3.1. Effect of Varying Cr–SiO2 Pairs on Absorption Characteristics and Optical Impedance
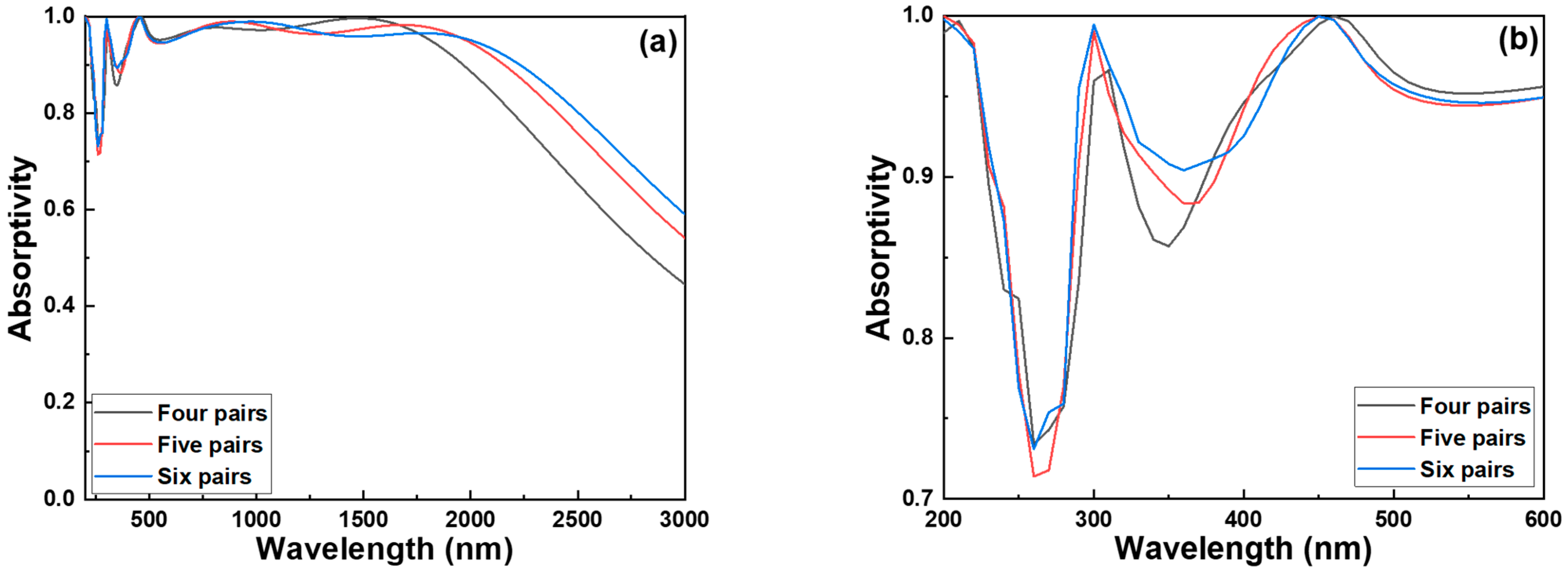
To evaluate the influence of layer configuration on absorption performance, we first compared the spectral characteristics of three absorber structures composed of different numbers of Cr–SiO
2 pairs. As the number of Cr–SiO
2 pairs increased from four to five, a trade-off phenomenon was observed: the average absorptivity experienced a slight decline; however, this was effectively offset by a substantial enhancement in operational bandwidth, as
Figure 3a shows. This behavior can be attributed to the increased optical path length and interference effects introduced by the additional layers, which enhance the constructive interference over a broader wavelength range while slightly affecting peak absorption. Further increasing the number of Cr–SiO
2 pairs from five to six yielded even more promising results. The average absorptivity remained nearly constant, while the absorption bandwidth continued to improve. This improvement is primarily due to the multilayer interference and impedance matching effects, which facilitate better suppression of reflection across a wider spectral range. At a wavelength of approximately 465 nm, these absorbers exhibit the characteristics of a perfect absorber (absorptivity~1), as shown in
Figure 3b. In the structure with four Cr–SiO
2 pairs, absorptivity began to drop at approximately 1900 nm, limiting the effective absorption range.
However, when the number of pairs increased to six, the high-absorptivity region (absorptivity > 0.900) extended from 1900 nm to around 2200 nm, indicating a redshift of the absorption band. This redshift can be explained by the increased effective optical thickness, which shifts the resonance conditions toward longer wavelengths. In addition to the improvements in the near-infrared region, increasing the number of Cr–SiO2 pairs also resulted in a noticeable enhancement in the ultraviolet to visible range (200–500 nm). Notably, the absorption dip at 360 nm—originally below the 0.900 threshold—was mitigated, with the absorptivity slightly exceeding 0.900. This indicates that the multilayer structure helps flatten the absorption spectrum and reduce spectral valleys through enhanced field confinement and multilayer interference. Based on these optimization studies, the six-pair Cr–SiO2 metamaterial absorber emerges as the most favorable configuration for achieving broadband absorption performance. Under carefully optimized parametric conditions, the absorber maintains high absorptivity (>0.900) across a wide wavelength range from 500 nm to 2160 nm, demonstrating excellent spectral coverage. Nevertheless, the current six-pair design has not yet achieved optimal performance in the sub-500 nm region. Further structural optimization—such as fine-tuning layer thicknesses or introducing additional nanoresonant features—will be necessary for enhancing absorptivity beyond 0.900 in this short-wavelength range, ensuring truly uniform ultra-broadband absorption.
In the field of optical absorber research and applications, an absorptivity threshold greater than 0.900 is commonly adopted as the standard criterion for defining the effective absorption bandwidth. This definition arises primarily from the high-performance requirements in practical applications such as infrared stealth, solar thermal conversion, and photodetection. In these scenarios, achieving high absorptivity (A > 0.900) ensures that the majority of the incident electromagnetic energy is effectively absorbed, minimizing reflection, transmission, and signal loss. Such a level of performance typically corresponds to the behavior of a near-perfect absorber, which serves as a benchmark in both experimental and simulation-based designs. Moreover, using 0.900 as a unified threshold facilitates consistent comparisons across different studies and absorber designs, enabling a standardized basis for evaluating performance. If the absorptivity falls below 0.900—such as 0.800—the absorber’s effectiveness is significantly compromised. At absorptivity = 0.800, up to 20% of the incident energy is not absorbed, leading to notable energy loss. This can negatively affect various applications: in solar thermal devices, it results in reduced heat conversion efficiency; in stealth technologies, the remaining reflected or transmitted signals may still be detectable by radar or infrared sensors; and in photovoltaic systems, it reduces light utilization and limits power conversion efficiency. Additionally, absorbers with lower absorptivity may fail to provide sufficient energy concentration or field enhancement in applications requiring strong resonance or thermal localization, such as sensors or thermal emitters. The optical selectivity is also weakened, as the absorber may no longer exhibit high absorption in the target band while maintaining low reflectance elsewhere, thereby diminishing its utility in filtering and stealth-related applications.
Achieving an ultra-broadband absorption range spanning from 200 to 2160 nm using a simple bilayer structure is extremely rare and scarcely reported in the current literature. In this work, we demonstrate that by utilizing straightforward material combinations such as Cr–SiO
2, it is possible to realize such an exceptionally wide absorption bandwidth without the need for complex structural designs. While several previous studies have attempted to enhance the absorption bandwidth using Cr–SiO
2 [
22,
23] or W–SiO
2 [
24,
25] systems, either alone or in combination with other materials, these configurations generally fall short of extending absorption into the deep-ultraviolet region. To achieve broader coverage, especially in the short-wavelength infrared or deep-ultraviolet ranges, these approaches often rely on additional layers or nanostructured geometries, which significantly complicate fabrication. Although various sophisticated designs have been proposed to push absorption boundaries further, extending the spectral response down to 200 nm typically requires intricate multi-layered or resonant architectures. In contrast, our ability to attain this broadband performance through a relatively simple and fabrication-friendly design highlights both the novelty and the practical potential of our approach. To demonstrate the critical role of the top Cr metal cylinder in our absorber design, we modified the original structure shown in
Figure 1 by removing this top component, resulting in a new configuration illustrated in
Figure 2a.
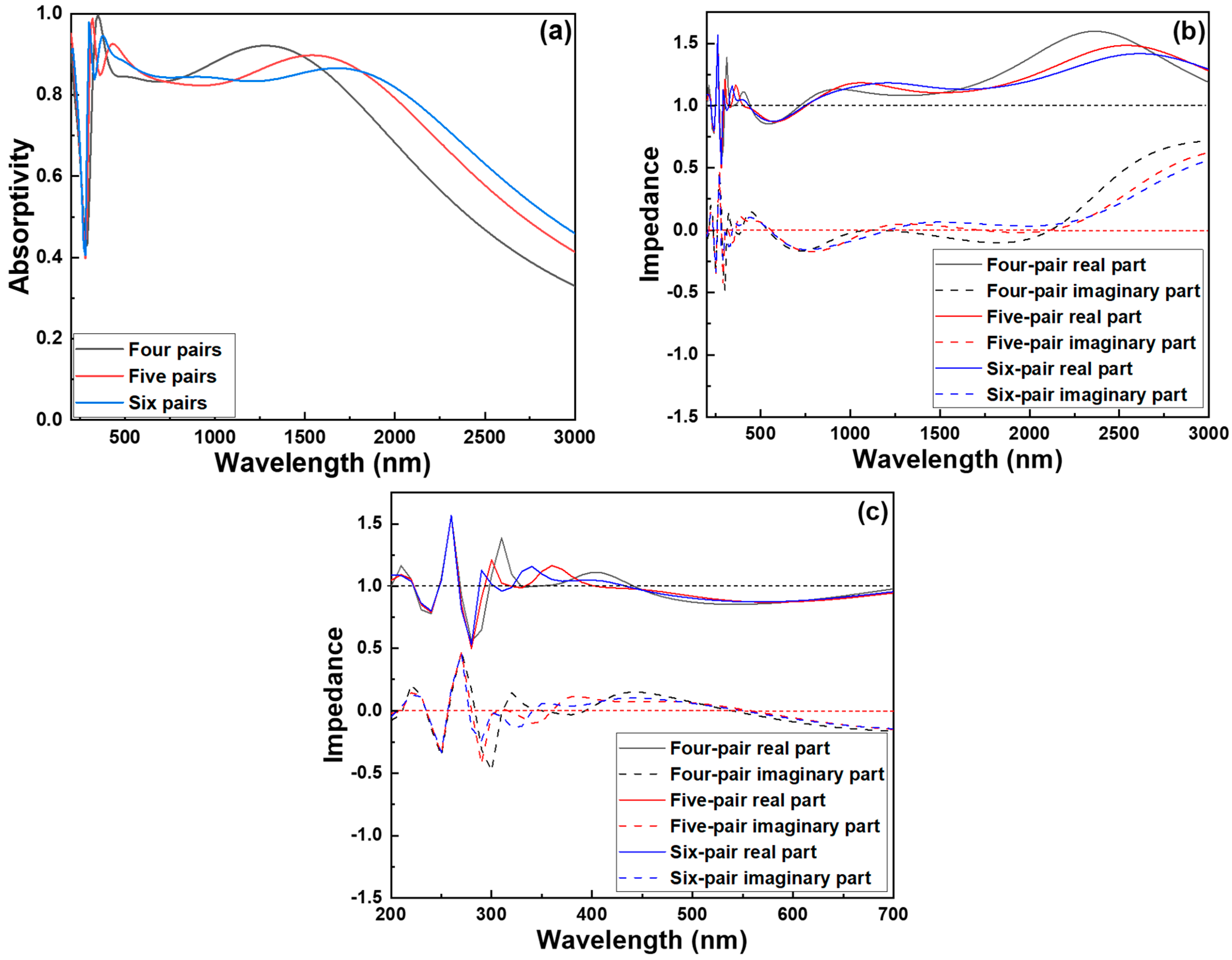
We then evaluated and compared the absorptivity of three different absorber types without the top Cr cylinder to the original structures that retained it. As shown in the simulation results in
Figure 4a, the removal of the top Cr cylinder causes a substantial drop in absorptivity across all three structures. Notably, in the ultraviolet wavelength range of 200–300 nm, the absorptivity sharply declines to around 0.4, indicating that the top Cr metal cylinder plays a pivotal role in enhancing the absorption characteristics of the system, especially in the UV region. This significant reduction in absorptivity can be attributed to the disruption of optical and electromagnetic interactions within the structure. The top Cr metal cylinder likely supports plasmonic resonance and facilitates impedance matching with free space, both of which are essential for efficient light trapping and energy dissipation. Its removal eliminates these beneficial effects, leading to increased reflection and reduced energy coupling into the absorber. To investigate the underlying physical mechanism responsible for this performance degradation, we conducted an optical impedance analysis of the structure depicted in
Figure 4a. The results, shown in
Figure 4b, reveal that the real part of the optical impedance deviates substantially from 1, while the imaginary part diverges significantly from 0 after the top Cr cylinder is removed. The real part of the impedance characterizes the degree of impedance matching with free space. When this value strays far from unity, it indicates a strong mismatch, causing considerable reflection of incident light at the interface and reducing the amount of energy that penetrates the absorber.
This mismatch disrupts the critical coupling condition necessary for optimal energy absorption. The imaginary part of the impedance represents the phase relationship between the electric and magnetic fields within the material and is closely associated with intrinsic loss or reactive energy storage. A large deviation from zero in the imaginary component suggests the presence of strong capacitive or inductive behavior, leading to excess energy being stored rather than dissipated as heat. Such behavior not only prevents efficient energy conversion but also contributes to further internal reflections, exacerbating the overall loss. Taken together, these results confirm that the absence of the top Cr metal cylinder undermines the absorber’s ability to effectively capture and dissipate incident electromagnetic energy. The Cr cylinder contributes not only to enhancing absorption through localized field enhancement and resonance effects but also to maintaining favorable impedance conditions. Its removal severely impairs the optical properties of the absorber, underscoring its indispensable role in achieving high-performance absorption, particularly in the ultraviolet regime. Nevertheless, even with the inclusion of the top Cr metal cylinder and six pairs of Cr–SiO2 layers, the absorber still fails to achieve high absorptivity in the ultraviolet range (200–400 nm) under the current parameter settings.
Figure 4c shows an expanded view of the impedance spectra of the investigated absorbers in the 200–700 nm wavelength range. As illustrated, in the region below 280 nm, the impedance values for all three structures remain nearly constant, indicating minimal spectral variation in that range. However, once the wavelength exceeds 280 nm, the six-pair structure designed in this study exhibits a real part of impedance that is closer to 1 and an imaginary part closer to 0 compared to the other two configurations. This behavior suggests that the six-pair structure achieves more effective impedance matching with free space across a wider portion of the spectrum, starting from relatively short wavelengths. Such improved matching is critical for enhancing absorption efficiency, particularly in the deep ultraviolet to visible regions, and demonstrates the advantage of the six-pair design in achieving ultra-broadband absorption. Therefore, we aim to perform parameter optimization on the six-pair Cr–SiO
2 structure to enhance its absorption performance, with the goal of achieving high absorptivity within the UV spectrum.
3.2. Analysis of the Optimal Structural Parameters for Each Layer of Cr–SiO2 Pairs and the Top Cr Cylinders
To determine the optimal thickness for each individual layer in the multilayer structure, we conducted a step-by-step simulation process. In this procedure, we varied the thickness of one specific layer at a time while keeping the thicknesses of all other layers fixed. This approach allowed us to isolate and examine the influence of each layer on the overall optical absorptivity. We began by analyzing the bottommost layer, designated as the h2 SiO
2 layer.
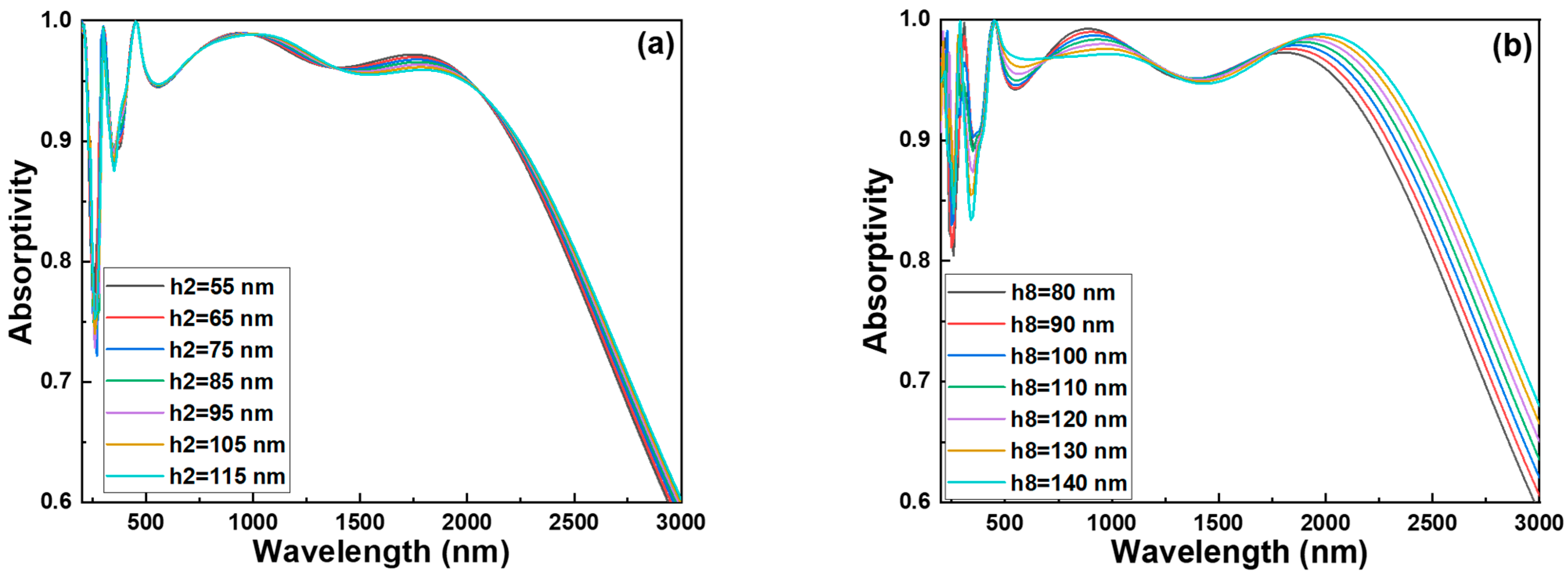
Figure 5a displays the absorption spectra as a function of layer thickness (y-axis) and wavelength (x-axis). The color scale indicating the absorptivity is typically shown on the right side of each figure. For example, in
Figure 5a, the right-side color bar shows that dark red represents an absorptivity of 1.000, while the color gradually transitions to blue, indicating an absorptivity of 0.500. All subsequent similar figures follow the same convention. As shown in
Figure 5a, changes in the thickness of this layer had only a minor impact on the overall absorptivity. A slight variation was observed near a wavelength of approximately 250 nm. When the thickness was adjusted to 55 nm, the 2D absorptivity map indicated a clear reduction in the yellow region near 250 nm, which corresponds to higher absorption. Therefore, we selected 55 nm as the optimal thickness for the h2 SiO
2 layer to enhance absorption performance at this specific wavelength. With the h2 SiO
2 layer fixed at 55 nm, we proceeded to examine the effect of the h4 SiO
2 layer, positioned higher in the structure. Similar to h2, the h4 SiO
2 layer had a relatively subtle influence on the total absorptivity, while the detailed data are not presented in this section. However, a closer look revealed that a thickness of 70 nm resulted in a noticeable improvement in absorption near 270 nm, making 70 nm the most suitable thickness for the h4 SiO
2 layer.
Next, we analyzed the h6 SiO
2 layer, which is even closer to the air interface and to the direction of incident light. Although we do not present the detailed results here, the trends observed were generally consistent with those of the h2 and h4 layers—mainly affecting localized absorption but having a limited impact on the overall profile. Interestingly, the behavior of the h6 layer resembled that of the h8 SiO
2 layer, whose performance is illustrated in
Figure 5b. In the case of h8, which lies just beneath the outermost SiO
2 layer, the influence on absorptivity was far more pronounced, especially in both the short and long wavelength regimes. Specifically, for wavelengths below 500 nm, two distinct absorption valleys were observed. Around 380 nm, the absorptivity exceeded 0.9 when the thickness of the h8 SiO
2 layer reached or surpassed 110 nm. In the long-wavelength range—particularly beyond 2000 nm—absorptivity increased progressively with the thickness of the h8 SiO
2 layer. However, this trend was not consistent across all spectral regions. For instance, an absorption peak near 880 nm exhibited a decrease in absorptivity as the thickness increased. Based on this analysis, we selected 110 nm as the optimal thickness for both the h6 and h8 SiO
2 layers.
The more significant influence of upper layers such as h6 and h8, particularly in the short and long wavelength regions, can be attributed to their physical proximity to the incident light source and the air interface. At shorter wavelengths, the optical penetration depth of incident light is relatively shallow, meaning that the interaction between the light and the topmost layers is stronger. Any change in thickness in these layers can significantly alter the conditions for interference and reflection, affecting how efficiently light is absorbed or reflected. In the long-wavelength regime, while light penetrates deeper into the structure, the top layers still play a critical role due to the phase accumulation over the optical path. Constructive and destructive interference effects become more pronounced as the optical path length increases with wavelength. Therefore, even subtle variations in the thickness of the upper layers can shift the phase conditions for these longer wavelengths, enhancing or suppressing absorption depending on the specific structural configuration. Moreover, the fact that the upper SiO2 layers lie beneath one more SiO2 layer (i.e., they are not the very topmost layer but still near the top) means that their optical effect is influenced by multi-layer interference within a semi-transparent stack. This layered interference can enhance the field localization at certain resonant wavelengths, thereby increasing the optical energy density and leading to enhanced absorption either in underlying lossy layers or at specific resonant points. These complex interactions explain why the h6 and h8 layers exhibit greater influence across both spectral extremes, and why their optimization is crucial for achieving high-performance broadband absorptivity.
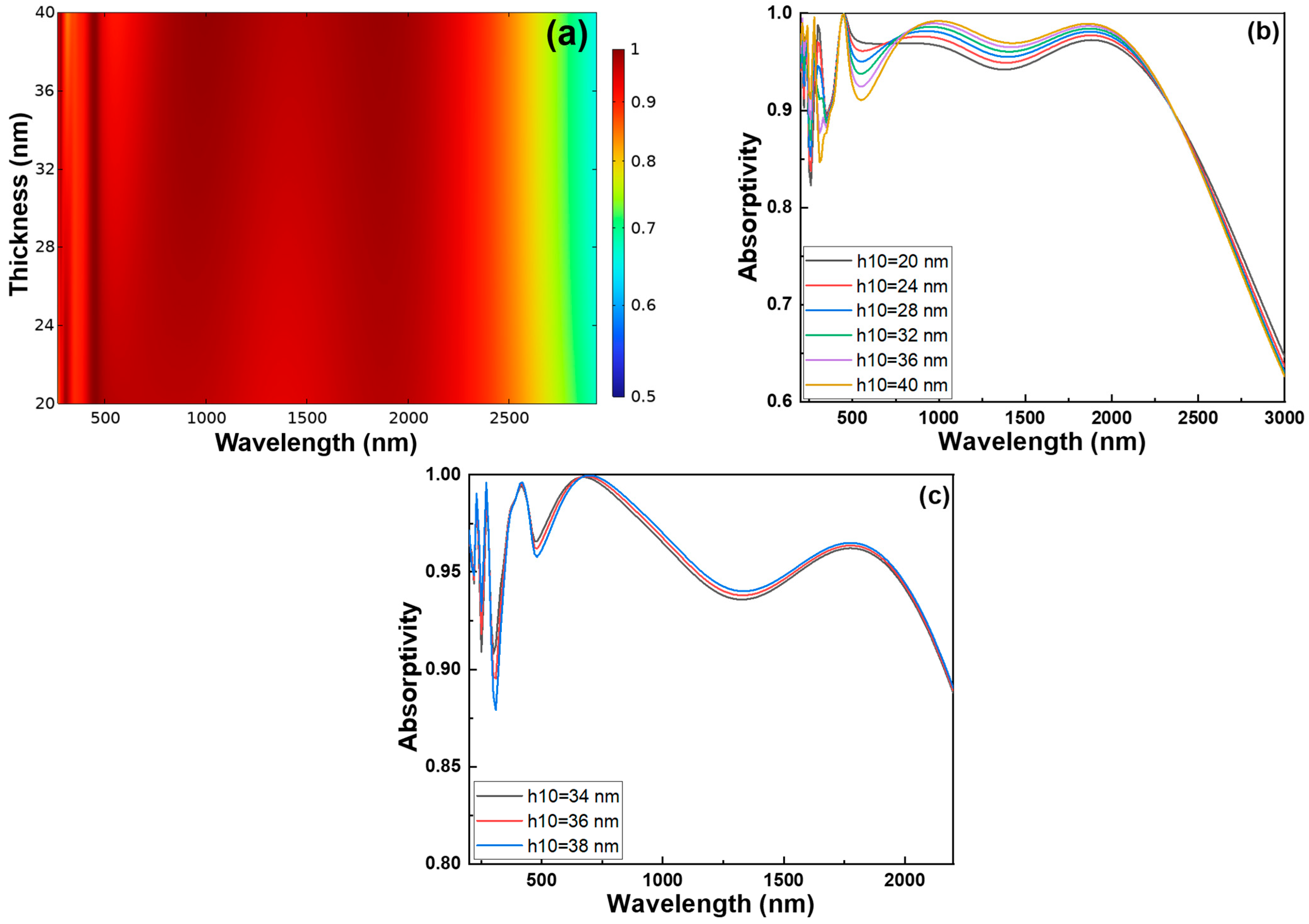
Finally, we performed a thickness sweep of the h10 SiO
2 layer, which is located beneath the Cr matrix-arranged cylindrical structures. We conducted a detailed thickness sweep of the h10 SiO
2 layer, which lies beneath the Cr cylindrical matrix structure, and observed distinct variations in absorptivity across different wavelength regions. As shown in
Figure 6a, when the thickness of the h10 SiO
2 layer increases, absorptivity decreases in the 300–370 nm and 500–850 nm ranges, but increases within the 200–290 nm ultraviolet region. This contrasting behavior is primarily attributed to the interference effects and the optical path length within the multilayer structure. At shorter wavelengths (200–290 nm), light penetration depth is shallow, and the increasing thickness of the SiO
2 layer enhances the near-field coupling between the Cr nanocylinders and the underlying layers, as well as constructive interference, thereby improving absorption. In contrast, at longer wavelengths (300–370 nm and 500–850 nm), destructive interference becomes more dominant with increasing SiO
2 thickness, resulting in reduced absorptivity due to phase mismatches and weakened field confinement at the absorber-active interfaces. The goal of adjusting the h10 SiO
2 thickness was to maximize absorption in the deep UV range while minimizing the loss of absorption in the visible range, as shown in
Figure 6b. However,
Figure 6b also reveals that in some cases, even with thickness optimization, the absorptivity fails to exceed 0.9, which is the target threshold for high-efficiency absorbers in the UV region. To finetune the optimal thickness, we performed a refined sweep in the 34–38 nm range, incrementing by 2 nm. As shown in
Figure 6c, within this range, increasing thickness still leads to improved absorption in the 200–290 nm UV region, due to enhanced field localization and better resonance conditions, but simultaneously causes a drop in visible light absorption, likely due to increased reflection and destructive interference. Considering the trade-off between enhancing UV absorptivity and maintaining acceptable performance in the visible range, a final thickness of 34 nm was selected as the optimal configuration, achieving better balance across the spectrum.
Next, we analyzed the influence of different Cr metal layers on the absorption properties of our designed absorbers. We began by investigating the effect of varying the thickness of the h3 Cr layer. As shown in
Figure 7a, increasing the thickness of the h3 Cr layer has a relatively minor impact on the overall absorptivity. However, as the metal thickness increases, a gradual decrease in absorptivity is observed around the wavelengths of 250 nm and 360 nm. Based on this trend, a thickness of 6 nm was determined to be the optimal parameter for the h3 Cr layer. We then examined the effect of varying the h5 Cr layer thickness on absorption. According to
Figure 7b, increasing the h5 Cr thickness results in a noticeable decrease in absorptivity beyond 2000 nm, indicating a blue shift in the spectral region where absorptivity remains above 0.9. Additionally, absorptivity near 270 nm and 360 nm also gradually decreases with increasing thickness. Therefore, 6 nm was again selected as the optimal thickness for the h5 Cr layer. Although we do not present the detailed results here, the analysis of the h7 Cr layer also reveals that increasing the Cr thickness leads to a broader region of low absorptivity at longer wavelengths, represented by expanded blue and yellow areas in the absorption maps—further evidence of a pronounced blue shift. Around 250 nm, absorptivity also decreases progressively, with the yellow (low absorptivity) regions becoming more prominent. Among the data, the 6 nm thickness clearly provides the best absorptivity performance, making it the most suitable choice for the h7 Cr layer as well.
Finally,
Figure 7c,d present the absorptivity distribution map and absorption spectra for different h9 Cr layer thicknesses. These figures clearly demonstrate that the thickness of the h9 Cr layer significantly influences the absorption behavior. As the Cr layer becomes thicker, regions of low absorptivity at longer wavelengths expand, again showing a marked blue shift. Based on the results in both figures, the h9 Cr layer also achieves optimal absorption performance at a 6 nm thickness. The observed variations in absorptivity with respect to different Cr layer thicknesses can be directly attributed to the optical characteristics of the multilayer structure, which consists of alternating SiO
2 and Cr layers on a 300 nm Cr substrate (h1). In this configuration, the optimal thicknesses of four SiO
2 layers of h2, h4, h6, and h8 are found to be 55, 70, 110, and 110 nm, while the corresponding Cr layers (h3, h5, h7, and h9) are set to the same thicknesses of 6 nm, and the topmost SiO
2 layer (h10) is thinner at 34 nm. Additionally, Cr nanocylinders are patterned on the top surface. This layered design creates multiple interference interfaces and plasmonic interactions that govern the absorption characteristics. As Cr is a lossy metal, its thickness plays a crucial role in modulating both reflection and absorption across different wavelengths.
When the Cr layers (h3, h5, h7, and h9) are thin (around 6 nm), they contribute to constructive interference and enhanced field confinement, leading to high absorption across a broad spectral range. However, increasing their thickness reduces the transmission of incident light into deeper layers and leads to stronger reflection or damping, particularly at specific wavelengths. For instance, the decreasing absorptivity near 250 nm and 360 nm, observed with increasing Cr thickness in h2 and h4 regions, is due to the enhanced reflectivity and phase mismatches introduced by thicker metallic layers. The blue shift in high absorptivity regions (noted beyond 2000 nm) occurs because increased metal thickness alters the effective optical path and shifts the resonance conditions, especially in the h4, h6, and h8 Cr layers. This effect is amplified in upper layers closer to the incident light, as they more directly modulate the interaction of light with the structure. Thus, maintaining the Cr layer thickness at 6 nm ensures an optimal balance between sufficient absorption and minimal reflection or damping losses, enabling strong absorptivity over both short and long wavelength ranges.
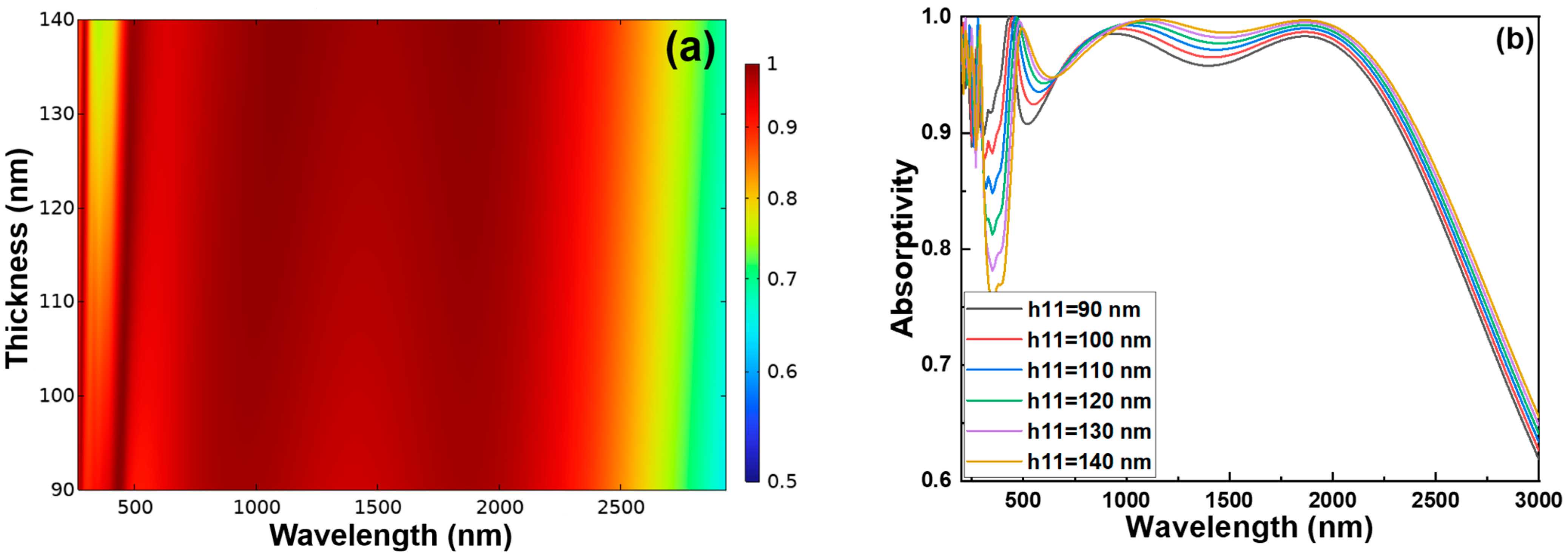
An optimization analysis was conducted on the cylinder height of the H11 Cr layer. As the height of the cylindrical structure increases, notable changes in optical absorption characteristics are observed. According to the 2D color map shown in
Figure 8a, an increase in cylinder height leads to a decrease in absorptivity within the wavelength range of 300–500 nm. This is visually represented by the expansion of the yellow low absorptivity region on the map. Furthermore, the absorption spectra in
Figure 8b confirm this trend: as the cylinder height increases, the overall absorption significantly decreases, particularly in the targeted wavelength range. Based on these observations, a cylinder height of 90 nm is determined to be the optimal value, balancing structural design with efficient optical performance.
Finally, we conducted an optimal width analysis for the cylindrical diameter d of the h11 Cr layer. As the cylinder diameter increases from 90 nm to 170 nm, it is evident that absorptivity improves significantly in the wavelength ranges of approximately 200–320 nm and 600–1350 nm. However, when the diameter exceeds 120 nm, a decreasing trend in absorptivity is observed in the 430–700 nm range. This trend is also visually apparent in the 2D color map, where the red regions become lighter, as shown in
Figure 9a,b, further illustrating the variation in the absorption spectra for diameters ranging from 110 nm to 150 nm. It is clear that as the diameter increases, the absorption in the longer wavelength region—where absorptivity exceeds 0.9—expands. However, this improvement comes with a trade-off: a noticeable drop in absorptivity around the 500 nm valley. For diameters of 110 nm and 120 nm, absorptivity at the 310 nm absorption dip remains below 0.9. In contrast, when the diameter increases to between 130 nm and 150 nm, absorptivity in the ultraviolet region surpasses 0.9 across the entire UV range. According to
Figure 9b, the configuration with a diameter of 130 nm demonstrates the most balanced and highest average absorptivity across the broad wavelength range of 200–2160 nm, indicating the most efficient overall absorption performance. Therefore, a cylinder diameter of 130 nm is identified as the optimal design parameter for the H11 Cr layer.
In this study, the topmost array of Cr cylinders plays a crucial role in enhancing absorption within the 200–350 nm wavelength range. By tuning the height and diameter of these cylinders, the structure can achieve an effective optical impedance with a real part close to 1 and an imaginary part near 0. This improvement in absorption arises from several key mechanisms. First, the Cr cylinder array behaves as an effective medium layer; by adjusting the diameter and spacing of the cylinders, the effective refractive index of the layer can be finely controlled, enabling a gradual impedance transition from air (n = 1) to the underlying layers. Second, the Cr cylinders support localized surface plasmon resonances within the ultraviolet range. When the cylinder dimensions resonate with the target wavelengths, strong electromagnetic field localization occurs, intensifying the light–matter interaction. Third, the periodic cylinder array induces strong scattering, which increases the optical path length within the structure. The scattered light undergoes multiple reflections within the multilayer architecture, creating a light-trapping effect that prolongs the interaction time between light and the absorbing materials. Fourth, the alternating SiO2 and Cr layers themselves form an interference cavity, enhancing absorption at specific wavelengths through constructive interference. The metallic Cr cylinders on top further couple with this interference field, boosting local field intensity and absorption. Lastly, the lower SiO2/Cr multilayer acts as a Fabry–Perot cavity, where the thicknesses of the SiO2 layers are precisely designed to match the target wavelengths.
3.3. Comparison of Absorption Spectra Before and After Structural Modification and the Principle of Optical Impedance
Based on the preceding series of analyses, the optimal thicknesses for the SiO
2 layers—h2, h4, h6, h8, and h10—were determined to be 55 nm, 70 nm, 110 nm, 110 nm, and 34 nm, respectively. The corresponding Cr layers—h3, h5, h7, and h9—were each optimized to a uniform thickness of 6 nm. Additionally, the cylindrical Cr nanostructures positioned on the topmost h11 Cr layer exhibited a height of 90 nm and a diameter d of 130 nm.
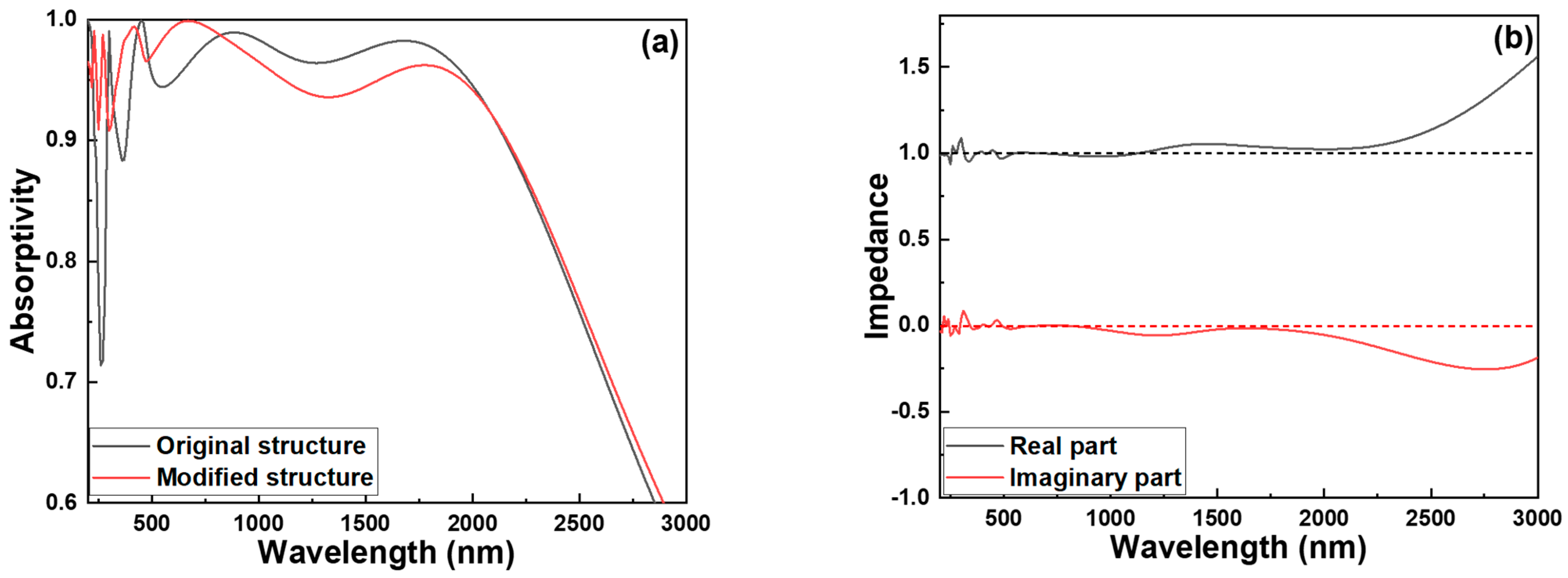
Figure 10a compares the absorption spectra of the original structure with that of the fully optimized absorber. Although the optimized absorber exhibits slightly reduced absorption in the longer wavelength range of 1000–2000 nm, it shows significant enhancement in absorptivity for wavelengths below 900 nm. This improvement is particularly prominent in the ultraviolet region, where the enhanced multilayer configuration—especially the addition of precisely tuned metal–dielectric pairs and the Cr cylindrical nanostructures—promotes stronger light confinement, increased resonance effects, and more efficient energy dissipation. These optimizations collectively contribute to superior performance in the UV and visible spectra, which are critical for applications such as photodetectors and solar absorbers.
Figure 10b presents the optical impedance analysis of the absorber with the optimized structural parameters. Compared with the impedance profile shown in
Figure 4b, it is evident that the imaginary part of the impedance in the optimized absorber remains close to zero until approximately 2050 nm, after which it begins to shift noticeably toward negative values.
Similarly, the real part of the impedance remains close to 1 until around 2300 nm, beyond which it starts to deviate significantly and bends toward higher values. This behavior indicates that the optimized absorber maintains well-matched impedance with free space (Z
0 ≈ 1) across a broader spectral range, thereby minimizing reflection and maximizing absorption in the UV to near-infrared regions. The delayed deviation of the imaginary part toward negative values suggests that the structure effectively suppresses reactive (non-dissipative) components of the impedance over a wide wavelength range, reducing phase mismatch and enhancing light coupling. Meanwhile, the shift in the real part toward higher values at longer wavelengths reflects increased resistive losses and impedance mismatching, which reduce absorption efficiency in the far-infrared range. These changes are primarily caused by the cumulative effects of material dispersion, interference within the multilayer stack, and the frequency-dependent effective permittivity and permeability of the engineered nanostructure. To elucidate the perfect absorption mechanism of the proposed ultra-broadband solar absorber based on Cr–SiO
2 metamaterial planar stacked structures, impedance matching theory is employed. The effective impedance Z is calculated using the following expression, where S
11 and S
21 represent the reflection and transmission coefficients, respectively [
26,
27]:
The absorptivity (A) can be expressed as follows:
When the structure is designed such that Re(Z) ≈ 1 and Im(Z) ≈ 0, the effective impedance of the absorber matches that of free space. This eliminates impedance mismatch, minimizing reflection and enabling near-perfect absorption of the incident electromagnetic waves.
Figure 10b shows that the designed absorber exhibits characteristics of Re(Z) ≈ 1 and Im(Z) ≈ 0 in the 200–2160 nm range. As a result, the absorber achieves an absorptivity greater than 0.900 within this wavelength range.
3.4. Simulated Spatial Distributions of Electric and Magnetic Field Intensities
In this study, the ultra-wideband optical absorber under investigation is specifically engineered to achieve high light absorption across an extensive spectral range, from 200 nm in the ultraviolet-C region to 2160 nm in the near-infrared region.
Figure 11 presents the simulated electric and magnetic field distributions at selected wavelengths of 420, 670, and 1780 nm, as shown in
Figure 11a and
Figure 11b, respectively. First,
Figure 11a,b clearly demonstrate the high absorptivity characteristics of the top Cr cylinder layer at wavelengths of 420 nm and 670 nm, and the low absorption characteristic at a wavelength of 1780 nm. This observation supports our earlier assertion that the top Cr cylindrical structure plays a crucial role in enhancing the absorption performance of the entire design, particularly in the shorter wavelength range. The electric field intensity distribution in
Figure 11a indicates strong field localization, correlating with high absorption at shorter wavelengths. Furthermore, as the wavelength increases, the regions of peak electric field intensity—and thus strong absorption—tend to shift toward the upper layers of the structure. This spatial shift is consistent with the wavelength-dependent nature of surface plasmon resonances (SPRs), which are more tightly confined at shorter wavelengths and more delocalized at longer wavelengths. Moreover, although the electric field intensity—and by extension, the absorptivity—slightly decreases as the wavelength increases from 420 to 1780 nm, the drop is not drastic.
Notably, at a wavelength of 1780 nm, the electric field intensity within the Cr cylinder becomes weaker than that in the surrounding dielectric regions. This phenomenon can be attributed to the wavelength-dependent behavior of SPRs. At longer wavelengths, such as 1780 nm, the excitation of localized surface plasmons is less efficient, resulting in reduced confinement of the electromagnetic field within the metallic nanostructure. Additionally, the increased skin depth at longer wavelengths allows the incident electromagnetic wave to penetrate deeper into the structure, causing the field to spread more broadly into the surrounding layers rather than being concentrated within the Cr cylinder itself. Despite the weaker field localization at this wavelength, the absorber still maintains considerable absorptivity, as energy dissipation continues to occur in the dielectric–metal interfaces and other lossy components of the multilayer structure. However, the magnetic field analysis in
Figure 11b reveals a more noticeable trend: as the wavelength increases, the overall region within the structure exhibiting high absorption becomes narrower and more superficial. In particular, between 670 and 1780 nm, the high-absorptivity zones not only become shallower but also decrease in spatial extent, as evidenced by the expansion of regions with lower field intensity (i.e., “origin regions”). These findings indicate a gradual reduction in the absorber’s efficiency at longer wavelengths.
Nevertheless, the results confirm that the dominant mechanism enabling broadband absorption across the full 200–2160 nm range is SPR. SPR arises from the collective oscillation of free electrons at the interface between the metal (Cr) and dielectric materials, which strongly enhances local electromagnetic fields and, consequently, absorption. The observed decline in absorptivity at longer wavelengths can be attributed to the shift in resonance conditions; specifically, as the incident wavelength approaches the mid-infrared region, the efficiency of SPR diminishes due to the lower confinement of plasmonic modes and reduced coupling strength. To address the possibility of non-negligible transmission due to the relatively thin multilayer structure and Cr substrate, we provide additional clarification based on the field absorption distribution results shown in
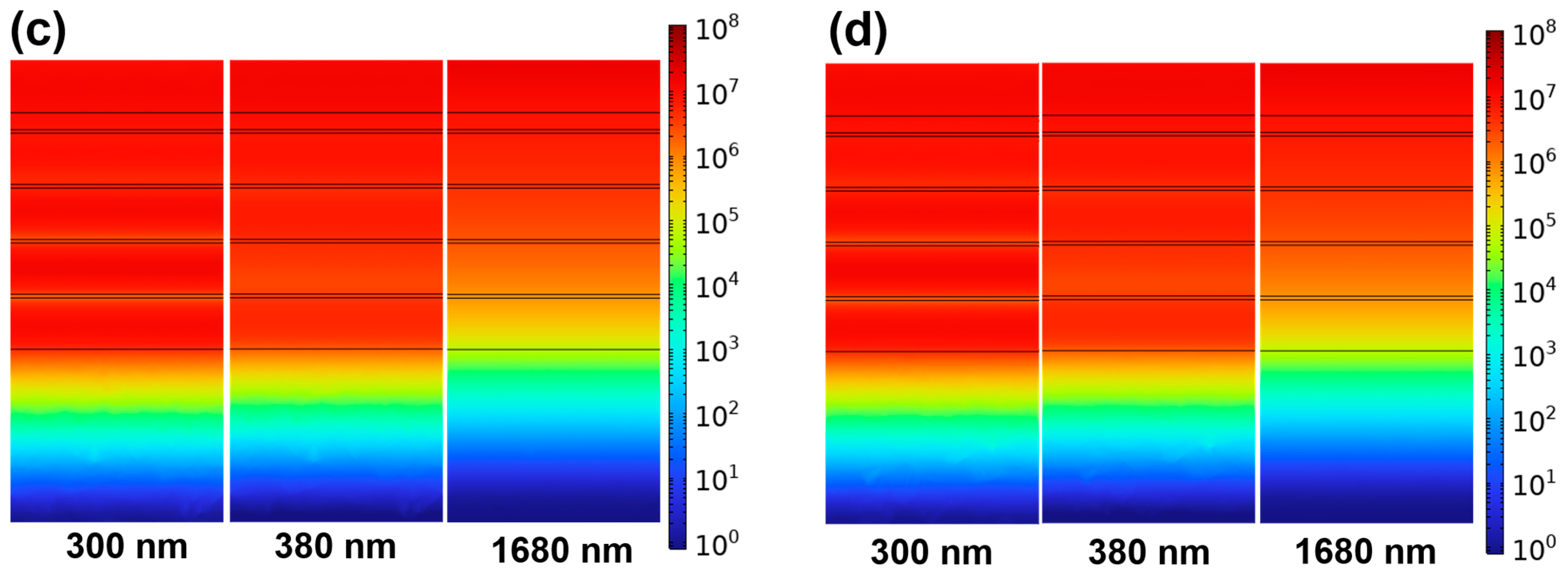
Figure 11a,b. It can be clearly observed that the bottom h1 Fe layer exhibits consistently low absorptivity across all wavelengths, as indicated by the prominent blue regions. This suggests that the incident electromagnetic waves are effectively absorbed by the upper layers before reaching the bottom layer, and that almost no significant energy penetrates through the entire structure. Therefore, the transmission through the absorber can be considered negligible. These results validate that the high absorptivity is primarily attributed to the efficient absorption and resonance effects within the multilayer system, rather than any transmitted component. The electric and magnetic field intensities for the six-pair structure without the topmost Cr cylinder (as shown in
Figure 2a) are presented in
Figure 11c,d. Notably, these intensities are significantly weaker compared to those observed in the six-pair structure with the topmost Cr cylinder.
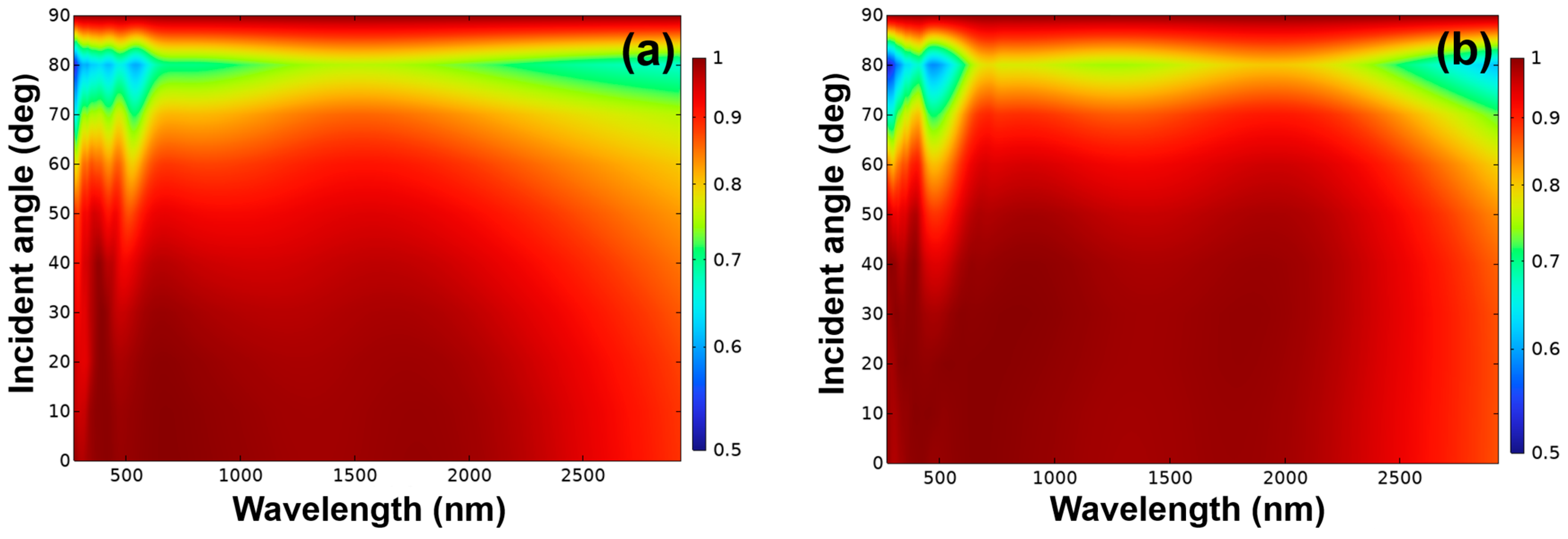
We conducted a comprehensive evaluation of the absorption performance of the proposed metamaterial absorber across the full electromagnetic spectrum, spanning from the ultraviolet-C band at 200 nm to the near-infrared region up to 2160 nm. The assessment considered incident angles ranging from 0° to 90°, and included both transverse electric (TE) polarization, as shown in
Figure 12a, and transverse magnetic (TM) polarization, as shown in
Figure 12b. As illustrated in
Figure 12, there is no significant difference in absorptivity between TE and TM polarizations, indicating polarization-insensitive behavior. The simulation results further reveal the variation in absorptivity as a function of both incident angle and wavelength. The absorber structure is composed of 11 alternating layers. The bottom layer (h1) is a continuous planar Cr film, where layers h3, h5, h7, and h9 consist of continuous planar Cr structures, while h2, h4, h6, and h8 are continuous s SiO
2 dielectric layers. The topmost layer features an array of discontinuous cylindrical Cr nanostructures. Absorptivity is angle-dependent and varies as the incident light angle changes from 0° to 90°. For both TE and TM polarizations, a lower absorptivity is observed across most angles in the broadband wavelength range of 200–2500 nm. However, a high absorptivity is consistently observed within the wavelength range of 200 nm to approximately 2300 nm, particularly for incident angles between 0° and 45°. These results demonstrate that the proposed ultra-broadband solar absorber, based on Cr–SiO
2 metamaterial planar stacked structures, exhibits excellent angular insensitivity. This makes it a promising candidate for practical solar energy harvesting applications where variable light incidence is a key consideration.