Carbon Quantum Dot-Embedded SiO2: PMMA Hybrid as a Blue-Emitting Plastic Scintillator for Cosmic Ray Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Carbon Quantum Dots
- 20 mL ethanol + 1 g urea
- 20 mL ethanol + 1 g urea + 0.05 g citric acid
2.2. Synthesis of Hybrid Organic–Inorganic Matrix (PMMA–SiO2)
- A silica-based matrix via sol–gel processing.
- A PMMA-based matrix via MMA polymerization.
- A combined PMMA–SiO2 hybrid matrix.
- A matrix composed solely of SiO2.
2.3. Characterization of Luminescent Nanomaterials and Hybrid Matrices
2.3.1. X-Ray Diffraction (XRD)
2.3.2. UV-VIS–NIR Spectroscopy
2.3.3. Photoluminescence and Quantum Yield
2.3.4. Transmission Electron Microscopy (TEM)
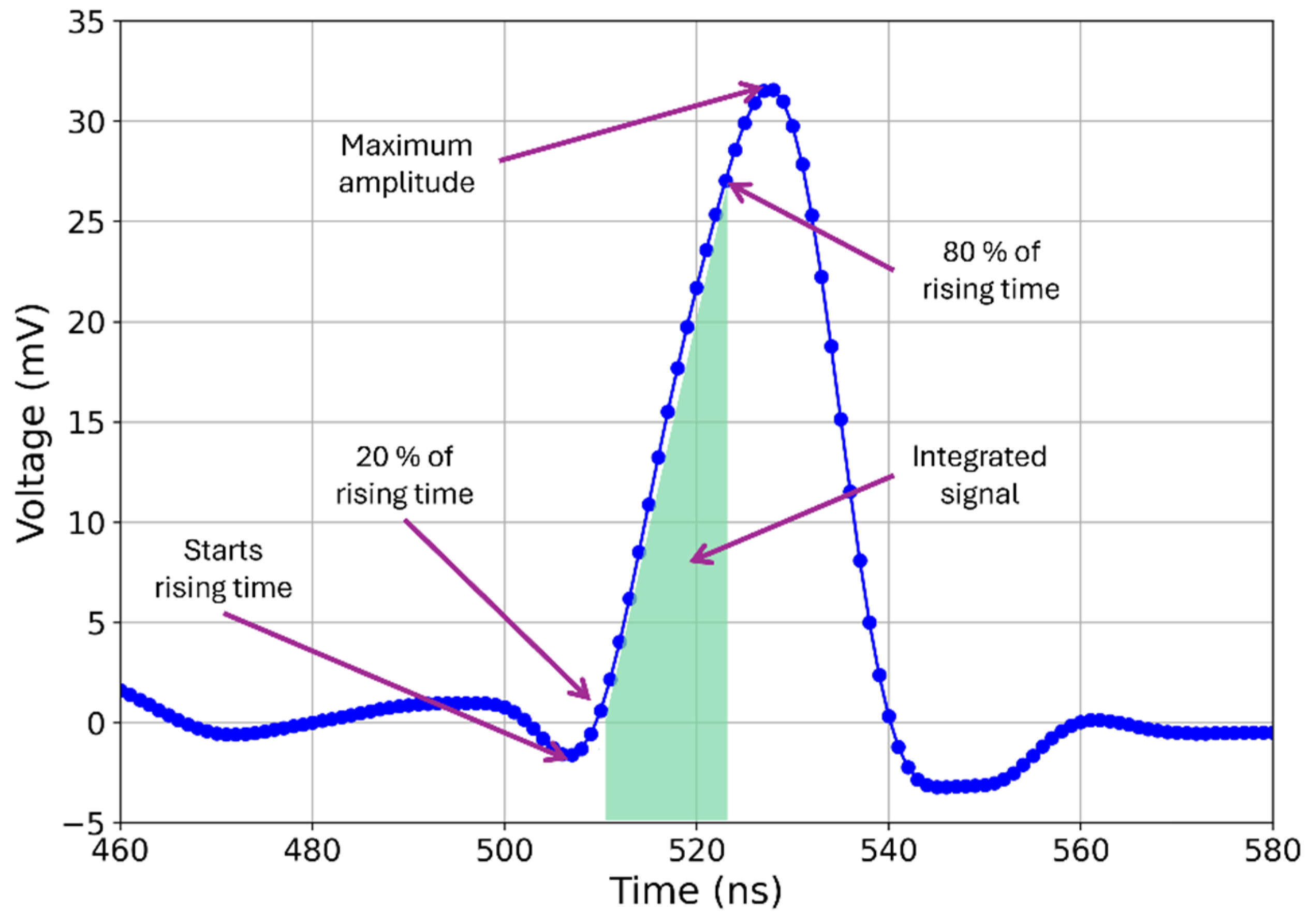
2.3.5. Scintillation Testing with Cosmic Ray Detector
3. Results and Discussion
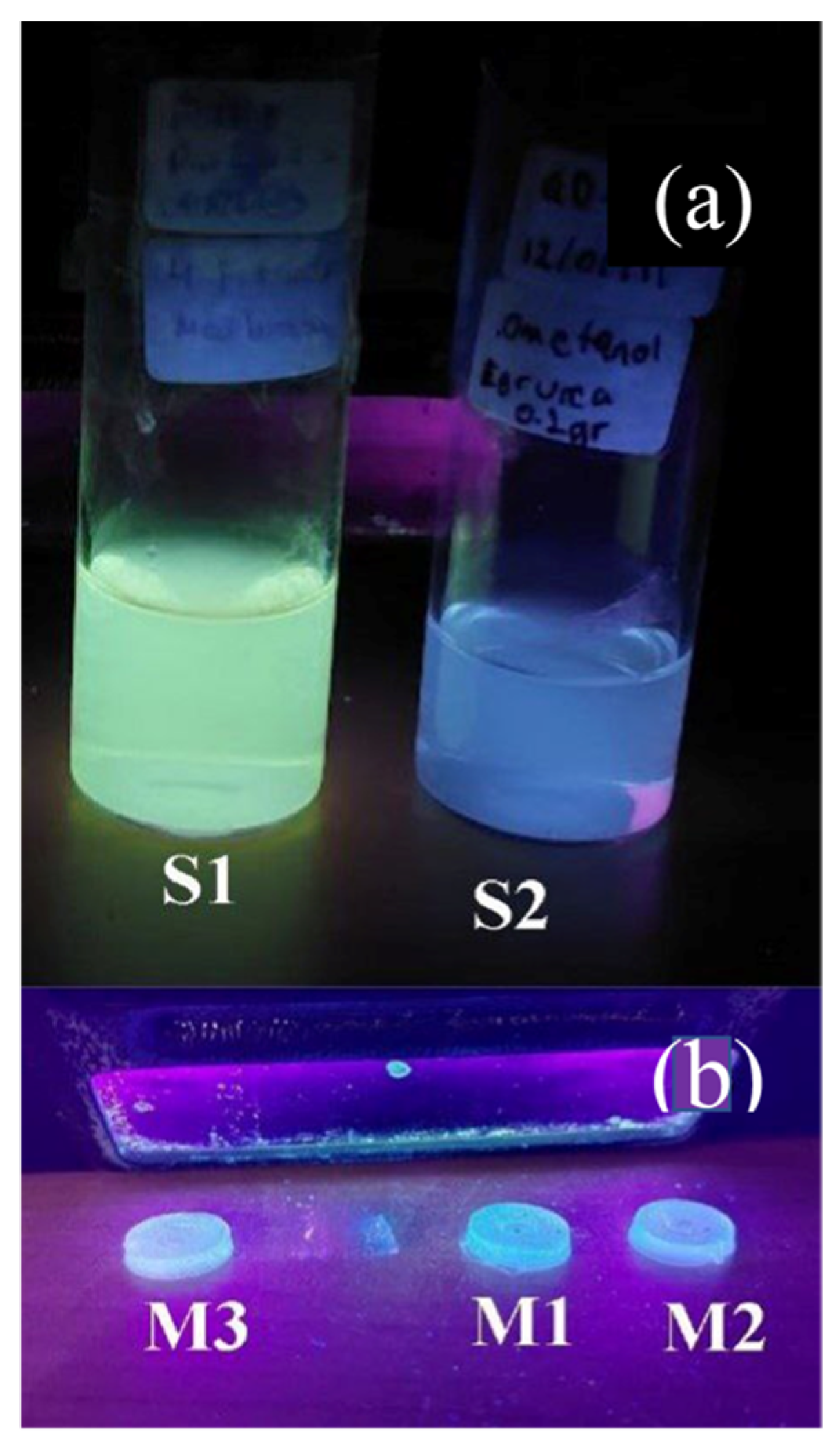
3.1. Synthesis of Carbon Quantum Dots and Hybrid Organic–Inorganic Matrix (PMMA–SiO2)
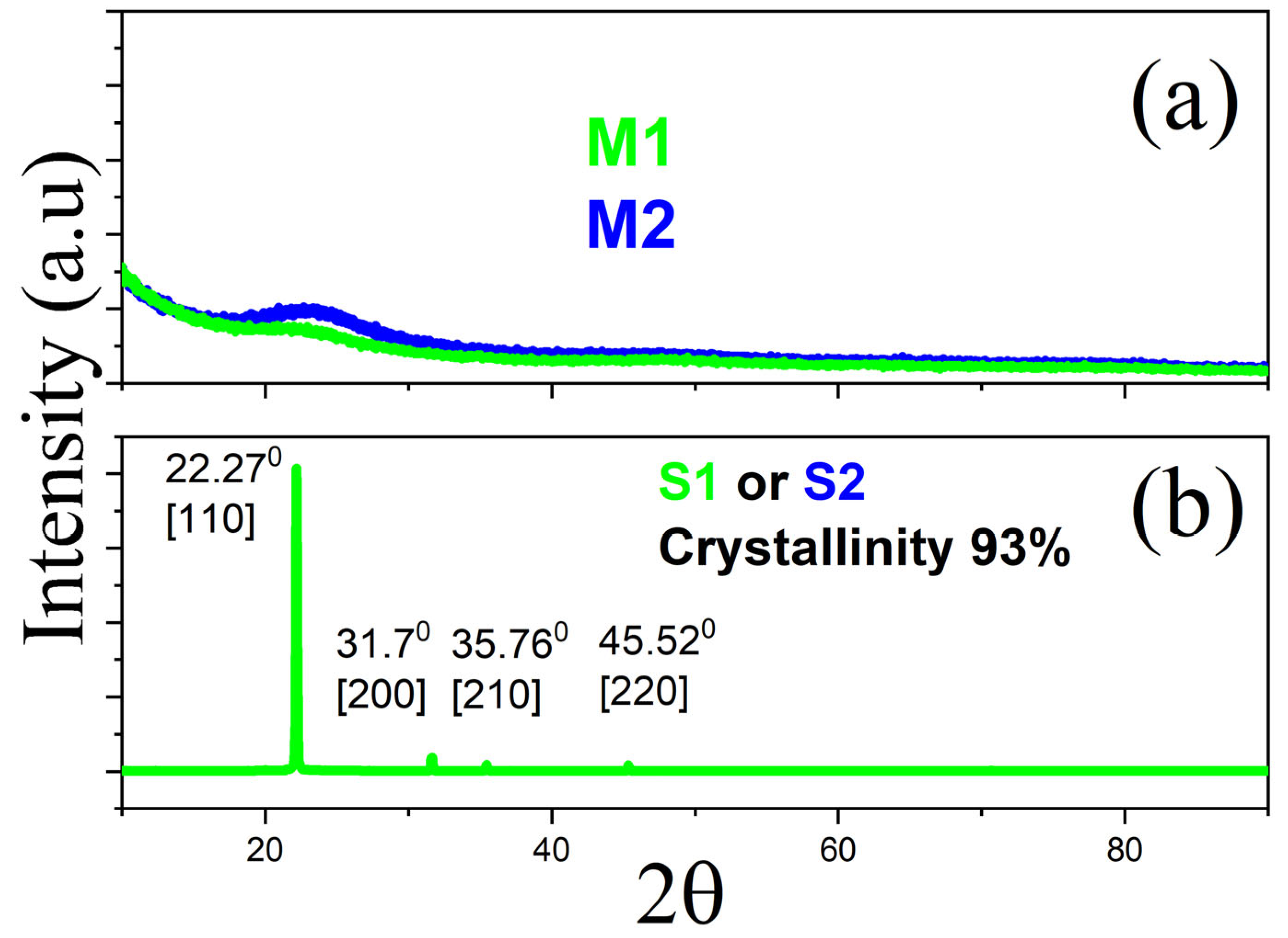
3.2. X-Ray Diffraction (XRD) Discussion
3.3. Transmission Electron Microscopy (TEM) Discussion
3.4. UV-VIS–NIR Spectroscopy Discussion
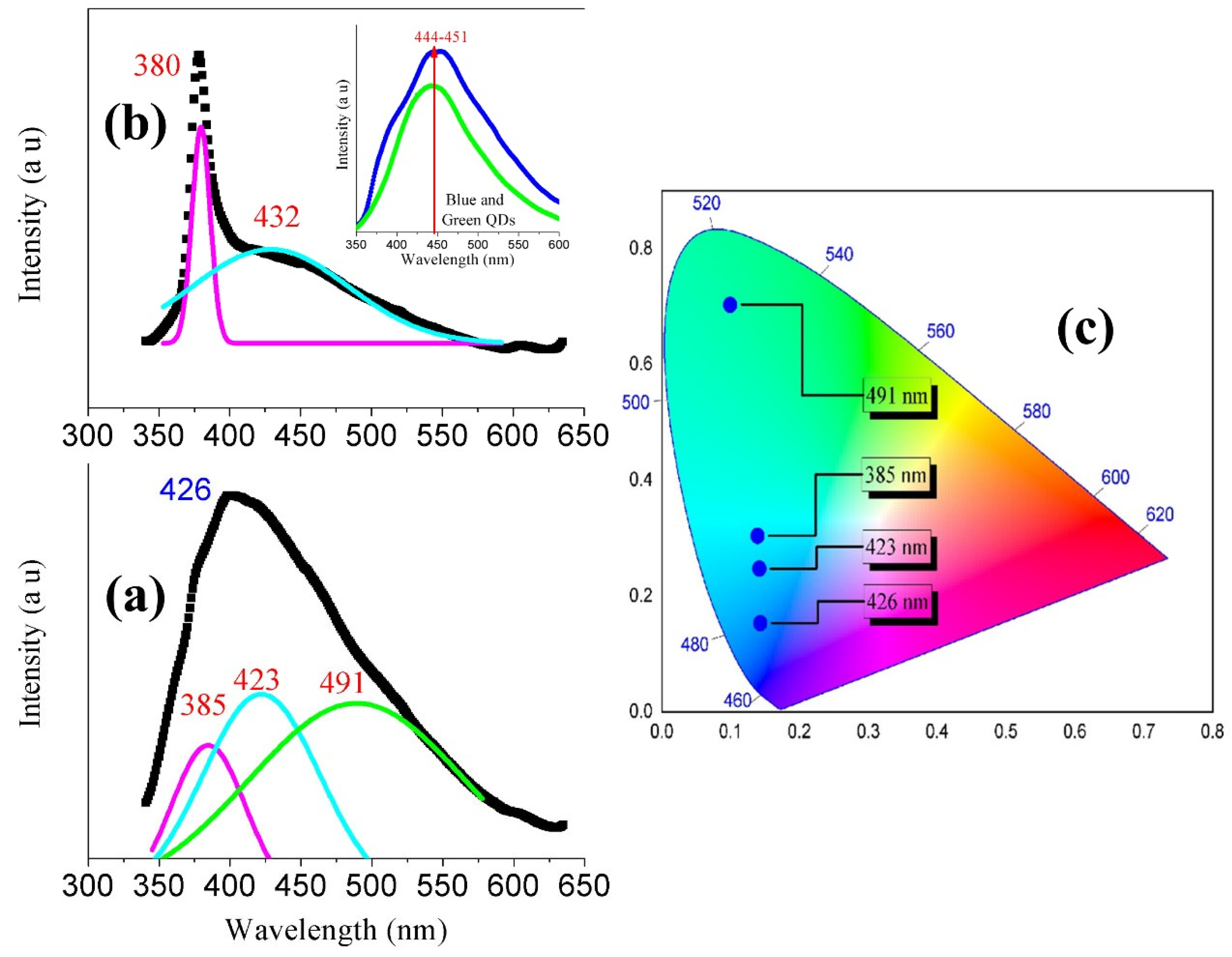
3.5. Photoluminescence
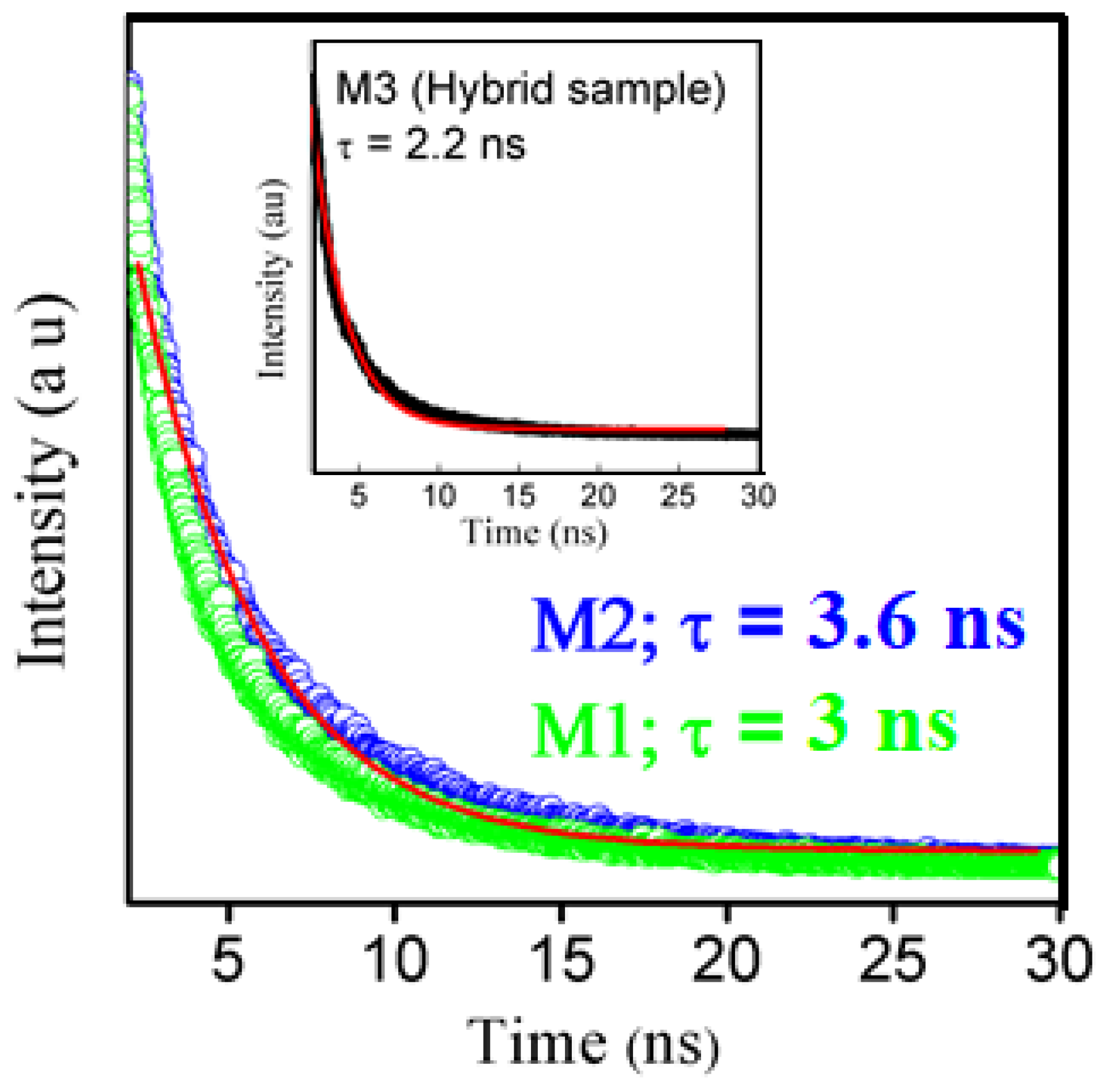
3.5.1. Time Decay
- (y) is the emitted light intensity,
- (y0) is the baseline intensity,
- (A1) is a normalization constant,
- (t − t0) is the time elapsed since excitation,
- (τ) is the decay time constant.
3.5.2. Optical Quantum Yield (OQY)
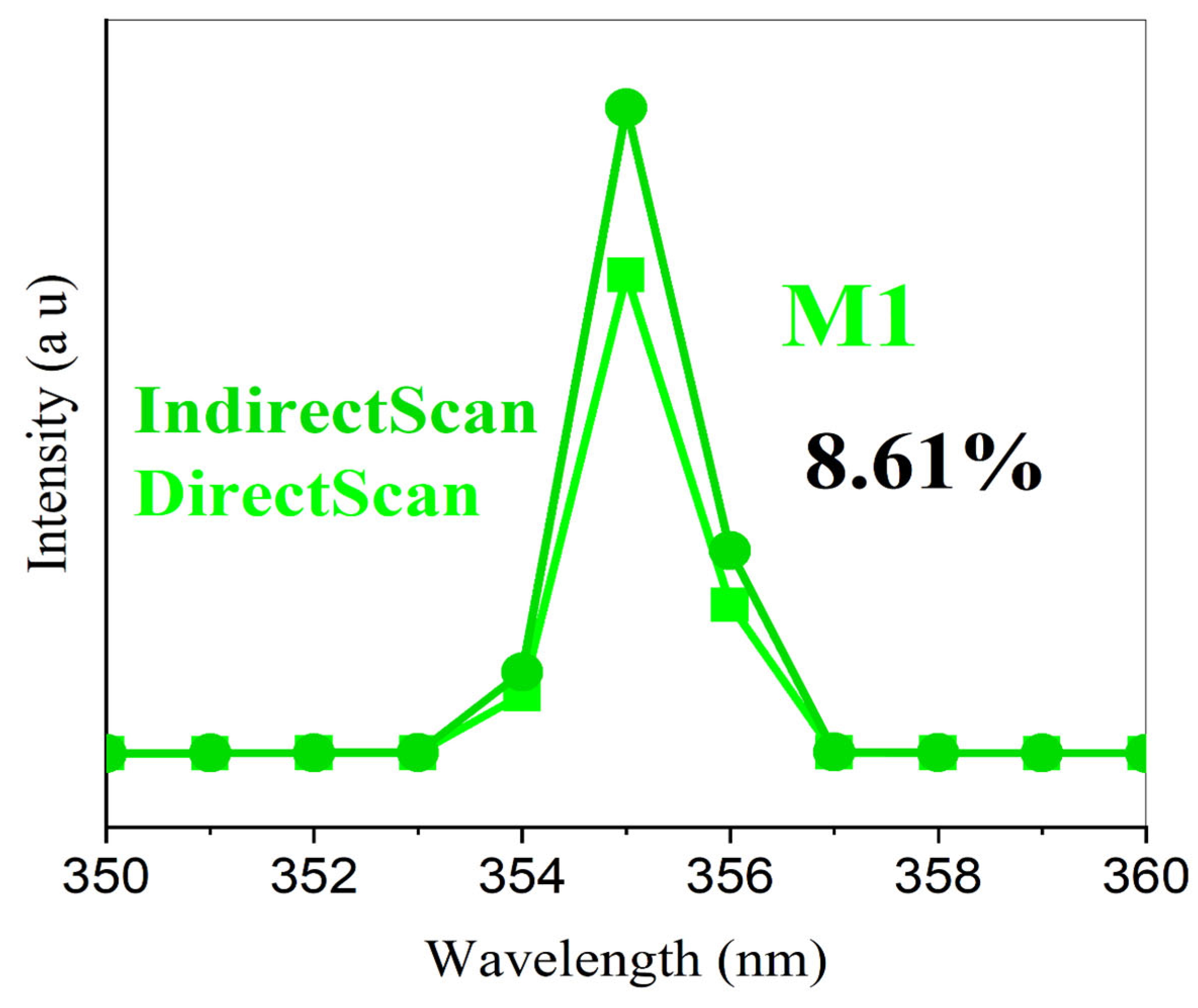
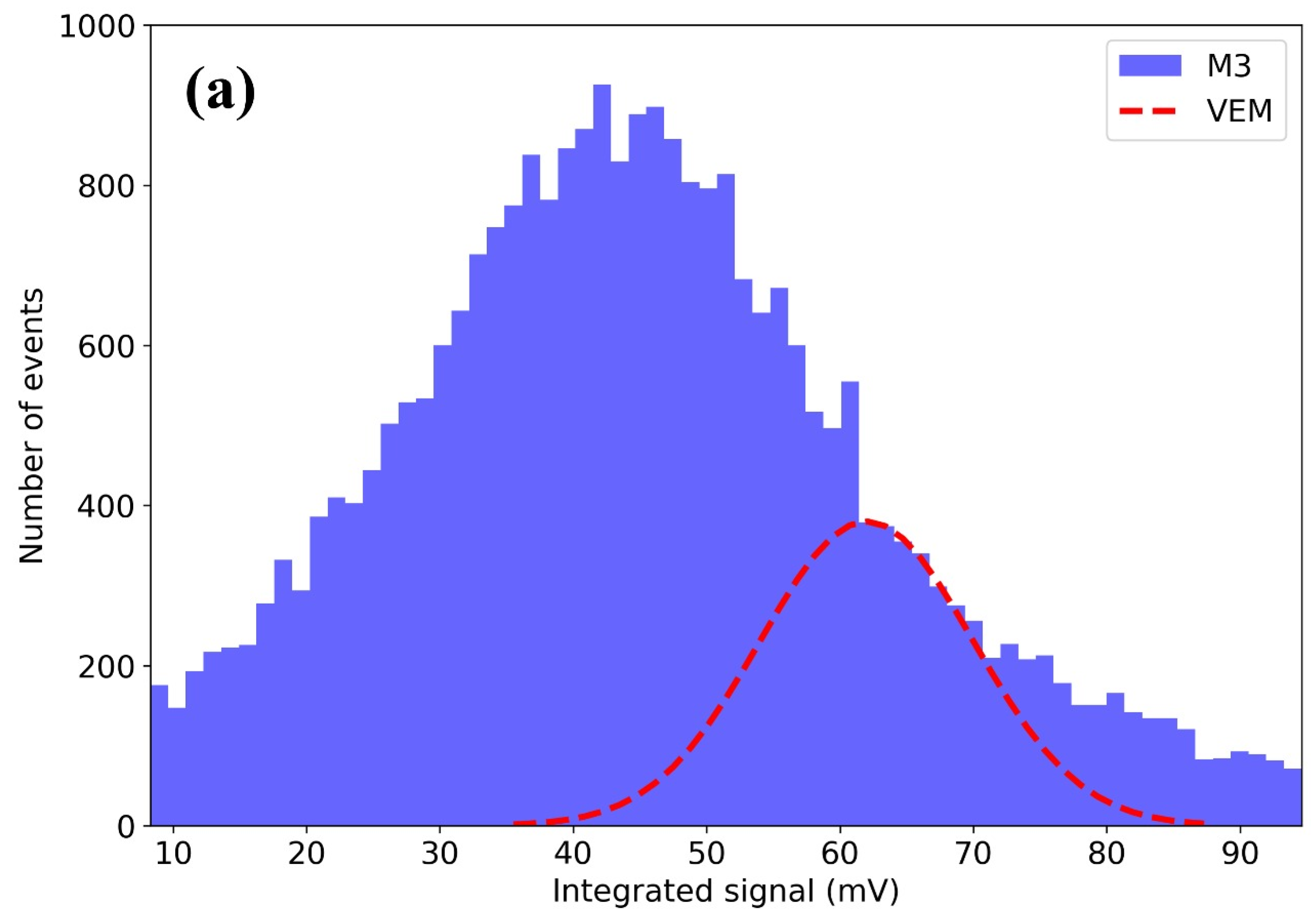
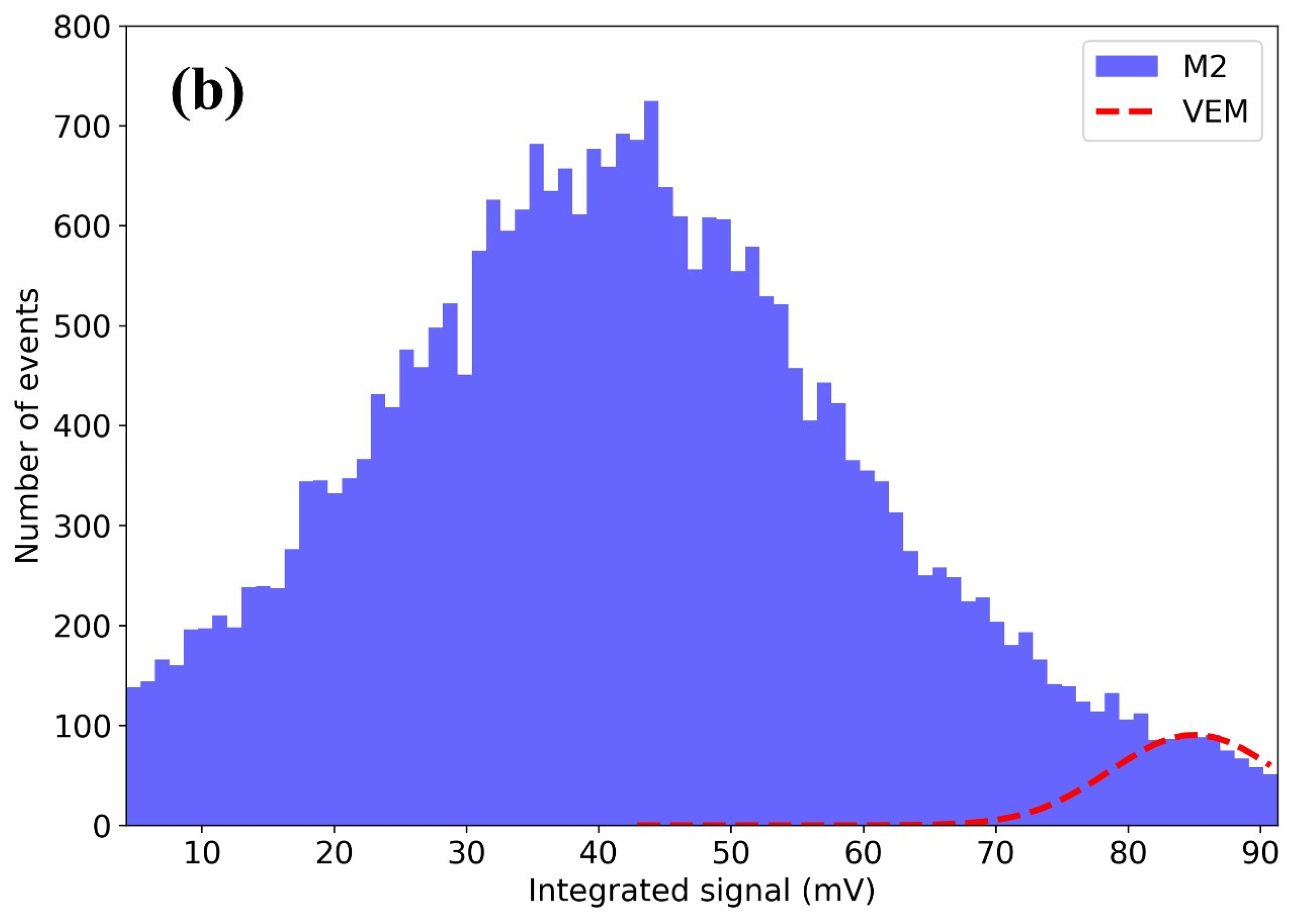
3.6. Scintillation Testing with Cosmic Ray Detector Discussion
- M3: Amplitude = 380 events, Mean = 62 mV, Sigma = 8 mV
- M2: Amplitude = 90 events, Mean = 85 mV, Sigma = 6.3 mV
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.; Fu, Q.; Zhang, K.; Sun, S.; Yue, M. Research on light-responsive luminescence properties of carbon dots and their applications. Mater. Horiz. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Wang, X.; Hao, Y.; Yang, J.; Wang, H.; Qu, S. Recent advances in highly luminescent carbon dots. Adv. Funct. Mater. 2025, 35, 2420587. [Google Scholar] [CrossRef]
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Huang, Z.; Ren, L. Large scale synthesis of carbon dots and their applications: A review. Molecules 2025, 30, 774. [Google Scholar] [CrossRef]
- Bhatt, J.P.; Godha, N. Quantum dots: Fundamentals, synthesis and applications. In Hydrothermal Synthesis of Quantum Dots; Elsevier: Amsterdam, The Netherlands, 2023; pp. 15–34. [Google Scholar] [CrossRef]
- Zhu, Z.; Niu, H.; Li, R.; Yang, Z.; Wang, J.; Li, X.; Pan, P.; Liu, J.; Zhou, B. One-pot hydrothermal synthesis of fluorescent carbon quantum dots with tunable emission color for application in electroluminescence detection of dopamine. Biosens. Bioelectron. X 2022, 10, 100141. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Kickelbick, G. (Ed.) Hybrid Materials: Synthesis, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2007; ISBN 978-3-527-31299-3. [Google Scholar]
- Gomez-Romero, P.; Pokhriyal, A.; Rueda-García, D.; Bengoa, L.N.; González-Gil, R.M. Hybrid materials: A metareview. Chem. Mater. 2024, 36, 8–27. [Google Scholar] [CrossRef]
- Olabisi, O.; Adewale, K. (Eds.) Handbook of Thermoplastics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 3: 978-1-4665-7723-7. [Google Scholar]
- Yuan, X.W.; Jung, D.D.; Bhattacharyya, D.; Easteal, A.J. Hybrid thermoplastic composites using ceramic reinforcements. Plast. Rubber Compos. 2005, 34, 85–92. [Google Scholar] [CrossRef]
- Park, W.; Shin, H.; Choi, B.; Rhim, W.K.; Na, K.; Han, D.K. Advanced hybrid nanomaterials for biomedical applications. Prog. Mater. Sci. 2020, 114, 100686. [Google Scholar] [CrossRef]
- Song, J.; Vikulina, A.S.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of hybrid materials. Part-II: The place of organics-on-inorganics in it, their composition and applications. Front. Chem. 2023, 11, 1078840. [Google Scholar] [CrossRef]
- Rojas-Valencia, O.G.; Regules-Carrasco, M.; Hernández-Fuentes, J.; Reza-San Germán, C.M.; Estrada-Flores, M.; Villagarcía-Chávez, E. Synthesis of blue emissive carbon quantum dots from Hibiscus Sabdariffa flower: Surface functionalization analysis by FT-IR spectroscopy. Materialia 2021, 19, 101182. [Google Scholar] [CrossRef]
- Yalshetti, S.; Thokchom, B.; Bhavi, S.M.; Singh, S.R.; Patil, S.R.; Harini, B.P.; Sillanpää, M.; Manjunatha, J.G.; Srinath, B.S.; Yarajarla, R.B. Microwave-assisted synthesis, characterization and in vitro biomedical applications of Hibiscus rosa-sinensis Linn.-mediated carbon quantum dots. Sci. Rep. 2024, 14, 9915. [Google Scholar] [CrossRef]
- Kundu, A.; Maity, B.; Basu, S. Orange pomace-derived fluorescent carbon quantum dots: Detection of dual analytes in the nanomolar range. ACS Omega 2023, 8, 22178–22189. [Google Scholar] [CrossRef]
- Gholipour, A.; Rahmani, S. The green synthesis of carbon quantum dots through one-step hydrothermal approach by orange juice for rapid, and accurate detection of dopamine. J. Fluoresc. 2024, 34, 2665–2677. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Younis, M.A.; Alsharidah, M.; Al Rugaie, O.; Tawfeek, H.M. Biomedical applications of quantum dots: Overview, challenges, and clinical potential. Int. J. Nanomed. 2022, 17, 1951–1970. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Lisi, F.; Sawayama, J.; Gautam, S.; Rubanov, S.; Duan, X.; Kirkwood, N. Re-Examination of the Polymer Encapsulation of Quantum Dots for Biological Applications. ACS Appl. Nano Mater. 2023, 6, 4046–4055. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, R.; Lin, G.; Roy, I.; Yong, K.-T. Functionalized Quantum Dots for Biosensing and Bioimaging and Concerns on Toxicity; ACS Appl. Mater. Interfaces 2013, 5, 2786–2799. [Google Scholar] [CrossRef]
- Korzhik, M.; Tamulaitis, G.; Vasil’ev, A.N. Physics of Fast Processes in Scintillators; Springer: Berlin/Heidelberg, Germany, 2020; Volume 49. [Google Scholar] [CrossRef]
- Lecoq, P. Development of new scintillators for medical applications. Nucl. Instrum. Methods Phys. Res. A 2016, 809, 130–139. [Google Scholar] [CrossRef]
- Weninger, L.; Morana, A.; Ouerdane, Y.; Marin, E.; Boukenter, A.; Girard, S. Distributed optical fiber-based radiation detection using an ultra-low-loss optical fiber. Radiation 2024, 4, 167–182. [Google Scholar] [CrossRef]
- Ntoupis, V.; Michail, C.; Kalyvas, N.; Bakas, A.; Kandarakis, I.; Fountos, G.; Valais, I. Luminescence efficiency and spectral compatibility of cerium fluoride (CeF3) inorganic scintillator with various optical sensors in the diagnostic radiology X-ray energy range. Inorganics 2024, 12, 230. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, K.; Chen, W.; Li, D.; Lei, L. Controlled synthesis of Cs2NaYF6: Tb nanoparticles for high-resolution X-ray imaging and molecular detection. Nanomaterials 2025, 15, 728. [Google Scholar] [CrossRef]
- Gong, S.; Ma, Z.; Li, X. UMFNet: Frequency-guided multi-scale fusion with dynamic noise suppression for robust low-light object detection. Appl. Sci. 2025, 15, 5362. [Google Scholar] [CrossRef]
- Buchner, A.; Hadrath, S.; Burkard, R.; Kolb, F.M.; Ruskowski, J.; Ligges, M.; Grabmaier, A. Analytical evaluation of signal-to-noise ratios for avalanche- and single-photon avalanche diodes. Sensors 2021, 21, 2887. [Google Scholar] [CrossRef]
- Sun, J.; Akdogan, E.K.; Klein, L.C.; Safari, A. Characterization and optical properties of sol–gel processed PMMA/SiO2 hybrid monoliths. J. Non-Cryst. Solids 2007, 353, 2807–2812. [Google Scholar] [CrossRef]
- Gil-Kowalczyk, M.; Łyszczek, R.; Jusza, A.; Piramidowicz, R. Thermal, Spectroscopy and Luminescent Characterization of Hybrid PMMA/Lanthanide Complex Materials. Materials 2021, 14, 3156. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, S.A.; Surin, N.M.; Borshchev, O.V.; Luponosov, Y.N.; Akimov, D.Y.; Alexandrov, I.S.; Burenkov, A.A.; Kovalenko, A.G.; Stekhanov, V.N.; Kleymyuk, E.A.; et al. Nanostructured organosilicon luminophores and their application in highly efficient plastic scintillators. Sci. Rep. 2014, 4, 6549. [Google Scholar] [CrossRef]
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical analysis by X-ray diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Madhurambal, G.; Mariappan, M. Growth and characterization of urea-thiourea non-linear optical organic mixed crystal. Indian J. Pure Appl. Phys. 2010, 48, 264–270. [Google Scholar]
- Adams, R.; Balyuzi, H.H.M.; Burge, R.E. X-ray diffraction studies of aqueous solutions of urea. J. Appl. Cryst. 1977, 10, 256–261. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice-Hall Inc.: Wilmington, DE, USA, 2001; pp. 96–102. ISBN 0-201-61091-4. [Google Scholar]
- Gómez-Rodríguez, A.; Beltrán-Del-Río, L.M.; Herrera-Becerra, R. SimulaTEM: Multislice simulations for general objects. Ultramicroscopy 2010, 110, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Trejo-García, P.M.; Palomino-Merino, R.; De la Cruz, J.; Espinosa, J.E.; Aceves, R.; Moreno-Barbosa, E.; Portillo Moreno, O. Luminescent properties of Eu3+-doped hybrid SiO2–PMMA material for photonic applications. Micromachines 2018, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, J.; Palomino Merino, R.; Trejo-García, P.; Espinosa, J.E.; Aceves Torres, R.; Moreno-Barbosa, E.; Gervacio-Arciniega, J.J.; Soto, E. Luminescent properties of a hybrid SiO2–PMMA matrix doped with terbium. Opt. Mater. 2019, 87, 42–47. [Google Scholar] [CrossRef]
- Mergen, Ö.B.; Arda, E.; Kara, S.; Pekcan, Ö. Effects of GNP addition on optical properties and band gap energies of PMMA films. Polym. Compos. 2019, 40, 1862–1869. [Google Scholar] [CrossRef]
- Wardani, D.A.P.; Hariyanto, B.; Kurniawati, N.; Har, N.P.; Darmawan, N. Irzaman, Functional groups, band gap energy, and morphology properties of annealed silicon dioxide (SiO2). Egypt. J. Chem. 2023, 66, 529–535. [Google Scholar] [CrossRef]
- Alibe, I.M.; Matori, K.A.; Saion, E.; Alibe, A.M.; Zaid, M.H.M.; Engku, E.A.A.G. A facile synthesis of amorphous silica nanoparticles by simple thermal treatment route. Dig. J. Nanomater. Biostruct. 2016, 11, 1155–1164. [Google Scholar]
- Rai, C.; Pandey, P.; Haque, F.Z. Optical, dielectric and impedance studies of SiO2/MWCNT nanocomposite synthesized through in-situ ultrasonication-assisted sol–gel method. J. Adv. Phys. 2014, 3, 179–193. [Google Scholar] [CrossRef]
- Seguini, G.; Schamm-Chardon, S.; Pellegrino, P.; Perego, M. The energy band alignment of Si in SiO2. Appl. Phys. Lett. 2011, 99, 082107. [Google Scholar] [CrossRef]
- Trukhin, A.; Truhins, K. Luminescence of α Quartz. arXiv 2012, arXiv:1209.4200. [Google Scholar] [CrossRef]
- Yan, Y.-L.; Liu, Y.; Fang, C.-H.; Zhu, J.-J.; Lin, S.-T.; Liu, S.-K.; Tang, C.-J.; Wang, C.-L.; Xing, H.-Y. Preparation and characterization of polystyrene-based plastic scintillators as a self-vetoing structural material. J. Polym. Res. 2025, 32, 19. [Google Scholar] [CrossRef]
- Salimgareeva, V.N.; Kolesov, S.V. Plastic scintillators based on polymethyl methacrylate: A review. Instrum. Exp. Tech. 2005, 48, 273–282. [Google Scholar] [CrossRef]
- Bhakta, S.; Raman, A.; Ajish, J.K. Development of plastic scintillator with better performance than the commercial counterpart (UPS-923A) and effect of fluorophore composition on light output. Appl. Radiat. Isot. 2024, 212, 111453. [Google Scholar] [CrossRef]
- Efremova, O.A.; Brylev, K.A.; Vorotnikov, Y.A.; Vejsadova, L.; Shestopalov, M.A.; Chimonides, G.F.; Mikes, P.; Topham, P.D.; Kim, S.-J.; Kitamura, N.; et al. Photoluminescent materials based on PMMA and a highly-emissive octahedral molybdenum metal cluster complex. J. Mater. Chem. C 2016, 4, 497–503. [Google Scholar] [CrossRef]
- Luxium Solutions, Solid Plastic Scintillators, Data Sheets. Available online: https://luxiumsolutions.com/radiation-detection-scintillators/plastic-scintillators/bc400-bc404-bc408-bc412-bc416 (accessed on 5 May 2025).
- Saint Gobain Crystals, BC400, BC404, BC408, BC412, BC416 Premium Plastic Scintillators, Data Sheet. Available online: https://www.hep.phy.cam.ac.uk/~lester/teaching/SparkChamber/SGC_BC400_404_408_412_416_Data_Sheet.pdf (accessed on 5 May 2025).
- Grieder, P.K.F. Extensive Air Showers—High Energy Phenomena and Astrophysical Aspects, in Cosmic Rays at Earth. Volume 1. 2010. Available online: http://www.sciencedirect.com/science/article/pii/B9780444507105500038 (accessed on 10 May 2025).
- Tomassetti, N. Direct measurements of galactic cosmic rays. In Proceedings of the 27th European Cosmic Ray Symposium (ECRS), Nijmegen, The Netherlands, 25–29 July 2022; Volume 423, p. 007. [Google Scholar] [CrossRef]
- Gaisser, T.K.; Engel, R.; Resconi, E. (Eds.) Atmospheric Muons and Neutrinos, in Cosmic Rays and Particle Physics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2016; pp. 126–148. [Google Scholar] [CrossRef]
- Procureur, S. Muon imaging: Principles, technologies and applications. Nucl. Instrum. Methods Phys. Res. A 2017, 878, 169–179. [Google Scholar] [CrossRef]
- Etchegoyen, A.; Bauleo, P.; Bertou, X.; Bonifazi, C.B.; Filevich, A.; Medina, M.C.; Melo, D.G.; Rovero, A.C.; Supanitsky, A.D.; Tamashiro, A. Muon-track studies in a water Cherenkov detector. Nucl. Instrum. Methods Phys. Res. A 2005, 545, 602–612. [Google Scholar] [CrossRef]
- Bertou, X.; Allison, P.S.; Bonifazi, C.; Bauleo, P.; Grunfeld, C.M.; Aglietta, M.; Arneodo, F.; Barnhill, D.; Beatty, J.J.; Busca, N.G.; et al. Calibration of the surface array of the Pierre Auger Observatory. Nucl. Instrum. Methods Phys. Res. A 2006, 568, 839–846. [Google Scholar] [CrossRef]


| Liquid Samples | Label |
|---|---|
| QDs Urea Green | S1 |
| QDs Urea Blue | S2 |
| Solid Samples | |
| Hybrid + QDs Urea Green | M1 |
| Hybrid + QDs Urea Blue | M2 |
| Hybrid Sample | M3 |
| PMMA | M4 |
| SiO2 | M5 |
| Plastic Scintillator | Time Decay (ns) | Quantum Yield (%) | Light Output (%) | Maximum Emission Wavelength (nm) | Reference |
|---|---|---|---|---|---|
| BC422Q | 0.7 | - | 11 | 370 | [42,43] |
| Polystyrene+ POPOP | 1.5 | 93 | - | - | [41] |
| BC408 | 2.1 | 22.9 | 64 | 425 | [42,43] |
| BC400 | 2.4 | - | 65 | 423 | [42,43] |
| M1 | 3.0 | 8.61 | - | 426 | This work |
| BC412 | 3.3 | - | 60 | 434 | [42,43] |
| M2 | 3.6 | 8.61 | - | 426 | This work |
| PMMA + 4’vinyl-2,5-diphenyloxadiazole-1,3,4 | 4.2 | 68 | - | - | [41] |
| PMMA+PPO | 12.4 | 42 | - | - | [41] |
| BC444 | 285 | - | 41 | 428 | [42,43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León, L.C.; Palomino Merino, M.R.; Rosales, J.E.E.; Cuapa, S.T.; de Celis Alonso, B.; Martínez Bravo, O.M.; Ruiz-Hernandez, O.I.; Suárez García, J.G.; Toledo-Solano, M.; Lugo Arce, J.E. Carbon Quantum Dot-Embedded SiO2: PMMA Hybrid as a Blue-Emitting Plastic Scintillator for Cosmic Ray Detection. Photonics 2025, 12, 854. https://doi.org/10.3390/photonics12090854
León LC, Palomino Merino MR, Rosales JEE, Cuapa ST, de Celis Alonso B, Martínez Bravo OM, Ruiz-Hernandez OI, Suárez García JG, Toledo-Solano M, Lugo Arce JE. Carbon Quantum Dot-Embedded SiO2: PMMA Hybrid as a Blue-Emitting Plastic Scintillator for Cosmic Ray Detection. Photonics. 2025; 12(9):854. https://doi.org/10.3390/photonics12090854
Chicago/Turabian StyleLeón, Lorena Cruz, Martin Rodolfo Palomino Merino, José Eduardo Espinosa Rosales, Samuel Tehuacanero Cuapa, Benito de Celis Alonso, Oscar Mario Martínez Bravo, Oliver Isac Ruiz-Hernandez, José Gerardo Suárez García, Miller Toledo-Solano, and Jesús Eduardo Lugo Arce. 2025. "Carbon Quantum Dot-Embedded SiO2: PMMA Hybrid as a Blue-Emitting Plastic Scintillator for Cosmic Ray Detection" Photonics 12, no. 9: 854. https://doi.org/10.3390/photonics12090854
APA StyleLeón, L. C., Palomino Merino, M. R., Rosales, J. E. E., Cuapa, S. T., de Celis Alonso, B., Martínez Bravo, O. M., Ruiz-Hernandez, O. I., Suárez García, J. G., Toledo-Solano, M., & Lugo Arce, J. E. (2025). Carbon Quantum Dot-Embedded SiO2: PMMA Hybrid as a Blue-Emitting Plastic Scintillator for Cosmic Ray Detection. Photonics, 12(9), 854. https://doi.org/10.3390/photonics12090854





