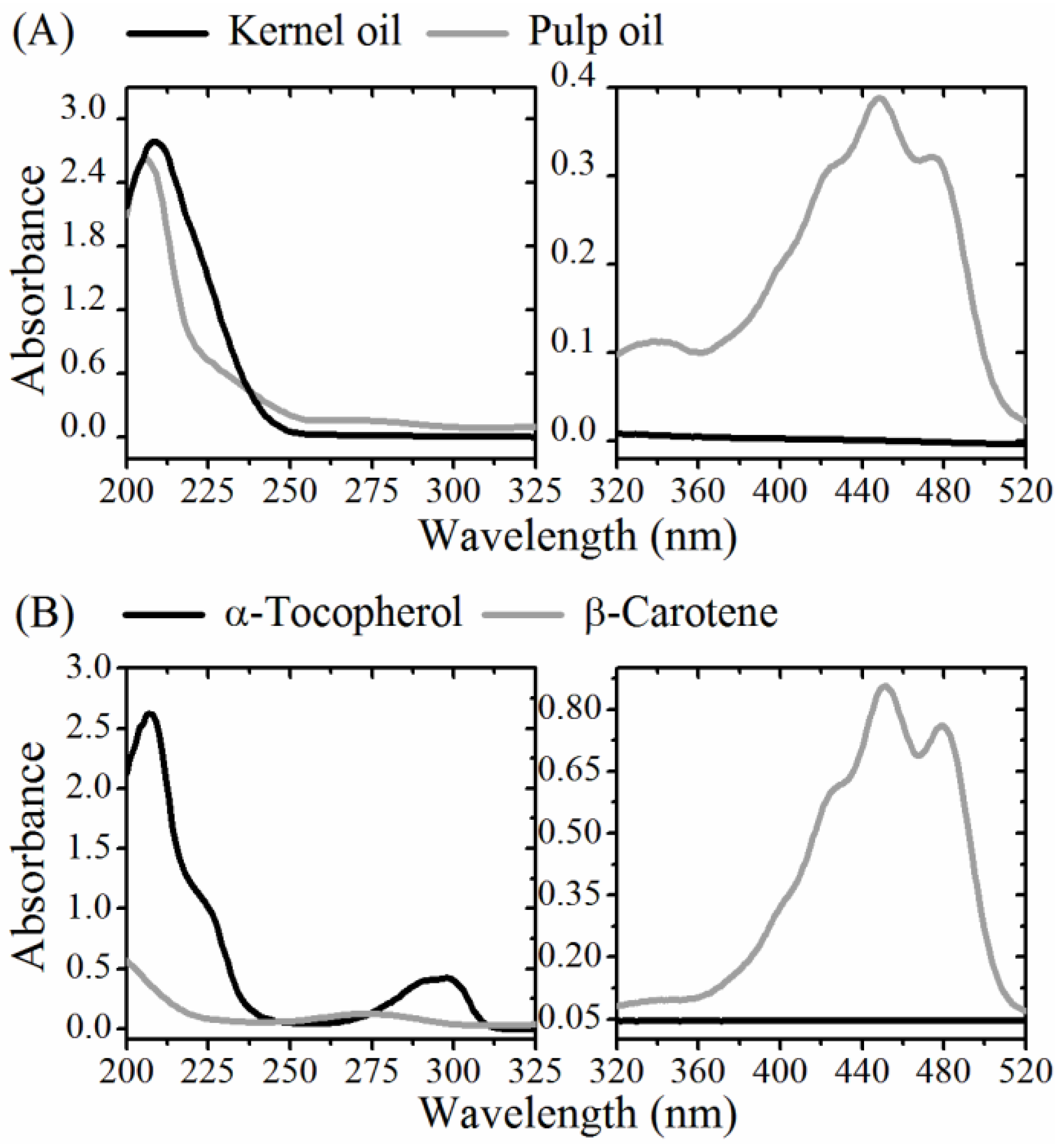
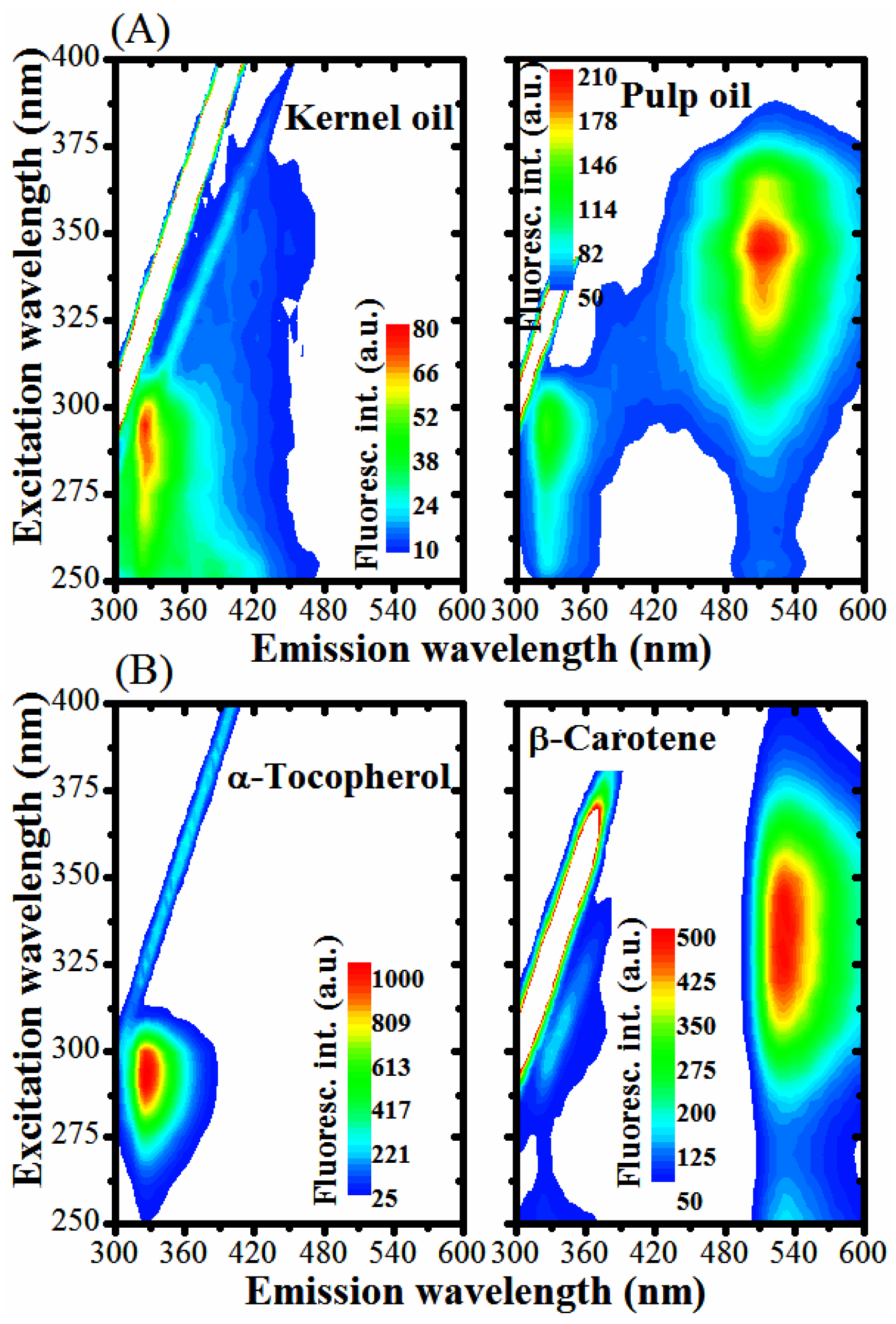
2.7. UV-Vis Absorption and Fluorescence
3. Results and Discussions
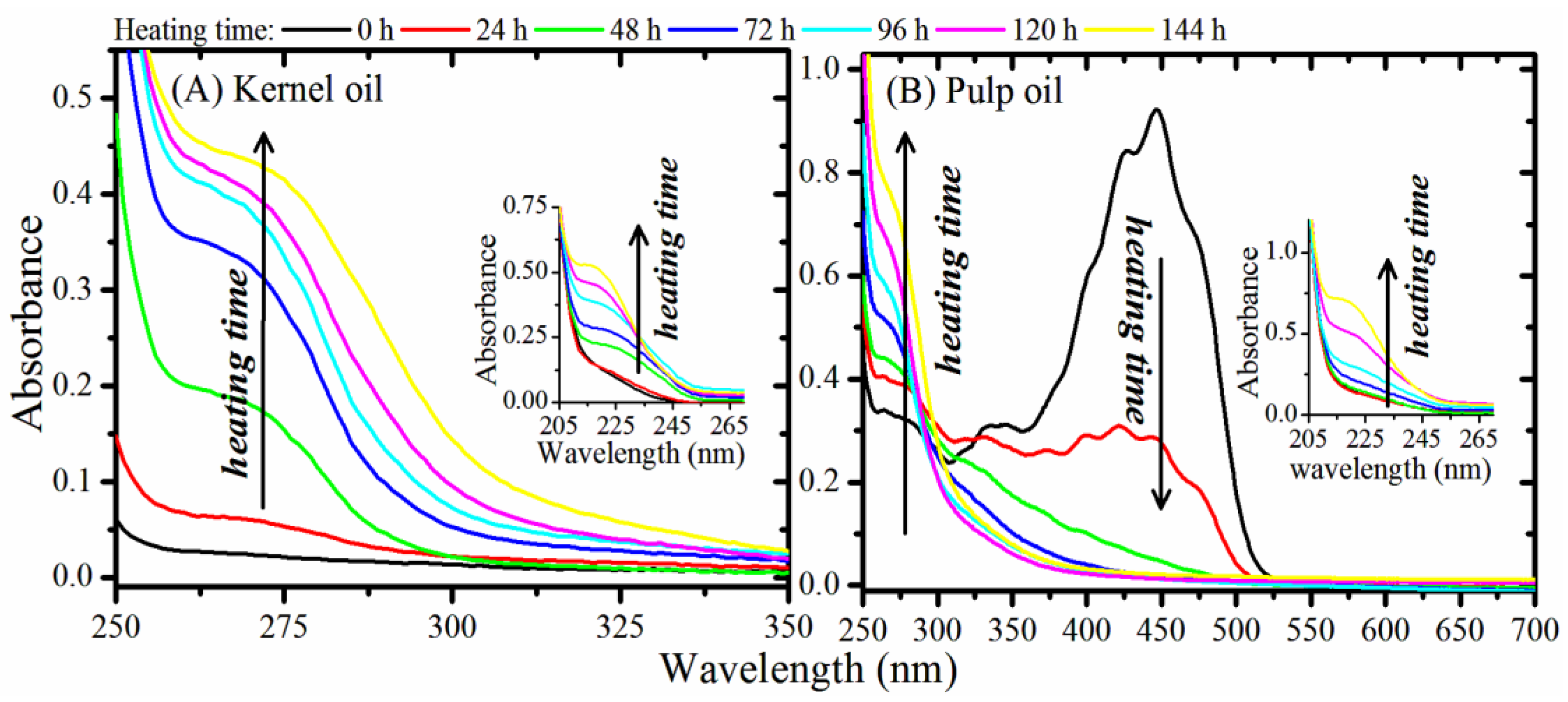
3.1. Oxidative Stability Determination
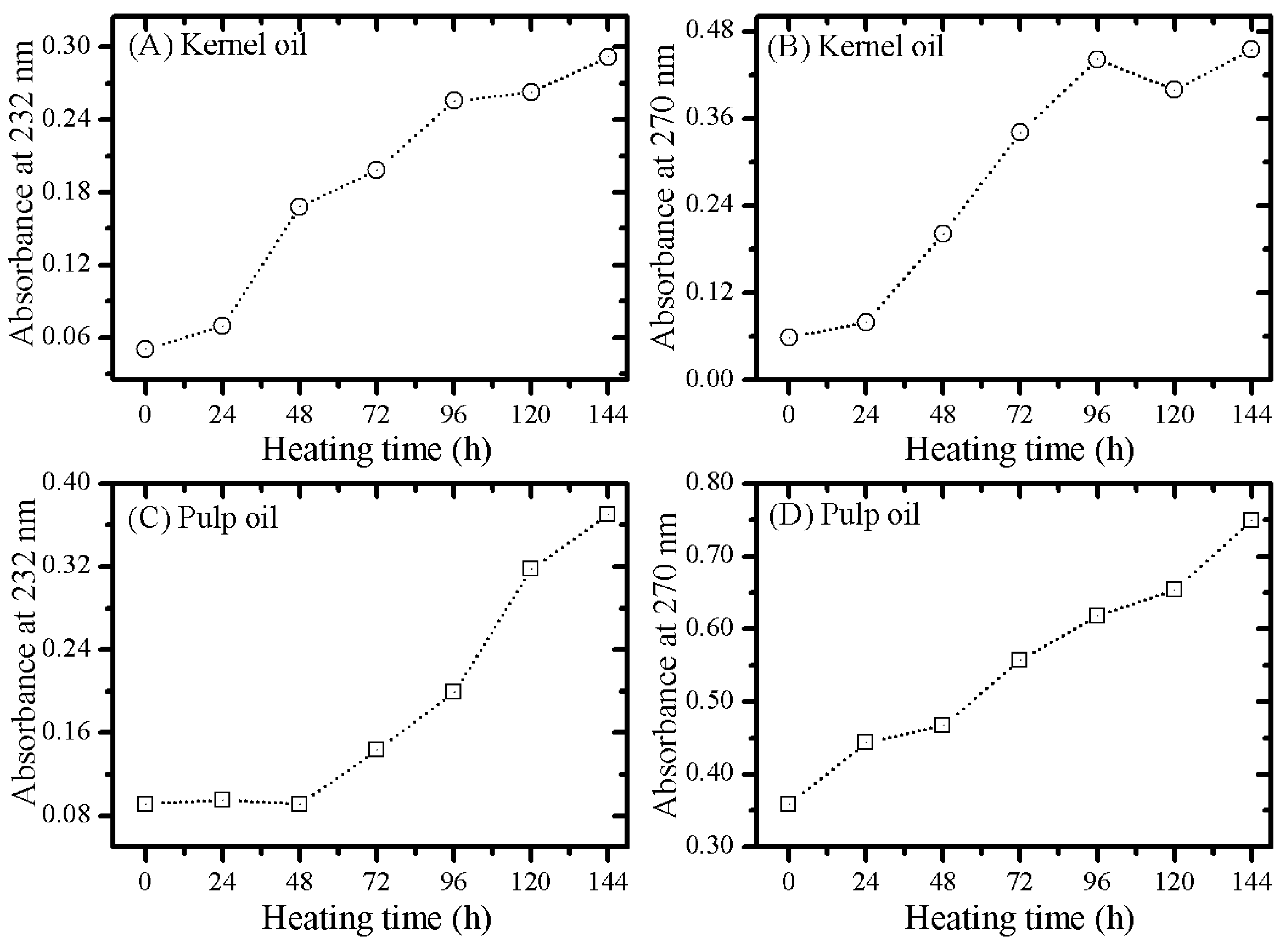
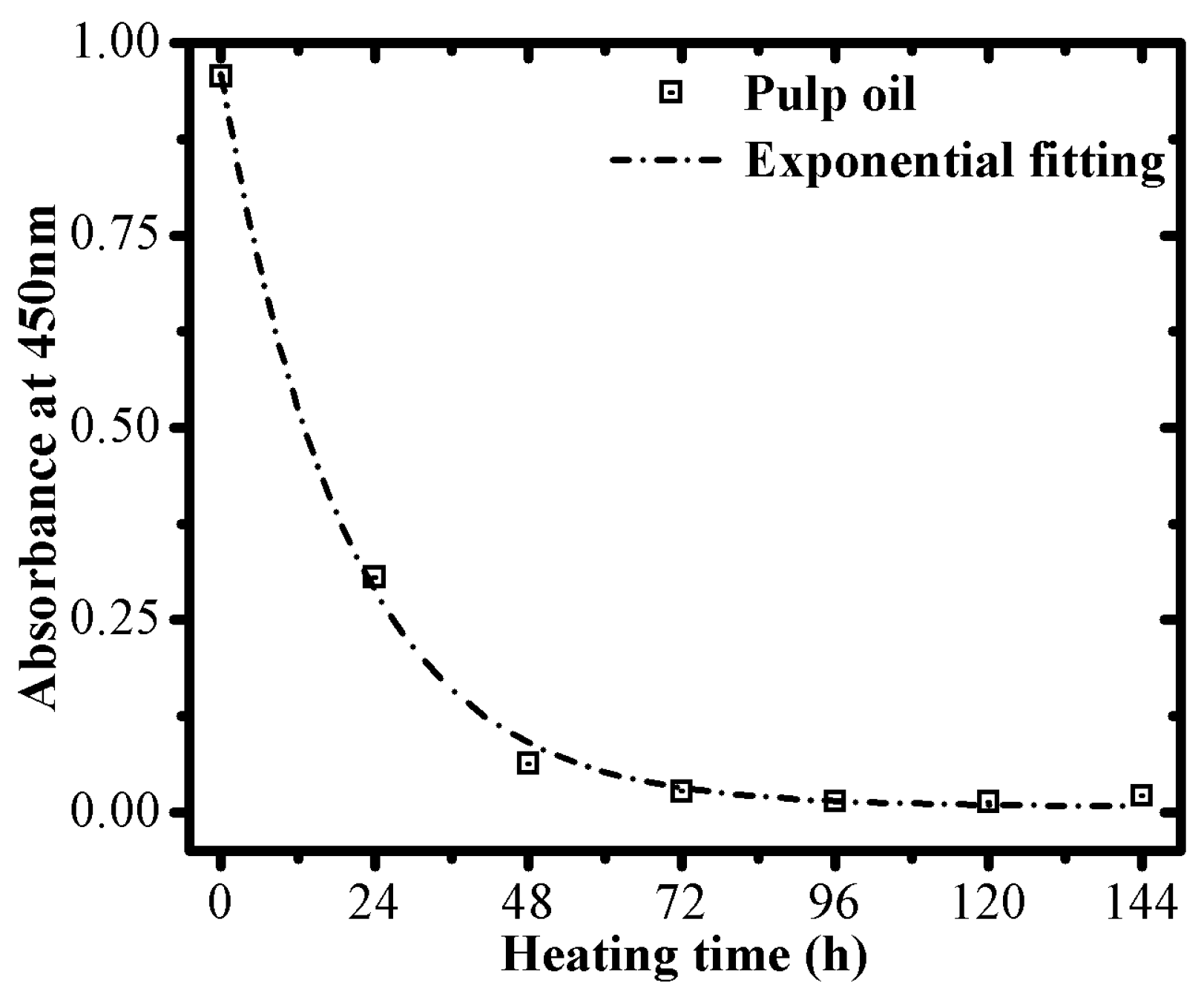
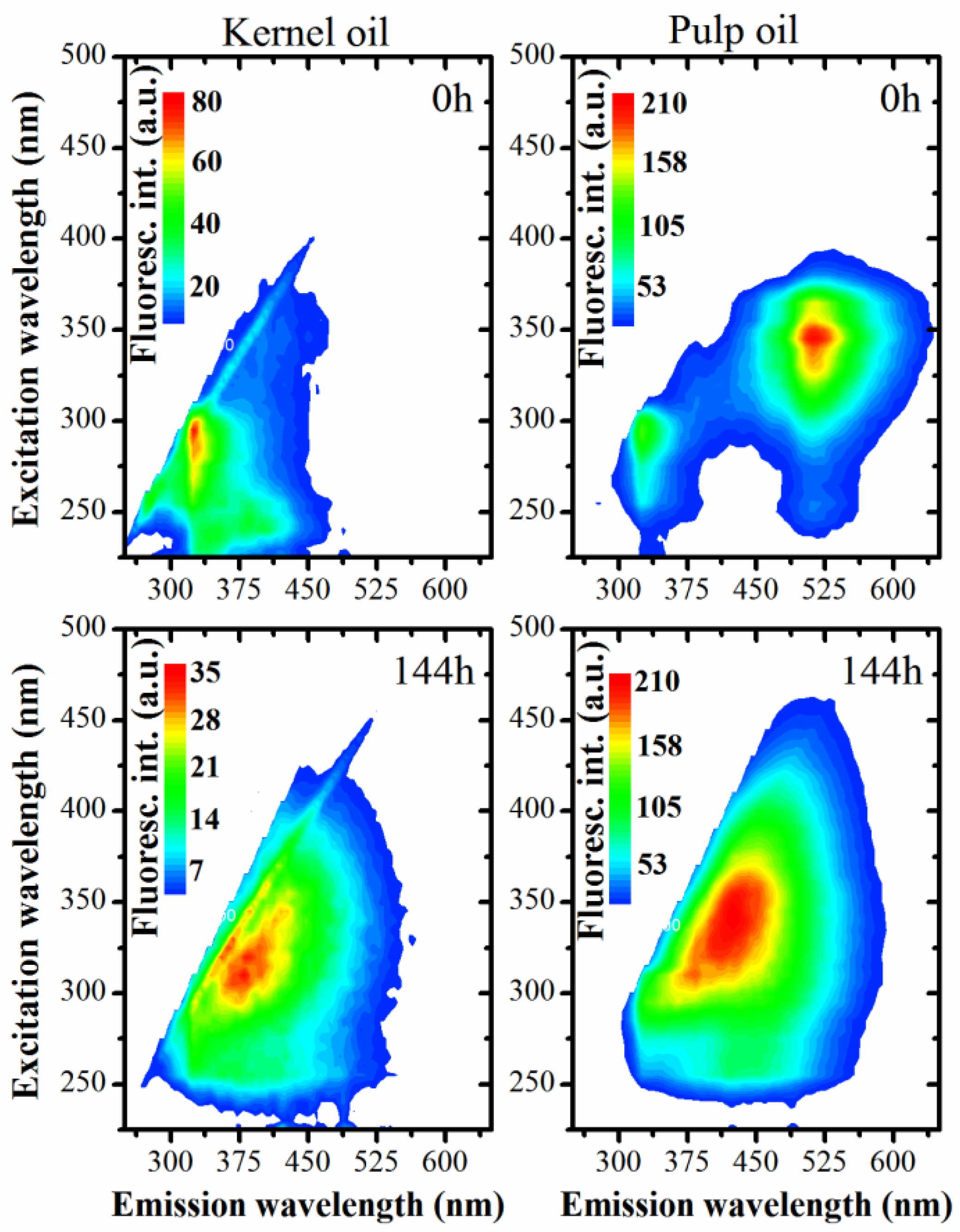
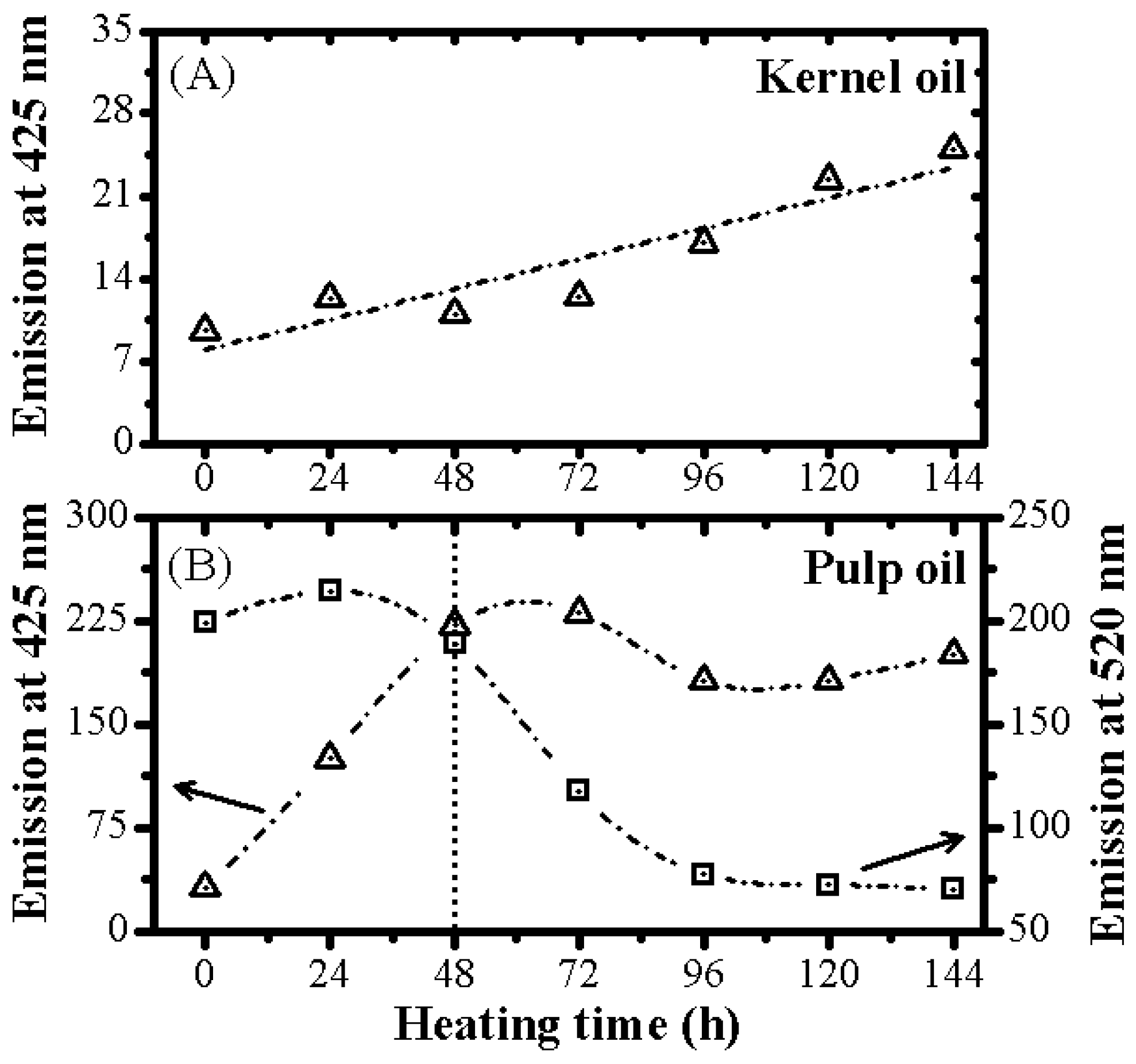
3.2. Optical Monitoring of Oil Degradation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oliveira, V.B.; Yamada, L.T.; Fagg, C.W.; Brandão, M.G.L. Native foods from Brazilian biodiversity as a source of bioactive compounds. Food Res. Int. 2012, 48, 170–179. [Google Scholar] [CrossRef]
- Ciconini, G.; Favaro, S.P.; Roscoe, R.; Miranda, C.H.B.; Tapeti, C.F.; Miyahira, M.A.M.; Bearari, L.; Galvani, F.; Borsato, A.V.; Colnago, L.A.; et al. Biometry and oil contents of Acrocomia aculeata fruits from the Cerrados and Pantanal biomes in Mato Grosso do Sul, Brazil. Ind. Crops Prod. 2013, 45, 208–214. [Google Scholar] [CrossRef]
- Ramos, M.I.L.; Siqueira, E.M.A.; Isomura, C.C.; Barbosa, A.M.J.; Arruda, S.F. Bocaiuva (Acrocomia aculeata (Jacq.) Lodd) improved vitamin A status in rats. J. Agric. Food Chem. 2007, 55, 3186–3190. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.C.; Jorge, N. Proximate composition of guariroba (Syagrus oleracea), jeriva (Syagrus romanzoffiana) and macauba (Acrocomia aculeata) palm fruits. Food Res. Int. 2011, 44, 2139–2142. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Jorge, N. Characterization of the Pulp and Kernel Oils from Syagrus oleracea, Syagrus romanzoffiana, and Acrocomia aculeata. J. Food Sci. 2011, 76, C1156–C1161. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.C.; Jorge, N. Fatty acids and bioactive compounds of the pulps and kernels of Brazilian palm species, guariroba (Syagrus oleraces), jeriva (Syagrus romanzoffiana) and macauba (Acrocomia aculeata). J. Sci. Food Agric. 2012, 92, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Traesel, G.K.; de Souza, J.C.; de Barros, A.L.; Souza, M.A.; Schmitz, W.O.; Muzzi, R.M.; Oesterreich, S.A.; Arena, A.C. Acute and subacute (28 days) oral toxicity assessment of the oil extracted from Acrocomia aculeata pulp in rats. Food Chem. Toxicol. 2014, 74, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Aguieiras, E.C.G.; Cavalcanti-Oliveira, E.D.; De Castro, A.M.; Langone, M.A.P.; Freire, D.M.G. Biodiesel production from Acrocomia aculeata acid oil by (enzyme/enzyme) hydroesterification process: Use of vegetable lipase and fermented solid as low-cost biocatalysts. Fuel 2014, 135, 315–321. [Google Scholar] [CrossRef]
- Lescano, C.H.; Oliveira, I.P.; Silva, L.R.; Baldivia, D.S.; Sanjinez-Argandoña, E.J.; Arruda, E.J.; Moraes, I.C.F.; Lima, F.F. Nutrients content, characterization and oil extraction from Acrocomia aculeata (Jacq.) Lodd. fruits. Afr. J. Food Sci. 2015, 9, 113–119. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Gomes, T.; Nasti, R.; Caponio, F.; Summo, C. Effects of free fatty acids on the oxidative processes in purified olive oil. Food Res. Int. 2010, 43, 1389–1394. [Google Scholar] [CrossRef]
- Pinheiro, I.; Luis, S.; Ferreira, C. Biodiesel: Parâmetros de qualidade e métodos analíticos. Quim. Nova 2009, 32, 1596–1608. [Google Scholar]
- Franco, M.N.; Galeano-Díaz, T.; Sánchez, J.; De Miguel, C.; Martín-Vertedor, D. Antioxidant capacity of the phenolic fraction and its effect on the oxidative stability of olive oil varieties grown in the southwest of Spain. Grasas y Aceites 2014, 65, e004. [Google Scholar]
- Mariod, A.; Ismail, M.; Rahman, N.F.A.; Matthaus, B. Stability of rice bran oil extracted by SFE and soxhlet methods during accelerated shelf-life storage. Grasas y Aceites 2014, 65, 1–10. [Google Scholar] [CrossRef]
- Gimeno, E.; Castellote, A.I.; Lamuela-Raventos, R.M.; De la Torre, M.C.; Lopez-Sabater, M.C. The effects of harvest and extraction methods on the antioxidant content (phenolics, alpha-tocopherol, and beta-carotene) in virgin olive oil. Food Chem. 2002, 78, 207–211. [Google Scholar] [CrossRef]
- Torres, M.; Martínez, M.; Pierantozzi, P.; Albanese, M.; Nasjleti, A.; Maestri, D. Contribution of compositional parameters to the oxidative stability of olive and walnut oil blends. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 755–762. [Google Scholar] [CrossRef]
- Dantas, M.B.; Albuquerque, A.R.; Barros, A.K.; Rodrigues Filho, M.G.; Antoniosi Filho, N.R.; Sinfrônio, F.S.M.; Rosenhaim, R.; Soledade, L.E.B.; Santos, I.M.G.; Souza, A.G. Evaluation of the oxidative stability of corn biodiesel. Fuel 2011, 90, 773–778. [Google Scholar] [CrossRef]
- Tena, N.; García-gonzález, D.L.; Aparicio, R. Evaluation of virgin olive oil thermal deterioration by fluorescence spectroscopy. J. Agric. Food Chem. 2009, 57, 10505–10511. [Google Scholar] [CrossRef] [PubMed]
- Nehdi, I.A. Cupressus sempervirens var. horizentalis seed oil: Chemical composition, physicochemical characteristics, and utilizations. Ind. Crops Prod. 2013, 41, 381–385. [Google Scholar] [CrossRef]
- Cheikhousman, R.; Zude, M.; Bouveresse, D.J.R.; Léger, C.L.; Rutledge, D.N.; Birlouez-Aragon, I. Fluorescence spectroscopy for monitoring deterioration of extra virgin olive oil during heating. Anal. Bioanal. Chem. 2005, 382, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Priego-López, E.; Velasco, J.; Dobarganes, M.C.; Ramis-Ramos, G.; Luque De Castro, M.D. Focused microwave-assisted Soxhlet extraction: An expeditive approach for the isolation of lipids from sausage products. Food Chem. 2003, 83, 143–149. [Google Scholar] [CrossRef]
- Almeida, P.P.; Mezzomo, N.; Ferreira, S.R.S. Extraction of Mentha spicata L. Volatile Compounds: Evaluation of Process Parameters and Extract Composition. Food Bioprocess Technol. 2012, 5, 548–559. [Google Scholar] [CrossRef]
- Li, Z.; Yang, D.; Huang, M.; Hu, X.; Shen, J.; Zhao, Z.; Chen, J. Chrysomya megacephala (Fabricius) larvae: A new biodiesel resource. Appl. Energy 2012, 94, 349–354. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, L.M.J.; Gomes, P.B.; de Oliveira Godoy, R.L.; Pacheco, S.; do Monte, P.H.F.; de Carvalho, J.L.V.; Nutti, M.R.; Neves, A.C.L.; Vieira, A.C.R.A.; Ramos, S.R.R. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Vasconcelos, A.F.F.; de Godinho, O.E.S. Uso de métodos analíticos convencionados no estudo da autenticidade do óleo de copaíba. Quim. Nova 2002, 25, 1057–1060. [Google Scholar] [CrossRef]
- Cardoso, S.L. Photophysics of carotenoids and the antioxidant role of β-carotene. Quim. Nova 1997, 20, 535–540. [Google Scholar] [CrossRef]
- Romero, N.; Robert, P.; Masson, L.; Ortiz, J.; González, K.; Tapia, K.; Dobaganes, C. Effect of α-tocopherol, α-tocotrienol and Rosa mosqueta shell extract on the performance of antioxidant-stripped canola oil (Brassica sp.) at high temperature. Food Chem. 2007, 104, 383–389. [Google Scholar] [CrossRef]
- Liang, Y.C.; May, C.Y.; Foon, C.S.; Ngan, M.A.; Hock, C.C.; Basiron, Y. The effect of natural and synthetic antioxidants on the oxidative stability of palm diesel. Fuel 2006, 85, 867–870. [Google Scholar] [CrossRef]
- De Sousa, L.S.; de Moura, C.V.R.; de Oliveira, J.E.; de Moura, E.M. Use of natural antioxidants in soybean biodiesel. Fuel 2014, 134, 420–428. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Li, X.; Hu, C.; Li, M.; Liu, Z. A study on photoinduced electronic transition in complex of vitamin E and benzoquinone. J. Mol. Struct. THEOCHEM 2004, 674, 257–266. [Google Scholar]
- DeCoster, B.; Christensen, R.L.; Gebhard, R.; Lugtenburg, J.; Farhoosh, R.; Frank, H.A. Low-lying electronic states of carotenoids. Biochim. Biophys. Acta 1992, 1102, 107–114. [Google Scholar] [CrossRef]
- Navarro-García, G.; Bringas-Alvarado, L.; Pacheco-Aguilar, R.; Ortega-García, J. Oxidative resistance, carotenes, tocopherols and lipid profile of liver oil of the ray Rhinoptera steindechneri. J. Food Compos. Anal. 2004, 17, 699–706. [Google Scholar] [CrossRef]
- Qiu, D.; Shao, S.X.; Zhao, B.; Wu, Y.C.; Shi, L.F.; Zhou, J.C.; Chen, Z.R. Stability of β-carotene in thermal oils. J. Food Biochem. 2012, 36, 198–206. [Google Scholar] [CrossRef]
- Murador, D.C.; da Cunha, D.T.; de Rosso, V.V. Effects of cooking techniques on vegetable pigments: A meta-analytic approach to carotenoid and anthocyanin levels. Food Res. Int. 2014, 65, 177–183. [Google Scholar] [CrossRef]
- Silva, V.D.; Conceição, J.N.; Oliveira, I.P.; Lescano, C.H.; Muzzi, R.M.; Filho, O.P.S.; Conceição, E.C.; Casagrande, G.A.; Caires, A.R.L. Oxidative stability of Baru (Dipteryx alata Vogel) oil monitored by fluorescence and absorption spectroscopy. J. Spectrosc. 2015, 2015, 803705. [Google Scholar]
- Christensen, R.L.; Barney, E.A.; Broene, R.D.; Galinato, M.G.I.; Frank, H.A. Linear polyenes: Models for the spectroscopy and photophysics of carotenoids. Arch. Biochem. Biophys. 2004, 430, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, K.F.; Caires, A.R.L.; Silva, M.S.; Alcantara, G.B.; Oliveira, S.L. Endogenous fluorescence of biodiesel and products thereof: Investigation of the molecules responsible for this effect. Fuel 2014, 119, 120–128. [Google Scholar] [CrossRef]
| Fatty Acids | Pulp Oil | Kernel Oil |
|---|---|---|
| Caprylic (C8:0) | 0.06 ± 0.06 | 1.7 ± 0.2 |
| Capric (C10:0) | 0.07 ± 0.06 | 2.4 ± 0.4 |
| Lauric (C12:0) | 0.4 ± 0.2 | 50 ± 16 |
| Myristic (C14:0) | 0.29 ± 0.05 | 10.4 ± 0.5 |
| Palmitic (C16:0) | 30 ± 1 | 6 ± 2 |
| Palmitoleic (C16:1) | 2.4 ± 0.4 | - |
| Stearic (C18:0) | 2.7 ± 0.3 | 4 ± 1 |
| Oleic (C18:1) | 61.4 ± 0.3 | 17 ± 8 |
| Linoleic (C18:2) | 2 ± 1 | 2.7 ± 0.8 |
| Arachidic (C20:0) | 0.9 ± 0.7 | - |
| Σ saturated | 34.4 | 74.5 |
| Σ unsaturated | 65.8 | 19.7 |
| Parameters | Pulp Oil | Kernel Oil |
|---|---|---|
| Iodine value (g I2/100 g) | 75 ± 9 | 36 ± 3 |
| Water content (mg/kg) | 464 ± 3 | 476 ± 3 |
| Induction period (h) | >65 | 43 ± 2 |
| Kinematic viscosity (mm2/s) | 6.34 ± 0.02 | 13.76 ± 0.01 |
| Dynamic viscosity (mPa·s) | 5.22 ± 0.01 | 12.09 ± 0.01 |
| Specific mass (kg/m3) | 823.4 ± 0.6 | 878.65 ± 0.07 |
| Antioxidant Activity | Pulp Oil | Kernel Oil |
|---|---|---|
| DPPH (g sample/g DPPH) | 63 ± 8 | 56.80 ± 0.09 |
| ABTS (µg trolox/g oil) | 52 ± 3 | 9 ± 4 |
| Total Polyphenols (mg gallic acid/100 g) | 3.9 ± 0.3 | 0.70 ± 0.02 |
| Total Carotenoids (µg/g) | (49 ± 2) × 10 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Oliveira, I.P.; Correa, W.A.; Neves, P.V.; Silva, P.V.B.; Lescano, C.H.; Michels, F.S.; Passos, W.E.; Muzzi, R.M.; Oliveira, S.L.; Caires, A.R.L. Optical Analysis of the Oils Obtained from Acrocomia aculeata (Jacq.) Lodd: Mapping Absorption-Emission Profiles in an Induced Oxidation Process. Photonics 2017, 4, 3. https://doi.org/10.3390/photonics4010003
De Oliveira IP, Correa WA, Neves PV, Silva PVB, Lescano CH, Michels FS, Passos WE, Muzzi RM, Oliveira SL, Caires ARL. Optical Analysis of the Oils Obtained from Acrocomia aculeata (Jacq.) Lodd: Mapping Absorption-Emission Profiles in an Induced Oxidation Process. Photonics. 2017; 4(1):3. https://doi.org/10.3390/photonics4010003
Chicago/Turabian StyleDe Oliveira, Ivan P., Willian A. Correa, Priscila V. Neves, Perla V. B. Silva, Caroline H. Lescano, Flávio S. Michels, Wilson E. Passos, Rozanna M. Muzzi, Samuel L. Oliveira, and Anderson R. L. Caires. 2017. "Optical Analysis of the Oils Obtained from Acrocomia aculeata (Jacq.) Lodd: Mapping Absorption-Emission Profiles in an Induced Oxidation Process" Photonics 4, no. 1: 3. https://doi.org/10.3390/photonics4010003
APA StyleDe Oliveira, I. P., Correa, W. A., Neves, P. V., Silva, P. V. B., Lescano, C. H., Michels, F. S., Passos, W. E., Muzzi, R. M., Oliveira, S. L., & Caires, A. R. L. (2017). Optical Analysis of the Oils Obtained from Acrocomia aculeata (Jacq.) Lodd: Mapping Absorption-Emission Profiles in an Induced Oxidation Process. Photonics, 4(1), 3. https://doi.org/10.3390/photonics4010003