Numerical Analysis of an Ultra-Sensitive Optical Fiber for Hemoglobin Concentration Detection
Abstract
1. Introduction
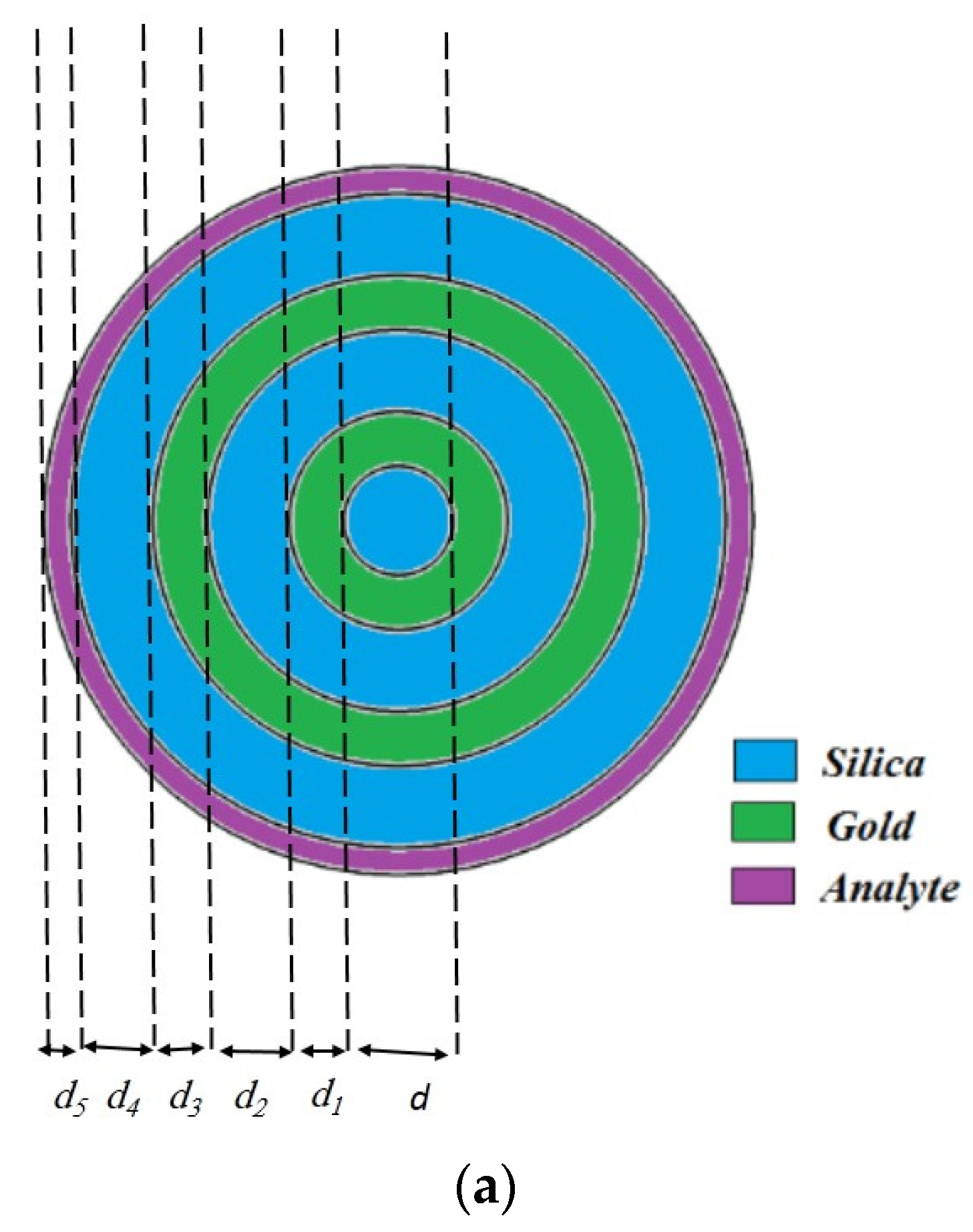
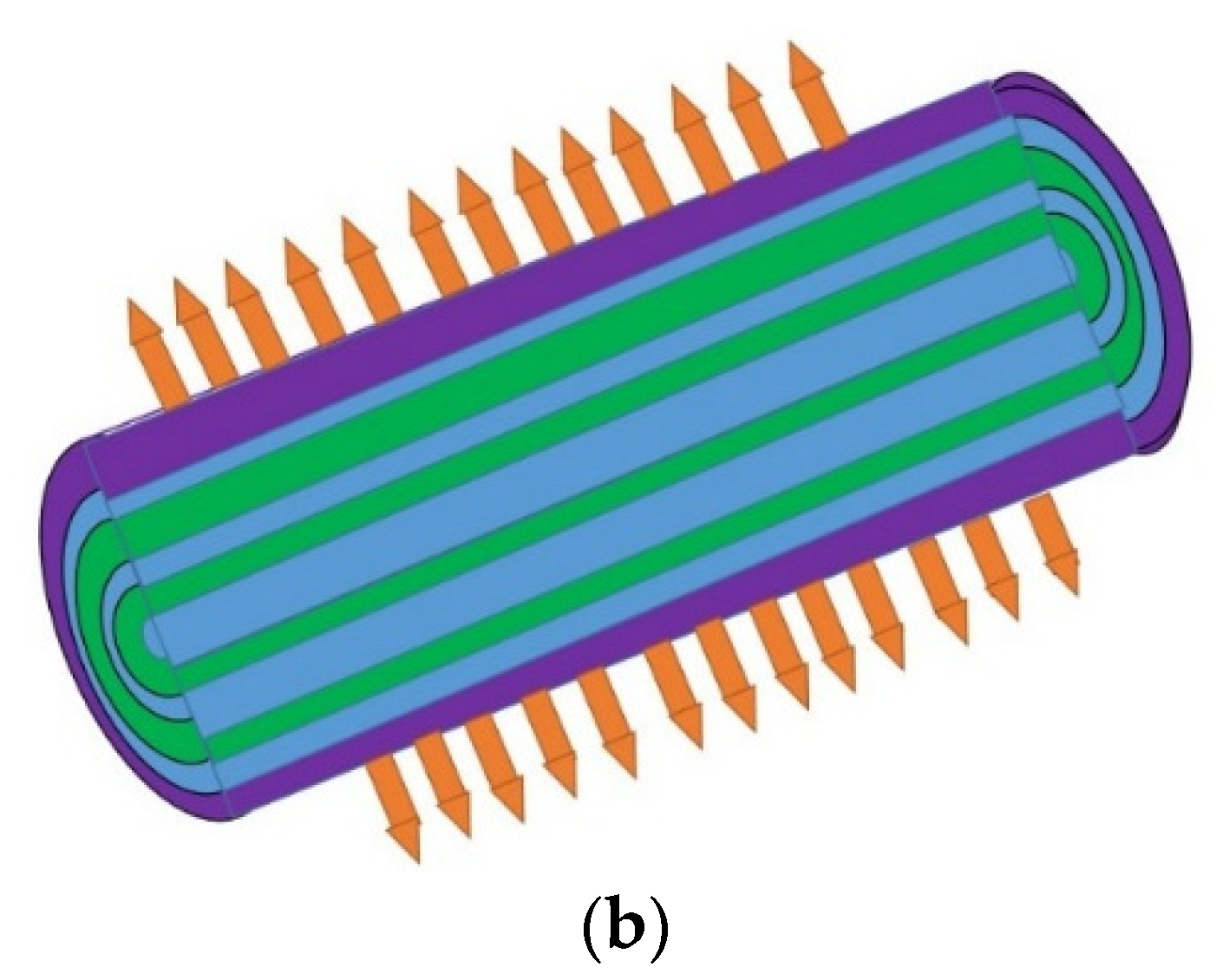
2. The Geometry of the Proposed OF
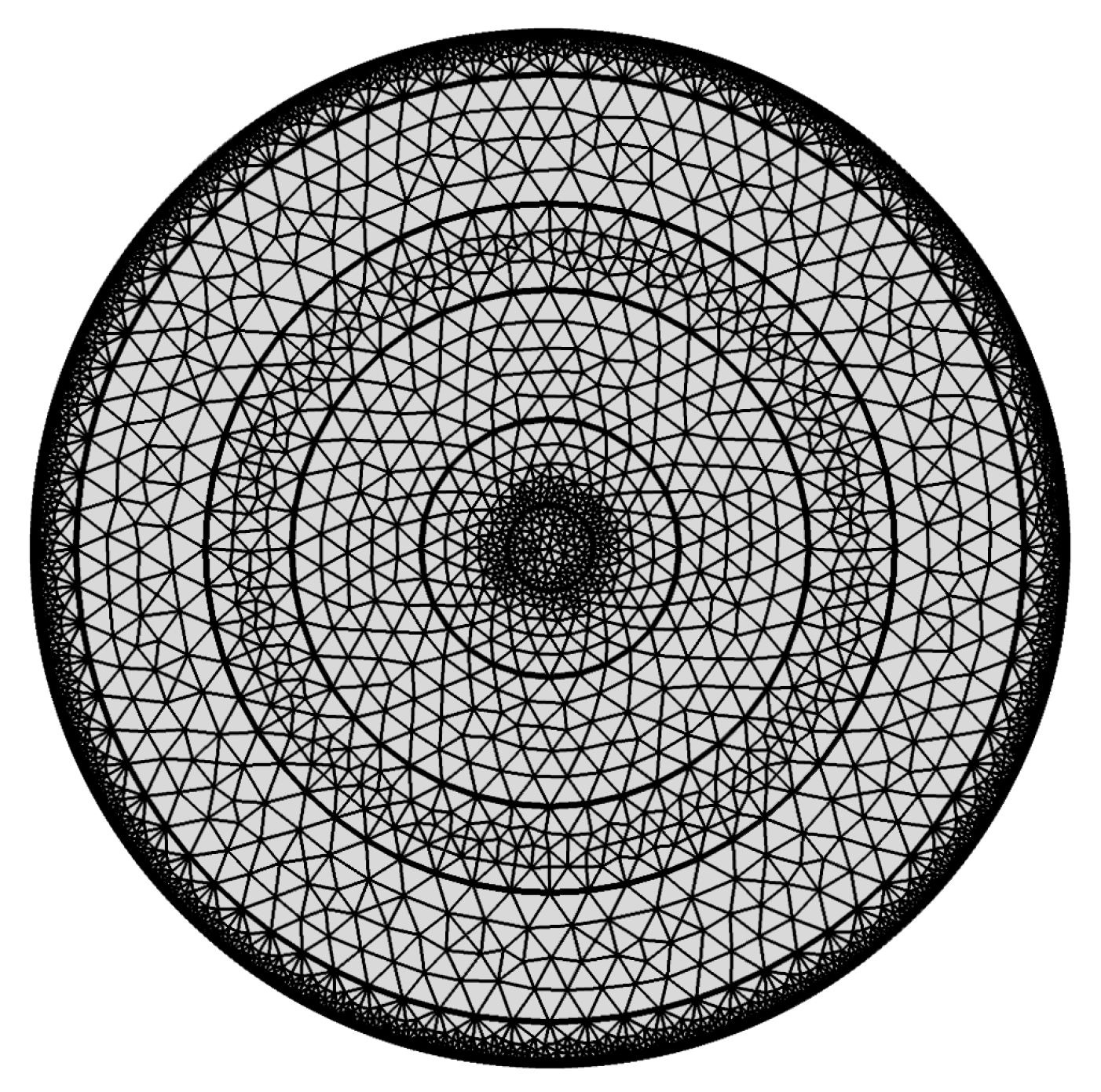
Refractive Index of the Layers and Meshing of the Proposed Structure
3. Mathematical Equations
4. Simulation Results
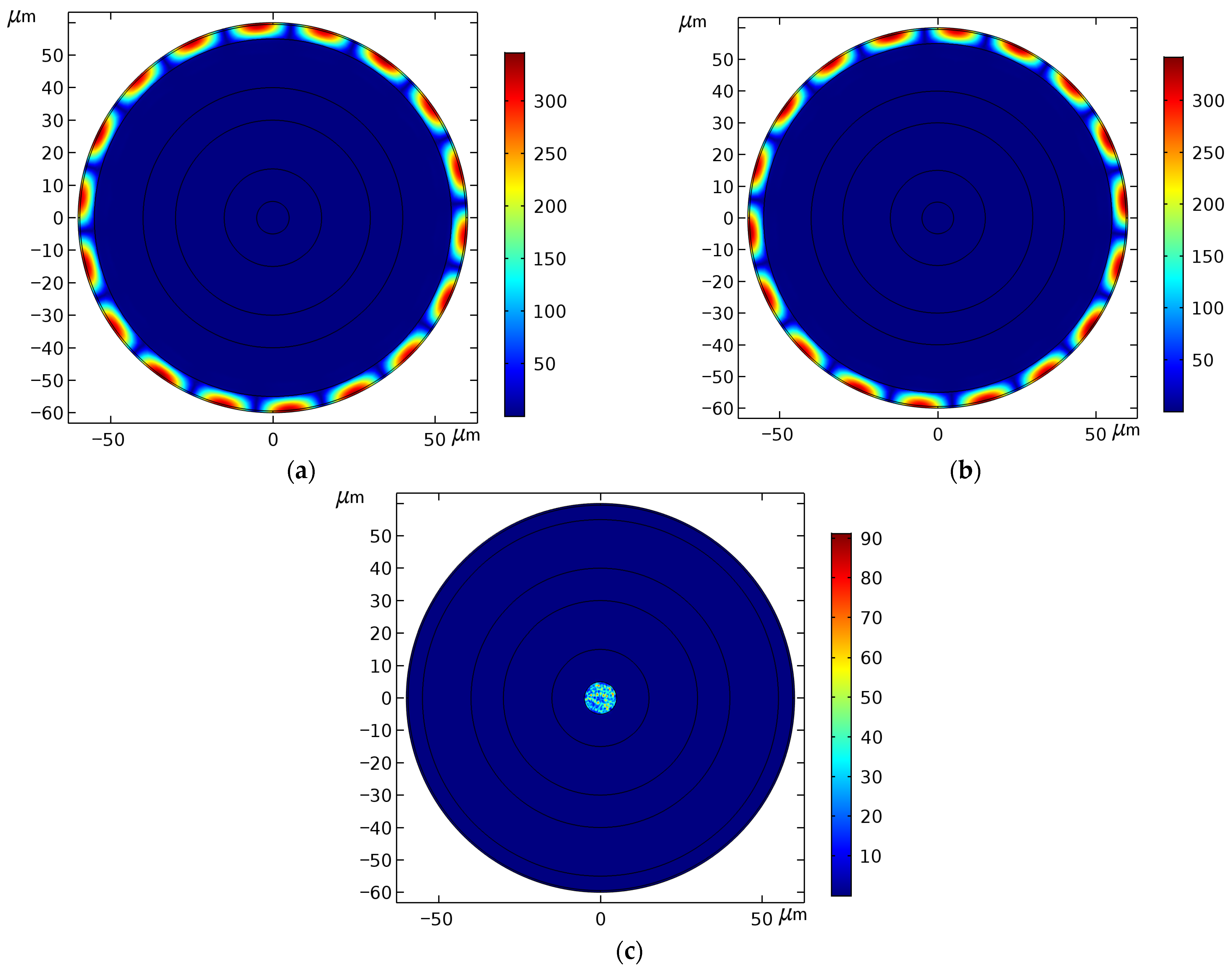
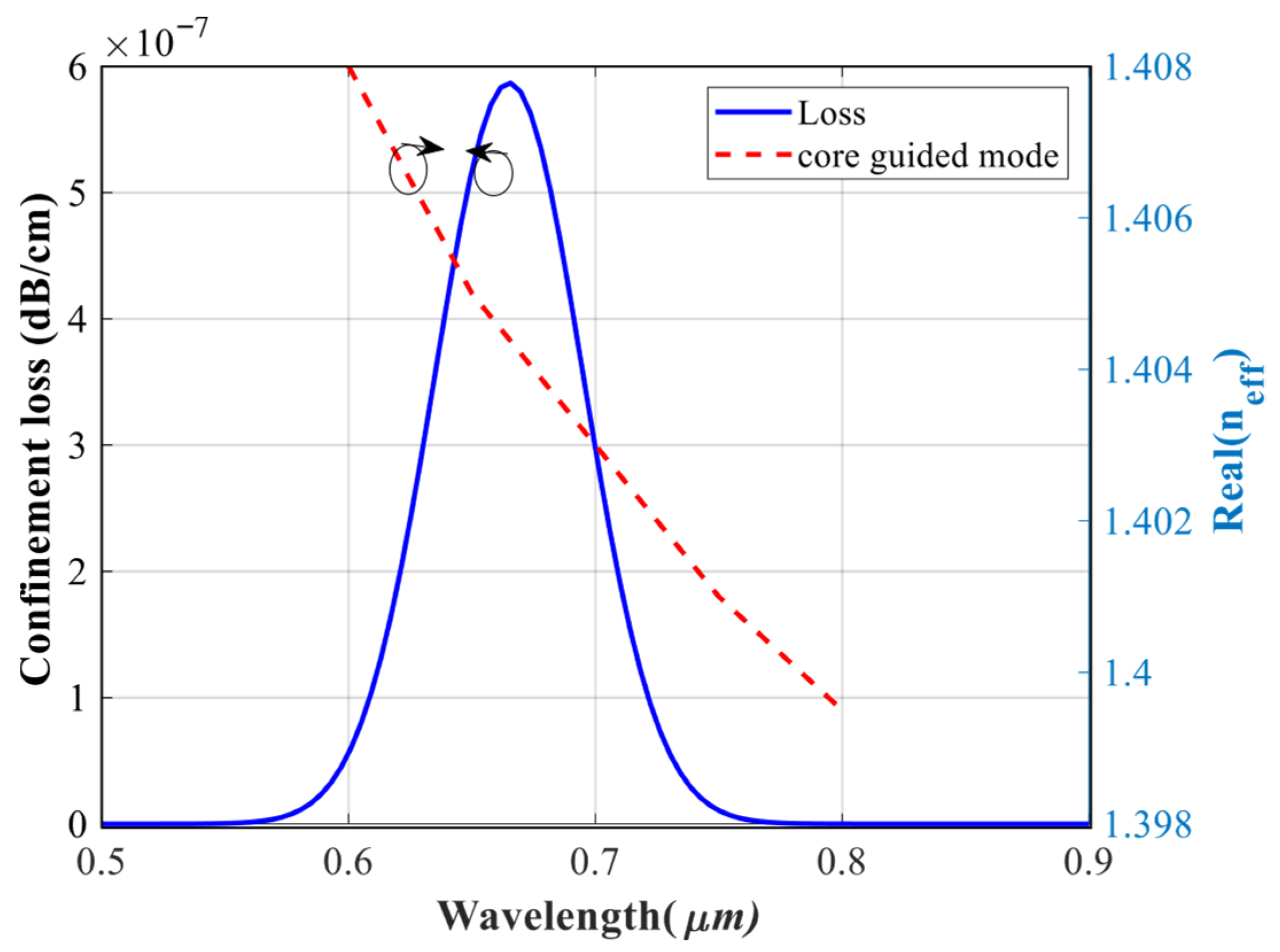
4.1. Evaluation of the Overall Performance of the Sensor
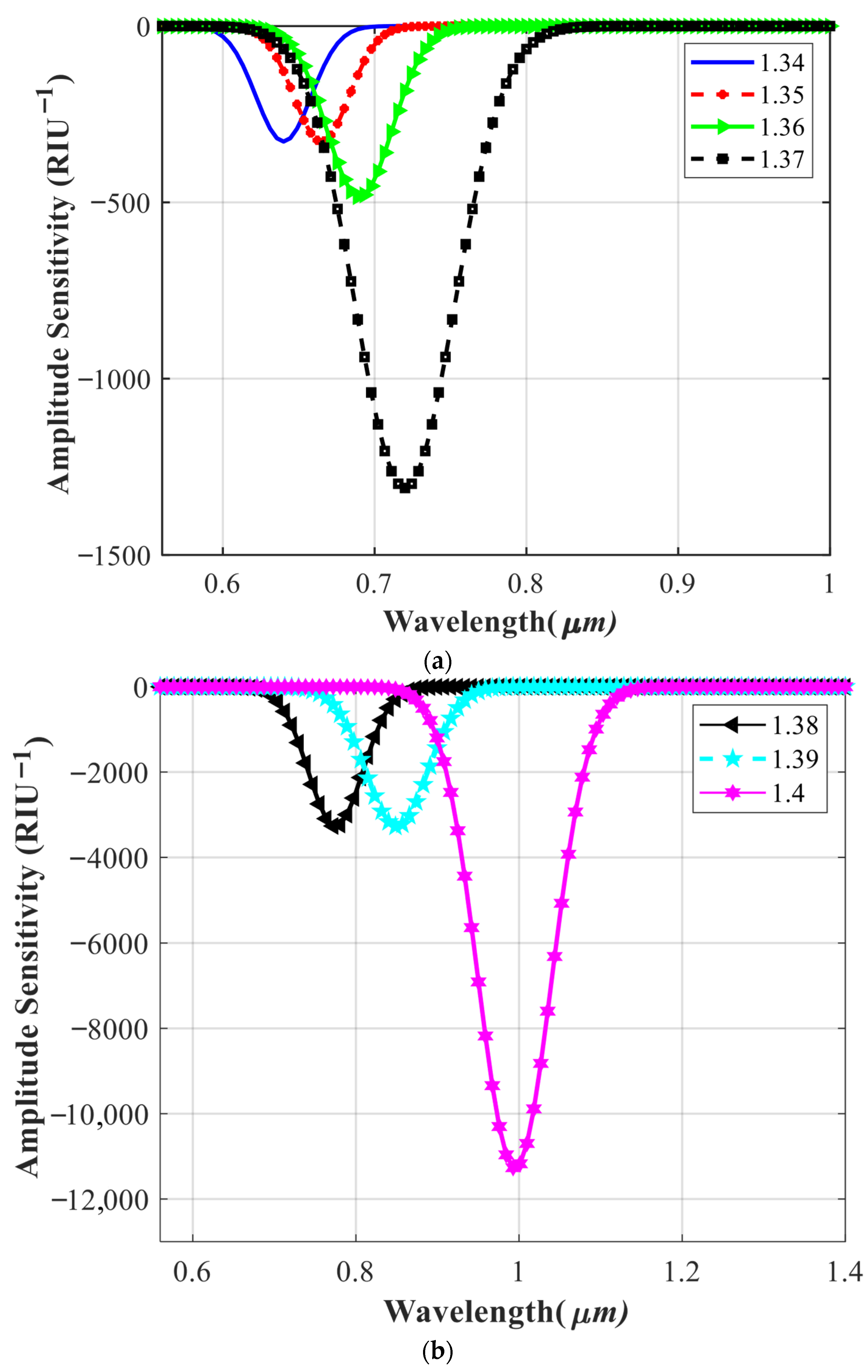
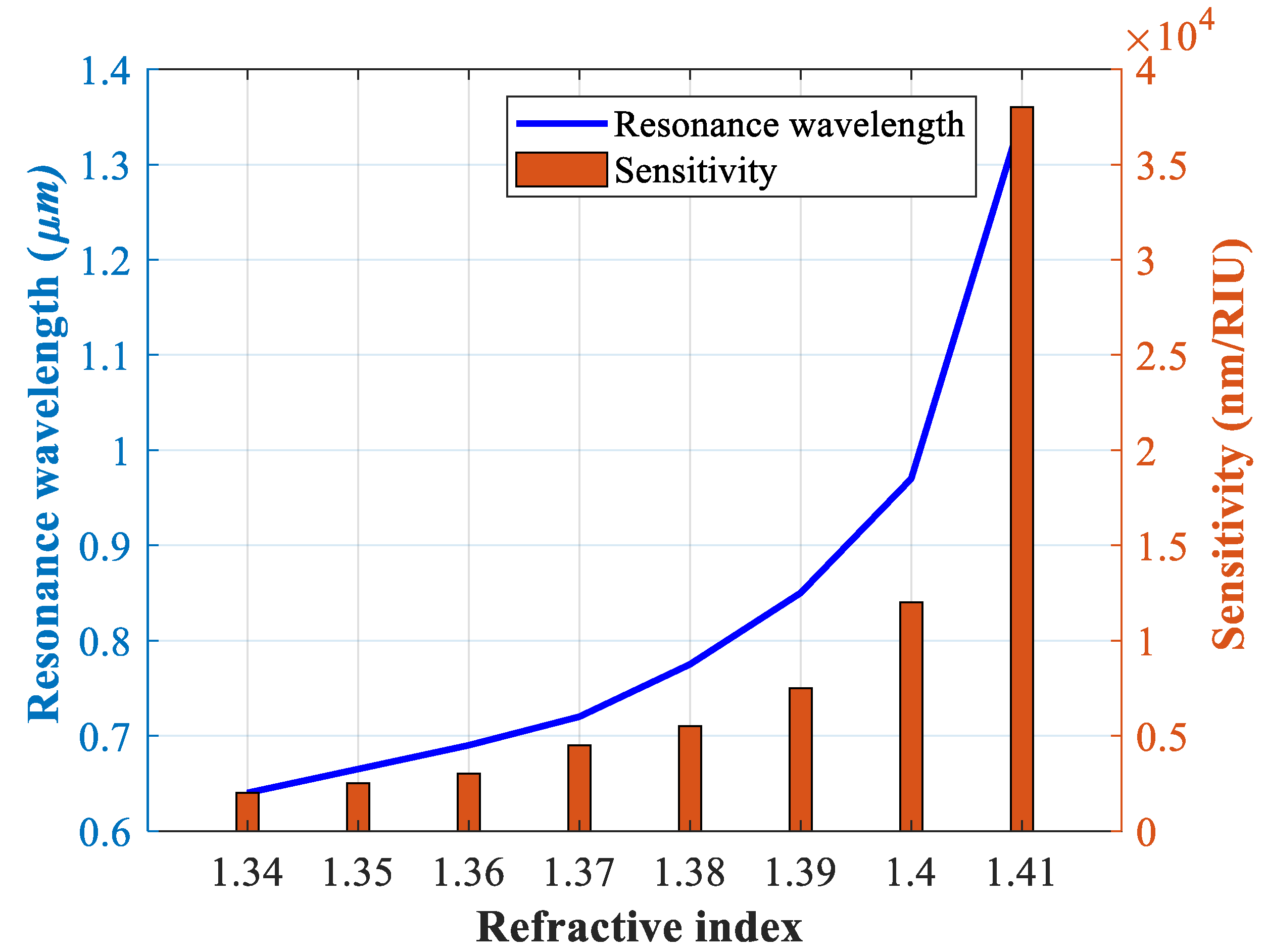
4.2. Investigating the Effect of Parameter Variations on Sensor Performance
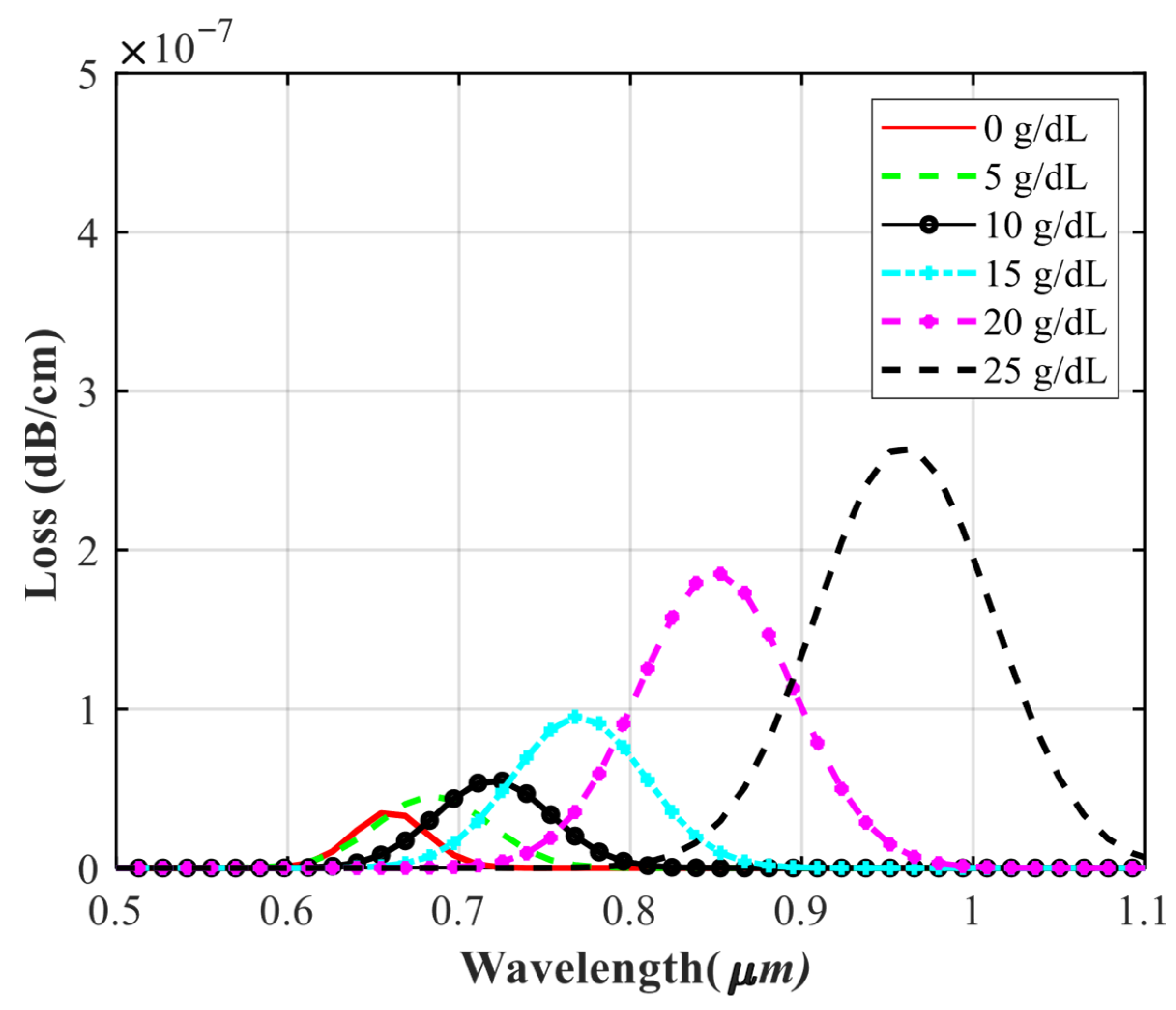
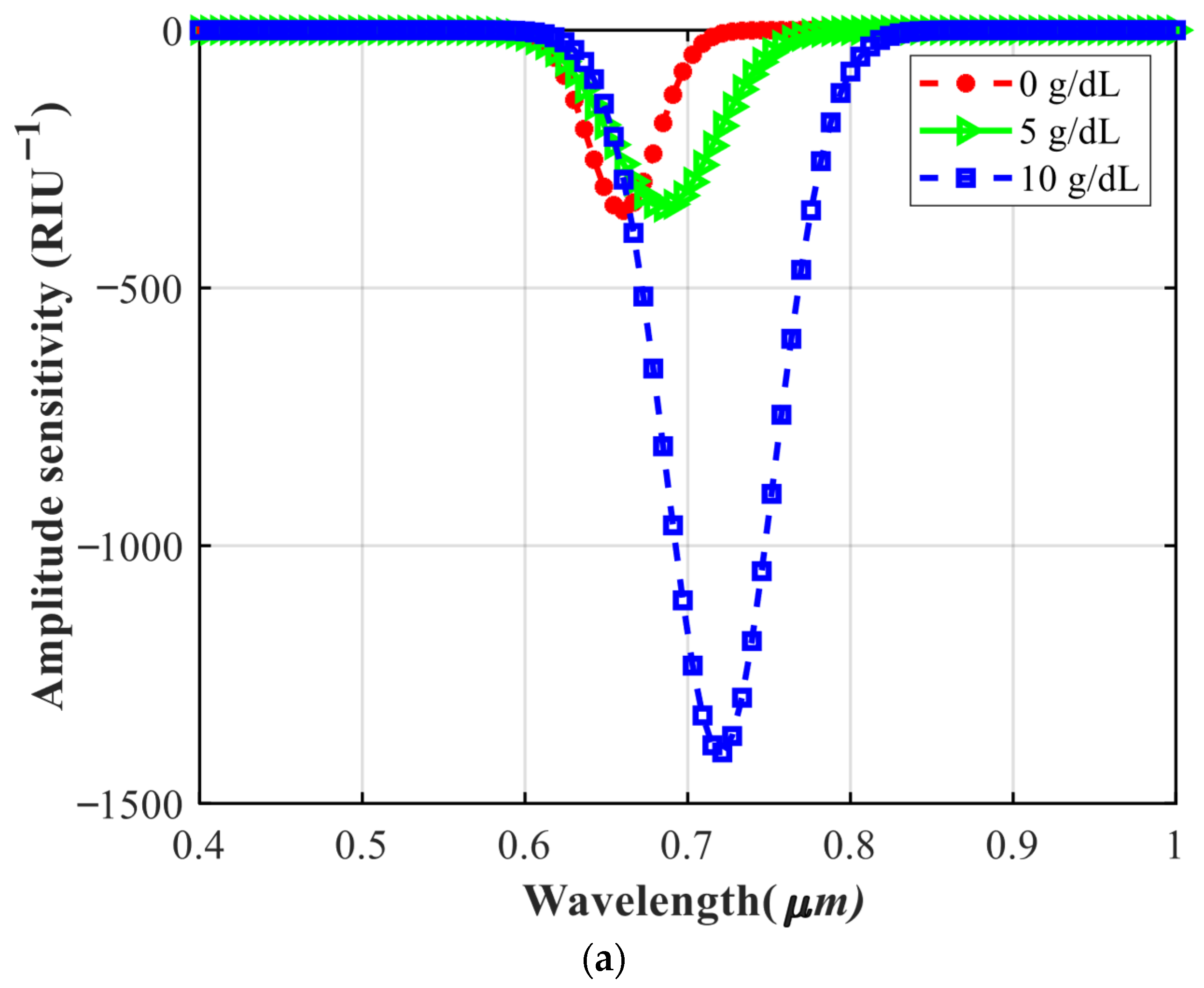
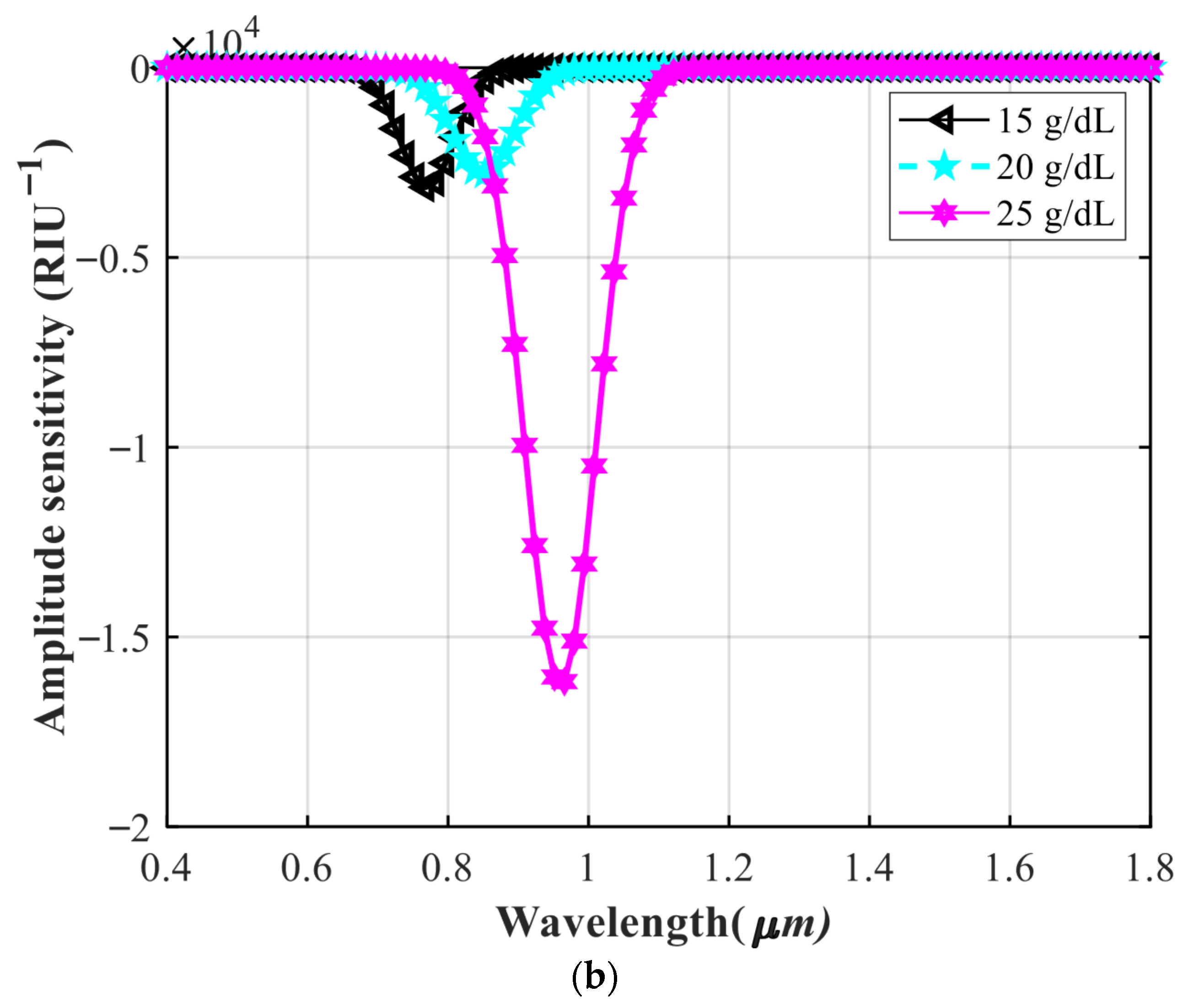
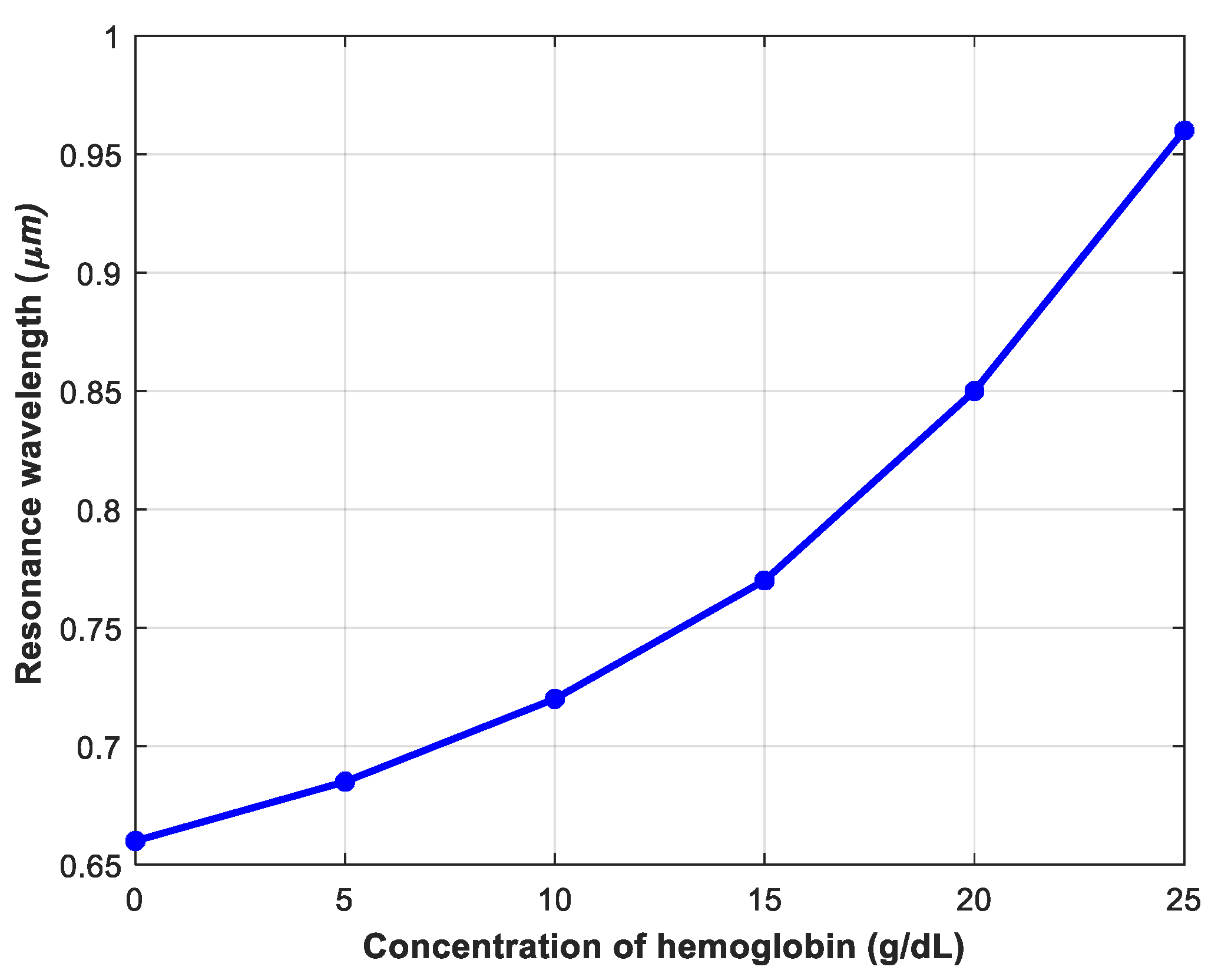
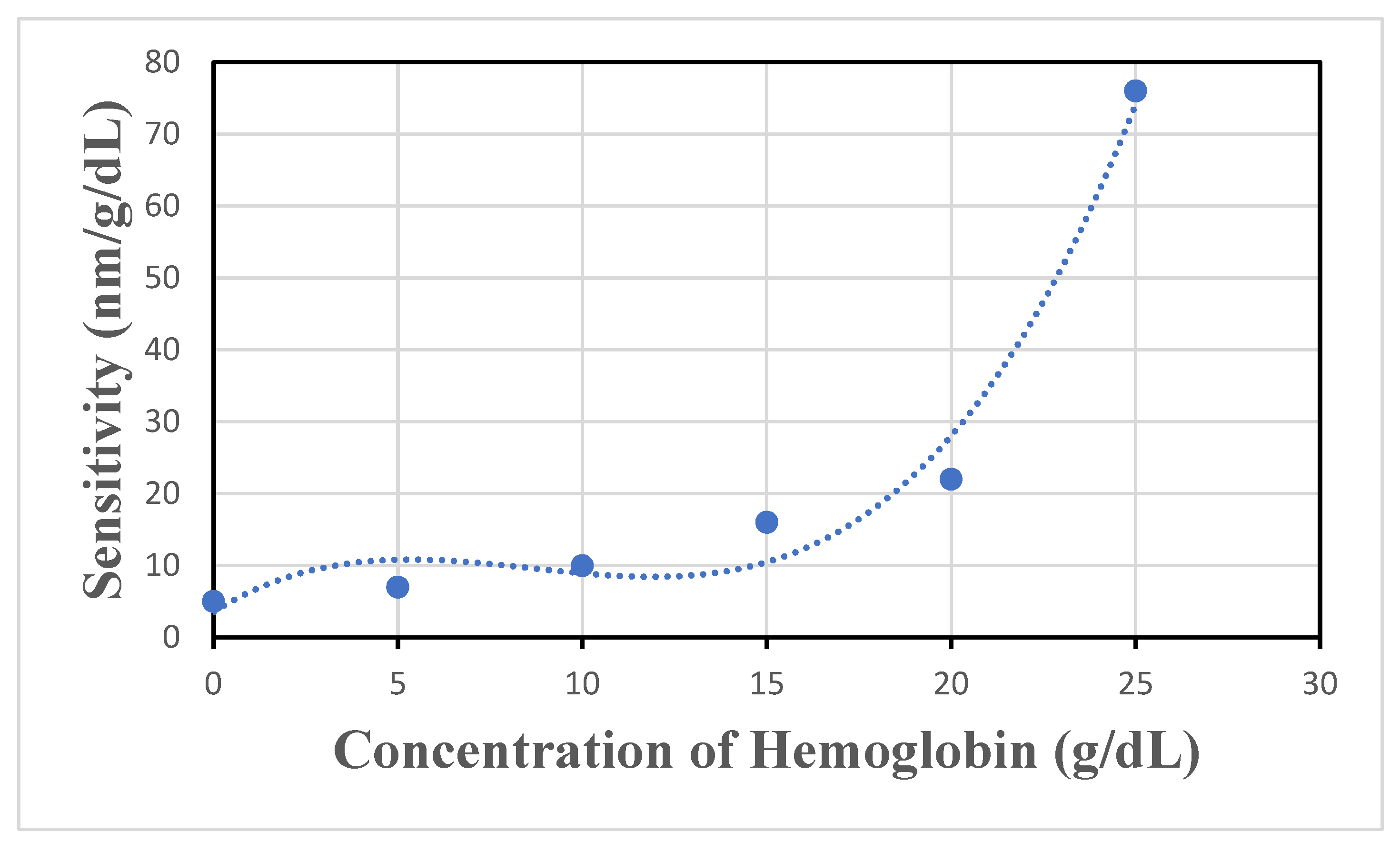
4.3. Investigating the Performance of the Sensor in Detecting Different Concentrations of Hemoglobin
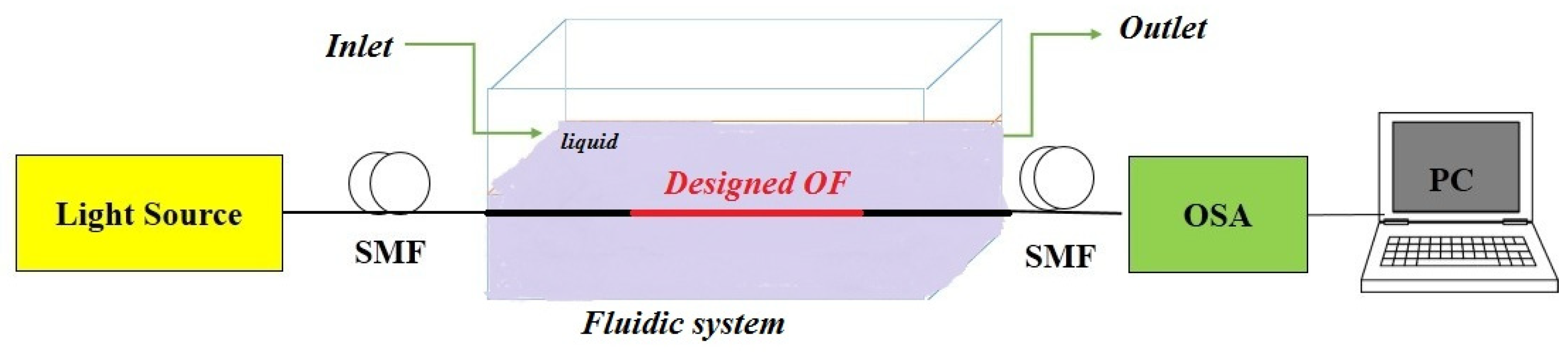
4.4. Investigating the Steps of How to Make the Proposed Sensor for Practical Work
4.5. Comparison of Proposed Sensor Parameters with Previous Works
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elsharkawy, N.B.; Abdelaziz, E.M.; Ouda, M.M.; Oraby, F.A. Effectiveness of health information package program on knowledge and compliance among pregnant women with anemia: A randomized controlled trial. Int. J. Environ. Res. Public Health 2022, 19, 2724. [Google Scholar] [CrossRef]
- Al-Jawaldeh, A.; Taktouk, M.; Doggui, R.; Abdollahi, Z.; Achakzai, B.; Aguenaou, H.; Al-Halaika, M.; Almamary, S.; Barham, R.; Coulibaly-Zerbo, F. Are countries of the eastern mediterranean region on track towards meeting the world health assembly target for anemia? A review of evidence. Int. J. Environ. Res. Public Health 2021, 18, 2449. [Google Scholar] [CrossRef]
- Hamouleh-Alipour, A.; Forouzeshfard, M.; Baghbani, R.; Vafapour, Z. Blood Hemoglobin Concentration Sensing by Optical Nano Biosensor-Based Plasmonic Metasurface: A Feasibility Study. IEEE Trans. Nanotechnol. 2022, 21, 620–628. [Google Scholar] [CrossRef]
- Han, G.-C.; Su, X.; Hou, J.; Ferranco, A.; Feng, X.-Z.; Zeng, R.; Chen, Z.; Kraatz, H.-B. Disposable electrochemical sensors for hemoglobin detection based on ferrocenoyl cysteine conjugates modified electrode. Sens. Actuators B Chem. 2019, 282, 130–136. [Google Scholar] [CrossRef]
- Meng, Z.; Tayyab, M.; Lin, Z.; Raji, H.; Javanmard, M.A. Smartphone-Based Disposable Hemoglobin Sensor Based on Colorimetric Analysis. Sensors 2023, 23, 394. [Google Scholar] [CrossRef] [PubMed]
- Chahkoutahi, A.; Emami, F.; Rafiee, E. Sensitive Hemoglobin Concentration Sensor Based on Graphene-Plasmonic Nano-structures. Plasmonics 2022, 17, 423–431. [Google Scholar] [CrossRef]
- Nida, M.H.; Al-Bassam, S.S. Coreless optical fiber for hemoglobin (HB) sensing with bilayer based on surface plasmon resonance. J. Opt. 2023, 52, 1724–1729. [Google Scholar] [CrossRef]
- Bharti, R.; Gupta, J.; Rajamani, P.; Moulick, R.G.; Bhattacharya, J. Iron oxide nanoparticles/PEDOT: PSS nanocomposite-based modification of both glassy carbon electrode and flexible cotton fiber OECT for highly sensitive multi-analytes detection. Appl. Nanosci. 2022, 12, 3823–3833. [Google Scholar] [CrossRef]
- Cheng, Z.Q.; He, J.; Zhou, L.; Li, Y.; Lin, P.; Guo, J.; Cai, S.; Xiong, X. Smart handheld device with flexible wrist and electrical bioimpedance sensor for tissue inspection. Proceedings of the Institution of Mechanical Engineers. Part H J. Eng. Med. 2022, 236, 416–426. [Google Scholar] [CrossRef]
- Yousuf, M.A.; Asiyanbola, B.A. A review of force and resonance sensors used in the clinical study of tissue properties. Proceedings of the Institution of Mechanical Engineers. Part H J. Eng. Med. 2013, 227, 1333–1340. [Google Scholar] [CrossRef]
- Dhara, P.; Singh, V.K.; Kumar, A.; Olivero, M.; Perrone, G. Reflection based silicon incorporated silver coated fiber optic SPR sensor for refractive index and temperature measurement. Microsyst. Technol. 2024, 30, 913–922. [Google Scholar] [CrossRef]
- Chew, J.W.; Gan, S.X.; Cui, J.; Chan, W.D.; Chu, S.T.; Tam, H.-Y. Recent Advancements in Optical Fiber Sensors for Non-Invasive Arterial Pulse Waveform Monitoring Applications: A Review. Photonics 2025, 12, 662. [Google Scholar] [CrossRef]
- Nizar, S.M.; Caroline, B.E.; Krishnan, P. Photonic crystal fiber sensor for the detection of hazardous gases. Microsyst. Technol. 2022, 28, 2023–2035. [Google Scholar] [CrossRef]
- Rossman, G.R. Optical spectroscopy. Rev. Mineral. Geochem. 2014, 78, 371–398. [Google Scholar] [CrossRef]
- Allsop, T.; Bhamber, R.; Lloyd, G.; Miller, M.R.; Dixon, A.; Webb, D.; Ania Castañón, J.D.; Bennion, I. Respiratory function monitoring using a real-time three-dimensional fiber-optic shaping sensing scheme based upon fiber Bragg gratings. J. Biomed. Opt. 2012, 17, 117001. [Google Scholar] [CrossRef]
- Singh, A.K.; Anwar, M.; Pradhan, R.; Ashar, M.S.; Rai, N.; Dey, S. Surface plasmon resonance based-optical biosensor: Emerging diagnostic tool for early detection of diseases. J. Biophotonics 2023, 16, e202200380. [Google Scholar] [CrossRef]
- Aray, A.; Chiavaioli, F.; Arjmand, M.; Trono, C.; Tombelli, S.; Giannetti, A.; Cennamo, N.; Soltanolkotabi, M.; Zeni, L.; Baldini, F. SPR-based plastic optical fibre biosensor for the detection of C-reactive protein in serum. J. Biophotonics 2016, 9, 1077–1084. [Google Scholar] [CrossRef]
- Eftimov, T.; Genova-Kalou, P.; Dyankov, G.; Bock, W.J.; Mankov, V.; Shoar Ghaffari, S.; Veselinov, P.; Arapova, A.; Makouei, S. Capabilities of Double-Resonance LPG and SPR Methods for Hypersensitive Detection of SARS-CoV-2 Structural Proteins: A Comparative Study. Biosensors 2023, 13, 318. [Google Scholar]
- Helmerhorst, E.; Chandler, D.J.; Nussio, M.; Mamotte, C.D. Real-time and Label-free Bio-sensing of Molecular Interactions by Surface Plasmon Resonance: A Laboratory Medicine Perspective. The Clinical biochemist. Reviews 2012, 33, 161–173. [Google Scholar]
- Bijalwan, A.; Rastogi, V. Gold–aluminum-based surface plasmon resonance sensor with a high quality factor and figure of merit for the detection of hemoglobin. Appl. Opt. 2018, 57, 9230–9237. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Q. A reflective optical fiber SPR sensor with surface modified hemoglobin for dissolved oxygen detection. Alex. Eng. J. 2021, 60, 4115–4120. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Shirkani, H. Highly sensitive mid-infrared SPR biosensor for a wide range of biomolecules and biological cells based on graphene-gold grating. Phys. E Low-Dimens. Syst. Nano. 2020, 119, 114005. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Makouei, S.; Meshgini, S. Ammonia measurement in exhaled human breath using PCF sensor for medical applications. Photonics Nano. Fundam. Appl. 2021, 44, 100917. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Makouei, S.; Meshgini, S. New hybrid photonic crystal fiber gas sensor with high sensitivity for ammonia gas detection. Can. J. Phys. 2022, 100, 129–137. [Google Scholar] [CrossRef]
- Amiri, I.; Yupapin, P.; Rashed, A.N.Z. Mathematical model analysis of dispersion and loss in photonic crystal fibers. J. Opt. Commun. 2023, 44, 139–144. [Google Scholar] [CrossRef]
- Fakhri, M.A.; Salim, E.T.; Sulaiman, G.M.; Albukhaty, S.; Ali, H.S.; Salim, Z.T.; Gopinath, S.C.; Hashim, U.; Al-aqbi, Z.T. Gold nanowires based on photonic crystal fiber by laser ablation in liquid to improve colon biosensor. Plasmonics 2023, 18, 2447–2463. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Rash-Ahmadi, S. A novel graphene-based circular dual-core photonic crystal fiber pressure sensor with high sensitivity. Appl. Phys. A 2023, 129, 570. [Google Scholar]
- Chao, C.-T.C.; Chen, S.-H.; Huang, H.J.; Kooh, M.R.R.; Lim, C.M.; Thotagamuge, R.; Mahadi, A.H.; Chau, Y.-F.C. Improving Temperature-Sensing Performance of Photonic Crystal Fiber via External Metal-Coated Trapezoidal-Shaped Surface. Crystals 2023, 13, 813. [Google Scholar]
- Singh, S.; Prajapati, Y.K. Dual-polarized ultrahigh sensitive gold/MoS2/graphene based D-shaped PCF refractive index sensor in visible to near-IR region. Opt. Quantum Electron. 2019, 52, 17. [Google Scholar] [CrossRef]
- Islam, M.S.; Cordeiro, C.M.B.; Sultana, J.; Aoni, R.A.; Feng, S.; Ahmed, R.; Dorraki, M.; Dinovitser, A.; Ng, B.W.H.; Abbott, D. A Hi-Bi Ultra-Sensitive Surface Plasmon Resonance Fiber Sensor. IEEE Access 2019, 7, 79085–79094. [Google Scholar] [CrossRef]
- Mahfuz, M.A.; Hossain, M.A.; Haque, E.; Hai, N.H.; Namihira, Y.; Ahmed, F. Dual-Core Photonic Crystal Fiber-Based Plasmonic RI Sensor in the Visible to Near-IR Operating Band. IEEE Sens. J. 2020, 20, 7692–7700. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K. Highly sensitive PCF based plasmonic biosensor for hemoglobin concentration detection. Photonics Nano. Fundam. Appl. 2022, 51, 101040. [Google Scholar] [CrossRef]
- Kaur, V.; Singh, S. Design of D-Shaped PCF-SPR sensor with dual coating of ITO and ZnO conducting metal oxide. Optik 2020, 220, 165135. [Google Scholar] [CrossRef]
- Soghra, G.; Jamal, B.; Bahar, M. Design and analysis of surface plasmon resonance based photonic crystal fiber sensor employing gold nanowires. Optik 2022, 260, 169026. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Xu, J.; Jiang, M.; Zou, H.A. D-Shaped SPR-Based PCF Sensor with an Extremely High-Amplitude Sensitivity for Measuring the Refractive Index. Micromachines 2023, 14, 1295. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Mollah, M.A.; Hassan, M.Z.; Gomez-Cardona, N.; Reyes-Vera, E. Graphene-coated highly sensitive photonic crystal fiber surface plasmon resonance sensor for aqueous solution: Design and numerical analysis. Photonics 2021, 8, 155. [Google Scholar] [CrossRef]
- Liu, W.; Hu, C.; Zhou, L.; Yi, Z.; Liu, C.; Lv, J.; Yang, L.; Chu, P.K. A square-lattice D-shaped photonic crystal fiber sensor based on SPR to detect analytes with large refractive indexes. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 138, 115106. [Google Scholar] [CrossRef]
- Dai, T.; Yan, J.; Zhu, W.; Bian, L.; Yi, Z.; Liu, M.; Tang, B.; Sun, T.; Li, G.; Yu, Z. Ultra-high sensitivity surface plasmon U-channel photonic crystal fiber for hemoglobin sensing. Sens. Actuators A Phys. 2024, 366, 115053. [Google Scholar] [CrossRef]
- Dagar, H.B.N.; Krishnan, P. High-Performance Dual-Core Bilateral Surface Optimized PCF SPR Biosensor for Early Detection of Six Distinct Cancer Cells. Plasmonics 2025, 20, 4799–4809. [Google Scholar] [CrossRef]
- Krishnan, P.; Khamaru, A.; Kumar, A. MXene coated concave shaped microchannel PCF SPR biosensor for the detection of HIV and sickle cell anaemia. Opt. Quantum Electron. 2025, 57, 270. [Google Scholar] [CrossRef]
- Bijalwan, A.; Singh, B.K.; Rastogi, V. Analysis of one-dimensional photonic crystal based sensor for detection of blood plasma and cancer cells. Optik 2021, 226, 165994. [Google Scholar] [CrossRef]
- Lazareva, E.; Tuchin, V. Measurement of refractive index of hemoglobin in the visible/NIR spectral range. J. Biomed. Opt. 2018, 23, 035004. [Google Scholar] [CrossRef]
- Elblbesy, M.A. The refractive index of human blood measured at the visible spectral region by single-fiber reflectance spectroscopy. AIMS Biophys. 2021, 8, 57–65. [Google Scholar] [CrossRef]
- Hassani, A.; Skorobogatiy, M. Photonic crystal fiber-based plasmonic sensors for the detection of biolayer thickness. J. Opt. Soc. Am. B 2009, 26, 1550–1557. [Google Scholar] [CrossRef]
- Erdmanis, M.; Viegas, D.; Hautakorpi, M.; Novotny, S.; Santos, J.L.; Ludvigsen, H. Comprehensive numerical analysis of a surface-plasmon-resonance sensor based on an H-shaped optical fiber. Opt Express 2011, 19, 13980–13988. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, G.K.M.; Sakib, T.M.; Paul, A.K. Gold coated surface plasmon resonance based biosensor: An hexagonal photonic crystal Fiber platform. Sens. Bio-Sens. Res. 2023, 42, 100582. [Google Scholar] [CrossRef]
- Chen, S.-H.; Lin, H.-B.; Wang, X.-Z.; Hu, S.-Q.; Luo, Y.-H. Enhanced sensitivity of a surface plasmon resonance biosensor utilizing Au/ITO hyperbolic metamaterial. Results Phys. 2023, 49, 106522. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Dai, W.; Cai, X.; Fu, H. A High Sensitivity Surface Plasmon Resonance Biosensor Based on Photonic Crystal Fibers for Refractive Index Sensing. In Proceedings of the 2022 Photonics & Electromagnetics Research Symposium (PIERS), Hangzhou, China, 25–29 April 2022. [Google Scholar] [CrossRef]
- Hasan, M.M.; El Hameed, A.A.A. Serum adipokine (apelin) in lean and obese polycystic ovary syndrome patients before and after metformin treatment. Middle East Fertil. Soc. J. 2018, 23, 315–318. [Google Scholar] [CrossRef]
- Monro, T.; Belardi, W.; Furusawa, K.; Flanagan, J.; Broderick, N.G.R.; Richardson, D.J. Sensing with microstructured optical fibres. Meas. Sci. Technol. 2001, 12, 854. [Google Scholar] [CrossRef]
- Friebel, M.; Meinke, M. Model function to calculate the refractive index of native hemoglobin in the wavelength range of 250–1100 nm dependent on concentration. Appl. Opt. 2006, 45, 2838–2842. [Google Scholar] [CrossRef]
- Tamaki, S.; Yoshiki, W.; Tanabe, T. Characterization and fabrication of silica-gold composite toroidal optical microcavity. Front. Opt. 2015, jw2a.11. [Google Scholar] [CrossRef]
- Homola, J.; Dostálek, J.; Chen, S.; Rasooly, A.; Jiang, S.; Yee, S.S. Spectral surface plasmon resonance biosensor for detection of staphylococcal enterotoxin B in milk. Int. J. Food Microbiol. 2002, 75, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Dostálek, J.; Vaisocherová, H.; Homola, J. Multichannel surface plasmon resonance biosensor with wavelength division multiplexing. Sens. Actuators B Chem. 2005, 108, 758–764. [Google Scholar] [CrossRef]



| Stages of Life | Normal Level of Hb (g/dL) |
|---|---|
| Adult men | 13.0–17.5 |
| Adult women | 12.0–15.3 |
| Neonates | 14.0–24.0 |
| Parameters | Values (µm) |
|---|---|
| d | 10 |
| d1 | 10 |
| d2 | 15 |
| d3 | 10 |
| d4 | 15 |
| d5 | 5 |
| Region | Maximum Element Size (µm) | Minimum Element Size | Maximum Growth Rate | Curvature Factor |
|---|---|---|---|---|
| Gold | 4.44 | 0.015 | 1.25 | 0.25 |
| Silica | 2.4 | 0.009 | 1.2 | 0.25 |
| Analyte | 1.2 | 0.0024 | 1.1 | 0.2 |
| RI | λpeak | SW (nm/RIU) | SA (RIU−1) | FWHM (nm) | FOM (RIU−1) | R (RIU) |
|---|---|---|---|---|---|---|
| 1.34 | 640 | 2000 | −327.52 | 18.25 | 109.58 | 4 × 10−5 |
| 1.35 | 665 | 2500 | −327.52 | 21.53 | 116.11 | 4 × 10−5 |
| 1.36 | 690 | 3000 | −484.41 | 32.65 | 91.88 | 3.33 × 10−5 |
| 1.37 | 720 | 4500 | −1310.10 | 33.45 | 134.52 | 1.81 × 10−5 |
| 1.38 | 775 | 5500 | −3275.20 | 38.46 | 143.00 | 1.33 × 10−5 |
| 1.39 | 850 | 7500 | −3395.50 | 45.16 | 166.07 | 6.89 × 10−6 |
| 1.4 | 970 | 12,000 | −3385.10 | 51.32 | 282.54 | 2.59 × 10−6 |
| 1.41 | 1350 | 38,000 | −11280 | 52.27 | 736.56 | 1.85 × 10−6 |
| References | RI Range | Sw (nm/RIU) a | SA (RIU−1) | Resolution (RIU) | FOM (RIU−1) |
|---|---|---|---|---|---|
| [29] | 1.33–1.4 | 14,933.34 | - | 6.69 × 10−6 | 401.05 |
| [30] | 1.33–1.38 | 25,000 | 1411 | 4 × 10−6 | 502 |
| [31] | 1.33–1.42 | 28,000 | 6829 | 5 × 10−6 | 2800 |
| [32] | 1.34–1.41 | 34,800 | - | 2.86 × 10−6 | 229 |
| [38] | 1.26–1.42 | 7,500 | - | 6 × 10−6 | 634.1 |
| Proposed OF | 1.34–1.41 | 38,000 | 11,280 | 1.85 × 10−6 | 736.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbaszadeh, A.; Makouei, S.; Rash-Ahmadi, S.; Danishvar, S. Numerical Analysis of an Ultra-Sensitive Optical Fiber for Hemoglobin Concentration Detection. Photonics 2025, 12, 933. https://doi.org/10.3390/photonics12090933
Abbaszadeh A, Makouei S, Rash-Ahmadi S, Danishvar S. Numerical Analysis of an Ultra-Sensitive Optical Fiber for Hemoglobin Concentration Detection. Photonics. 2025; 12(9):933. https://doi.org/10.3390/photonics12090933
Chicago/Turabian StyleAbbaszadeh, Aryan, Somayeh Makouei, Samrand Rash-Ahmadi, and Sebelan Danishvar. 2025. "Numerical Analysis of an Ultra-Sensitive Optical Fiber for Hemoglobin Concentration Detection" Photonics 12, no. 9: 933. https://doi.org/10.3390/photonics12090933
APA StyleAbbaszadeh, A., Makouei, S., Rash-Ahmadi, S., & Danishvar, S. (2025). Numerical Analysis of an Ultra-Sensitive Optical Fiber for Hemoglobin Concentration Detection. Photonics, 12(9), 933. https://doi.org/10.3390/photonics12090933






