Exhaled Breath Analysis (EBA): A Comprehensive Review of Non-Invasive Diagnostic Techniques for Disease Detection
Abstract
1. Introduction

2. Methodology: A Systematic Approach to Literature Synthesis
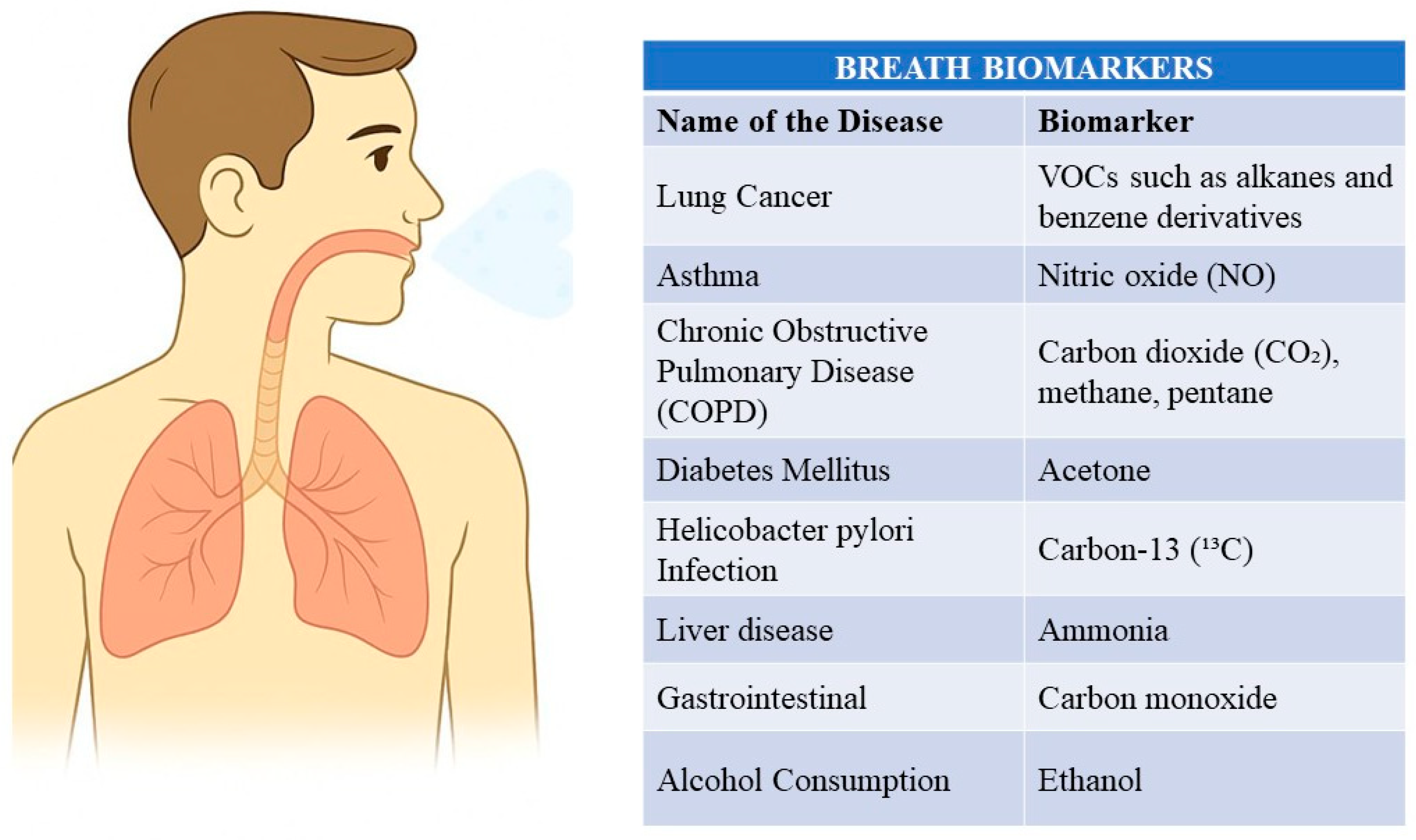
3. Common Diseases Diagnosed by Breath Analysis
| Name of the Disease | Exhaled Biomarker | Description | References of Studies | FDA-Approved Biomarkers for Disease Diagnosis |
|---|---|---|---|---|
| Lung Cancer | VOCs such as alkanes, benzene derivatives, aldehydes | Breath analysis for lung cancer involves detecting specific VOCs produced by cancerous cells. Elevated levels of n-pentane and isoprene are commonly observed. | [20,21] | None |
| Asthma | Nitric oxide (NO) | Elevated levels of NO in exhaled breath indicate airway inflammation, which is a hallmark of asthma. | [22,23] | Fractional exhaled nitric oxide (FeNO) |
| Chronic Obstructive Pulmonary Disease (COPD) | Carbon dioxide (CO2), methane (CH4), ethane | COPD diagnosis through breath analysis involves detecting elevated levels of specific gases such as ethane and pentane. | [24,25] | None |
| Diabetes Mellitus | Acetone | Elevated levels of acetone in breath correlate with blood glucose levels, indicating diabetes. | [1,26] | None |
| Helicobacter pylori Infection | Carbon-13 (13C) urea | The urea breath test (UBT) detects H. pylori infection by measuring labeled CO2 after ingestion of 13C-urea. | [24,27] | 13C-urea |
| Liver Disease | Ammonia, acetone | Elevated levels of ammonia and acetone indicate impaired liver function. | [28,29] | None |
| Neonatal Jaundice | Carbon monoxide (CO) | CO breath test detects elevated levels of CO, indicating jaundice in newborns. | [30,31] | Carbon monoxide test |
| Gastrointestinal Disorders | Hydrogen (H2), methane (CH4) | Breath tests for hydrogen and methane are used to diagnose conditions like fructose and lactose malabsorption and bacterial overgrowth. | [32,33] | Hydrogen and methane breath tests |
| Alcohol Consumption | Ethanol | Ethanol breath test measures blood alcohol levels. | [34] | Ethanol test |
4. Sensors Used in Exhaled Breath Analysis
4.1. Role of Nanomaterials in Sensor Development
4.2. Chemiresistive Gas Sensors
4.3. Electronic Nose Technology
- Metal Oxide Sensors (MOSs): These sensors rely on the change in electrical conductivity of a metal oxide semiconductor upon interaction with VOCs. When VOC molecules adsorb onto the sensor surface, they change the electron density, resulting in a detectable variation in resistance [67]. MOSs are among the most widely used sensors in eNose technology due to their high sensitivity, fast response times, and relatively low cost [68]. These sensors are based on a semiconducting metal oxide layer (e.g., tin dioxide and zinc oxide) deposited on a substrate. The gas molecules adsorb onto the sensor surface upon exposure to VOCs, changing the metal oxide’s electrical conductivity. This change in conductivity is directly proportional to the concentration of the VOCs and can be measured as a change in resistance. The selectivity of MOSs can be tuned by adjusting the operating temperature, the type of metal oxide, and the addition of dopants or catalysts [69].
- Conducting Polymer (CP) Sensors: Similar to MOSs, CP sensors also exhibit changes in electrical conductivity upon exposure to VOCs. The interaction of VOCs with the polymer matrix causes swelling or contraction, resulting in a change in resistance. CP sensors offer an alternative approach to VOC detection, leveraging changes in the electrical conductivity of a polymer film upon interaction with VOCs. These sensors often comprise a polymer matrix (e.g., polypyrrole and polyaniline) embedded with conductive particles (e.g., carbon black). The adsorption of VOCs onto the polymer matrix can cause it to swell or contract, resulting in a change in the distance between the conductive particles and, consequently, a change in resistance. CP sensors are known for their flexibility in design, ease of fabrication, and potential for miniaturization [70].
- Quartz Crystal Microbalance (QCM) Sensors: These sensors utilize the piezoelectric effect of a quartz crystal resonator. When VOCs adsorb onto the crystal surface, the mass of the crystal changes, leading to a shift in its resonant frequency. This frequency shift is proportional to the mass of the adsorbed VOCs [71]. QCM sensors exploit the piezoelectric properties of quartz crystals to detect VOCs. These sensors consist of a quartz crystal resonator coated with a selective material that adsorbs specific VOCs. The adsorption of VOCs onto the crystal surface increases its mass, causing a decrease in the crystal’s resonant frequency. This frequency shift is proportional to the mass of the adsorbed VOCs and can be used to quantify their concentration. QCM sensors are highly sensitive and can detect even trace amounts of VOCs [72].
- Mass Spectrometry (MS) Sensors: MS sensors ionize VOC molecules and separate them according to their mass-to-charge ratio, producing a mass spectrum that serves as a unique fingerprint for identifying the VOCs in the sample [73]. While not as common as MOS, CP sensors, or QCM sensors, MS sensors offer unparalleled selectivity and sensitivity in VOC detection. In MS sensors, VOCs are ionized and separated based on their mass-to-charge ratio. The resulting mass spectrum provides a unique fingerprint of the VOCs present in the sample. However, MS sensors are typically bulky, expensive, and require complex operation, limiting their widespread adoption in eNose technology [74]. A comparative summary of these sensor technologies, highlighting their principles of operation, advantages, and disadvantages, is provided in Table 3.
4.4. Chromatography
- Gas Chromatography (GC): GC is the workhorse of breath gas analysis, separating volatile organic compounds (VOCs) based on their boiling points and polarity. The sample is vaporized and carried through a column by an inert carrier gas. Different VOCs interact differently with the column’s stationary phase, leading to separation. This method is frequently combined with mass spectrometry (GC-MS) and offers excellent sensitivity and specificity for detecting and measuring VOCs [79].
- Liquid Chromatography (LC): LC is used for less volatile or polar analytes, such as metabolites. In this process, the sample is dissolved in a liquid mobile phase and then injected into a column containing a solid stationary phase. Separation occurs due to differences in the analytes’ affinity for the stationary and mobile phases. LC-MS is a powerful combination for analyzing complex biological samples [80].
- Ion Mobility Spectrometry (IMS): IMS is a rapid technique where ionized gas-phase molecules are separated based on their size, shape, and charge in an electric field. IMS is becoming increasingly popular for its speed, portability, and sensitivity in detecting VOCs, including those in exhaled breath [81].
Challenges and Limitations
- Gas Chromatography (GC)
- Liquid Chromatography (LC)
- Ion Mobility Spectrometry (IMS)
4.5. Infrared Spectroscopy
Measurement Process
- Sample Collection:
- 2.
- Mid-Infrared Laser Source:
- 3.
- Photoacoustic Effect:
- 4.
- Detection:
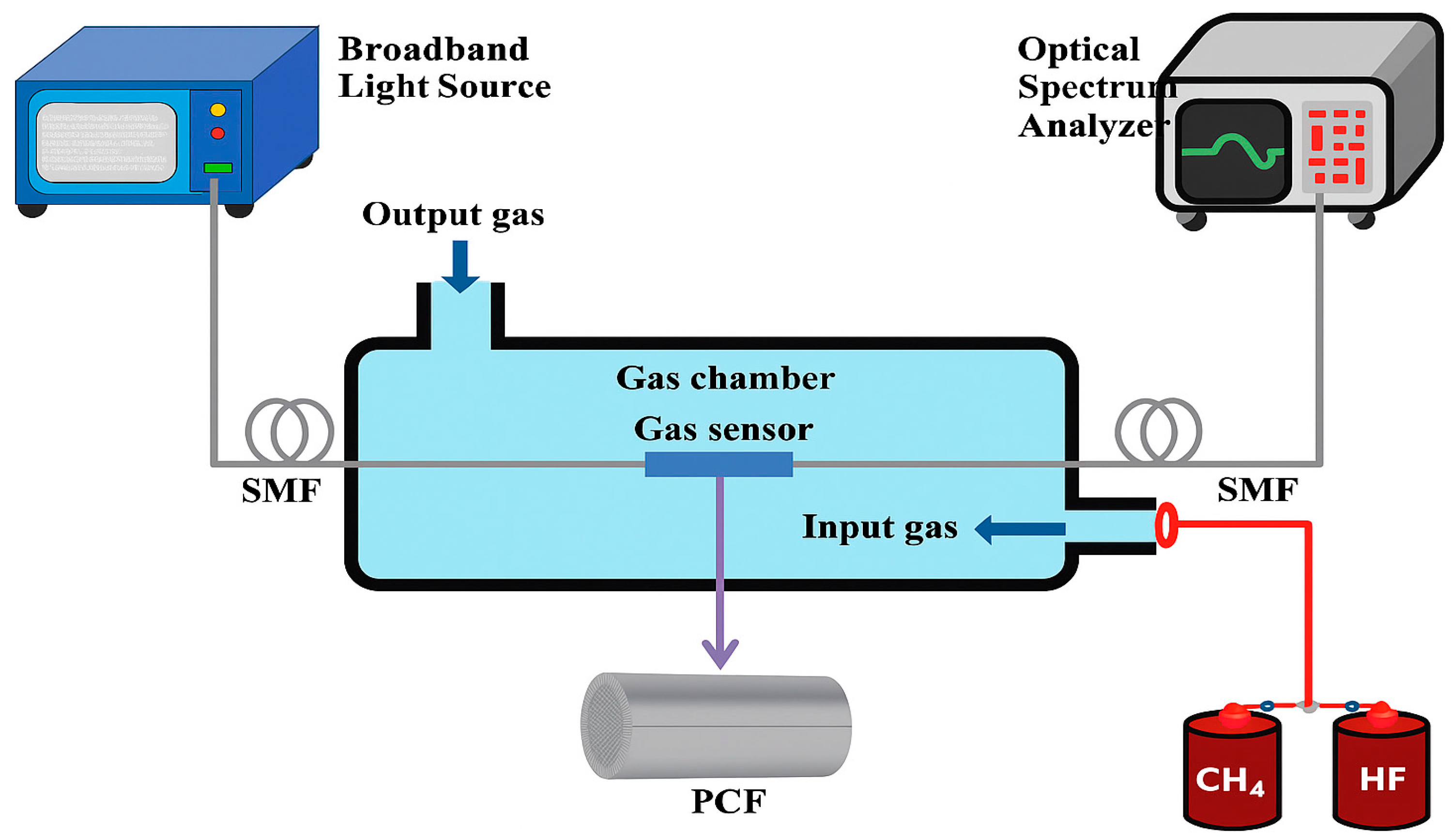
4.6. Photonic Crystal Fiber Sensors
Outstanding Features of PCF Sensors
- High Sensitivity: PCF sensors can detect very low concentrations of gases due to their enhanced light-gas interaction. Sensitivities as high as 75% have been reported, making them suitable for detecting trace amounts of gases such as ammonia, hydrogen, and methane [94].
- Fast Response Time: The design of PCF sensors ensures rapid response to changes in gas concentration, which is critical for real-time monitoring and breath analysis applications. The fast response is due to the efficient gas diffusion within the hollow core and the swift interaction with the guided light [95].
- Compact and Lightweight: PCF sensors are inherently compact and lightweight, which makes them easy to integrate into portable devices for on-site and real-time gas monitoring. This compactness is advantageous for applications in medical diagnostics, environmental monitoring, and industrial safety [96].
- Wide Wavelength Range: These sensors can operate over a wide range of wavelengths, from ultraviolet to infrared, allowing the detection of various gases with different absorption characteristics. This versatility makes PCF sensors suitable for multiple applications [97].
- Robustness and Durability: PCF sensors are engineered for durability and robustness, allowing them to perform reliably even under harsh environmental conditions. Their structural integrity ensures long-term reliability and consistent performance, essential for continuous monitoring applications [98]. A comprehensive comparison of these sensor technologies, outlining their respective advantages, disadvantages, and operational features, is presented in Table 4.
5. PCF Sensors in EBA
Types of Photonic Crystal Fiber Sensors
- Hollow-Core PCF Sensors: These sensors confine the light within a hollow core surrounded by a microstructured cladding. The interaction between light and gas molecules takes place within the hollow core, resulting in enhanced sensitivity and swift response times [108].
- Solid-Core PCF Sensors: These sensors use the evanescent field that extends into the microstructured cladding filled with gas. Although they generally have lower sensitivity than hollow-core PCFs, they are more straightforward to fabricate and highly effective for specific applications [109].
6. Challenges, Interdisciplinary Roles, and Future Perspectives
6.1. Standardization and Confounding Factors
6.2. Sensor Performance and Clinical Validation
6.3. The Essential Role of Interdisciplinary Collaboration
6.4. Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, T.; Liu, T.; Li, T.; Zhao, H.; Chen, Q. Exhaled breath analysis in disease detection. Clin. Chim. Acta 2021, 515, 61–72. [Google Scholar] [CrossRef]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Probert, C.S.; Ahmed, I.; Khalid, T.; Johnson, E.; Smith, S.; Ratcliffe, N. Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J. Gastrointest. Liver Dis. 2009, 18, 337–343. [Google Scholar]
- Mule, N.M.; Patil, D.D.; Kaur, M. A comprehensive survey on investigation techniques of exhaled breath (EB) for diagnosis of diseases in human body. Inform. Med. Unlocked 2021, 26, 100715. [Google Scholar] [CrossRef]
- Phillips, M. Breath tests in medicine. Sci. Am. 1992, 267, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Biagini, D.; Lomonaco, T.; Ghimenti, S.; Bellagambi, F.G.; Onor, M.; Scali, M.C.; Barletta, V.; Marzilli, M.; Salvo, P.; Trivella, M.G.; et al. Determination of volatile organic compounds in exhaled breath of heart failure patients by needle trap micro-extraction coupled with gas chromatography-tandem mass spectrometry. J. Breath Res. 2017, 11, 047110. [Google Scholar] [CrossRef] [PubMed]
- Issitt, T.; Wiggins, L.; Veysey, M.; Sweeney, S.T.; Brackenbury, W.J.; Redeker, K. Volatile compounds in human breath: Critical review and meta-analysis. J. Breath Res. 2022, 16, 024001. [Google Scholar] [CrossRef]
- Issitt, T.; Sweeney, S.T.; Brackenbury, W.J.; Redeker, K.R. Sampling and analysis of low-molecular-weight volatile metabolites in cellular headspace and mouse breath. Metabolites 2022, 12, 599. [Google Scholar] [CrossRef]
- Mazzone, P.J. Exhaled breath volatile organic compound biomarkers in lung cancer. J. Breath Res. 2012, 6, 027106. [Google Scholar] [CrossRef]
- Buszewski, B.; Kęsy, M.; Ligor, T.; Amann, A. Human exhaled air analytics: Biomarkers of diseases. Biomed. Chromatogr. 2007, 21, 553–566. [Google Scholar] [CrossRef]
- Aresta, A.M.; De Vietro, N.; Picciariello, A.; Rotelli, M.T.; Altomare, D.F.; Dezi, A.; Martines, G.; Di Gilio, A.; Palmisani, J.; De Gennaro, G.; et al. Volatile organic compounds determination from intestinal polyps and in exhaled breath by gas chromatography–mass spectrometry. Appl. Sci. 2023, 13, 6083. [Google Scholar] [CrossRef]
- Rahimpour, E.; Khoubnasabjafari, M.; Jouyban-Gharamaleki, V.; Jouyban, A. Non-volatile compounds in exhaled breath condensate: Review of methodological aspects. Anal. Bioanal. Chem. 2018, 410, 6411–6440. [Google Scholar] [CrossRef] [PubMed]
- Horvath, I.; Lazar, Z.; Gyulai, N.; Kollai, M.; Losonczy, G. Exhaled biomarkers in lung cancer. Eur. Respir. J. 2009, 34, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Gashimova, E.; Osipova, A.; Temerdashev, A.; Porkhanov, V.; Polyakov, I.; Perunov, D.; Dmitrieva, E. Exhaled breath analysis using GC-MS and an electronic nose for lung cancer diagnostics. Anal. Methods 2021, 13, 4793–4804. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, A.A.; Almangush, A.; Youssef, O.; Metsälä, M.; Silen, S.; Nixon, I.J.; Haigentz, M., Jr.; Rodrigo, J.P.; Saba, N.F.; Vander Poorten, V.; et al. Exhaled breath analysis in the diagnosis of head and neck cancer. Head Neck 2020, 42, 787–793. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, D.; Zhang, L.; Lu, G. Non-invasive blood glucose monitoring for diabetics by means of breath signal analysis. Sens. Actuators B Chem. 2012, 173, 106–113. [Google Scholar] [CrossRef]
- Schnabel, R.; Fijten, R.; Smolinska, A.; Dallinga, J.; Boumans, M.L.; Stobberingh, E.; Boots, A.; Roekaerts, P.; Bergmans, D.; van Schooten, F.J. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Sci. Rep. 2015, 5, 17179. [Google Scholar] [CrossRef]
- Amann, A.; Smith, D. Breath analysis for clinical diagnosis and therapeutic monitoring. Siriraj Med. Thurs. Dec 20 2005, 64, 201218. [Google Scholar]
- Zheng, W.; Min, Y.; Pang, K.; Wu, D. Sample collection and processing in volatile organic compound analysis for gastrointestinal cancers. Diagnostics 2024, 14, 1563. [Google Scholar] [CrossRef]
- Amann, A.; Miekisch, W.; Schubert, J.; Buszewski, B.; Ligor, T.; Jezierski, T.; Pleil, J.; Risby, T. Analysis of exhaled breath for disease detection. Annu. Rev. Anal. Chem. 2014, 7, 455–482. [Google Scholar]
- Amor, R.E.; Nakhleh, M.K.; Barash, O.; Haick, H. Breath analysis of cancer in the present and the future. Eur. Respir. Rev. 2019, 28, 190002. [Google Scholar] [CrossRef]
- Smith, A.D.; Cowan, J.O.; Filsell, S.; McLachlan, C.; Monti-Sheehan, G.; Jackson, P.; Taylor, D.R. Diagnosing asthma: Comparisons between exhaled nitric oxide measurements and conventional tests. Am. J. Respir. Crit. Care Med. 2004, 169, 473–478. [Google Scholar] [CrossRef]
- Silkoff, P.E.; Carlson, M.; Bourke, T.; Katial, R.; Ögren, E.; Szefler, S.J. The Aerocrine exhaled nitric oxide monitoring system NIOX is cleared by the US Food and Drug Administration for monitoring therapy in asthma. J. Allergy Clin. Immunol. 2004, 114, 1241–1256. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. TrAC Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Westhoff, M.; Litterst, P.; Maddula, S.; Bödeker, B.; Baumbach, J.I. Statistical and bioinformatical methods to differentiate chronic obstructive pulmonary disease (COPD) including lung cancer from healthy control by breath analysis using ion mobility spectrometry. Int. J. Ion Mobil. Spectrom. 2011, 14, 139–149. [Google Scholar] [CrossRef]
- Pradhan, U.U.; Bhat, P. Breathe analysis for medical diagnostics—A review. Int. J. Innov. Res. Dev. 2015, 4, 240–246. [Google Scholar]
- Cao, W.; Duan, Y. Breath analysis: Potential for clinical diagnosis and exposure assessment. Clin. Chem. 2006, 52, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef]
- Schubert, J.K.; Miekisch, W.; Geiger, K.; Nöldge–Schomburg, G.F. Breath analysis in critically ill patients: Potential and limitations. Expert Rev. Mol. Diagn. 2004, 4, 619–629. [Google Scholar] [CrossRef]
- Osborne, J.; Sobh, M.; Trudel, G. Carbon monoxide as a clinical marker of hemolysis. Am. J. Hematol. 2023, 98, 1127–1159. [Google Scholar] [CrossRef] [PubMed]
- Morimatsu, H.; Takahashi, T.; Maeshima, K.; Inoue, K.; Kawakami, T.; Shimizu, H.; Takeuchi, M.; Yokoyama, M.; Katayama, H.; Morita, K. Increased heme catabolism in critically ill patients: Correlation among exhaled carbon monoxide, arterial carboxyhemoglobin, and serum bilirubin IXα concentrations. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 290, L114–L119. [Google Scholar] [CrossRef]
- Di Stefano, M.; Corazza, G.R. Role of hydrogen and methane breath testing in gastrointestinal diseases. Dig. Liver Dis. Suppl. 2009, 3, 40–43. [Google Scholar] [CrossRef]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American consensus. Off. J. Am. Coll. Gastroenterol.|ACG 2017, 112, 775–784. [Google Scholar] [CrossRef]
- Lindberg, L.; Brauer, S.; Wollmer, P.; Goldberg, L.; Jones, A.W.; Olsson, S.G. Breath alcohol concentration determined with a new analyzer using free exhalation predicts almost precisely the arterial blood alcohol concentration. Forensic Sci. Int. 2007, 168, 200–207. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kim, K.H.; Kim, B.S.; Byun, Y.T. Characteristics of highly sensitive and selective nitric oxide gas sensors using defect-functionalized single-walled carbon nanotubes at room temperature. Appl. Surf. Sci. 2021, 550, 149250. [Google Scholar] [CrossRef]
- Yin, F.; Yue, W.; Li, Y.; Gao, S.; Zhang, C.; Kan, H.; Niu, H.; Wang, W.; Guo, Y. Carbon-based nanomaterials for the detection of volatile organic compounds: A review. Carbon 2021, 180, 274–297. [Google Scholar] [CrossRef]
- Luo, Y.; Ly, A.; Lahem, D.; Zhang, C.; Debliquy, M. A novel low-concentration isopropanol gas sensor based on Fe-doped ZnO nanoneedles and its gas sensing mechanism. J. Mater. Sci. 2021, 56, 3230–3245. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Y.; Wang, Z.; Wang, Z.; Yang, J.; Chen, M.; Qi, M.; Ur Rehman, S.; Shum, P.P.; Zhu, L.; et al. Ultrasensitive exhaled breath sensors based on anti-resonant hollow core fiber with in situ grown ZnO-Bi2O3 nanosheets. Adv. Mater. Interfaces 2021, 8, 2001978. [Google Scholar] [CrossRef]
- Nath, N.; Kumar, A.; Chakroborty, S.; Soren, S.; Barik, A.; Pal, K.; de Souza, F.G., Jr. Carbon nanostructure embedded novel sensor implementation for detection of aromatic volatile organic compounds: An organized review. ACS Omega 2023, 8, 4436–4452. [Google Scholar] [CrossRef]
- Sajor, N.J.; Foronda, J.R.; Olarve, R.S.; Torre, H.D.; Santos, M.G.; Lopez, T.B.; Haygood, K.J.; Santos, G.N.; Koledov, V.; Gratowski, S.V. Synthesis of metal oxide nanomaterials for early lung disease detection. J. Phys. Conf. Ser. 2020, 1461, 012149. [Google Scholar] [CrossRef]
- Salimi, M.; Hosseini, S.M.R.M. Smartphone-based detection of lung cancer-related volatile organic compounds (VOCs) using rapid synthesized ZnO nanosheet. Sens. Actuators B Chem. 2021, 344, 130127. [Google Scholar] [CrossRef]
- Ray, B.; Desai, S.M.; Parmar, S.; Datar, S. Polymer-Modified Quartz Tuning Forks for Breath Biomarker Sensing. Eng. Proc. 2021, 6, 62. [Google Scholar]
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-based gas sensors used for breath diagnosis. J. Mater. Chem. B 2020, 8, 3231–3248. [Google Scholar] [CrossRef]
- Maciel, M.; Sankari, S.; Woollam, M.; Agarwal, M. Optimization of metal oxide nanosensors and development of a feature extraction algorithm to analyze VOC profiles in exhaled breath. IEEE Sens. J. 2023, 23, 16571–16578. [Google Scholar] [CrossRef]
- Sun, J.Y.; Salahuddin, U.; Zhu, C.; Gao, P.X. Medical Diagnosis Using Volatile Organic Compounds Sensors. Int. J. High Speed Electron. Syst. 2022, 31, 2240004. [Google Scholar] [CrossRef]
- Velumani, M.; Prasanth, A.; Narasimman, S.; Chandrasekhar, A.; Sampson, A.; Meher, S.R.; Rajalingam, S.; Rufus, E.; Alex, Z.C. Nanomaterial-Based Sensors for Exhaled Breath Analysis: A Review. Coatings 2022, 12, 1989. [Google Scholar] [CrossRef]
- Saidi, T.; Palmowski, D.; Babicz-Kiewlicz, S.; Welearegay, T.G.; El Bari, N.; Ionescu, R.; Smulko, J.; Bouchikhi, B. Exhaled breath gas sensing using pristine and functionalized WO3 nanowire sensors enhanced by UV-light irradiation. Sens. Actuators B Chem. 2018, 273, 1719–1729. [Google Scholar] [CrossRef]
- Postica, V.; Vahl, A.; Santos-Carballal, D.; Dankwort, T.; Kienle, L.; Hoppe, M.; Cadi-Essadek, A.; De Leeuw, N.H.; Terasa, M.I.; Adelung, R.; et al. Tuning ZnO sensors reactivity toward volatile organic compounds via Ag doping and nanoparticle functionalization. ACS Appl. Mater. Interfaces 2019, 11, 31452–31466. [Google Scholar] [CrossRef]
- Hanh, N.H.; Ngoc, T.M.; Van Duy, L.; Hung, C.M.; Van Duy, N.; Hoa, N.D. A comparative study on the VOCs gas sensing properties of Zn2SnO4 nanoparticles, hollow cubes, and hollow octahedra towards exhaled breath analysis. Sens. Actuators B Chem. 2021, 343, 130147. [Google Scholar] [CrossRef]
- Lagopati, N.; Valamvanos, T.F.; Proutsou, V.; Karachalios, K.; Pippa, N.; Gatou, M.A.; Vagena, I.A.; Cela, S.; Pavlatou, E.A.; Gazouli, M.; et al. The role of nano-sensors in breath analysis for early and non-invasive disease diagnosis. Chemosensors 2023, 11, 317. [Google Scholar] [CrossRef]
- Kalidoss, R.; Umapathy, S.; Anandan, R.; Ganesh, V.; Sivalingam, Y. Comparative study on the preparation and gas sensing properties of reduced graphene oxide/SnO2 binary nanocomposite for detection of acetone in exhaled breath. Anal. Chem. 2019, 91, 5116–5124. [Google Scholar] [CrossRef]
- Andre, R.S.; Sanfelice, R.C.; Pavinatto, A.; Mattoso, L.H.; Correa, D.S. Hybrid nanomaterials designed for volatile organic compounds sensors: A review. Mater. Des. 2018, 156, 154–166. [Google Scholar] [CrossRef]
- Yang, D.; Gopal, R.A.; Lkhagvaa, T.; Choi, D. Metal-oxide gas sensors for exhaled-breath analysis: A review. Meas. Sci. Technol. 2021, 32, 102004. [Google Scholar] [CrossRef]
- Zonta, G.; Rispoli, G.; Malagù, C.; Astolfi, M. Overview of gas sensors focusing on chemoresistive ones for cancer detection. Chemosensors 2023, 11, 519. [Google Scholar] [CrossRef]
- Kim, J.N.; Kim, H.J. A Chemoresistive Gas Sensor Readout Integrated Circuit with Sensor Offset Cancellation Technique. IEEE Access 2023, 11, 85405–85413. [Google Scholar] [CrossRef]
- Vajhadin, F.; Mazloum-Ardakani, M.; Amini, A. Metal oxide-based gas sensors for the detection of exhaled breath markers. Med. Devices Sens. 2021, 4, e10161. [Google Scholar] [CrossRef] [PubMed]
- Aroutiounian, V.M. Microelectronic Gas sensors for Non-invasive Analysis of Exhaled Gases. Med. Sci. Armen. 2020, 60, 3–15. [Google Scholar]
- Wu, T.C.; De Luca, A.; Zhong, Q.; Zhu, X.; Ogbeide, O.; Um, D.S.; Hu, G.; Albrow-Owen, T.; Udrea, F.; Hasan, T. Inkjet-printed CMOS-integrated graphene–metal oxide sensors for breath analysis. npj 2D Mater. Appl. 2019, 3, 42. [Google Scholar] [CrossRef]
- Barreca, D.; Maccato, C.; Gasparotto, A. Metal oxide nanosystems as chemoresistive gas sensors for chemical warfare agents: A focused review. Adv. Mater. Interfaces 2022, 9, 2102525. [Google Scholar] [CrossRef]
- Janfaza, S.; Banan Nojavani, M.; Nikkhah, M.; Alizadeh, T.; Esfandiar, A.; Ganjali, M.R. A selective chemiresistive sensor for the cancer-related volatile organic compound hexanal by using molecularly imprinted polymers and multiwalled carbon nanotubes. Microchim. Acta 2019, 186, 137. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; de León-Martínez, L.D.; Gorocica-Rosete, P.; Pérez-Padilla, R.; Domínguez-Reyes, C.A.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Mehta, G.; Zamora-Mendoza, B.N.; Flores-Ramírez, R. Application of chemoresistive gas sensors and chemometric analysis to differentiate the fingerprints of global volatile organic compounds from diseases. Preliminary results of COPD, lung cancer and breast cancer. Clin. Chim. Acta 2021, 518, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Gong, C.; Bian, W.; Tian, Q.; Zhang, Y.; Chen, N.; Xu, C.; Sun, N.; Wang, X.; Li, C.; et al. Ultrasensitive chemiresistive gas sensor can diagnose asthma and monitor its severity by analyzing its biomarker H2S: An experimental, clinical, and theoretical study. ACS Sens. 2022, 7, 2243–2252. [Google Scholar] [CrossRef]
- Shang, G.; Dinh, D.; Mercer, T.; Yan, S.; Wang, S.; Malaei, B.; Luo, J.; Lu, S.; Zhong, C.J. Chemiresistive sensor array with nanostructured interfaces for detection of human breaths with simulated lung cancer breath VOCs. ACS Sens. 2023, 8, 1328–1338. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
- Dragonieri, S.; Schot, R.; Mertens, B.J.; Le Cessie, S.; Gauw, S.A.; Spanevello, A.; Resta, O.; Willard, N.P.; Vink, T.J.; Rabe, K.F.; et al. An electronic nose in the discrimination of patients with asthma and controls. J. Allergy Clin. Immunol. 2007, 120, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- van Geffen, W.H.; Lamote, K.; Costantini, A.; Hendriks, L.E.; Rahman, N.M.; Blum, T.G.; Van Meerbeeck, J. The electronic nose: Emerging biomarkers in lung cancer diagnostics. Breathe 2020, 15, e135–e141. [Google Scholar] [CrossRef]
- Tripathy, P.; Biswas, S. Mechanical and thermal properties of mineral fiber based polymeric nanocomposites: A review. Polym.-Plast. Technol. Mater. 2022, 61, 1385–1410. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Xing, H.; Bai, P.; Liu, B.; Wang, C.; Lei, K.; Wang, H.; Peng, S.; Yang, S. Gas sensing of ordered and disordered structure SiO2 and their adsorption behavior based on quartz crystal microbalance. Sens. Actuators B Chem. 2020, 305, 127479. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 2005, 24, 661–700. [Google Scholar] [CrossRef]
- Su, R.; Yang, T.; Zhang, X.; Li, N.; Zhai, X.; Chen, H. Mass spectrometry for breath analysis. TrAC Trends Anal. Chem. 2023, 158, 116823. [Google Scholar] [CrossRef]
- Röck, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef]
- Kalidoss, R.; Surya, V.J.; Sivalingam, Y. Recent progress in graphene derivatives/metal oxides binary nanocomposites based chemi-resistive sensors for disease diagnosis by breath analysis. Curr. Anal. Chem. 2022, 18, 563–576. [Google Scholar] [CrossRef]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L.; et al. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano 2017, 11, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C. Quantitative Chemical Analysis; Macmillan: New York, NY, USA, 2010. [Google Scholar]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Ligor, M.; Ligor, T.; Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Denz, H.; Fiegl, M.; Hilbe, W.; Weiss, W.; et al. Determination of volatile organic compounds in exhaled breath of patients with lung cancer using solid phase microextraction and gas chromatography mass spectrometry. Clin. Chem. Lab. Med. 2009, 47, 550–560. [Google Scholar] [CrossRef]
- Eiceman, G.A.; Karpas, Z. Ion Mobility Spectrometry; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Selvaraj, R.; Vasa, N.J.; Nagendra, S.S.; Mizaikoff, B. Advances in mid-infrared spectroscopy-based sensing techniques for exhaled breath diagnostics. Molecules 2020, 25, 2227. [Google Scholar] [CrossRef]
- Hannemann, M.; Antufjew, A.; Borgmann, K.; Hempel, F.; Ittermann, T.; Welzel, S.; Weltmann, K.D.; Völzke, H.; Röpcke, J. Influence of age and sex in exhaled breath samples investigated by means of infrared laser absorption spectroscopy. J. Breath Res. 2011, 5, 027101. [Google Scholar] [CrossRef]
- Wojtas, J.; Tittel, F.K.; Stacewicz, T.; Bielecki, Z.; Lewicki, R.; Mikolajczyk, J.; Nowakowski, M.; Szabra, D.; Stefanski, P.; Tarka, J. Cavity-enhanced absorption spectroscopy and photoacoustic spectroscopy for human breath analysis. Int. J. Thermophys. 2014, 35, 2215–2225. [Google Scholar] [CrossRef]
- Petersen, J.C.; Lamard, L.; Feng, Y.; Focant, J.F.; Lassen, M. Quartz-enhanced photoacoustic spectroscopy as a platform for non-invasive trace gas analyser targeting breath analysis. arXiv 2017, arXiv:1704.07442. [Google Scholar]
- Yi, H.; Laurent, O.; Schilt, S.; Ramonet, M.; Gao, X.; Dong, L.; Chen, W. Simultaneous Monitoring of Atmospheric CH4, N2O, and H2O Using a Single Gas Sensor Based on Mid-IR Quartz-Enhanced Photoacoustic Spectroscopy. Anal. Chem. 2022, 94, 17522–17532. [Google Scholar] [CrossRef]
- Wojtas, J.; Gluszek, A.; Hudzikowski, A.; Tittel, F.K. Mid-infrared trace gas sensor technology based on intracavity quartz-enhanced photoacoustic spectroscopy. Sensors 2017, 17, 513. [Google Scholar] [CrossRef] [PubMed]
- Rabee, A.S.H.; Hameed, M.F.O.; Heikal, A.M.; Obayya, S.S.A. Highly sensitive photonic crystal fiber gas sensor. Optik 2019, 188, 78–86. [Google Scholar] [CrossRef]
- Yang, J.; Che, X.; Shen, R.; Wang, C.; Li, X.; Chen, W. High-sensitivity photonic crystal fiber long-period grating methane sensor with cryptophane-A-6Me absorbed on a PAA-CNTs/PAH nanofilm. Opt. Express 2017, 25, 20258–20267. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.; Hameed, M.F.O.; Heikal, A.M.; Obayya, S.S.A. Novel optical gas sensor based on photonic crystal fiber. In Proceedings of the Optical Components and Materials XVI, San Francisco, CA, USA, 2–7 February 2019; Volume 10914, pp. 354–360. [Google Scholar]
- Mishra, G.P.; Kumar, D.; Chaudhary, V.S.; Kumar, S. Design and sensitivity improvement of microstructured-core photonic crystal fiber based sensor for methane and hydrogen fluoride detection. IEEE Sens. J. 2021, 22, 1265–1272. [Google Scholar] [CrossRef]
- Wu, B.; Lu, Y.; Hao, C.; Duan, L.; Musideke, M.; Yao, J. A photonic crystal fiber sensor based on differential optical absorption spectroscopy for mixed gases detection. Optik 2014, 125, 2909–2911. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Makouei, S.; Meshgini, S. High sensitive triangular photonic crystal fiber sensor design applicable for gas detection. Adv. Electromagn. 2021, 10, 1–5. [Google Scholar] [CrossRef]
- Britto, E.C.; Nizar, S.M.; Krishnan, P. A highly sensitive photonic crystal fiber gas sensor for the detection of sulfur dioxide. Silicon 2022, 14, 12665–12674. [Google Scholar] [CrossRef]
- Islam, M.I.; Ahmed, K.; Sen, S.; Chowdhury, S.; Paul, B.K.; Islam, M.S.; Miah, M.B.A.; Asaduzzaman, S. Design and optimization of photonic crystal fiber based sensor for gas condensate and air pollution monitoring. Photonic Sens. 2017, 7, 234–245. [Google Scholar] [CrossRef]
- Rifat, A.A.; Ahmed, K.; Asaduzzaman, S.; Paul, B.K.; Ahmed, R. Development of photonic crystal fiber-based gas/chemical sensors. Comput. Photonic Sens. 2019, 287–317. [Google Scholar]
- Cubillas, A.M.; Unterkofler, S.; Euser, T.G.; Etzold, B.J.; Jones, A.C.; Sadler, P.J.; Wasserscheid, P.; Russell, P.S.J. Photonic crystal fibres for chemical sensing and photochemistry. Chem. Soc. Rev. 2013, 42, 8629–8648. [Google Scholar] [CrossRef]
- Hu, H.F.; Zhao, Y.; Zhang, Y.N.; Yang, Y. Characterization of infrared gas sensors employing hollow-core photonic crystal fibers. Instrum. Sci. Technol. 2016, 44, 495–503. [Google Scholar] [CrossRef]
- Yoon, J.W.; Lee, J.H. Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: Recent progress and future perspectives. Lab A Chip 2017, 17, 3537–3557. [Google Scholar] [CrossRef]
- Li, Z.; Sie, S.H.; Lee, J.L.; Chen, Y.R.; Chou, T.I.; Wu, P.C.; Chuang, Y.T.; Lin, Y.T.; Chen, I.C.; Lu, C.C.; et al. A miniature electronic nose for breath analysis. In Proceedings of the 2021 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 11–15 December 2021; IEEE: New York, NY, USA, 2021; pp. 35.2.1–35.2.4. [Google Scholar]
- Kononov, A.; Korotetsky, B.; Jahatspanian, I.; Gubal, A.; Vasiliev, A.; Arsenjev, A.; Nefedov, A.; Barchuk, A.; Gorbunov, I.; Kozyrev, K.; et al. Online breath analysis using metal oxide semiconductor sensors (electronic nose) for diagnosis of lung cancer. J. Breath Res. 2019, 14, 016004. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- Scarlata, S.; Pennazza, G.; Santonico, M.; Pedone, C.; Antonelli Incalzi, R. Exhaled breath analysis by electronic nose in respiratory diseases. Expert Rev. Mol. Diagn. 2015, 15, 933–956. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Makouei, S.; Meshgini, S. Ammonia measurement in exhaled human breath using PCF sensor for medical applications. Photonics Nanostruct.-Fundam. Appl. 2021, 44, 100917. [Google Scholar] [CrossRef]
- Chow, K.K.; Short, M.; Lam, S.; McWilliams, A.; Zeng, H. A Raman cell based on hollow core photonic crystal fiber for human breath analysis. Med. Phys. 2014, 41, 092701. [Google Scholar] [CrossRef]
- Rifat, A.A.; Haider, F.; Ahmed, R.; Mahdiraji, G.A.; Mahamd Adikan, F.R.; Miroshnichenko, A.E. Highly sensitive selectively coated photonic crystal fiber-based plasmonic sensor. Opt. Lett. 2018, 43, 891–894. [Google Scholar] [CrossRef]
- Hanf, S.; Bögözi, T.; Keiner, R.; Frosch, T.; Popp, J. Fast and highly sensitive fiber-enhanced Raman spectroscopic monitoring of molecular H2 and CH4 for point-of-care diagnosis of malabsorption disorders in exhaled human breath. Anal. Chem. 2015, 87, 982–988. [Google Scholar] [CrossRef]
- Mortazavi, S.; Makouei, S.; Garamaleki, S.M. Hollow core photonic crystal fiber based carbon monoxide sensor design applicable for hyperbilirubinemia diagnosis. Opt. Eng. 2023, 62, 066105. [Google Scholar] [CrossRef]
- Akowuah, E.K.; Gorman, T.; Ademgil, H.; Haxha, S.; Robinson, G.K.; Oliver, J.V. Numerical analysis of a photonic crystal fiber for biosensing applications. IEEE J. Quantum Electron. 2012, 48, 1403–1410. [Google Scholar] [CrossRef]
- De, M.; Gangopadhyay, T.K.; Singh, V.K. Prospects of photonic crystal fiber as physical sensor: An overview. Sensors 2019, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- Al Mahfuz, M.; Mollah, M.A.; Momota, M.R.; Paul, A.K.; Masud, A.; Akter, S.; Hasan, M.R. Highly sensitive photonic crystal fiber plasmonic biosensor: Design and analysis. Opt. Mater. 2019, 90, 315–321. [Google Scholar] [CrossRef]
- Mehaney, A.; Elsayed, H.A.; Ahmed, A.M. Detection of Isoprene Traces in Exhaled Breath by Using Photonic Crystals as a Biomarker For Chronic Liver Fibrosis Disease. 2021. Available online: https://scispace.com/pdf/detection-of-isoprene-traces-in-exhaled-breath-by-using-39ago6x9ep.pdf (accessed on 21 August 2025).
- Zhou, D.; Wang, Q.; Lan, Z.; Chen, Y.; Peng, Z.; Zhang, L.; Liu, Y. Liquid-crystal-based fiber laser sensor for non-invasive gas detection. Opt. Lett. 2023, 48, 4508–4511. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, S.; Shen, Z.; Niu, P.; Cheng, W.; Xu, F. Respiration monitoring using antiresonant reflecting guidance in selectively infiltrated hollow core photonic crystal fiber. IEEE Sens. J. 2023, 23, 26004–26011. [Google Scholar] [CrossRef]
- Nizar, S.M.; Kesavaraman, B.; Priyanka, E.; Jayasri, R. Detection of immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies using circular photonic crystal fiber sensor. J. Phys. Conf. Ser. 2021, 1717, 012039. [Google Scholar] [CrossRef]
- Shao, M.; Sun, H.; Liang, J.; Han, L.; Feng, D. In-fiber Michelson interferometer in photonic crystal fiber for humidity measurement. IEEE Sens. J. 2020, 21, 1561–1567. [Google Scholar] [CrossRef]
- Chi, Z.; Li, M.; Xu, J.; Yang, L. A photonic crystal fiber–based fluorescence sensor for simultaneous and sensitive detection of lactic acid enantiomers. Anal. Bioanal. Chem. 2022, 414, 1641–1649. [Google Scholar] [CrossRef]
- Shirmohammad, M.; Short, M.A.; Zeng, H. A new gas analysis method based on single-beam excitation stimulated Raman scattering in hollow core photonic crystal fiber enhanced Raman spectroscopy. Bioengineering 2023, 10, 1161. [Google Scholar] [CrossRef]
- Ferdous, A.H.M.I.; Mynuddin, M.; Noor, K.S. High-performance sulphur dioxide sensor: Unveiling the potential of photonic crystal fibre technology. IET Nanodielectr. 2024, 7, 262–272. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, D.; Sahu, A.; Chaudhary, V.S.; Singh, G.; Kumar, S. Photonic Crystal Fiber Based Sensors for Various Cancer Detection in Human Body-A Review. IEEE Sens. J. 2025, 25, 5956–5968. [Google Scholar] [CrossRef]
- Sharif, V.; Saberi, H.; Pakarzadeh, H. Designing a terahertz optical sensor based on helically twisted photonic crystal fiber for toxic gas sensing. Sci. Rep. 2025, 15, 2268. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Zeng, F.; Cheng, H.; Jiang, X.; Huang, Z.; Yao, Q.; Tang, J. Performance of Hollow-Core Photonic Crystal Fiber-Based Trace C2H2 Detection System. IEEE Trans. Instrum. Meas. 2025, 74, 9503410. [Google Scholar] [CrossRef]


| Application | Sensor Type | Nanomaterials | Target Gas | Key Features | References |
|---|---|---|---|---|---|
| Early-stage disease diagnosis | Chemoresistive VOC sensors | Nanomaterials (general) | VOCs | High sensitivity, real-time, non-invasive diagnosis | [45] |
| Lung cancer detection | Resistive gas sensor | ZnO nanosheets | Diethyl ketone, acetone, isopropanol | High sensitivity, smartphone integration | [41] |
| Diabetes diagnosis | Chemiresistive sensors | ZnO-Bi2O3 nanosheets | Acetone | High sensitivity and selectivity | [38] |
| General disease diagnosis | Various sensor technologies | Nanomaterials (general) | VOCs | Non-invasive, highly selective, sensitive, robust sensors | [46] |
| Non-invasive medical diagnostics | Metal oxide semiconductor sensors | WO3 nanowires | VOCs | Enhanced response with UV-light irradiation, selective towards VOCs | [47] |
| VOC detection for health and environmental applications | Gas sensors | Ag-doped ZnO | Propanol, acetone, methane | High sensitivity at low operating temperatures | [48] |
| Diabetic diagnosis via exhaled breath | Gas sensors | Zn2SnO4 nanoparticles | Acetone | High sensitivity, good selectivity, and stability | [49] |
| General disease diagnosis via breath analysis | NM-based gas sensors | Nanomaterials (general) | VOCs | High surface-to-volume ratio, controllable morphology, potential for miniaturization | [43] |
| Early and non-invasive disease diagnosis | Gas sensors | Nanomaterials (general) | VOCs | Accurate detection, potential for commercial use as disease self-test kits | [50] |
| VOC detection in human breath for diabetes and respiratory diseases | Binary nanocomposites | Reduced graphene oxide/SnO2 | Acetone | Enhanced acetone sensing performance distinguishes between healthy and diabetic subjects | [51] |
| Portable, low-cost sensors for environmental and health applications | Hybrid nanomaterial sensors | Conducting polymers, metal oxides, graphenes | VOCs | Superior sensitivity, low detection limits, potential for miniaturization, and versatility | [52] |
| Sensor Type | Principle of Operation | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Metal Oxide Sensor (MOS) | Change in electrical conductivity upon VOC adsorption | High sensitivity, fast response, low cost, mature technology | Limited selectivity, cross-sensitivity, susceptibility to humidity and temperature variations | [68,69] |
| Conducting Polymer (CP) Sensor | Change in electrical conductivity upon VOC adsorption | Flexibility in design, ease of fabrication, miniaturization potential | Low sensitivity compared to MOSs, potential for drift and aging | [70] |
| Quartz Crystal Microbalance (QCM) Sensor | Change in resonant frequency due to mass change upon VOC adsorption | High sensitivity, ability to detect trace amounts of VOCs | Requires selective coatings, susceptibility to interference from other gases | [72] |
| Mass Spectrometry (MS) Sensor | Ionization and separation of VOCs based on mass-to-charge ratio | Unparalleled selectivity and sensitivity | Bulky, expensive, complex operation | [74] |
| Sensor Type | Advantages | Disadvantages | Cost | Features | Gas Detection | Limitations | Response Time | Sensitivity | References |
|---|---|---|---|---|---|---|---|---|---|
| Nanomaterial-Based | High sensitivity and selectivity, low cost, portable, non-invasive, low power consumption | Sensitive to humidity, may require pre-treatment, stability issues | Low | Uses metal oxides, carbon nanotubes, graphene; can be miniaturized | NO, NH3, H2S, acetone, other VOCs | Humidity sensitivity, stability issues in varying conditions | Seconds to minutes | High sensitivity (ppb level) | [99] |
| Chemiresistive Gas | High sensitivity, capable of detecting multiple gases, pattern recognition, portable | May require calibration, influenced by environmental conditions, moderate cost | Moderate | Uses an array of sensors integrated with AI and pattern recognition systems | H2S, NH3, NO, VOCs | Environmental sensitivity, potential need for recalibration | Seconds to minutes | High sensitivity (ppb level) | [100,101] |
| Electronic Nose (E-nose) | High sensitivity, can detect multiple gases, pattern recognition, portable | May require calibration, influenced by environmental conditions, moderate cost | Moderate | Uses an array of sensors integrated with AI and pattern recognition systems | H2S, NH3, NO, VOCs | Environmental sensitivity, potential need for recalibration | Seconds to minutes | High sensitivity (ppb level) | [100,101] |
| Infrared Spectroscopy | High precision and accuracy, can detect a wide range of gases, non-invasive | Expensive, requires skilled operation, large equipment | Very high | High precision, capable of detecting a wide range of gases | Multiple VOCs, CO2, CH4 | Expensive, not easily portable | Seconds to minutes | High sensitivity (ppb to ppm level) | [102] |
| Chromatography | High accuracy, can detect very low concentrations of gases, gold standard for analysis | Expensive, time-consuming, requires skilled operation, non-portable | Very high | High accuracy and sensitivity, capable of comprehensive gas analysis | Wide range of VOCs | Expensive, non-portable, requires skilled operation | Minutes to hours | Very high sensitivity (ppb level) | [103] |
| Photonic Crystal Fiber | High sensitivity, selectivity, low interference from humidity, non-invasive, highly stable | High cost, requires specialized equipment and knowledge for operation | Moderate | Uses photonic crystals, highly selective and stable, less affected by humidity | Multiple VOCs, NOx | High cost, requires specialized operation | Seconds to minutes | Very high sensitivity (ppb level) | [43] |
| Title | Gas/Analyte | Disease Diagnosed | Sensitivity | Advantages & Features | Wavelength/Band | References |
|---|---|---|---|---|---|---|
| Ammonia Measurement via PCF | Ammonia | Kidney disease | 63.18% | Moisture-resistant, FEM-optimized | 1.544 µm | [104] |
| CO Detection via Hollow-Core PCF | Carbon monoxide (CO) | Hyperbilirubinemia | 64.28% | Early jaundice detection | 1.567 µm | [108] |
| Isoprene Detection via PC | Isoprene | Liver fibrosis | 0.321 nm/ppm | Tamm plasmon-based, non-invasive | Visible-NIR | [112] |
| Liquid Crystal PCF Sensor | Acetone | Diabetes | 65 ppm (LOD) | Compact, temp-compensated | N/A | [113] |
| Antiresonant PCF for Respiration | Water vapor | Respiration monitoring | — | Wearable, tracks breathing | ~1.5 µm | [114] |
| Circular PCF for IgG/IgM | Antibodies | COVID-19 | High (qualitative) | Low loss, blood-serum compatible | Not stated | [115] |
| PCF Humidity Sensor | Humidity | Respiratory rate | −0.166 dB/%RH | Low temp cross-sensitivity | ~1.55 µm | [116] |
| PCF Fluorescence for Lactic Acid | Lactic acid | Sepsis/cancer | 0.8–9.5 µM (LOD) | Dual-channel, enzymatic | Fluorescence | [117] |
| Raman-Enhanced HC-PCF | VOCs (propene, H2, CO2) | Lung cancer | SRS-based | High enhancement, broadband | Visible–NIR | [118] |
| High-Performance SO2 PCF Sensor | Sulfur dioxide | Respiratory risk | 87.39% | Low loss, THz-compatible | 1.8 THz | [119] |
| PCF Sensors for Cancer Detection (Review) | Various biomarkers | Multiple cancers | — (review) | SPR/SERS/Interferometry Overview | Various | [120] |
| Helically Twisted PCF Sensor | CO, NOx, SOx | Toxic gas exposure | 3000 RIU−1 | 100% relative sensitivity, simple build | 0.2–3.0 THz | [121] |
| Trace C2H2 Detection via HC-PCF | Acetylene (C2H2) | VOCs in breath | 49 ppm (LOD) | Linear response, real-time sensing | 1–500 Hz | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortazavi, S.; Makouei, S.; Abbasian, K.; Danishvar, S. Exhaled Breath Analysis (EBA): A Comprehensive Review of Non-Invasive Diagnostic Techniques for Disease Detection. Photonics 2025, 12, 848. https://doi.org/10.3390/photonics12090848
Mortazavi S, Makouei S, Abbasian K, Danishvar S. Exhaled Breath Analysis (EBA): A Comprehensive Review of Non-Invasive Diagnostic Techniques for Disease Detection. Photonics. 2025; 12(9):848. https://doi.org/10.3390/photonics12090848
Chicago/Turabian StyleMortazavi, Sajjad, Somayeh Makouei, Karim Abbasian, and Sebelan Danishvar. 2025. "Exhaled Breath Analysis (EBA): A Comprehensive Review of Non-Invasive Diagnostic Techniques for Disease Detection" Photonics 12, no. 9: 848. https://doi.org/10.3390/photonics12090848
APA StyleMortazavi, S., Makouei, S., Abbasian, K., & Danishvar, S. (2025). Exhaled Breath Analysis (EBA): A Comprehensive Review of Non-Invasive Diagnostic Techniques for Disease Detection. Photonics, 12(9), 848. https://doi.org/10.3390/photonics12090848






