Abstract
Surface-enhanced Raman scattering (SERS) technology, leveraging its single-molecule-level detection sensitivity, molecular fingerprint recognition capability, and capacity for rapid, non-destructive analysis, has emerged as a pivotal analytical tool in food science, life sciences, and environmental monitoring. This review systematically summarizes recent advancements in SERS technology, encompassing its enhancement mechanisms (synergistic effects of electromagnetic and chemical enhancement), innovations in high-performance substrates (noble metal nanostructures, non-noble metal substrates based on semiconductors/graphene, and hybrid systems incorporating noble metals with functional materials), and its interdisciplinary applications. In the realm of food safety, SERS has enabled the ultratrace detection of pesticide residues, mycotoxins, and heavy metals, with flexible substrates and intelligent algorithms significantly enhancing on-site detection capabilities. Within biomedicine, the technique has been successfully applied to the rapid identification of pathogenic microorganisms, screening of tumor biomarkers, and viral diagnostics. For environmental monitoring, SERS platforms offer sensitive detection of heavy metals, microplastics, and organic pollutants. Despite challenges such as matrix interference and insufficient substrate reproducibility, future research directions aimed at developing multifunctional composite materials, integrating artificial intelligence algorithms, constructing portable devices, and exploring plasmon-catalysis synergy are poised to advance the practical implementation of SERS technology in precision diagnostics, intelligent regulation, and real-time monitoring.
1. Introduction
Amid rapid economic and technological advancement, public expectations for quality of life have been rising steadily. The fulfillment of basic livelihood needs has progressively shifted toward demands for safety and quality in critical areas such as food and the environment. Issues ranging from livestock products contaminated with prohibited substances to agricultural products exceeding pesticide residue limits, and heavy metal contamination caused by wastewater discharge, all pose serious threats to public health and safety. Therefore, efficient and accurate detection of hazardous substances in food and the environment is crucial. Current commonly used detection methods include chromatography [1,2], fluorescence spectroscopy [3,4], and enzyme inhibition assays [5,6]. Although these techniques offer excellent detection sensitivity and reliability, they exhibit significant limitations, complex pre-processing procedures, high detection costs, and lengthy analysis cycles, making them unsuitable for on-site instant detection requirements. Consequently, the development of novel detection platforms capable of highly sensitive and rapid analysis of trace substances is imperative.
Raman spectroscopy is a spectroscopic analysis technique based on the interaction between incident light and the internal chemical bonds of a substance. Characteristic peaks in the Raman spectrum provide information about defects, phase composition, stress, and molecular structure of the analyte. Each substance possesses a unique Raman spectrum, making it a fingerprinting technique. Raman spectroscopy offers broad analytical applicability, non-destructive testing, rapid and straightforward operation, and suitability for aqueous samples, making it particularly suitable for the detection and analysis of biological specimens. However, the Raman scattering cross-section for most molecules is very small, resulting in extremely weak Raman scattering intensity. Additionally, Raman signals are susceptible to interference from fluorescent backgrounds. To overcome these limitations of conventional Raman spectroscopy, novel Raman techniques have emerged alongside advancements in laser technology, among which Surface-Enhanced Raman Scattering (SERS) is the most extensively studied. The signal enhancement in SERS primarily originates from the Localized Surface Plasmon Resonance (LSPR) effect of metallic nanostructures [7,8]. Compared to ordinary Raman scattering signals, the Raman signals of molecules located near these metallic structures can experience immense enhancement, with enhancement factors reaching 1010 to 1012, enabling single-molecule detection. Leveraging its significant advantages—such as ultra-high detection sensitivity, unique molecular fingerprint characteristics, and non-destructive analytical capabilities—SERS demonstrates substantial application potential across multiple critical fields, including food safety, medical diagnostics, and environmental pollution monitoring.
The development and application of SERS technology rely heavily on the advancement of high-performance SERS substrates. Traditional SERS substrates primarily consist of noble metal nanoparticles or nanostructures. Their localized surface plasmon resonance (LSPR) absorption peaks typically span the spectral range from 400 to 2500 nm. The excitation light sources (532–785 nm wavelength) commonly configured in standard Raman detection systems are precisely aligned with this spectral coverage, providing the essential physical foundation for SERS. Additionally, noble metal substrates offer tunable LSPR absorption properties and relatively high detection sensitivity.
Despite the excellent plasmonic performance of noble metals—especially the high stability of Au—certain nanostructures (e.g., Ag-based) may suffer from issues such as oxidation or limited reproducibility, which can affect their long-term performance in complex matrices. Constructing multifunctional composite SERS substrates by integrating noble metal nanoparticles with functional materials (such as graphene, semiconductors, and metal-organic frameworks (MOFs)) can maintain the inherent advantages of both components while overcoming the limitations of single-component noble metal SERS substrates. Functional materials can provide additional chemical enhancement, generating a synergistic effect with the electromagnetic enhancement from the noble metal to further amplify the Raman signal of target molecules. Simultaneously, they can enhance molecular adsorption and improve the substrate’s stability and reproducibility.
2. Surface-Enhanced Raman Scattering: An Introduction
2.1. Raman Scattering
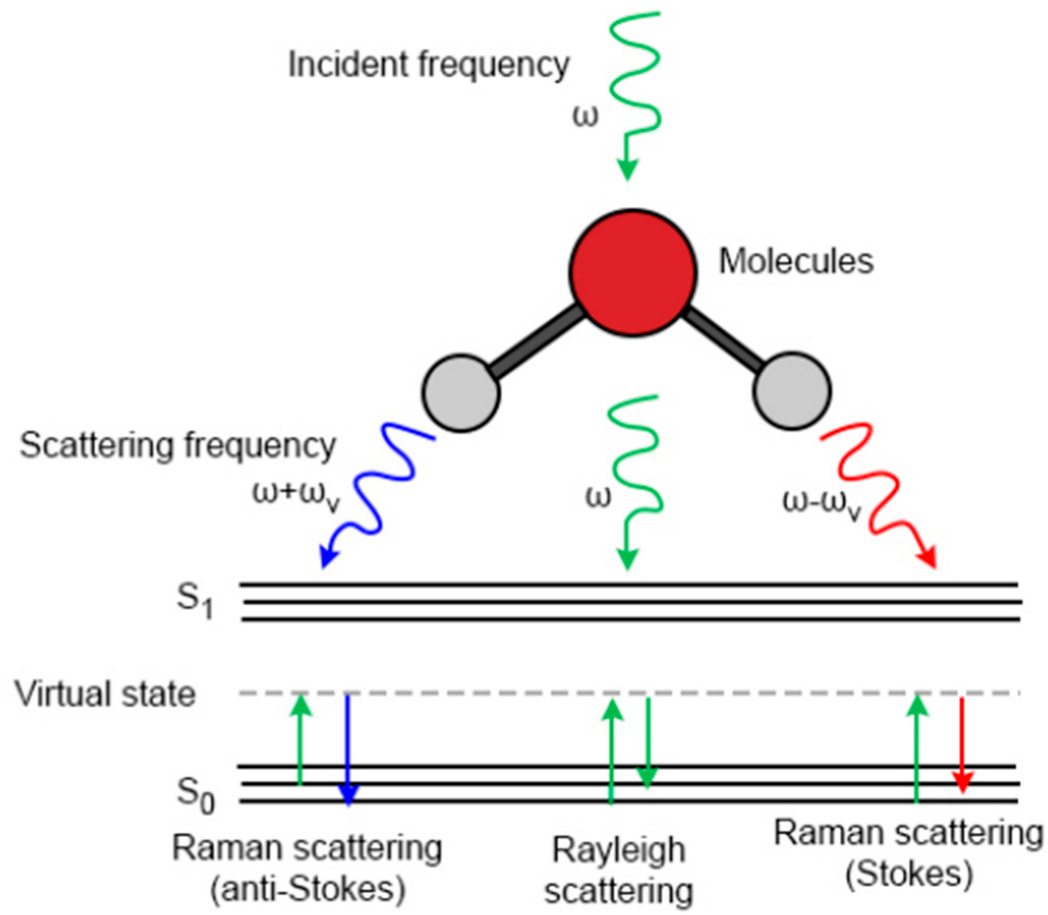
The physical essence of Raman scattering lies in the inelastic energy exchange process between photons and molecular systems. This phenomenon was first experimentally observed by the Indian physicist C.V. Raman in 1928 [9]. This breakthrough discovery significantly advanced the development of quantum theory and earned him the Nobel Prize in Physics in 1930. When incident photons interact with matter, scattering occurs. Depending on the energy conservation state, this scattering can be classified into two types, elastic scattering and inelastic scattering, as illustrated in Figure 1 [10].

Figure 1.
Diagram of Rayleigh scattering and Raman scattering [10].
During elastic scattering, an incident photon undergoes an elastic collision with a molecule in its ground state or first excited state. The molecule transitions to a high-energy virtual state—an unstable state—and rapidly releases a photon to return to the ground state. In this collision process, neither the photon’s nor the molecule’s energy changes, meaning the frequency of the incident light matches that of the scattered light. This type of elastic scattering, where the particle diameter is significantly smaller than the wavelength of the incident light, is defined as Rayleigh scattering.
In contrast, the inelastic scattering process involves changes in the wavelength and frequency of both the molecule and the photon, resulting in non-conserved energy. This scattering is defined as Raman scattering. Raman scattering is further categorized into Stokes scattering and anti-Stokes scattering. The distinction between them lies in the energy change—Stokes scattering involves a loss of photon energy, resulting in scattered light with a lower frequency than the incident light; anti-Stokes scattering exhibits the opposite behavior. During Raman scattering, incident photons undergo inelastic collisions with molecules, inducing vibrational or rotational transitions within the molecule. Consequently, the characteristic peaks in the Raman spectrum provide information about the molecular structure of the substance. Each molecule exhibits its own distinctive set of absorption peaks, where the peak positions and profiles reflect specific internal vibrational modes. This unique spectral fingerprint enables molecular fingerprint identification, making Raman spectroscopy a vital technique for component identification and structural analysis in modern analytical chemistry, renowned as a powerful non-destructive detection method.
As a molecular vibrational (and rotational) spectroscopy, Raman spectroscopy offers unique advantages. The technique enables rapid and non-destructive detection of substances with high resolution. The inherently weak Raman scattering of water allows Raman spectroscopy to be effectively applied to aqueous systems. Furthermore, its broad wavenumber coverage facilitates the detection of both organic and inorganic compounds and is suitable for analyzing samples in solid, liquid, and gaseous states. Leveraging these strengths, Raman spectroscopy finds extensive applications in chemical analysis, biomedicine, and environmental monitoring.
However, substances with small Raman scattering cross-sections exhibit weak scattering intensity, and their detection is susceptible to fluorescence interference, making Raman signals difficult to detect. These limitations constrain the broader application and development of conventional Raman spectroscopy. To overcome these challenges, Surface-Enhanced Raman Spectroscopy (SERS) technology has emerged.
2.2. Development of Surface-Enhanced Raman Scattering
In 1974, Fleischmann et al. observed a dramatic enhancement in the Raman signal intensity of pyridine molecules adsorbed on a roughened silver electrode surface [11]. Initially, they attributed this signal enhancement solely to increased molecular adsorption resulting from the enlarged surface area. Three years later, however, Van Duyne and Jeanmaire et al. made a breakthrough discovery, the Raman signal of pyridine molecules adsorbed on the rough silver electrode surface was enhanced by 5 to 6 orders of magnitude compared to its solution state [12]. Consequently, they concluded that the signal enhancement arose not from increased molecular adsorption, but from a novel surface enhancement effect. This phenomenon was designated Surface-Enhanced Raman Scattering (SERS).
In 1978, American scientist Moskovits first elucidated the relationship between the localized surface plasmon resonance (LSPR) effect on roughened Ag electrodes and electromagnetic field enhancement, identifying this mechanism as the key factor responsible for Raman signal amplification. He further predicted that similar enhancement effects could occur in Ag and Cu colloids [13]. This breakthrough was experimentally confirmed in 1979 by Creighton et al. [14], thereby extending SERS research from roughened electrode surfaces to metal colloids and laying the foundation for studies on plasmonic nanostructure-based SERS systems. With advances in nanotechnology and Raman instrumentation, SERS technology has become an indispensable technique across diverse fields due to its ultra-high sensitivity.
3. Surface-Enhanced Raman Scattering Enhancement Mechanisms
The currently widely accepted enhancement mechanisms are the Electromagnetic Mechanism (EM) and the Chemical Mechanism (CM). The EM primarily arises from the amplified electromagnetic fields generated at the surface of metallic nanostructures, while the CM involves changes in the electronic structure of molecules upon adsorption. In practical SERS scenarios, these two mechanisms generally coexist and exhibit synergistic interactions, collectively contributing to the overall signal enhancement.
3.1. Electromagnetic Enhancement Mechanism
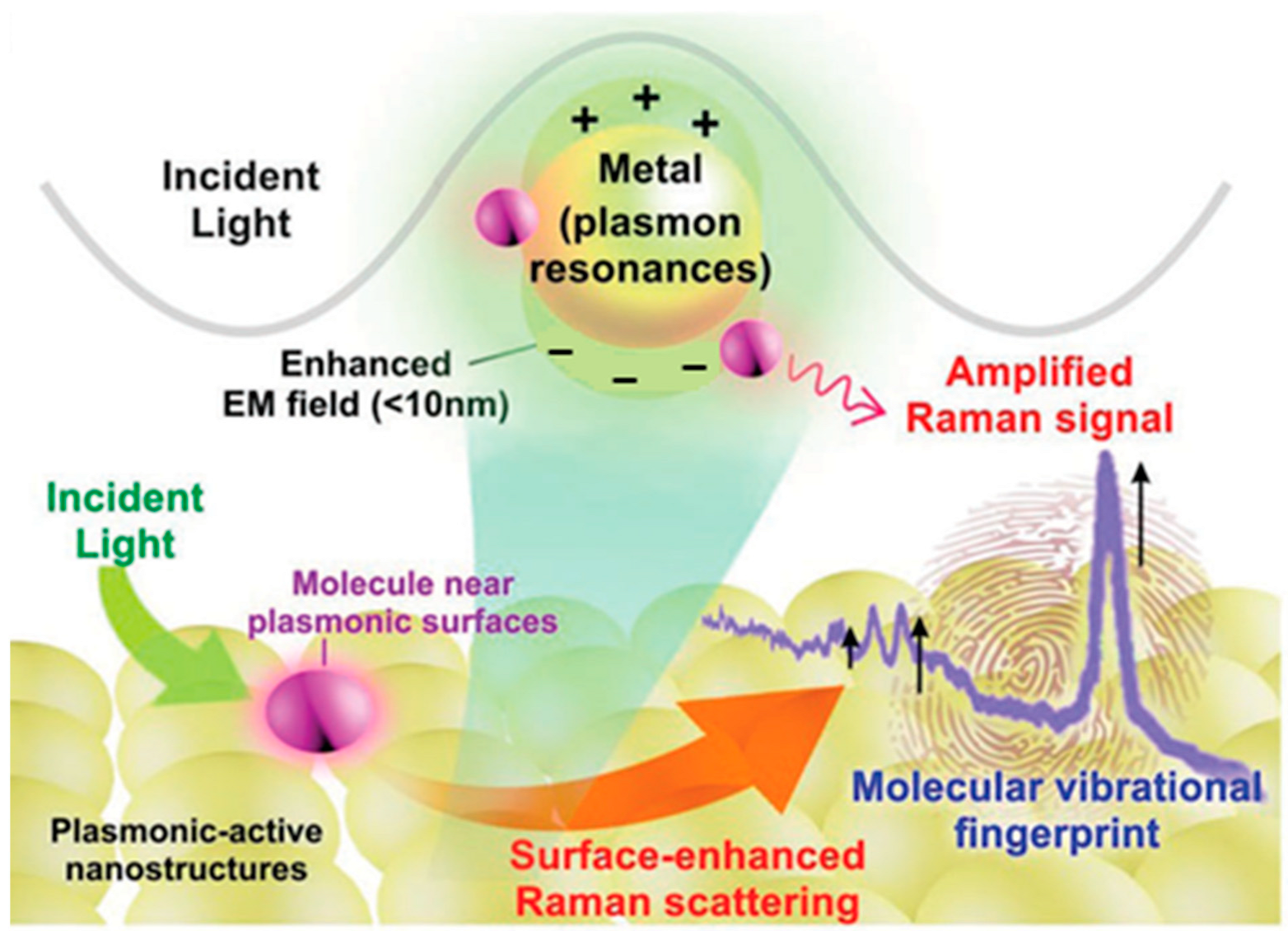
The electromagnetic (EM) enhancement mechanism in SERS primarily involves three effects—the image field effect [15], surface plasmon resonance (SPR) [16], and the lightning rod effect [17], with SPR playing the dominant role. When incident light irradiates metallic nanostructures, free electrons on the metal surface undergo collective oscillations, coupling to form propagating electromagnetic waves. Surface plasmon resonance is triggered when the incident light frequency matches the intrinsic oscillation frequency of these free electrons [18].
Surface plasmon resonance (SPR) is categorized into propagating surface plasmon polaritons (SPPs) and localized surface plasmon resonance (LSPR).
Propagating SPPs typically occur on smooth metal surfaces, where surface plasmon waves exhibit distinctive propagation characteristics—the electromagnetic field reaches its maximum intensity at the interface and decays exponentially along the normal direction into both the metal and dielectric media. The interference between incident light and SPR-generated electromagnetic fields further amplifies incident photons, thereby enhancing the Raman signals of target molecules on the metal surface.
LSPR arises when the incident light frequency matches the oscillation frequency of free electrons in noble metals, inducing collective electron oscillations. This generates spatially confined plasmon resonance that undergoes interference and coupling with incident light, producing a secondary electric field that amplifies the local electromagnetic field. Consequently, the Raman signals of molecules near metallic nanostructures are dramatically enhanced (Figure 2) [19].
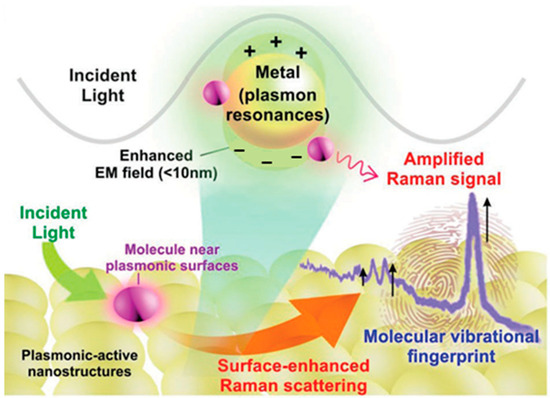
Figure 2.
Schematic diagram of localized surface plasmon resonance of metal nanostructure [19]. The plus and minus signs represent the distribution of positive and negative charges induced by the oscillation of conduction electrons under incident light.
The SERS signal enhancement primarily originates from electromagnetic effects, with intensity proportional to the fourth power of the localized electromagnetic field strength. The magnitude of EM enhancement is governed by metal composition, nanostructure morphology, nanogap dimensions and excitation wavelength.
Thus, optimization of these parameters is critical for maximizing electromagnetic field enhancement.
3.2. Chemical Enhancement Mechanism
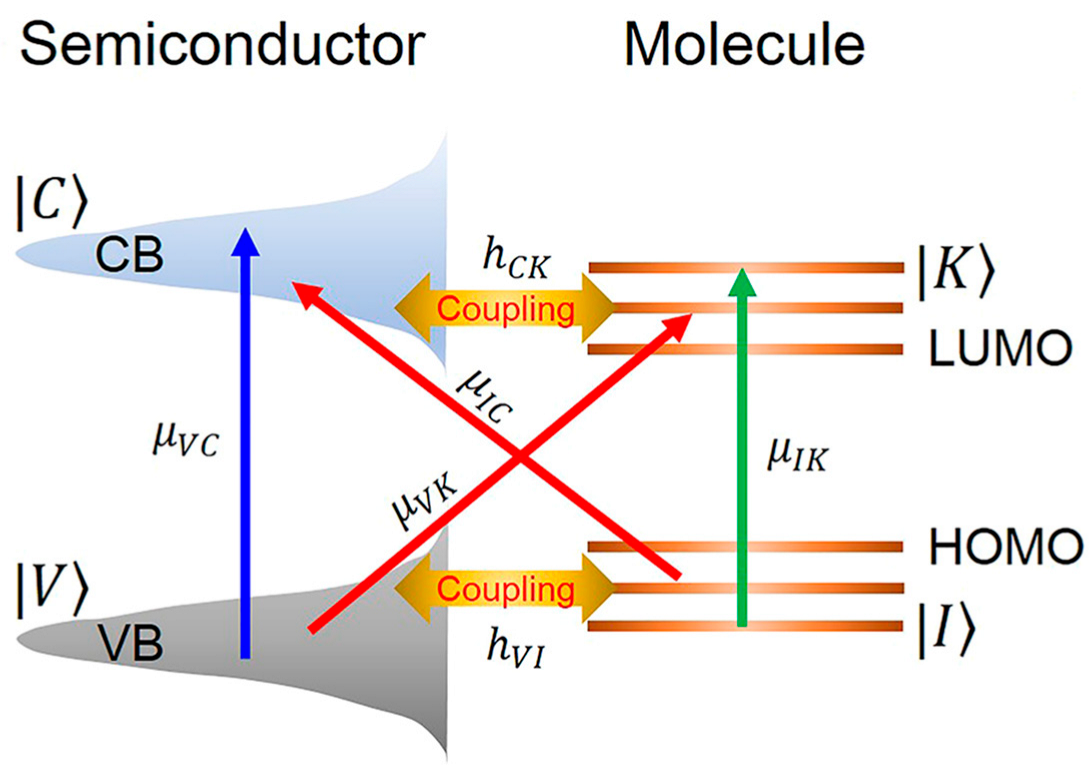
The chemical enhancement (CM) contribution to SERS is relatively modest, typically yielding enhancement factors of 10–102. In practical detection, electromagnetic and chemical mechanisms act synergistically to effectively amplify molecular Raman signals. CM es from chemical interactions between the substrate and target molecules, categorized into non-resonant enhancement, resonant enhancement, and pre-resonant enhancement [20]. Non-resonant enhancement arises from chemical bonding interactions between molecules and the metal substrate, inducing alterations in electronic structures. Resonant enhancement occurs when adsorbed molecules form new adsorbate-substrate complexes with surface adatoms, creating additional electronic transition pathways. Pre-resonant enhancement, primarily driven by charge transfer between the substrate and target molecules, typically yields the most significant contribution among chemical enhancement mechanisms [21]. In the charge transfer enhancement model, adsorption of analyte molecules onto SERS-active substrates generates new charge-transfer excited states. When the incident light wavelength resonates with these excited states—corresponding to resonant electron transitions between the metal’s Fermi level and molecular orbitals (or vice versa)—the effective polarizability of the analyte molecules is substantially enhanced, thus amplifying SERS signals.
Figure 3 schematically illustrates the charge transfer pathways between semiconductors and adsorbed molecules. In the chemical enhancement mechanism, the SERS effect is predominantly determined by the chemical properties of both target molecules and metal surfaces. This includes the nature of molecule-metal interactions (e.g., chemisorption affinity) and interaction strength. Molecules such as thiols, amines, and carboxylic acids exhibit strong affinity for metal surfaces. While chemical enhancement contributes to signal amplification, electromagnetic enhancement constitutes the primary contribution in most scenarios, with chemical enhancement factors (typically ranging from 10 to 102) being significantly weaker than electromagnetic enhancement (104–1011).
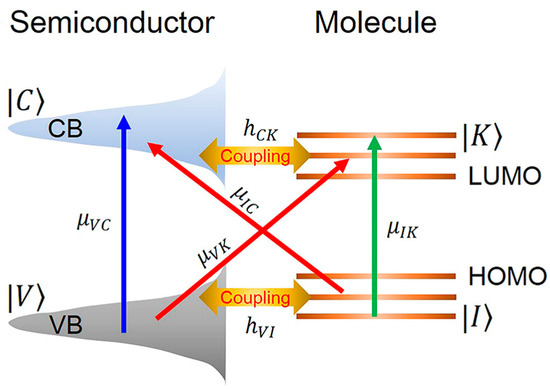
Figure 3.
Schematic diagram of charge transfer enhancement mechanism between semiconductor and molecule [22].
4. Surface-Enhanced Raman Scattering Substrates
Research on SERS-active substrates plays a pivotal role in advancing SERS technology. Significant progress has been achieved in developing substrates with high-sensitivity, high-stability, and high-uniformity properties. Traditional SERS substrates rely on noble metal nanostructures (Au, Ag, Cu). To enhance substrate performance—including sensitivity, reproducibility, stability, and specificity—while expanding applicability and enabling cost-effective fabrication, novel substrate architectures continue to emerge. These advancements have extended beyond single-material noble metal substrates to encompass non-noble metal materials and composite architectures.
4.1. Noble Metal SERS Substrates
Noble metal SERS substrates—typically comprising gold (Au), silver (Ag), or copper (Cu) with engineered nanostructures—leverage strong localized surface plasmon resonance (LSPR) under light excitation to significantly amplify Raman signals from surface-adsorbed target molecules. Owing to their exceptional SERS activity and well-established fabrication techniques, these substrates represent the most widely adopted and extensively studied class of SERS platforms [23].
Among these, gold has emerged as the premier material for high-performance SERS substrates, capitalizing on its superior oxidation resistance, exceptional biocompatibility, unique band structure, and tunable LSPR absorption characteristics [24]. These properties collectively enable robust detection in complex environments. Silver, while exhibiting outstanding SERS activity, cost-effective synthesis, and facile processability, suffers from oxidation susceptibility and compromised stability. Copper demonstrates comparatively weaker SERS performance relative to Au and Ag.
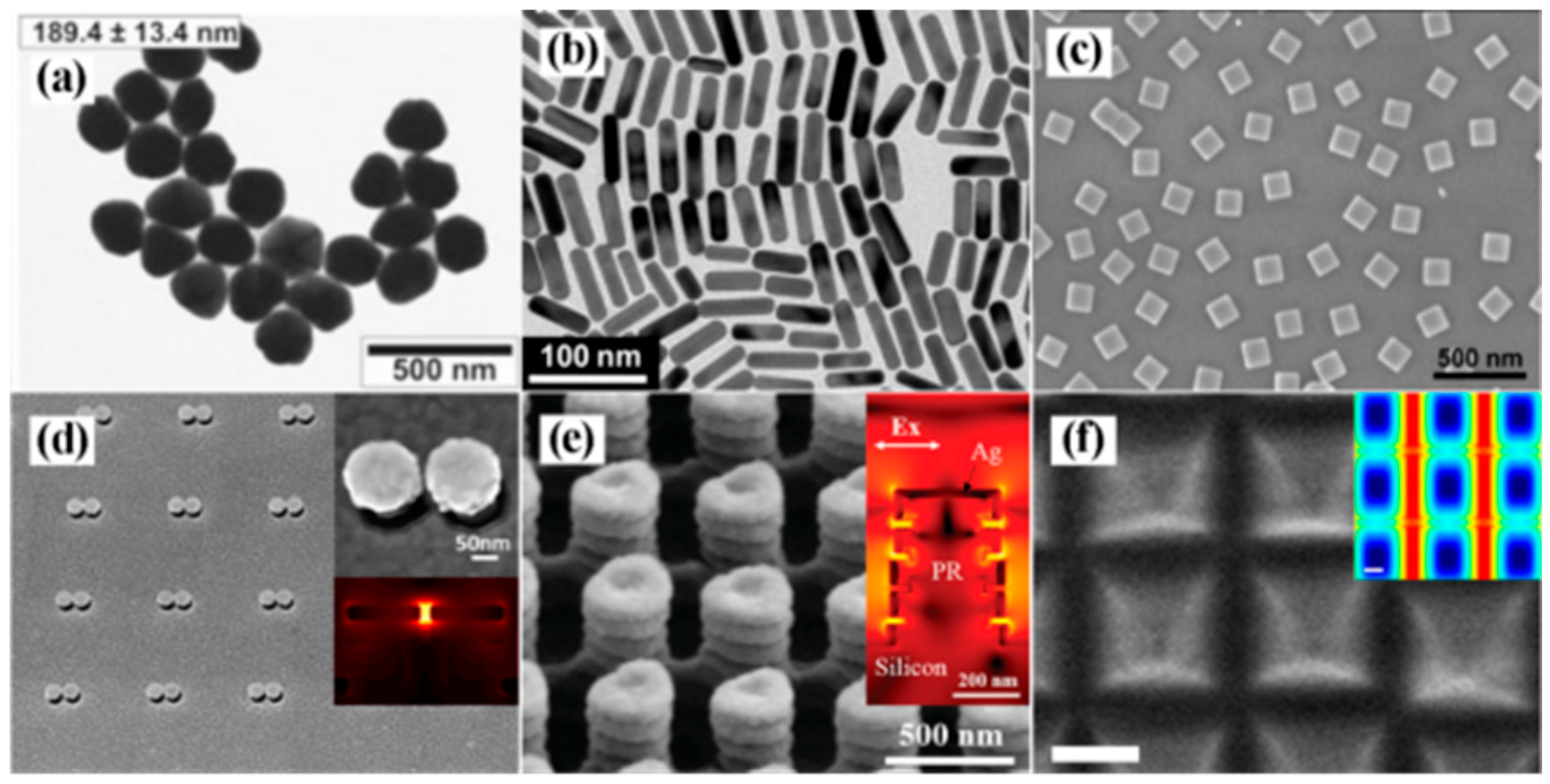
Noble metal substrates are categorized by nanostructure design. Colloidal nanoparticles, synthesized via chemical reduction methods, allow geometric control (e.g., morphology and size) through parameter optimization, thereby tuning LSPR properties for SERS enhancement. Figure 4a–c exemplifies this diversity, presenting silver nanoparticles [25], gold nanorods [26], and silver nanocubes [27]. Despite advantages in scalable production, colloidal systems exhibit uncontrolled “hotspot” distributions, limiting signal uniformity.
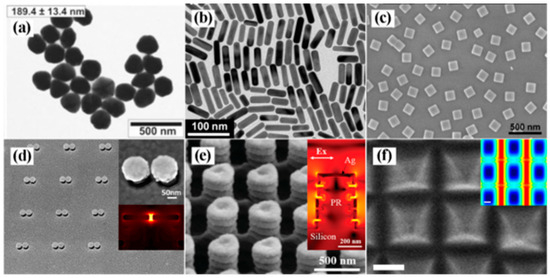
Figure 4.
(a) TEM images of silver nanoparticles [25]; (b) TEM image of gold nanorods [26]; (c) SEM images of silver nanocubes [27]; (d) SEM image of mushroom-type gold nanostructures [28]; (e) SEM image of pagoda-like silver nanopillars [29]; (f) SEM image of silver octahedral arranged as square superlattice [30].
With advancements in nanotechnology, researchers have developed ordered arrays and three-dimensional SERS substrates with controllable “hotspots” using techniques such as electron-beam lithography (EBL), nanoimprinting, and self-assembly. As exemplified in Figure 4d, Xiang et al. fabricated mushroom-shaped gold nanoarrays via EBL overlay technology, achieving an average interparticle gap of 8.38 nm that effectively amplifies electromagnetic fields [28]. Figure 4e demonstrates a pagoda-like silver nanopillar array created by photolithography, where vertically aligned nanogaps generate abundant hotspot regions for enhanced SERS detection [29]. Lee et al. further guided the self-assembly of silver octahedrons at liquid/liquid interfaces into distinct 2D plasmonic superlattices, with the square superlattice configuration exhibiting dense hotspots as an efficient SERS substrate (Figure 4f) [30].
4.2. Non-Noble Metal SERS Substrates
Non-noble metal materials (e.g., graphene, semiconductors, metal-organic frameworks) have emerged as research frontiers in SERS owing to their tunable optical properties, enhanced stability, superior biocompatibility, and significantly lower cost. These substrates substantially broaden SERS application horizons.
Extensively studied non-noble SERS platforms include:
- Metal oxides—ZnO [31,32], TiO2 [33,34], MoO3 [35,36], Nb2O5 [37,38];
- Elemental semiconductors—Graphene [39,40], Si [41,42], Ge [43];
- Transition metal dichalcogenides—MoS2 [44,45], NbS2 [46,47];
- Organic semiconductors [48,49].
Despite demonstrated SERS activity in non-noble metals, their enhancement factors (EFs) typically remain modest. Researchers employ four primary strategies to optimize performance:
- 1.
- Morphological Engineering
Analogous to noble metal nanostructures, semiconductor SERS performance can be enhanced through tailored morphology designs. Specific configurations induce structural resonances and intensify light-matter interactions [50], while sharp tips and engineered nanogaps create abundant hotspots for signal amplification [51].
- 2.
- Dimensional Control
Quantum confinement in semiconductor nanostructures regulates energy-level alignment and charge transfer efficiency [52]. Alternatively, Mie resonance effects in large, high-refractive-index nanoparticles boost SERS responses [53].
- 3.
- Crystallinity and Phase Manipulation
Modulating crystalline phases optimizes charge transfer pathways to enhance SERS activity [54].
- 4.
- Defect Engineering
Introducing defects (e.g., oxygen vacancies, elemental doping) facilitates charge transfer between substrates and analytes. Crucially, defects blueshift LSPR peaks from far-infrared to visible regions, enabling plasmonic behavior in semiconductors [55].
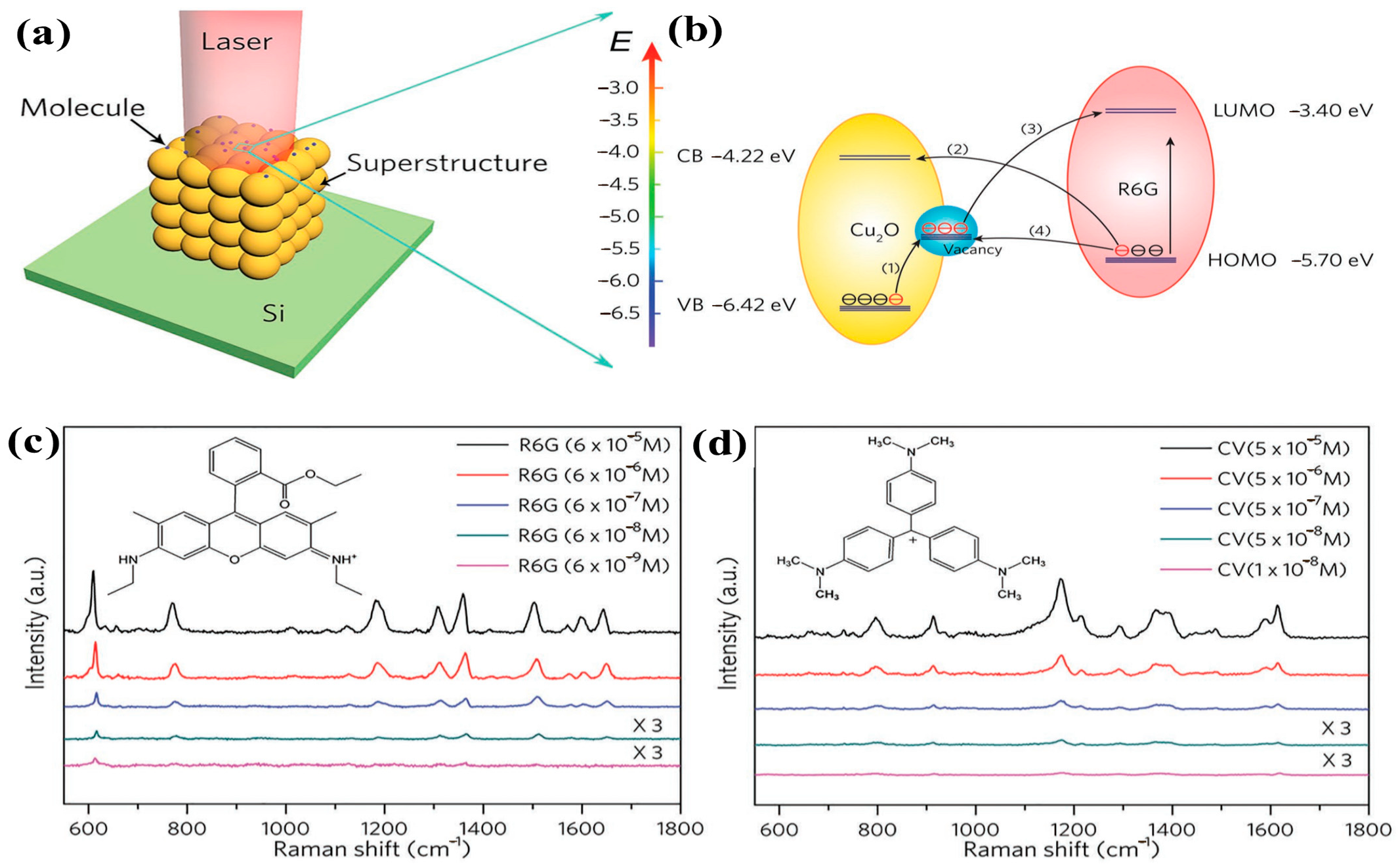
As illustrated in Figure 5, Lin et al. constructed 3D cubic Cu2O through recrystallization-induced self-assembly (RISA). During assembly, lattice fusion of adjacent Cu2O mesocrystals generated substantial defects. The resulting surface states facilitated photoinduced electron transfer, while copper vacancy defects enabled electrostatic adsorption of positively charged targets. This synergy endowed individual Cu2O particles with exceptional SERS performance—enhancement factors reaching 8 × 105 and detection limits as low as 10−9 M [56].

Figure 5.
(a) Schematic diagram of SERS detection target molecule by single Cu2O superstructure particle; (b) Schematic diagram of the photoinduced charge transfer process between Cu2O and R6G under excitation at 647 nm; (c,d) SERS spectra of R6G and CV molecules adsorbed on single Cu2O superstructure particles with different concentrations [56].
The extension of SERS beyond the visible spectrum, particularly into the deep-UV range (UV-SERS), has emerged as a significant advancement, where non-noble plasmonic materials such as Al, Al-Mg alloys, Rh, and Ga demonstrate remarkable performance—enabling ultrasensitive biomolecule detection, tunable substrates, and enhanced spectroscopic applications compared to traditional noble metals [57,58,59,60,61,62].
4.3. Noble Metal/Functional Material Composite SERS Substrates
Although noble metal SERS substrates provide substantial electromagnetic enhancement, they suffer from limitations including poor stability, oxidation susceptibility, inadequate reproducibility, and weak specificity. To overcome these constraints of single-component noble metal substrates and endow SERS platforms with enriched functionality and superior performance, researchers have integrated noble metals with functional materials to construct novel composite SERS substrates. These hybrid systems synergistically combine the electromagnetic enhancement from noble metal nanostructures with the chemical enhancement contributed by functional materials. This cooperative interaction significantly amplifies the substrate’s SERS activity, enabling detection with heightened sensitivity. Furthermore, composite substrates harness the complementary properties of both materials, transcending the limitations of singular noble metal systems. For instance, they exhibit enhanced stability and reproducibility while strengthening adsorption capacity for target molecules.
In composite SERS substrates, functional materials commonly include graphene, semiconductors, metal-organic frameworks (MOFs), and piezoelectric polymers. Graphene’s hexagonal honeycomb crystal lattice offers exceptional chemical stability and large specific surface area, enabling high nanoparticle loading capacity. Its biocompatibility and unique electronic structure facilitate efficient adsorption of aromatic molecules through π-π interactions [63]. Semiconductors achieve SERS enhancements of 10–103 via multiple mechanisms—surface plasmons, charge-transfer resonance, excitonic resonance, and molecular resonance. Within composite architectures, semiconductors bridge electron transfer between metals and target molecules [64]. MOFs—porous materials characterized by high porosity, extensive surface area, and robust stability—efficiently adsorb analytes. Their tunable pore sizes further enable selective molecular adsorption/detection in complex matrices [65]. Piezoelectric polymers convert mechanical energy into electrical energy under stress. When integrated with metals, deformation-induced negative potentials inject electrons into metallic structures, amplifying local electric fields to boost SERS intensity [66].
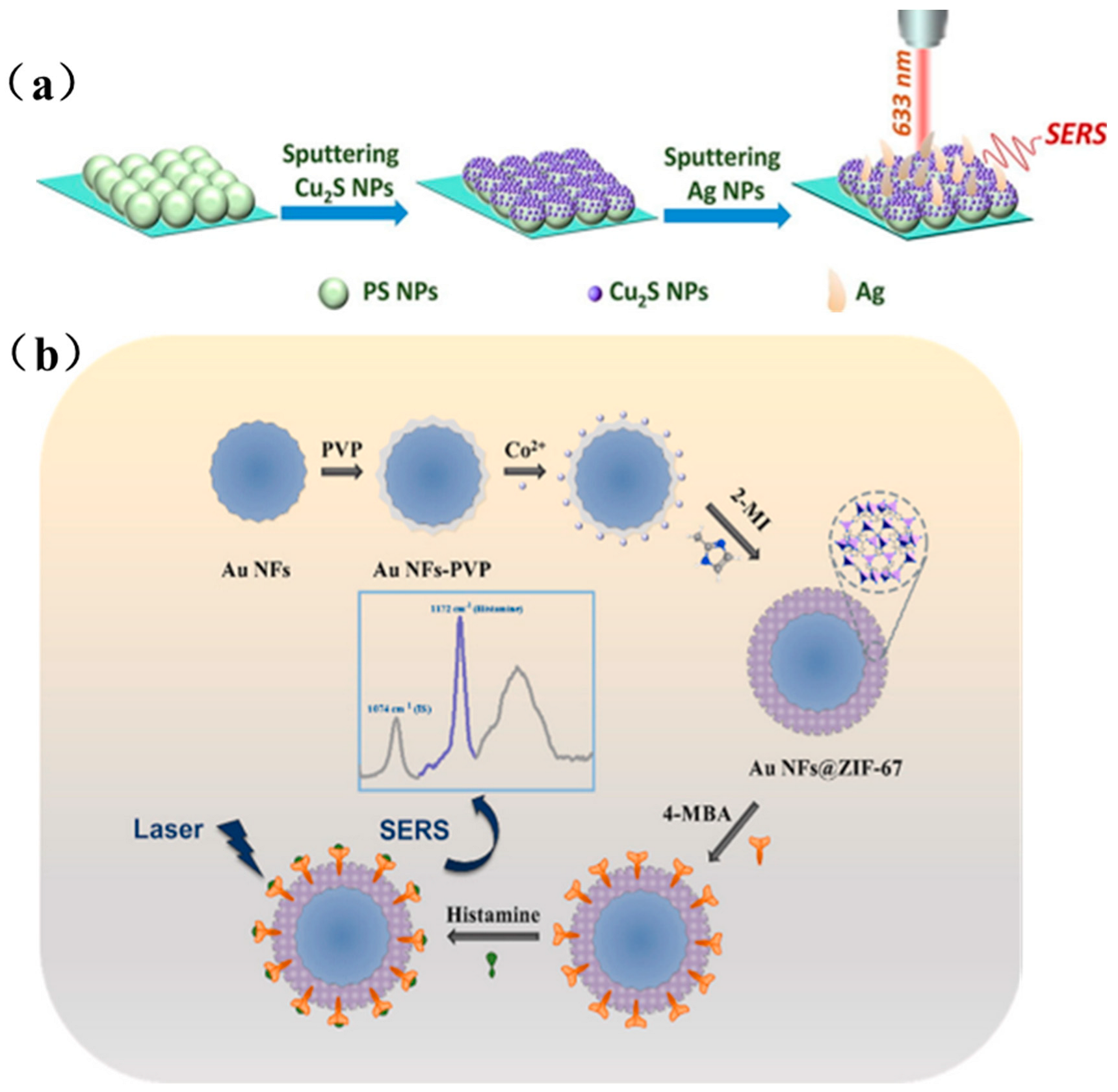
Composite noble metal/functional material structures are typically fabricated by depositing noble metal nanoparticles onto functional material surfaces, either via a direct-contact “sandwich” configuration with analyte molecules as bridges or through core–shell encapsulation. Figure 6a outlines Xue et al.’s stepwise construction of a PS(polystyrene)/Cu2S/Ag SERS substrate [67], first, a monolayer of 200 nm PS spheres is self-assembled on a silicon wafer; next, a uniform Cu2S shell is deposited by magnetron sputtering, forming PS@Cu2S core–shell particles; finally, Ag is sputtered for an optimized duration to yield a cashew-like PS/Cu2S/Ag architecture rich in electromagnetic hot spots, achieving an ultralow LOD of 10−13 M for 4-MBA. Figure 6b illustrates Xu et al.’s MOF-based SERS sensor for histamine detection [68], PVP-capped Au nanoflowers serve as cores for in situ heterogeneous nucleation of ZIF-67 shells, and 4-MBA is pre-grafted as both a specific receptor and internal standard, enabling sensitive histamine quantification down to 0.87 × 10−7 M.
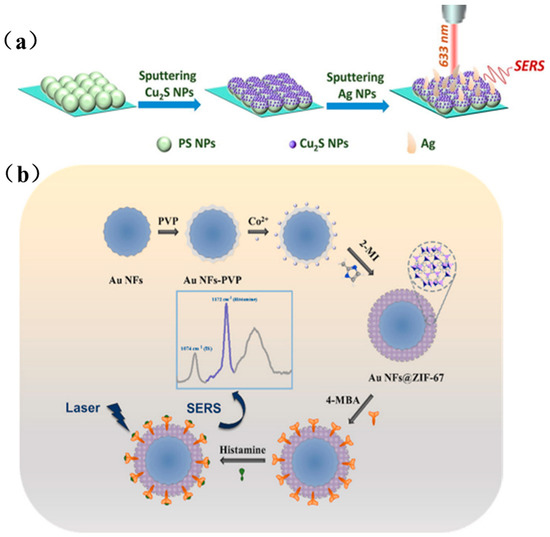
Figure 6.
(a) Preparation process of PS/Cu2S/Ag SERS substrate [67]; (b) Preparation of Au NFs@ZIF-67 SERS substrate and schematic diagram of histamine detection [68].
5. Applications of Surface-Enhanced Raman Scattering
Capitalizing on its ultra-high sensitivity, rapid non-destructive analysis, and molecular fingerprinting capabilities, surface-enhanced Raman scattering technology transcends the constraints of conventional detection methods, demonstrating broad application potential across food safety, biomedical diagnostics, and environmental monitoring.
5.1. Applications of SERS in Food Safety
Contemporary food safety faces multifaceted challenges including illegal additives in processing, pesticide residues in agricultural products, inadequate risk assessment for genetically modified foods, and chain transmission of mycotoxin contamination. These issues constitute critical threats to food quality and public health security. Consequently, developing highly sensitive and rapid detection technologies has become imperative for safeguarding food integrity. With continuous advancements in surface-enhanced Raman spectroscopy, SERS demonstrates significant potential for detecting illegal additives, pesticide residues, and related hazards. Flexible SERS substrates—adaptable to irregular sample surfaces—enable non-destructive in situ detection, further enhancing their practicality for food safety applications.
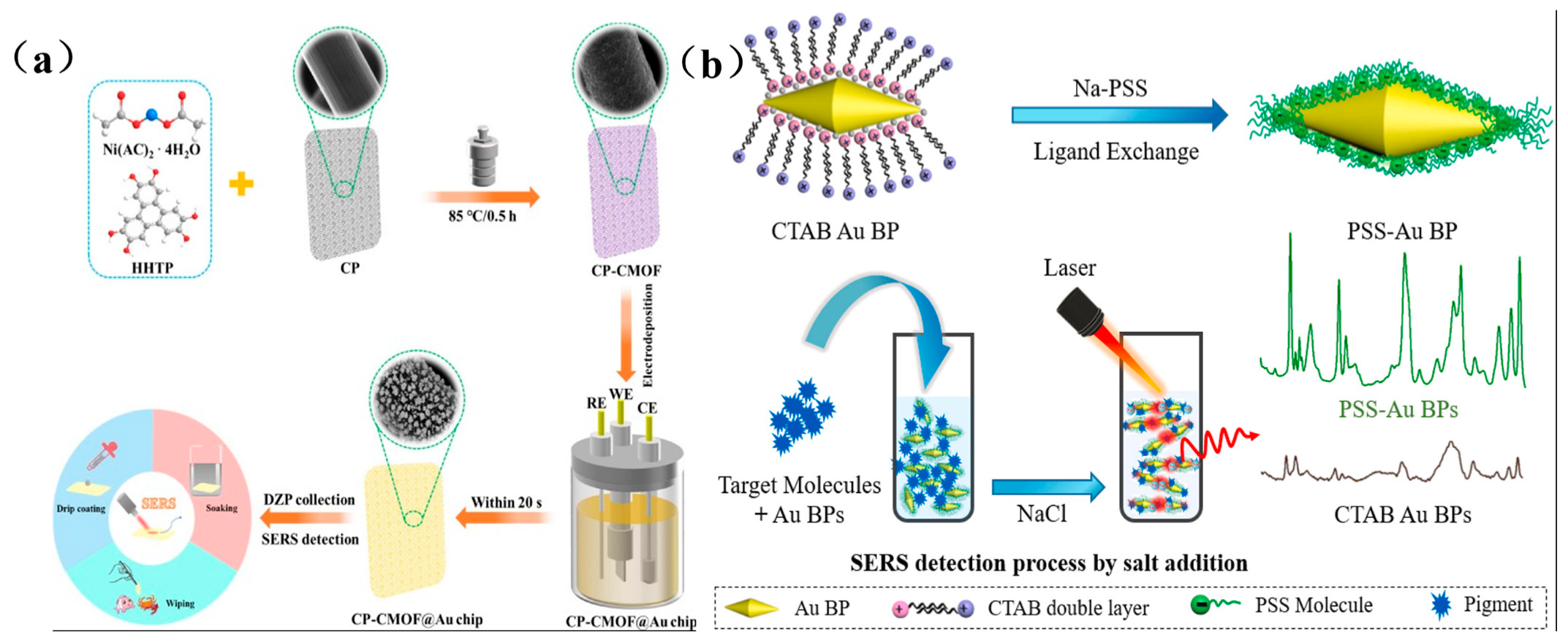
For instance, Xu et al. developed a flexible FP/AgNWs@ZIF-8 substrate using filter paper (FP) as support, enabling pesticide residue extraction and detection in river water, fruit juices, and apple surfaces through immersion, filtration, and swabbing techniques [69]. Similarly, Zhu et al. integrated conductive metal–organic frameworks (CMOFs) with electrodeposited gold nanoparticles (Au NPs) on flexible carbon paper, creating a versatile CP-CMOF@Au SERS chip for detecting diazepam (DZP) in aquatic products (Figure 7a) [70]. The CMOF scaffold preconcentrates DZP molecules via adsorption, while its conductivity enables uniform growth of flower-like Au NPs, whose sharp edges generate intense electromagnetic “hot spots” for signal amplification. Additionally, Amin et al. functionalized gold bipyramids (Au BPs) with polystyrene sulfonate (PSS) via ligand exchange, yielding a negatively charged SERS substrate (Figure 7b) [71]. This surface facilitates electrostatic adsorption of cationic illegal additives (e.g., MG, MB, R6G), while controlled salt-induced aggregation creates interparticle nanogaps that further enhance sensitivity via plasmonic coupling, enabling ultratrace detection.
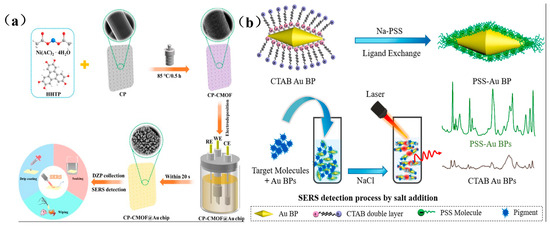
Figure 7.
(a) Schematic diagram of CP-CMOF@Au SERS sensor preparation and DZP detection [70]; (b) Schematic diagram of preparation and SERS detection of PSS-AuBPs [71].
Recent progress in surface-enhanced Raman scattering (SERS) technology has significantly advanced food safety detection across multiple domains, including pesticide screening, microbial analysis, heavy metal monitoring, mycotoxin identification, and other compositional analyses. Key developments in these areas will be systematically reviewed with selected representative studies.
5.1.1. Pesticide Residue Detection
Leveraging its high-sensitivity, molecular fingerprinting capability, and rapid detection advantages, surface-enhanced Raman scattering has emerged as a cornerstone technology for pesticide residue analysis. Recent cutting-edge research focuses on innovative substrate design, multiplex residue screening, artificial intelligence algorithm integration, on-site field detection, and complex matrix pretreatment innovations—collectively advancing detection efficacy and expanding application horizons.
- 1.
- Innovative design of high sensitivity SERS substrate
The development of novel nanostructured substrates is pivotal for enhancing detection performance. Au@Ag core-shell nanoparticles (Au@Ag NPs), leveraging bimetallic synergistic enhancement, have been widely employed in pesticide detection for tea. Zhu et al. (2018) integrated Au@Ag NPs with a Genetic Algorithm-Partial Least Square (GA-PLS) algorithm to quantitatively analyze chlorpyrifos in tea (LOD = 0.29 μg/mL), achieving accuracy comparable to Gas chromatography-mass spectrometry (GC-MS) [72]. Hassan et al. (2019) further combined this substrate with solid-phase extraction (SPE) to simultaneously detect acetamiprid and 2,4-D in matcha, reaching detection limits at the 10−4 μg/mL level [73]. Subsequent research in 2021 utilized silver nanoflowers (AgNFs) for ultratrace detection of methomyl, acetamiprid, and 2,4-D in tea, capitalizing on their high enhancement factor (EF = 1.39 × 106) and multi-hotspot geometry [74]. Most recently, Li et al. (2022) engineered AgNFs with electromagnetic field-focusing surface crevices, enabling synchronous detection of chlorpyrifos and carbendazim in rice at 0.01 μg/mL [75].
- 2.
- Breakthrough in simultaneous multi-residue detection technology
Simultaneous detection of multiple pesticides in complex food matrices remains challenging for SERS applications. Hassan (2019) pioneered an SPE-SERS hyphenated platform integrating GA-PLS modeling for quantitative analysis of acetamiprid and 2,4-D in matcha [73]. Ma et al. (2023) achieved dual-pesticide screening in apple juice using Au@Ag NPs with portable Raman systems, identifying characteristic peaks (acetamiprid 635 cm−1, thiram 1385 cm−1) at 0.076 μM LOD [76]. Li et al. (2023) enhanced sensitivity for thiram and pymetrozine in tea (LOD = 0.286 ppb) through Au-Ag octahedral hollow cages (OHCs) coupled with CNN algorithms [77].
Novel multiplex strategies continue to emerge, Zhu et al. (2021) applied Au@Ag nanoflowers to detect 2,4-D and imidacloprid in milk, where Uninformative Variable Elimination-Partial Least Squares (UVE-PLS) modeling significantly improved prediction accuracy (Residual Predictive Deviation (RPD) = 6.32) [78]; concurrently, Adade et al. (2023) implemented Au@Ag bimetallic nanoflowers with genetic algorithms for quadrupole screening of Sudan dyes in palm oil (LOD = 8.8 × 10−4 ppm) [79].
- 3.
- Intelligent Algorithm-Driven Quantitative Analysis
Chemometrics and artificial intelligence have substantially enhanced SERS data interpretation capabilities. Zhu (2018) optimized chlorpyrifos quantification using Genetic Algorithm-Partial Least Squares (GA-PLS) and synergy interval PLS-GA (siPLS-GA) models (r2 = 0.98) [72]. Subsequently, Li et al. (2021) developed a Competitive Adaptive Reweighted Sampling-Extreme Learning Machine (CARS-ELM) nonlinear model to resolve nonlinear signal issues for thiabendazole in apples (Rp2 = 0.9406) [80]. Yang et al. (2024) further advanced detection sensitivity by comparing Monte Carlo Uninformative Variable Elimination (MCUVE) and CARS algorithms, achieving chlorpyrifos detection at sub-ng/mL levels [81].
Deep learning and novel variable selection methods represent current frontiers, Li et al. (2022) integrated flower-like AgNPs with Python-Scikit-learn algorithms for high-accuracy Recursive Feature Elimination-Support Vector Machine (RFE-SVM) prediction of thiabendazole (RPD = 6.477) [82]; Huang et al. (2022) employed Bootstrapping Soft Shrinkage-PLS (BOSS-PLS) modeling to optimize carbendazim detection with 116% recovery [83]; concurrently, Adade (2023) leveraged Random Frog (RF) algorithms to enhance acetamiprid screening efficiency in palm oil (Rc = 0.990) [84].
- 4.
- Innovations in on-site rapid testing programmes
Flexible substrates and portable instrumentation are driving the advancement of on-site SERS detection. Li (2021) engineered a paper-based flexible SERS sensor for rapid chloromycin screening (LOD = 10−5 μg/mL) [85]. Subsequently, Chen et al. (2023) developed a PDADMAC/PSS/Au@Ag NRs filter paper system enabling pesticide capture from fruit/vegetable surfaces via three-minute stick-peel operation [86].
Innovative field technologies continue to evolve, Zhang et al. (2018) pioneered AgNR@Al2O3 tape substrates for integrated “stick-peel-detect” pesticide screening on fruit surfaces [87]; Kim et al. (2019) created TEMPO-oxidized cellulose/AuNRs nanopaper wrap for direct produce surface analysis (LOD = 60 ng/cm2) [88]; meanwhile, Zhang et al. (2022) engineered an AgMNPs magnetic fluidic chip with microfluidic channels, achieving picomolar sensitivity for malachite green in fish tissue [89].
- 5.
- Innovations in complex matrix pre-treatment
Addressing complex food matrices, advanced pretreatment techniques significantly enhance SERS specificity. Hassan (2019) employed solid-phase extraction (SPE) to eliminate matcha pigment interference [73]. Wei et al. (2023) engineered a flexible aptamer sensor for specific carbendazim recognition on apple surfaces via nucleic acid capture (LOD = 1.20 ng/cm2) [90].
Cutting-edge pretreatment strategies continue to evolve, Zhang et al. (2019) constructed Fe3O4/chitosan@Ag magnetic microspheres for thiram detection in apple juice using SMSPE-SERS (LOD = 1.2 ng/cm2) [91]; concurrently, Zhang et al. (2023) utilized laccase-mediated capsaicin degradation to overcome matrix effects in hotpot condiments [92]; while Qu et al. (2023) designed hierarchical SiO2 microspheres@AuNPs (HSM@AuNPs) floating substrates coupled with acetonitrile extraction for thiabendazole screening in tea beverages (LOD = 102 ppb) [93].
Table 1 summarizes representative analytes, methodologies, and performance metrics for SERS-based pesticide detection. Current SERS technologies face critical challenges including;
Complex matrix interference (e.g., tea pigments obscuring spectral signals [73,79]), insufficient substrate reproducibility (relative standard deviation RSD > 5% [94]), and lack of standardized quantification protocols.
Future research should prioritize, developing high-specificity anti-interference substrates (e.g., Fe-BTC MOF suppressing capsaicin interference [92], graphene-encapsulated AgNPs enhancing selectivity [95]); advancing automated microfluidic-SPE integration (exemplified by HSM@AuNPs floating platforms enabling extraction-detection unification [93]); implementing deep learning algorithms with portable systems (e.g., Au-Ag OHCs coupled with CNN models for precision prediction [96]); and expanding novel application scenarios such as soil pesticide monitoring via magnetic enrichment (3D magnetically assembled substrates [97]) and in situ aquatic drug residue tracking (magnetofluidic SERS chips [80]). Multidisciplinary synergy will ultimately establish SERS as a standardized on-site screening tool for pesticide regulation.

Table 1.
Representative Applications of SERS in Pesticide Detection.
Table 1.
Representative Applications of SERS in Pesticide Detection.
| Detection Target | Substrate Scheme | LOD | Detection Range | Key Features | Ref. |
|---|---|---|---|---|---|
| Chlorpyrifos | Au@Ag NPs | 0.29 μg/mL | N/A | GA-PLS/siPLS-GA models; GC-MS equivalent accuracy | [72] |
| Silver nanoflowers (AgNFs) | 0.01 μg/mL | 0.01–1000 μg/mL | Simultaneous detection with carbendazim; ICPA-PLS algorithm | [74] | |
| Acetamiprid | Au@Ag NPs | 10−4 μg/mL | N/A | First dual-pesticide detection; SPE pretreatment | [73] |
| SERS sensor + RF algorithm | 10 ng/g | 5–100 ng/g | Palm oil matrix; RF-PLS model | [86] | |
| Carbendazim | Flexible aptasensor | 1.20 ng/cm2 | N/A | PVDF/CQDs flexible substrate; non-destructive apple surface detection | [98] |
| Fe3O4/CS@Ag magnetic microspheres | 1.2 ng/cm2 | N/A | SMSPE-SERS integration; magnetic separation enrichment | [83] | |
| Thiabendazole | Flower-like AgNPs | 0.24 μg/mL | 0.5–50 mg/L | CARS-ELM nonlinear model | [76] |
| HSM@AuNPs floating substrate | 102 ppb | N/A | Liquid-liquid microextraction integration; tea beverage matrix | [99] | |
| Thiram | Au@Ag NPs | 0.076 μM | 0.5–10 μM | Portable Raman; dual-pesticide simultaneous detection | [75] |
| Fe3O4/CS@Ag magnetic microspheres | 1.2 ng/cm2 | N/A | Direct detection in apple juice; magnetic separation | [83] | |
| Methomyl | Flower-like AgNPs | 5.58 × 10−4 μg/mL | N/A | SPE pretreatment; tea matrix | [100] |
| Cadmium | Sodium alginate-reduced AgNPs | 2.36 × 10−5 μg/L | N/A | Green synthesis; edge enrichment technology | [85] |
| Sudan dyes | Au@Ag bimetallic nanoflowers | 0.00088 ppm (Type I) | 0.001–4.0 ppm | Four-dye synchronous screening; genetic algorithm optimization | [82] |
* N/A = Not available or not applicable.
5.1.2. Advances in Mycotoxin Detection via SERS
Since its discovery in the 1970s, the application of surface-enhanced Raman scattering for mycotoxin detection has evolved from fundamental exploration to practical implementation. Early research primarily focused on noble metal nanoparticle substrates and laboratory-based single-toxin analysis, exemplified by silica-encapsulated gold nanoparticles for aflatoxin B1 immunosensing [101] and portable SERS validation of deoxynivalenol (DON) [102].
Post-2015, advancements in nanosynthesis catalyzed significant progress, with novel substrates like core-shell architectures [103,104] and magnetic composites [105,106] markedly enhancing detection sensitivity and anti-interference capabilities.
In the past five years, convergence of three cutting-edge strategies—aptamer-functionalized probes [107,108,109], multiplexed synchronous analysis [105,110,111], and deep learning algorithms [112]—has accelerated the transition toward rapid on-site analysis and high-throughput multi-mycotoxin screening.
In the realm of food safety, SERS technology has evolved bespoke detection strategies for diverse mycotoxins. For aflatoxin B1 (AFB1) analysis, Si–Au–Ag Janus nanocomposites deliver stable, high-sensitivity detection (LOD = 0.1 ng/mL) [113]; magnetic core–shell Fe3O4@PDA/Au particles coupled with multivariate analysis achieve simultaneous enrichment and identification in maize matrices (LOD ≈ 1 ng/mL) [106]; and magnetic SERS lateral-flow test strips built on rough silver nanoparticle substrates enable concurrent determination of AFB1 and zearalenone [105,110]. In zearalenone sensing, aptamer-based assays predominate, mesoporous silica-supported Au nanocomposites exploit the aptamer-masking effect for ultra-trace detection (LOD = 0.0064 ng/mL) [107]; core-shell Au@AgNP-embedded test strips attain an LOD of 3.6 μg/kg in maize with excellent agreement to High Performance Liquid Chromatography (HPLC) results [103]; bilayer-stacked Raman reporters assembled with Au nanostars further push the detection limit down to 3 μg/kg [114]; and deep-learning models markedly improve quantitative precision in corn oil (LOD = 6.81 × 10−4 μg/mL) [112].
In ochratoxin analysis, multivariate, simultaneous detection has become a highlight, rough silver nanoparticles coupled with a genetic-algorithm-partial-least-squares model achieve high-precision, concurrent quantification of OTA and OTB in rice (LOD = 1.13–1.18 μg/kg) [110], while single-toxin assays using pH-tuned AgNP substrates reach ultrahigh sensitivity (LOD = 2.63 pg/mL) [115]. Key advances in patulin sensing focus on anti-interference designs, a GO@Au nanosheet-film aptasensor suppresses matrix effects via competitive binding (LOD = 0.46 ng/mL) [108]; a signal-off assay based on metal-organic-framework-DNA nanoassemblies attains an LOD of 0.0281 ng/mL in apple juice [109,116]; and magnetic enrichment techniques deliver recoveries of 96.3–108% with a sensitivity of 0.0384 ng/mL [104]. For alternariol (AOH) and mixed-toxin screening, the coffee-ring effect combined with chemometrics enables high-throughput, simultaneous screening of patulin (PAT) and AOH in apples (LOD = 1 μg/L) [117], and a microarray SERS immunosensor can multiplex three toxins with recoveries of 83.8–108.1% [111].
Table 2 summarizes the typical analytes, detection strategies, and performance metrics for SERS-based mycotoxin assays. Current efforts must overcome complex matrix interferences; the use of selective adsorbent materials such as metal–organic frameworks (MOFs) [118,119] and the optimization of aptamers represent key solutions. Advances in noble-metal nanoparticle assemblies [120], deep-learning algorithms [112], and the development of portable devices [121] will continue to drive this technology toward on-site, intelligent monitoring, providing ever-more powerful tools for ensuring food safety.

Table 2.
Summary of Representative SERS-Based Detection Methods for Major Mycotoxins.
5.1.3. Heavy Metal Detection
In recent years, surface-enhanced Raman scattering (SERS) has achieved remarkable progress in the realm of foodborne heavy-metal analysis, leveraging its single-molecule-level detection sensitivity [122] and distinctive fingerprint-recognition capability to tackle trace-level contamination challenges [123]. Contemporary research has centered on the design of novel nanostructured substrates, innovations in molecular-recognition mechanisms, and the integration of multi-modal strategies [124,125,126], collectively yielding substantial improvements in the detection performance for critical contaminants such as mercury, arsenic, cadmium, lead, and chromium [123,127].
In mercury-ion sensing, core-shell nanoprobe architectures exhibit exceptional performance. Au@Ag nanoparticles combined with Rhodamine 6G probes leverage Hg2+-induced signal-attenuation effects to achieve an LOD of 0.48 pM [128]. DNA molecular-switch assays based on the T–Hg2+–T specific coordination motif deliver ultra-high sensitivity down to 1.35 × 10−15 M in blood samples [129]. Dual-mode paper-based chips, which integrate colorimetric readout with SERS detection, maintain a stable LOD of 0.48 pM in tea matrices [130]. On-site detection platforms—such as coffee-ring-effect microfluidic chips [131] and handheld lateral-flow test strips [132]—further shorten analysis times to just three minutes while preserving femtomolar-level sensitivity [133,134].
In the realm of arsenic sensing, dual advances have been made in both species-specific and total arsenic monitoring. 3-Aminophenylboronic acid-functionalized silver nanoparticle sensors harness As–O–Ag bond formation to generate a “signal-on” effect, achieving a total arsenic LOD of 0.0273 μg/g [135]. A label-free strategy employs Cu2O/Ag heterojunction substrates for direct arsenic capture, coupled with a competitive adaptive reweighted sampling–PLS (CARS-PLS) algorithm to attain a prediction correlation coefficient of 0.9935 [136]. Additionally, immunoassay test strips using monoclonal-antibody-functionalized Au@Ag nanorods enable As(III)-specific screening with an LOD of 7.62 μg/mL [137].
In cadmium-contamination monitoring, green synthesis approaches have delivered major advances, sodium-alginate-reduced Ag nanoparticles combine eco-friendliness with a high enhancement factor of 3.48 × 105, and when paired with edge-enrichment techniques, the LOD is pushed down to 2.36 × 10−5 μg/L [138]. Lead sensing has benefited from multi-path optimization strategies, including aptamer-modulated Au nanoparticle growth-kinetics methods [139] and self-assembled Au@Ag nanorod aggregation schemes [140], achieving detection sensitivities of 0.1 μg/L and 0.021 μg/L, respectively. Multiplex heavy-metal platforms—such as paper-chromatography–SERS—enable simultaneous analysis of Cd2+, Cu2+, and Ni2+ in rice, each with LODs around 1 μM [141].
In chromium sensing, systems have been developed that combine speciation discrimination with on-site screening capabilities. Carbimazole-functionalized Au@Ag nanoparticles achieve selective recognition of Cr6+ via characteristic peak-intensity changes, reaching an LOD of 0.945 mg/kg in tea leaves [142]. AgNP-based immunochromatographic test strips elevate Cr3+ detection sensitivity to 10−5 ng/mL [142,143]. Portable silicon-based chips coupled with handheld Raman spectrometers support rapid on-site screening of industrial wastewater [144]. For other heavy metals, manganese ion assays using a dual-ligand system of 6-mercaptonicotinic acid and melamine attain an LOD of 4.0 × 10−6 mol/L [145]. Multiplex heavy-metal analysis techniques—such as Au/graphene sandwich-structured substrates [144] and combined SERS—fluorescence spectroscopy strategies [146]—further expand application scenarios.
Table 3 summarizes the typical analytes, detection strategies, and performance metrics for SERS-based heavy-metal assays. Current technical challenges are primarily centered on food-matrix interference effects [120], substrate reproducibility variations [124,125], and probe cross-reactivity in multi-target assays [147]. Future innovation directions include the optimization of heavy-metal classification via deep-learning models [147,148], the development of photocatalytically regenerable substrates [126], microfluidic-chip enrichment techniques [131], and in-situ monitoring systems in living plants [148]. These breakthroughs will propel SERS technology toward more intelligent, portable, and multiplexed simultaneous detection.

Table 3.
Representative SERS-Based Strategies for Heavy Metal Detection in Food Samples.
5.1.4. Detection of Other Constituents
Leveraging its high sensitivity, molecular-fingerprint recognition, and rapid analysis capabilities, surface-enhanced Raman scattering (SERS) has demonstrated broad application potential in the field of food-safety testing. Beyond its extensive use for pesticide residues, foodborne microorganisms, heavy-metal ions, and mycotoxins, SERS has also achieved significant advances in the detection of a variety of other food-risk factors and constituents, including antibiotic residues, illicit additives, naturally occurring toxins, polycyclic aromatic hydrocarbons (PAHs), and plastic-related contaminants.
- 1.
- Antibiotic Residue Detection
The overuse of antibiotics leading to resistance poses a serious threat to health, and SERS has emerged as a critical tool for rapid screening. Research has concentrated on the ultrasensitive detection of chloramphenicol (CAP). Li et al. fabricated a flexible paper-based SERS sensor using flower-like silver nanoparticles (AgNPs), coupled with a multivariate scattering-correction competitive adaptive-weighted partial least-squares (MSCCARS-PLS) model, achieving a limit of detection (LOD) of 10−5 μg/mL and recoveries of 90–102% in milk and other samples [85]. Hassan et al. developed a SERS sensor based on hollow Au/Ag nanoflowers (HAu/Ag NFs) combined with a first-derivative CARS-PLS model, demonstrating excellent linearity for CAP across 0.0001–1000 μg/mL in both milk and water matrices [149]. Subsequent work has optimized substrates and algorithms—Li et al. employed gold nanotriangles (AuNTs) assembled on a PDMS flexible film with a random-frog-jump PLS (RF-PLS) model for CAP in milk, yielding a prediction coefficient (Rp) of 0.9802 [150]. Yang et al. used Ag@Au core–shell nanoparticles with a variable-combination population-analysis PLS (VCPA-PLS) model to detect CAP in fish muscle, attaining an LOD of 10−5 μg/mL [151]. Hassan et al. further functionalized Au@AgNPs with ascorbic acid and applied an RF-PLS model to achieve an LOD of 2.73 × 10−5 μg/mL for CAP in milk, with results consistent with HPLC [152]. Michalowska et al. innovatively employed magnetic Fe3O4@Au nanoparticles coated with silica as a SERS substrate, integrating magnetic separation to detect penicillin G in milk below the EU maximum residue limit, with an LOD of 1 nmol/L [153].
- 2.
- Detection of Illicit Additives and Prohibited Drugs
SERS has proven highly effective in combating food adulteration and the presence of banned substances. Li et al. developed a diazo-coupling-based SERS assay for capsaicin, using it as a marker for gutter oil. In this method, para-aminothiophenol (4-ATP) reacts with capsaicin to form an azo dye that serves as the SERS reporter, with gold nanorods (Au NRs) as the substrate. The assay achieves an exceptionally broad linear range (10−11–10−4 M) and an ultralow detection limit of 3.24 × 10−12 M [154]. Jiang et al. designed a cuttable, recyclable TiO2 nanofiber-supported Ti@Ag nanoparticle (TiO2 NFSF/Ti@Ag NPs) SERS substrate for the detection of the banned aquaculture drug malachite green (MG) in seafood. This substrate combines a high enhancement factor (7.11 × 108) with excellent reusability (at least five cycles) [155].
- 3.
- Natural Active Compounds and Toxin Detection
SERS has also demonstrated robust quantitative capabilities for flavor compounds and natural toxins in food matrices. Peng et al. developed a tri-channel biosensor—integrating SERS, colorimetry, and image analysis—based on the catalytic activity of Fe3O4@Cu and aptamer recognition for on-site detection of the potent neurotoxin tetrodotoxin (TTX) in seafood; the SERS channel achieved an LOD as low as 0.055 ng/mL [156]. Sun et al. combined dynamic SERS (D-SERS) with a uniform gold nanorod (AuNR) substrate to qualitatively and quantitatively analyze the key flavor compound hydroxyl-sanshool (particularly α-sanshool) in Zanthoxylum (Sichuan pepper) pericarp, reaching an LOD of 0.03 mg/mL with results consistent with HPLC [157]. Wei et al. developed a magnetic-separation–based, competitive-ratiometric SERS aptasensor using 4-aminothiophenol (4-ATP) as an internal standard, enabling highly selective detection of diarrhetic shellfish toxin okadaic acid (OA) in seafood and environmental samples over the range 0.5–100 ng/mL, with significantly improved reproducibility and stability [158].
- 4.
- Polycyclic Aromatic Hydrocarbon (PAH) Detection
PAHs are a class of potent carcinogenic contaminants. Adade et al. developed a SERS sensor based on gold nanoparticles (AuNPs) coupled with chemometric models (CARS-PLS, LDA, KNN) for the qualitative and quantitative analysis of benzo[b]fluoranthene (BbF) in shrimp, achieving an LOD of 0.12 ng/mL and successfully discriminating between contaminated and uncontaminated samples [159]. The same group then introduced a flexible, hydrophobic paper-based AuNP SERS substrate integrated with deep-learning algorithms (CNN, LSTM) for rapid, highly sensitive detection of BbF and dibenzo[a,h]anthracene (DbA) in mussels, with LODs of 0.10 ng/mL and 0.09 ng/mL respectively, showing excellent agreement with HPLC-FLD results [160]. Wang et al. ingeniously employed AgNP/graphene hybrids as a SERS platform—graphene’s π–π stacking enriches PAH molecules (e.g., pyrene, anthracene, phenanthrene) at AgNP-generated hotspots, enabling ultrasensitive ppb-level detection without additional surface modification [161].
- 5.
- Other Additives and Contaminants
- Plasticizers (phthalate esters)
Rong et al. constructed a dual-mode nanosensor by integrating SERS with upconversion-fluorescence nanoparticles (UCNPs@AuNPs). Using aptamer recognition, this platform achieved ultrasensitive detection of phthalate esters (PAEs) in food and packaging materials, with LODs of 0.0108 ng/mL for SERS and 0.0087 ng/mL for fluorescence [162].
- Joint detection of preservatives and heavy metals
Jin et al. proposed a data-fusion strategy combining SERS and fluorescence spectra, leveraging machine-learning techniques such as deep forest (DF) and convolutional neural networks (CNN). This approach enabled simultaneous quantification of low-level potassium sorbate (a preservative) and lead in matsutake mushroom, with a decision-level-fused CNN model yielding the best predictive performance [149].
- Sulfur-containing gases
Zhu et al. synthesized a dual-probe nanocomposite (MOF-5-NH2-assembled Au@Ag NPs) that operates via two mechanisms. SERS signal modulation through the redox reaction between Au@Ag NPs and H2S, and fluorescence “turn-on” via charge transfer between SO2 and amino groups. This sensor achieved highly sensitive, selective detection of H2S (LOD = 2.26 nM) and SO2 in water and beer samples [163].
- 4-Aminothiophenol (4-ATP)
Xu et al. developed a superhydrophilic mesoporous Au–CuO substrate that suppresses the coffee-ring effect and enhances chemical amplification. Using 4-ATP both as an analyte and intrinsic SERS reporter, they demonstrated high sensitivity (LOD = 10−7 M) and reproducibility (RSD = 3.33%) for 4-ATP detection in real water samples [164].
Table 4 outlines representative analytes, detection strategies, and performance metrics for SERS-based assays targeting “other constituents” in food safety. In summary, SERS has been vigorously applied to detect antibiotics, illicit additives, natural toxins, PAHs, plasticizers, preservatives, and various small-molecule contaminants. Research efforts have focused on the development of high-performance, stable, reusable, or portable SERS substrates—such as diverse noble-metal nanostructures, flexible supports, core-shell architectures, magnetic composites, and semiconductor-metal hybrids—paired with advanced chemometric algorithms, machine-learning/deep-learning models, and multimodal sensing strategies (e.g., ratiometric assays, dual-probe platforms, tri-channel sensors, and data-fusion approaches). Together, these innovations have markedly enhanced sensitivity, specificity, accuracy, anti-interference capability, and field-deployability, providing a robust technological foundation for ensuring food safety.

Table 4.
Advanced SERS-Based Detection Strategies for Representative Food Contaminants: Substrate Design, Analytical Performance, and Technical Features.
5.2. Applications of SERS in the Biomedical Field
In the biomedical arena, the precise identification of molecular pathological biomarkers can dramatically improve the efficiency of early diagnosis. It is critically important for disease detection and for the dynamic regulation of drug dosing. By virtue of its ultra-high sensitivity, molecular-fingerprint specificity, and non-destructive detection capabilities, SERS offers unique advantages in disease diagnostics, bioimaging, pharmacological analysis, and drug delivery applications [165].
A cutting-edge application of SERS in biomedicine is single-molecule detection and sequencing of DNA/proteins, enabled by plasmonic nanopore platforms that integrate spectroscopic enhancement with electrical sensing for ultrahigh sensitivity and structural analysis at the sub-nanometer scale [166,167,168,169].
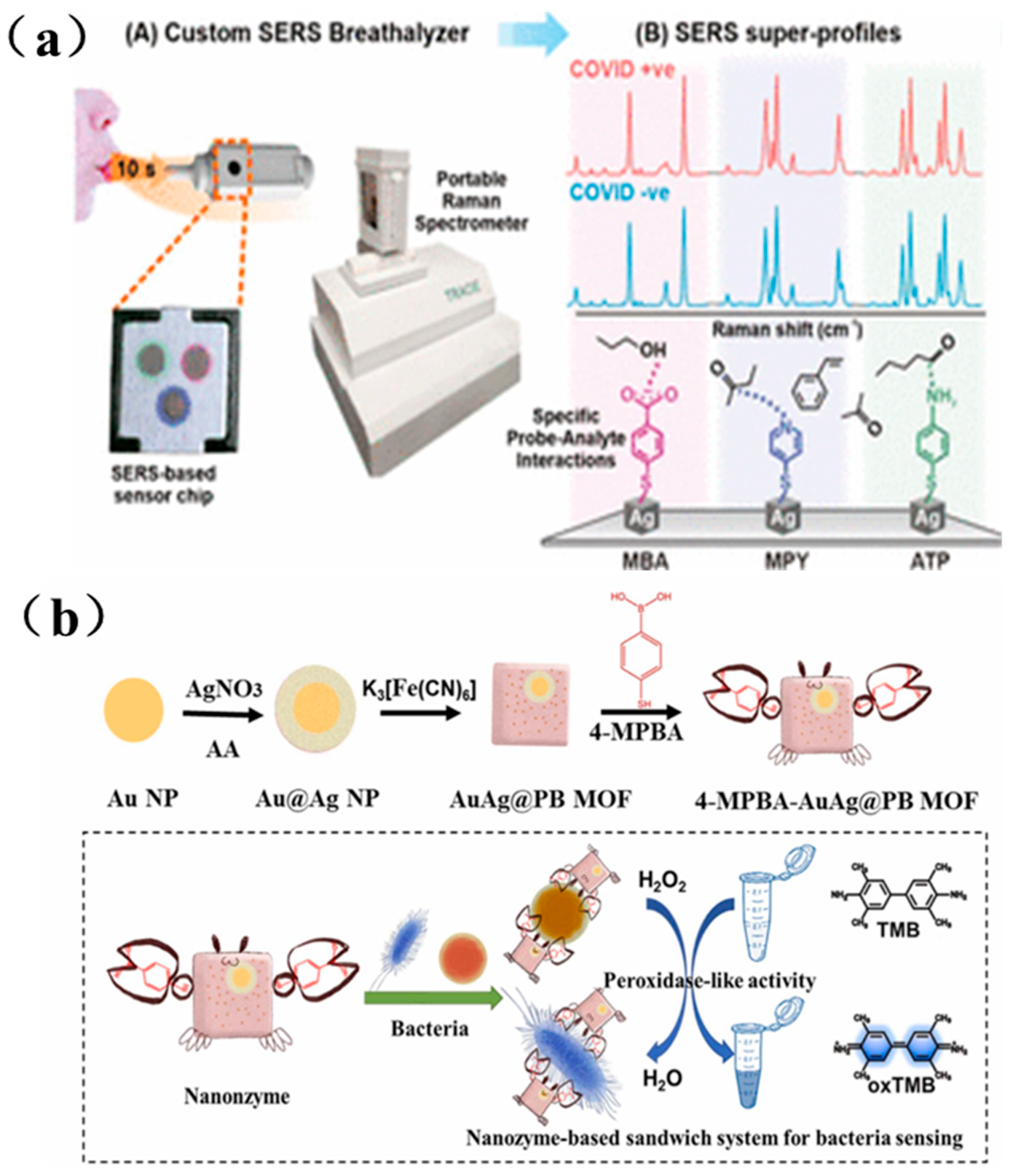
Since 2019, the global outbreak of COVID-19 has posed a serious threat to human health. Given the virus’s rapid transmission rate, the development of rapid and highly sensitive detection methods is critical. As shown in Figure 8a, Leong et al. designed a handheld SERS-based breath analyzer capable of identifying SARS-CoV-2 within 5 min, offering both high sensitivity and specificity. They designed multiple surface receptors on their SERS sensor to induce a myriad of complementary intermo-lecular interactions with the BVOCs present as the breath sample flows through the breath chamber [170].
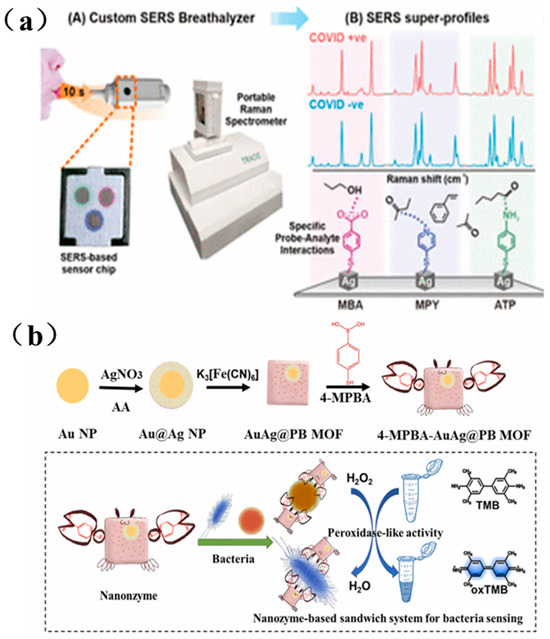
Figure 8.
(a) SERS-based respiratory detector and identification of COVID positive individuals schematic diagram [170]; (b) Schematic diagram of dual-mode sensor for bacterial colorimetric analysis and SERS detection based on bacteria/4-MPBA/AuAg@PBMOF [171].
Bacterial infections also present an escalating threat to global healthcare systems. Rapid identification of pathogens and quantification of bacterial load are essential for early diagnosis of bacterial infections. As illustrated in Figure 8b, Cai et al. prepared a AuAg@PB MOF substrate by growing a metal-organic framework (MOF) ex situ on Au@Ag nanoparticles, and further assembled it into a sandwich-structured nanosystem composed of nanozymes, SERS tags, and the AuAg@PB MOF substrate. This platform enabled highly sensitive bacterial detection, with a detection limit as low as 6 CFU/mL [171].
Surface-enhanced Raman scattering (SERS) technology, owing to its ultra-high sensitivity (down to the single-molecule level), fingerprint-spectrum recognition capability, and resistance to photobleaching, has demonstrated revolutionary potential in the life-science and biomedical fields. In recent years, research on SERS in biomedicine has been primarily focused on the following areas:
- 1.
- Rapid Detection of Pathogenic Microorganisms
For foodborne pathogen screening, SERS aptasensors have become the mainstream approach due to their high specificity. Duan et al. (2016) developed a Salmonella aptasensor based on Au@Ag core-shell nanoparticles, employing a “aptamer 1–target bacterium–aptamer 2–ROX reporter” sandwich structure to achieve an LOD of 15 CFU/mL, with results in strong agreement with plate-counting assays [172]. Li et al. (2017) further introduced a competitive-binding strategy. Salmonella cells compete with complementary DNA for aptamer binding, inducing aggregation of gold nanorods and converting this into a measurable SERS signal, lowering the LOD to 9 CFU/mL [173]. For fungal contamination, Guo et al. (2021) captured Raman spectra of spoilage-fungus spores on a gold-nanorod substrate and applied BP-ANN and PCA-LDA models to achieve classification accuracies exceeding 98% [174]. Notably, the synergy of SERS with chemometrics has greatly enhanced discrimination power, Liu et al. (2019) used cationic gold nanorods to boost Pseudomonas SERS signals and achieved 100% accuracy in classifying four strains via LDA and HCA [175]. Moreover, integrating SERS with microfluidics and artificial intelligence has accelerated on-site detection [176]. Fu et al. (2021) combined deep convolutional neural networks (CNNs) to identify urinary-tract–infection pathogens’ resistance profiles with 96% accuracy—substantially outperforming traditional PCA-KNN methods [177].
- 2.
- Tumor Biomarker and Exosome Analysis
The core advantage of SERS in cancer diagnostics lies in its ultrasensitive detection of low-abundance biomarkers. Wang et al. (2025) achieved uniform enhancement of exosome SERS signals by precisely tuning nanocone architectures [178], while Sun et al. (2024) combined MXene-coated Au@Ag core–shell substrates with deep-learning algorithms to detect thyroid-cancer–derived exosomes down to 1.7 × 109 particles/mL, attaining 96% diagnostic accuracy in clinical samples [179].
For protein biomarkers, Yang et al. (2019) designed an Au@Ag@SiO2–AuNP core–shell–satellite structure that pushed the detection limit for alpha-fetoprotein (AFP) to 0.3 fg/mL [180]; Lu et al. (2021) exploited self-assembled silver-coated gold nanoparticle oligomers to create abundant “hot spots,” enabling pg/mL-level detection of carcinoembryonic antigen (CEA) [181].
Notably, significant progress has been made in portable devices, Tong et al. (2019) developed a label-free SERS sensor on AuNRs/PMMA substrates for quantitative measurement of serum prostate-specific antigen (PSA) with an LOD of 0.06 ng/mL [182], and Das et al. (2021) implemented a “nano-popcorn” microarray platform coupled with a handheld Raman spectrometer for on-site screening of scrub typhus IgG/IgM antibodies [183].
- 3.
- Virus and Genetic Disease Diagnostics
In the realm of viral detection, SERS-PCR technology has dramatically shortened the thermal-cycling requirements of conventional PCR. Wu et al. (2022) employed a gold-nanoparticle–embedded, nanopatterned grooved substrate to reduce the number of cycles needed for SARS-CoV-2 detection from 25 to just 8 [184]. Lee et al. (2024) developed a focused-ion-beam–fabricated nanorod-array chip capable of dual, label-free detection of both the SARS-CoV-2 Delta variant and influenza virus [185].
For genetic markers, Pang et al. (2014) introduced a Molecular Sentinel approach, using hairpin DNA conformational switching to detect highly pathogenic avian-influenza viral RNA with a sensitivity of 2.67 attomoles [186]. Progress has also been made in Alzheimer’s-disease biomarker sensing, Eremina et al. (2024) utilized a chitosan-film–immobilized silver-nanoparticle SERS sensor to discriminate between monomeric and aggregated β-amyloid protein, achieving an LOD of 15 pM [187].
Despite its remarkable achievements, SERS faces multiple challenges in clinical translation:
- eliminating background interference in complex biological matrices requires incorporation of highly specific probe designs [188,189];
- achieving quantitative standardization demands the development of stable, homogeneous enhancement substrates [181,190];
- multiplexed detection necessitates the creation of high-throughput microarray platforms [183,191]. Future trends include the development of flexible and wearable SERS devices [176], the integration of artificial intelligence for intelligent spectral analysis [174,179], and the exploration of plasmon–catalytic synergistic effects for therapeutic monitoring [192,193]. Zhu et al. (2023) pointed out that integrating SERS with microfluidic and electrochemical platforms will accelerate the deployment of point-of-care testing (POCT) devices [188], while Yuan et al. (2022) emphasized that machine-learning algorithms’ ability to deconvolute complex biological spectra will be a critical breakthrough enabler [194].
5.3. Applications of SERS in Environmental Monitoring
Against the backdrop of accelerated global industrialization, environmental pollution has continued to worsen, with industrial effluent discharge, agricultural runoff, and the spread of emerging contaminants posing serious threats to human health and ecosystem integrity. In this context, SERS—thanks to its high sensitivity and molecule-specific fingerprinting capability—has offered novel solutions for environmental monitoring [195].
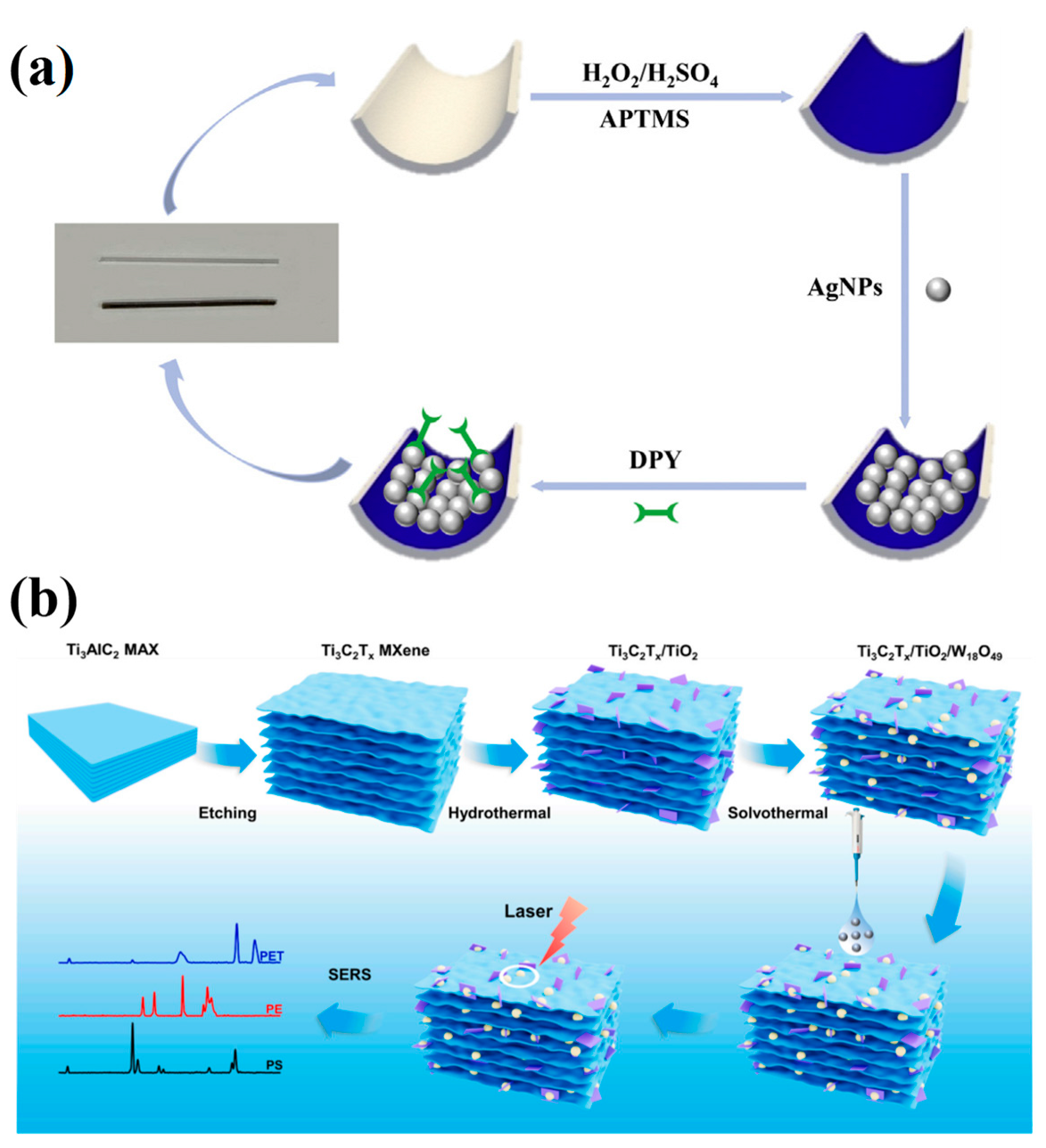
Mercury ions (Hg2+), as prototypical neurotoxic heavy metals, can inflict irreversible damage on the central nervous system and renal tissues. Zhao et al. fabricated a SERS sensor by modifying the inner wall of a capillary with silver nanoparticles (Ag NPs) and employing 4,4′-bipyridine (Dpy) as the probe molecule, the main advantage of this sensor is that it can collect samples directly by capillary force and carry out on-site analysis by combining portable Raman spectrometer. This platform was successfully applied to the detection of Hg2+ in real environmental water samples (Figure 9a) [196].
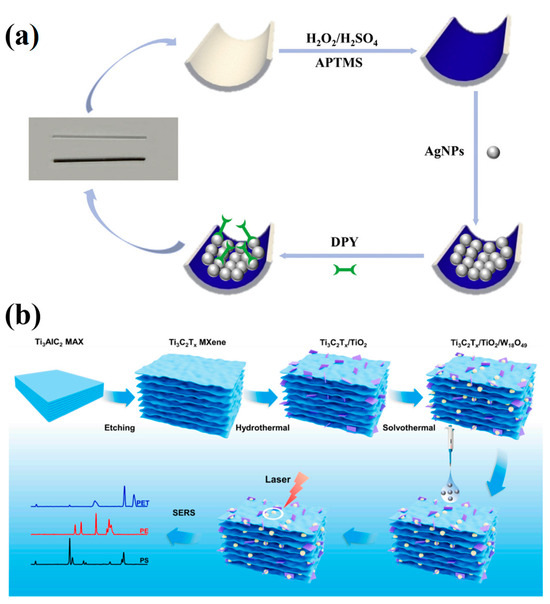
Figure 9.
(a) Schematic diagram of SERS capillary sensor preparation process [196]; (b) Ti3C2Tx/TiO2/W18O49 SERS sensor preparation process and SERS detection schematic diagram of MNPs [197].
Micro-/nanoplastics (MNPs), once introduced into the environment, can enter aquatic organisms, plants, and ultimately humans through multiple pathways, constituting a global environmental concern. Han et al. addressed this by in situ growing TiO2 and W18O49 on Ti3C2Tx MXene to create a novel 3D Ti3C2Tx/TiO2/W18O49 SERS sensor for environmental MNP detection, achieving a limit of detection of 25 μg/mL (Figure 9b) [197].
Surface-enhanced Raman scattering (SERS), with its ultra-high sensitivity (approaching single-molecule detection), molecular-fingerprint recognition, and rapid response, has become an indispensable tool for monitoring environmental contaminants. In recent years, significant breakthroughs have been made in substrate design, detection strategies, and real-world applications.
5.3.1. Design and Optimization of High-Performance SERS Substrates
Given the complexity and variability of environmental matrices, the development of highly sensitive, selective, and stable SERS substrates remains a core research focus.
- 1.
- Noble-metal nanostructures
Precise control over the morphology of gold and silver nanoparticles—such as star-shaped particles [198], core–shell architectures [199,200], and urchin-like structures [201]—as well as their organization into three-dimensional networks [202] or ordered arrays [203], can dramatically increase the density of electromagnetic “hot spots.” For example, a capillary-loaded 3D silver nanostructure achieved trace-level detection of Hg2+ with an LOD of 0.2 pM—well below the drinking-water standard of 10 nM [204]—while a nanoporous gold (NPG) substrate detected malachite green isothiocyanate at concentrations down to 10−16 M [198].
- 2.
- Composite Functional Materials
The synergistic integration of noble metals with magnetic nanoparticles [205], metal–organic frameworks (MOFs) [206], metal oxides (e.g., ZnO [207], Fe2O3 [204]), or carbonaceous supports can markedly enhance SERS performance. For instance, Au@Fe2O3 nanoflowers combine electromagnetic and chemical enhancement to achieve a 4-ATP LOD of 7.25 × 10−9 M [204]; AuNPs modified with UiO-66(Ce) MOF provide increased adsorption sites for efficient enrichment of ziram, yielding an LOD of 5.21 × 10−9 M [206]. Magnetic nanoparticles (MNPs) facilitate facile separation and preconcentration of analytes, improving detection efficiency in complex matrices [205].
- 3.
- Flexible and Wearable Substrates
Polymer nanofibers—such as polylactic acid (PLA) [208] and polyamide (PA) [209]—loaded with silver nanoparticles (Ag NPs) form pliable SERS substrates that can be directly adhered to fruit and vegetable surfaces for on-site pesticide-residue screening. For example, a PLA/AgNP nanofiber patch detected thiophanate-methyl with an LOD of 10−8 M [208]. Meanwhile, a three-dimensional, porous PA/Ag array achieved an LOD of 2.69 × 10−9 M for diquat, exhibiting good reproducibility (RSD < 20%) [209].
5.3.2. Strategies for Enhancing Detection Sensitivity and Selectivity
To overcome the challenges posed by trace-level pollutant concentrations and strong matrix interferences in environmental samples, researchers have devised several signal-enhancement approaches.
- 1.
- Integration of microfluidics and enrichment techniques
Coupling microfluidic chips with SERS enables continuous, real-time analysis. For example, a zigzag microfluidic channel combined with Ag NP probes achieved uninterrupted, flow-through detection of arsenite (As3+) with an LOD of 0.67 ppb [210]. Moreover, diffusive gradient thin-film (DGT) technology integrated with SERS (SERS-DGT)—utilizing Au@g-C3N4 nanosheets to perform in situ enrichment and detection—has been successfully applied to monitor sulfadimethoxine (SMT) in aqueous environments [211].
- 2.
- Portable Devices and On-Site Detection
Portable Raman systems built on Arduino or Raspberry Pi platforms, integrated with Internet-of-Things (IoT) connectivity, enable real-time pollutant monitoring in the field [212]. PDMS-based multi-channel sensors can simultaneously detect Hg2+, aniline, polychlorinated biphenyls (PCBs), and other contaminants, with individual channel LODs in the range of 10−10–10−7 M, making them well suited for emergency water-quality surveillance [213].
- 3.
- Machine-Learning–Assisted Analysis
Advanced machine-learning techniques—such as preprocessing SERS spectra with Fourier or Walsh transforms followed by deep-learning models—have been employed to deconvolute complex spectral data. These approaches substantially enhance both qualitative and quantitative accuracy for multi-component pollutant analysis (achieving >80% accuracy), effectively mitigating matrix-interference effects [214,215].
Although SERS offers significant advantages, it still faces challenges—such as complex matrix interferences, insufficient quantitative reproducibility, and cost control [212,216,217]. Future research should focus on—developing low-cost, reusable substrates; advancing multi-technology integration (e.g., dual-function platforms that combine SERS with photocatalytic degradation [214]); and promoting standardization and field validation to establish unified detection protocols. With cross-disciplinary innovations in nanotechnology, artificial intelligence, and materials science, SERS is poised to become a universal platform for environmental pollutant monitoring, providing critical technical support for environmental health risk management.
6. Summary
Surface-enhanced Raman scattering (SERS) has emerged as a highly promising analytical technique with broad application prospects across food safety, biomedical diagnostics and environmental monitoring. Looking ahead, three key areas will drive its further advancement. First, the development of novel substrates with enhanced stability and reusability—such as self-cleaning TiO2@Ag composites and recyclable magnetic nanomaterials—will not only lower per-test costs but also improve analytical throughput. Second, the rise of multimodal sensing platforms that integrate SERS with complementary techniques—such as fluorescence and electrochemistry—will enable more comprehensive and reliable analyses. Finally, the deep integration of artificial intelligence will revolutionize SERS data processing, advanced deep-learning algorithms will more accurately deconvolute complex Raman signatures in heterogeneous samples, while cloud-based spectral libraries will facilitate rapid result comparison and data sharing, paving the way for real-time, large-scale deployment of SERS-based detection.
In the field of food safety, the application of SERS is poised to break free from laboratory constraints and advance toward rapid, on-site detection. This technology not only enables highly sensitive monitoring of pesticide residues, mycotoxins, and heavy-metal contaminants, but can also be integrated with smart packaging systems to provide end-to-end oversight of food quality. In particular, supply-chain management stands to benefit from intelligent SERS-enabled sensor networks capable of real-time assessment of freshness and safety. Nonetheless, to overcome the challenges posed by complex food matrices, there remains a pressing need for the development of more selective recognition elements and more efficient sample-preparation techniques.
In the field of biomedical diagnostics, SERS is rapidly moving toward precision medicine. Its application in liquid biopsy is especially striking, ultra-sensitive SERS aptasensors enable the detection of early cancer biomarkers at exceptionally low concentrations. In infectious-disease diagnostics, the combination of SERS with emerging biotechnologies such as CRISPR has opened up new avenues for rapid pathogen screening. Looking forward, as nanotechnology continues to advance, SERS is poised to achieve single-molecule detection, providing an even more robust technical foundation for early disease diagnosis.
In the field of environmental monitoring, SERS is advancing toward intelligent, real-time applications. By integrating SERS detectors with drones and other mobile platforms, in-situ surveillance and rapid alerting of environmental contaminants become possible. This approach is especially powerful for emerging pollutants such as microplastics, where coupling SERS with Raman imaging allows for precise analysis of chemical composition and spatial distribution. Nevertheless, to meet the challenges posed by complex environmental samples, further enhancements in substrate stability and anti-interference performance are still needed.
Looking ahead, the evolution of SERS technology will be driven by the goals of ever-higher sensitivity, enhanced intelligence, and greater practical applicability. With the development of novel plasmonic materials and the miniaturization of detection systems, SERS is poised to make a leap from laboratory research to real-world deployment over the next decade. By deeply integrating with emerging technologies such as the Internet of Things and blockchain, SERS platforms will form a comprehensive safety-monitoring network that spans from on-site detection to traceability, providing powerful technical support for ensuring global food safety, advancing precision medicine, and protecting the environment. This progress will not only transform traditional analytical paradigms but also make a lasting contribution to human health and sustainable development.
Author Contributions
Conceptualization, M.-Y.C. and X.-J.W.; writing—review and editing, R.-S.X., J.-Y.D., X.-J.W. and M.-Y.C.; visualization, R.-S.X.; supervision, X.-J.W. and M.-Y.C.; project administration, M.-Y.C.; funding acquisition, X.-J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 62205132).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rick, S.; Matthias, P.; Dwight, R.; Bob, W. Recent trends in two-dimensional liquid chromatography. Anal. Chem. 2023, 166, 117166. [Google Scholar]
- Elliott, L.; Saumen, P.; Sazia, I.; Kyungah, S.; Ashley, G.; Susan, O.; Allegra, P.; Morgan, W.; Shae, L.; David, S. Affinity chromatography: A review of trends and developments over the past 50 years. J. Chromatogr. B 2020, 1157, 122332. [Google Scholar] [CrossRef]
- Fábio, H.; Gonzalo, G.; Thyerre, S.; Ljubica, T. Applications of fluorescence spectroscopy in protein conformational changes and intermolecular contacts. J. Fluoresc. 2023, 3, 100091. [Google Scholar]
- Despoina-Eleni, Z.; Ioannis, F.; Melina, K. Review of Fluorescence Spectroscopy in Environmental Quality Applications. Multidiscip. Digit. Publ. Inst. 2022, 27, 4801. [Google Scholar]
- Alessandro, P. Mixed and non-competitive enzyme inhibition: Underlying mechanisms andmechanistic irrelevance of the formal two-site model. J. Enzym. Inhib. Med. Chem. 2023, 38, 2245168. [Google Scholar]
- Rakhi, C.; Swarnendu, R. Angiotensin-converting enzyme inhibitors from plants: A review of their diversity, modes of action, prospects, and concerns in the management of diabetes-centric complications. J. Integr. Med. 2021, 19, 478–492. [Google Scholar]
- Yong, C.; Hai, M. Review of surface plasmon resonance and localized surface plasmon resonance sensor. Photonic Sens. 2012, 2, 37–49. [Google Scholar]
- Zhao, J.; Xue, S.; Ji, R.; Lia, B.; Li, J. Localized surface plasmon resonance for enhanced electrocatalysis. Chem. Soc. Rev. 2021, 50, 12070–12097. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.; Krishnan, K. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Lin, L.; Bi, X.; Gu, Y.; Wang, F.; Ye, J. Surface-enhanced Raman scattering nanotags for bioimaging. J. Appl. Phys. 2021, 129, 191101. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.; Mcquillan, A. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Moskovits, M. Surface roughness and the enhanced intensity of Raman scattering by molecules adsorbed on metals. J. Chem. Phys. 1978, 69, 4159–4161. [Google Scholar] [CrossRef]
- Creighton, J.; Blatchford, C.; Albrecht, M. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Chen, H.; Lin, M.; Wang, C.; Chang, Y.-M.; Gwo, S. Large-Scale Hot Spot Engineering for Quantitative SERS at the Single-Molecule Scale. J. Am. Chem. Soc. 2015, 137, 13698–13705. [Google Scholar] [CrossRef]
- Liao, P.; Wokaun, A. Lightning rod effect in surface enhanced Raman scattering. J. Chem. Phys. 1982, 76, 751–752. [Google Scholar] [CrossRef]
- Hao, F.; Sonnefraud, Y.; Van, D.; Maier, S.A.; Halas, N.J.; Nordlander, P. Symmetry Breaking in Plasmonic Nanocavities: Subradiant LSPR Sensing and a Tunable Fano Resonance. Nano Lett. 2008, 8, 3983–3988. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Koh, C.; Phan-Quang, G.C.; Han, X.; Lay, C.L.; Sim, H.Y.F.; Kao, Y.-C.; An, Q.; Ling, X.Y. Designing surface-enhanced Raman scattering (SERS) platforms beyond hotspot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756. [Google Scholar] [CrossRef]
- Wang, X. Fabrication and Application of Highly-Sensitive Surface-Enhanced Raman Scattering Sensors. Ph.D. Thesis, Hunan University, Changsha, China, 2020. (In Chinese). [Google Scholar]
- Ding, S.; Wu, D.; Yang, Z.; Ren, B.; Xu, X.; Tian, Z.Q. Some Progresses in Mechanistic Studies on Surface-Enhanced Raman Scattering. Chem. J. Chin. Univ. Chin. 2008, 29, 2569–2581. [Google Scholar]
- Wang, X.; Shi, W.; Wang, S.; Zhao, H.; Lin, J.; Yang, Z.; Chen, M.; Guo, L. Two-Dimensional Amorphous TiO2 Nanosheets Enabling High-Efficiency Photoinduced Charge Transfer for Excellent SERS Activity. J. Am. Chem. Soc. 2019, 141, 5856–5862. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Liu, B.; Wang, Z.C.; Hu, C.L.; Chen, J. Classification and Application of Surface-enhanced Raman Spectroscopy Substrates. Chin. J. Anal. Chem. 2024, 52, 910–924. (In Chinese) [Google Scholar]
- Li, M.; Qiu, Y.; Fan, C.; Cui, K.; Zhang, Y.; Xiao, Z. Design of SERS nanoprobes for Raman imaging: Materials, critical factors and architectures. Acta Pharm. Sin. B 2018, 8, 381–389. [Google Scholar] [CrossRef]
- Bastús, N.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Fernández-López, C.; Polavarapu, L.; Solís, D.; Taboada, J.M.; Obelleiro, F.; Contreras-Cáceres, R.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold Nanorod–pNIPAM Hybrids with Reversible Plasmon Coupling: Synthesis, Modeling, and SERS Properties. ACS Appl. Mater. Interfaces 2015, 7, 12530–12538. [Google Scholar] [CrossRef]
- Lee, H.; Koh, C.; Lee, Y.; Liu, C.; Phang, I.Y.; Han, X.; Tsung, C.K.; Ling, X.Y. Favoring the unfavored: Selective electrochemical nitrogen fixation using a reticular chemistry approach. Sci. Adv. 2018, 4, eaar3208. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Li, Z.; Zheng, M.; Liu, Q.; Chen, Y.; Yang, L.; Jiang, T.; Duan, H. Sensitive SERS detection at the single-particle level based on nanometer-separated mushroom-shaped plasmonic dimers. Nanotechnology 2018, 29, 105301. [Google Scholar] [CrossRef]
- Jeon, T.; Park, S.; Kim, D.; Kim, S.H. Standing-Wave-Assisted Creation of Nanopillar Arrays with Vertically Integrated Nanogaps for SERS-Active Substrates. Adv. Funct. Mater. 2015, 25, 4681–4688. [Google Scholar] [CrossRef]
- Lee, Y.; Shi, W.; Lee, H.; Jiang, R.; Phang, I.Y.; Cui, Y.; Isa, L.; Yang, Y.; Wang, J.; Li, S.; et al. Nanoscale surface chemistry directs the tunable assembly of silver octahedra into three two-dimensional plasmonic superlattices. Nat. Commun. 2015, 6, 6990. [Google Scholar] [CrossRef]
- Proniewicz, E.; Tąta, A.; Wójcik, A.; Starowicz, M.; Pacek, J.; Molenda, M. SERS activity and spectroscopic properties of Zn and ZnO nanostructures obtained by electrochemical and green chemistry methods for applications in biology and medicine. Phys. Chem. Chem. Phys. 2020, 22, 28100–28114. [Google Scholar] [CrossRef] [PubMed]
- Thu, T.; Xuan, H.; Thi, L.; Thi, T.; Dac, D.; Van, D. Enhanced Raman scattering based on a ZnO/Ag nanostructured substrate: An in-depth study of the SERS mechanism. Phys. Chem. Chem. Phys. 2023, 25, 15941–15952. [Google Scholar]
- Yang, L.; Peng, Y.; Yang, Y.; Liu, J.; Li, Z.; Ma, Y.; Zhang, Z.; Wei, Y.; Li, S.; Huang, Z.; et al. Green and Sensitive Flexible Semiconductor SERS Substrates: Hydrogenated Black TiO2 Nanowires. ACS Appl. Nano Mater. 2018, 1, 4516–4527. [Google Scholar] [CrossRef]
- Zalduendo, M.M.; Oestreicher, V.; Langer, J.; Liz-Marzán, L.M.; Angelomé, P.C. Monitoring Chemical Reactions with SERS-Active Ag-Loaded Mesoporous TiO2 Films. Anal. Chem. 2020, 92, 13656–13660. [Google Scholar] [CrossRef]
- Li, J.; Bai, H.; Zhai, J.; Li, W.; Fan, W.; Xi, G. Metallic and plasmonic MoO2 monocrystalline ultrathin mesoporous nanosheets for highly sensitive and stable surface-enhanced Raman spectroscopy. Chem. Commun. 2019, 55, 4679–4682. [Google Scholar] [CrossRef]
- Meng, X.; Yu, J.; Shi, W.; Qiu, L.; Qiu, K.; Li, A.; Liu, Z.; Wang, Y.; Wu, J.; Lin, J.; et al. SERS Detection of Trace Carcinogenic Aromatic Amines Based on Amorphous MoO3 Monolayers. Angew. Chem. 2024, 136, e202407597. [Google Scholar] [CrossRef]
- Yang, L.; Wei, Y.; Song, Y.; Peng, Y.; Yang, Y.; Huang, Z. Surface-enhanced Raman scattering from amorphous nanoflower-structural Nb2O5 fabricated by two-step hydrothermal technology. Mater. Des. 2020, 193, 108808. [Google Scholar] [CrossRef]
- Sirsendu, G.; Sanju, N.; Giri, P.K. Defect-Engineered Nb2O5 Nanoparticles for SERS Sensing through Suppressed Phonon-Assisted Recombination at Cryogenic Temperature. ACS Appl. Nano Mater. 2024, 7, 20484–20497. [Google Scholar]
- Pan, X.; Li, L.; Lin, H.; Tan, J.; Wang, H.; Liao, M.; Chen, C.; Shan, B.; Chen, Y.; Li, M. A graphene oxide-gold nanostar hybrid based-paper biosensor for label-free SERS detection of serum bilirubin for diagnosis of jaundice. Biosens. Bioelectron. 2019, 145, 111713. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cheng, Y.; Sun, M. Graphene-based SERS for sensor and catalysis. Appl. Spectrosc. Rev. 2023, 58, 1–38. [Google Scholar] [CrossRef]
- Cui, H.; Li, S.; Deng, S.; Chen, H.; Wang, C. Flexible, Transparent, and Free-Standing Silicon Nanowire SERS Platform for in Situ Food Inspection. ACS Sens. 2017, 2, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.; Liu, H.; Shieh, J.; Shieh, D.; Chen, S.; Chen, Y.; Lin, E. Using Si/MoS2 Core-Shell Nanopillar Arrays Enhances SERS Signal. Nanomaterials 2021, 11, 733. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; She, G.; Mu, L. Using Si and Ge Nanostructures as Substrates for Surface-Enhanced Raman Scattering Based on Photoinduced Charge Transfer Mechanism. J. Am. Chem. Soc. 2011, 133, 16518–16523. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhai, Y.; Ma, J.; Fang, W.; Zhang, Y. Development and application of MoS2 and its metal composite surface enhanced Raman scattering substrates. Acta Phys. Sin. 2019, 68, 133–144. (In Chinese) [Google Scholar] [CrossRef]
- Su, R.; Yang, S.; Han, D.; Hu, M.; Liu, Y.; Yang, J.; Gao, M. Ni and O co-modified MoS2 as universal SERS substrate for the detection of different kinds of substances. J. Colloid Interface Sci. 2023, 635, 1–11. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Zhao, F.; Li, Q.; Ta, H.Q.; Rümmeli, M.H.; Tully, C.G.; Li, Z.; Yin, W.J.; Yang, L.; et al. Plasmon-Free Surface-Enhanced Raman Spectroscopy Using Metallic 2D Materials. ACS Nano 2019, 13, 8312–8319. [Google Scholar] [CrossRef]
- Liang, C.; Sun, K.; Chen, M.; Xu, P. Crystal-Phase Engineering of Two-Dimensional Transition-Metal Dichalcogenides for Surface-Enhanced Raman Scattering: A Perspective. Langmuir 2023, 39, 11946–11953. [Google Scholar] [CrossRef]
- Demirel, G.; Usta, H.; Yilmaz, M.; Celik, M.; Alidagi, H.A.; Buyukserin, F. Surface-enhanced Raman spectroscopy (SERS): An adventure from plasmonic metals to organic semiconductors as SERS platforms. J. Mater. Chem. C 2018, 6, 5314–5335. [Google Scholar] [CrossRef]
- Swarna, G.; Krishnan, V.; Bo, T. Quantum scale organic semiconductors for SERS detection of DNA methylation and gene expression. Nat. Commun. 2020, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Li, L.; Guan, J.; Mu, M.; Song, W.; Sun, L.; Zhao, B.; Ozaki, Y. Hollow Multi-Shelled V2O5 Microstructures Integrating Multiple Synergistic Resonances for Enhanced Semiconductor SERS. Adv. Opt. Mater. 2021, 9, 2101866. [Google Scholar] [CrossRef]
- Wang, L.; Patskovsky, S.; Gauthier-Soumis, B.; Meunier, M. Porous Au–Ag Nanoparticles from Galvanic Replacement Applied as Single-Particle SERS Probe for Quantitative Monitoring. Small 2022, 18, 2105209. [Google Scholar] [CrossRef]
- Islam, S.; Sohel, M.; Lombard, J. Coupled Exciton and Charge-Transfer Resonances in the Raman Enhancement of Phonon Modes of CdSe Quantum Dots (QDs). J. Phys. Chem. C 2014, 118, 19415–19421. [Google Scholar] [CrossRef]
- Dmitriev, P.; Baranov, D.; Milichko, V.; Makarov, S.V.; Mukhin, I.S.; Samusev, A.K.; Krasnok, A.E.; Belov, P.A.; Kivshar, Y.S. Resonant Raman scattering from silicon nanoparticles enhanced by magnetic response. Nanoscale 2016, 8, 9721–9726. [Google Scholar] [CrossRef]
- Lin, J.; Ren, W.; Li, A.; Yao, C.; Chen, T.; Ma, X.; Wang, X.; Wu, A. Crystal–Amorphous Core–Shell Structure Synergistically Enabling TiO2 Nanoparticles’ Remarkable SERS Sensitivity for Cancer Cell Imaging. ACS Appl. Mater. Interfaces 2020, 12, 4204–4211. [Google Scholar] [CrossRef]
- Gu, C.; Li, D.; Zeng, S.; Jiang, T.; Shen, X.; Zhang, H. Synthesis and defect engineering of molybdenum oxides and their SERS applications. Nanoscale 2021, 13, 5620–5651. [Google Scholar] [CrossRef]
- Lin, J.; Shang, Y.; Li, X.; Yu, J.; Wang, X.; Guo, L. Ultrasensitive SERS Detection by Defect Engineering on Single Cu2O Superstructure Particle. Adv. Mater. 2016, 29, 1604797. [Google Scholar] [CrossRef]
- Sharma, B.; Cardinal, M.F.; Ross, M.B.; Zrimsek, A.B.; Bykov, S.V.; Punihaole, D.; Asher, S.A.; Schatz, G.C.; Van Duyne, R.P. Aluminum Film-Over-Nanosphere Substrates for Deep-UV Surface-Enhanced Resonance Raman Spectroscopy. Nano Lett. 2016, 16, 7968–7973. [Google Scholar] [CrossRef]
- Paolo, P.; Giorgia, G.; Sandro, C.; Remo, P.; Sergio, M.; Mirko, P.; Andrea, S.; Francesco, D.; Eugenio, C.; Francesco, D.; et al. Metallic Nanoporous Aluminum—Magnesium Alloy for UV-Enhanced Spectroscopy. J. Phys. Chem. C 2019, 123, 20287–20296. [Google Scholar]
- Shankar, K.; Zeeshan, A.; Mario, A.; Yasin, E.; Jörg, F. Deep-UV Surface-Enhanced Resonance Raman Scattering of Adenine on Aluminum Nanoparticle Arrays. J. Am. Chem. Soc. 2012, 134, 1966–1969. [Google Scholar]
- Tao, D.; Daniel, O.; Lars, O.; Daniel, W.; Jeremy, J. Nanoimprint Lithography of Al Nanovoids for Deep-UV SERS. ACS Appl. Mater. Interfaces 2014, 6, 17358–17363. [Google Scholar] [CrossRef]
- Shrobona, B.; Luca, M.; Nicolò, M.; Sandro, C.; Weng, S.; Ali, D.; German, L.; Anastasiia, S.; Francesco, D.; Ma, Q.; et al. Porous aluminum decorated with rhodium nanoparticles: Preparation and use as a platform for UV SERS. Mater. Adv. 2024, 5, 6248–6254. [Google Scholar] [CrossRef]
- Li, L.; Fang Lim, S.; Puretzky, A.A.; Riehn, R.; Hallen, H.D. Near-field enhanced ultraviolet resonance Raman spectroscopy using aluminum bow-tie nano-antenna. Appl. Phys. Lett. 2012, 101, 113116. [Google Scholar] [CrossRef]
- Fan, W.; Lee, Y.H.; Pedireddy, S.; Zhang, Q.; Liu, T.; Ling, X.Y. Graphene oxide and shape-controlled silver nanoparticle hybrids for ultrasensitive single-particle surface-enhanced Raman scattering (SERS) sensing. Nanoscale 2014, 6, 4843–4851. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, X.; Yin, D.; Li, X.; Yang, M.; Han, X.; Yang, L.; Zhao, B. Recyclable Au-TiO2 nanocomposite SERS-active substrates contributed by synergistic chargetransfer effect. Phys. Chem. Chem. Phys. 2017, 19, 11212–11219. [Google Scholar] [CrossRef]
- Rösler, C.; Fischer, R. Metal-organic frameworks as hosts for nanoparticles. Crystengcomm 2015, 17, 199–217. [Google Scholar] [CrossRef]
- Li, H.; Dai, H.; Zhang, Y.; Tong, W.; Gao, H.; An, Q. Surface-Enhanced Raman Spectra Promoted by a Finger Press in an All-Solid-State Flexible Energy Conversion and Storage Film. Angew. Chem. Int. Ed. Engl. 2017, 56, 2649–2654. [Google Scholar] [CrossRef]
- Xue, X.; Chen, L.; Wang, L.; Wang, C.; Qiao, Y.; Zhao, C.; Wang, H.; Nie, P.; Shi, J.; Chang, L. Facile fabrication of PS/Cu2S/Ag sandwich structure as SERS substrate for ultra-sensitive detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120370. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, P.; Lin, X.; Khan, I.M.; Ma, X.; Wang, Z. Controllable synthesis of flower-like AuNFs@ZIF-67 core-shell nanocomposites for ultrasensitive SERS detection of histamine in fish. Anal. Chim. Acta 2023, 1240, 340776. [Google Scholar] [CrossRef]
- Xu, N.; Tang, Z.; Jiang, Y.; Fang, J.; Zhang, L.; Lai, X.; Sun, Q.J.; Fan, J.M.; Tang, X.G.; Liu, Q.X.; et al. Highly sensitive detection of thiram residues on fruit peel surfaces using a filter paper-based SERS sensor with AgNWs@ZIF-8. J. Environ. Chem. Eng. 2023, 11, 109736. [Google Scholar] [CrossRef]
- Zhu, C.; Ren, C.; Jiang, W.; Liu, D.; Huang, Y.; Wang, W.; Chang, K.; Zhu, L.; Wang, Q. A versatile SERS platform based on conductive MOF-enforced carbon paper for rapidly and sensitively monitoring diazepam in aquatic products. Food Chem. 2024, 435, 137608. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Li, L.; Zhang, R.; Fang, J. Rapid and ultrasensitive solution-based SERS detection of drug additives in aquaculture by using polystyrene sulfonate modified gold nanobipyramids. Talanta 2023, 251, 123800. [Google Scholar] [CrossRef]
- Zhu, J.; Agyekum, A.; Kutsanedzie, F.; Li, H.; Chen, Q.; Ouyang, Q.; Jiang, H. Qualitative and quantitative analysis of chlorpyrifos residues in tea by surface-enhanced Raman spectroscopy (SERS) combined with chemometric models. LWT—Food Sci. Technol. 2018, 97, 760–769. [Google Scholar] [CrossRef]
- Hassan, M.; Li, H.; Ahmad, W.; Zareef, M.; Wang, J.; Xie, S.; Wang, P.; Ouyang, Q.; Wang, S.; Chen, Q. Au@Ag Nanostructure Based SERS Substrate for Simultaneous Determination of Pesticides Residue in Tea via Solid Phase Extraction Coupled Multivariate Calibration. LWT—Food Sci. Technol. 2019, 105, 290–297. [Google Scholar] [CrossRef]
- Hassan, M.; Zareef, M.; Jiao, T.; Liu, S.; Xu, Y.; Viswadevarayalu, A.; Li, H.; Chen, Q. Signal optimized rough silver nanoparticle for rapid SERS sensing of pesticide residues in tea. Food Chem. 2020, 338, 127796. [Google Scholar] [CrossRef]
- Li, H.; Hassan, M.; He, Z.; Haruna, S.A.; Chen, Q.; Ding, Z. A Sensitive Silver Nanoflower-Based SERS Sensor Coupled Novel Chemometric Models for Simultaneous Detection of Chlorpyrifos and Carbendazim in Food. LWT—Food Sci. Technol. 2022, 167, 113804. [Google Scholar] [CrossRef]
- Ma, L.; Han, E.; Yin, L.; Xu, Q.; Zou, C.; Bai, J.; Wu, W.; Cai, J. Simultaneous detection of mixed pesticide residues based on portable Raman spectrometer and Au@Ag nanoparticles SERS substrate. Food Control 2023, 153, 109951. [Google Scholar] [CrossRef]
- Li, H.; Luo, X.; Haruna, S.A.; Zareef, M.; Chen, Q.; Ding, Z.; Yan, Y. Au-Ag OHCs-Based SERS Sensor Coupled with Deep Learning CNN Algorithm to Quantify Thiram and Pymetrozine in Tea. Food Chem. 2023, 428, 136742. [Google Scholar] [CrossRef]
- Zhu, A.; Xu, Y.; Ali, S.; Ouyang, Q.; Chen, Q. Au@Ag Nanoflowers Based SERS Coupled Chemometric Algorithms for Determination of Organochlorine Pesticides in Milk. LWT—Food Sci. Technol. 2021, 150, 111000. [Google Scholar] [CrossRef]
- Adade, S.Y.-S.S.; Lin, H.; Haruna, S.A.; Johnson, N.A.N.; Barimah, A.O.; Zhu, A.; Chen, Z.; Ekumah, J.-N.; Wang, F.; Li, H.; et al. Multicomponent Prediction of Sudan Dye Adulteration in Crude Palm Oil Using SERS-Based Bimetallic Nanoflower Combined with Genetic Algorithm. J. Food Compos. Anal. 2023, 125, 105870. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.; Zou, X.; Meng, G.; Wu, N. Raman Spectroscopy for Food Quality Assurance and Safety Monitoring: A Review. Curr. Opin. Food Sci. 2022, 47, 100885. [Google Scholar] [CrossRef]
- Yang, H.; Qian, H.; Xu, Y.; Zhai, X. A Sensitive SERS Sensor Combined with Intelligent Variable Selection Models for Detecting Chlorpyrifos Residue in Tea. Foods 2024, 13, 2363. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, X.; Haruna, S.A.; Zhou, W.; Chen, Q. Rapid Detection of Thiabendazole in Food Using SERS Coupled with Flower-Like AgNPs and PSL-Based Variable Selection Algorithms. J. Food Compos. Anal. 2022, 115, 105013. [Google Scholar] [CrossRef]
- Hajikhani, M.; Kousheh, S.; Zhang, Y.; Lin, M.S. Design of a Novel SERS Substrate by Electrospinning for the Detection of Thiabendazole in Soy-Based Foods. Food Chem. 2024, 436, 137703. [Google Scholar] [CrossRef]
- Adade, S.Y.-S.S.; Lin, H.; Johnson, N.A.N.; Zhu, A.; Chen, Z.; Haruna, S.A.; Ekumah, J.-N.; Agyekum, A.A.; Li, H.; Chen, Q. Rapid Quantitative Analysis of Acetamiprid Residue in Crude Palm Oil Using SERS Coupled with Random Frog (RF) Algorithm. J. Food Compos. Anal. 2023, 125, 105818. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Hassan, M.; Zuo, M.; Wei, W.; Wu, X.; Ouyang, Q.; Chen, Q. Rapid Detection of Chloramphenicol in Food Using SERS Flexible Sensor Coupled Artificial Intelligent Tools. Food Control 2021, 128, 108186. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Shi, J.; Zhang, W.; Zhang, X.; Hang, X.; Li, Z.; Zou, X. Convenient Self-Assembled PDADMAC/PSS/Au@Ag NRs Filter Paper for Swift SERS Evaluate of Non-Systemic Pesticides on Fruit and Vegetable Surfaces. Food Chem. 2023, 424, 136232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tian, Y.; Liu, S.; Wang, Y.; Li, H.; Chen, Y.; Gao, Q.; Wang, X.; Chen, M. Multifunctional Surface Enhanced Raman Scattering Substrate Fe3O4@AgNPs@MIL-101 for Pretreatment and Rapid Detection of Pesticide Residues on the Surface of Fruit Peels. Luminescence 2025, 40, e70106. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ko, Y.; Kwon, G.; Kim, U.; Lee, J.; You, J. 2,2,6,6-Tetramethylpiperidine-1-oxy-Oxidized Cellulose Nanofiber-Based Nanocomposite Papers for Facile In Situ Surface-Enhanced Raman Scattering Detection. Acs Sustain. Chem. Eng. 2019, 7, 15640–15647. [Google Scholar]
- Zhang, M.; Liao, J.; Kong, X.; Yu, Q.; Zhang, M.; Wang, A.X. Ultra-Sensitive, Rapid and On-Site Sensing Harmful Ingredients Used in Aquaculture with Magnetic Fluid SERS. Biosensors 2022, 12, 169. [Google Scholar] [CrossRef]
- Wei, X.; Song, W.; Fan, Y.; Sun, Y.; Li, Z.; Chen, S.; Shi, J.; Zhang, D.; Zou, X.; Xu, X. A SERS aptasensor based on a flexible substrate for interference-free detection of carbendazim in apple. Food Chem. 2023, 431, 137120. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Liu, W.; Hao, R.; Jia, H.; Dai, Y.; Amin, M.; You, H.; Li, T.; Fang, J. Highly active Au NP microarray films for direct SERS detection. J. Mater. Chem. C 2019, 7, 15259–15268. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, Y.; Sun, X.; Wei, X.; Lin, Z.; Zhang, X.; Shi, J.; Battino, M.; Gong, Y.; Shi, B.; et al. SERS determination of hydroxy-α-sanshool in spicy hotpot seasoning: The strategy to restrain the interference of capsaicin and its mechanism. Food Chem. 2023, 413, 135644. [Google Scholar] [CrossRef]
- Qu, Q.; Zeng, C.; Peng, X.; Qi, W.; Wang, M. Sensitive SERS detection of pesticide residues in beverages based on an extraction integrated plasmonic platform. Sens. Actuators B Chem. 2022, 376, 133042. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Hassan, M.; Ali, S.; Chen, Q. SERS based artificial peroxidase enzyme regulated multiple signal amplified system for quantitative detection of foodborne pathogens. Food Control 2021, 123, 107733. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, Y.; Sun, J.; Yin, H.; Yin, J. SERS detection of thiram using a 3D sea cucumber-like composite flexible porous substrate. Analyst 2024, 149, 5041–5051. [Google Scholar] [CrossRef]
- Pan, H.; Ahmad, W.; Jiao, T.; Zhu, A.; Ouyang, Q.; Chen, Q. Label-free Au NRs-based SERS coupled with chemometrics for rapid quantitative detection of thiabendazole residues in citrus. Food Chem. 2022, 375, 131681. [Google Scholar] [CrossRef]
- Xiang, Z.; He, M.; Li, L.; Bobokalonov, J.; Dzhonmurodov, A.; Ji, X. A xylan assisted surface-enhanced Raman scattering substrate for rapid food safety detection. Front. Bioeng. Biotechnol. 2022, 10, 1031152. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, N.; Li, Z.; Shi, J.; Tahir, H.; Sun, Y.; Zhang, Y.; Zhang, X.; Holmes, M.; Zou, X. Rapid Detection of Carbendazim Residue in Apple Using Surface-Enhanced Raman Scattering and Coupled Chemometric Algorithm. Foods 2022, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, X.; Wang, H.; Yan, M.; Chen, Z.; Yang, Q.; Shao, Y. Research advances of SERS analysis method based on silent region molecules for food safety detection. Microchim. Acta 2023, 190, 387. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, W.; Hassan, M.; Zhang, Z.; Chen, Q. A facile and sensitive SERS-based biosensor for colormetric detection of acetamiprid in green tea based on unmodified gold nanoparticles. J. Food Meas. Charact. 2019, 13, 259–268. [Google Scholar] [CrossRef]
- Ko, J.; Lee, C.; Choo, J. Highly sensitive SERS-based immunoassay of aflatoxin B1 using silica-encapsulated hollow gold nanoparticles. J. Hazard. Mater. 2015, 285, 11–17. [Google Scholar] [CrossRef]
- Yuan, J.; Sun, C.; Guo, X.; Yang, T.; Wang, H.; Fu, S.; Li, C.; Yang, H. A rapid Raman detection of deoxynivalenol in agricultural products. Food Chem. 2017, 221, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; You, T.; El-Seedi, H.; El-Garawani, I.; Guo, Z.; Zou, X.; Cai, J. Rapid and sensitive detection of zearalenone in corn using SERS-based lateral flow immunosensor. Food Chem. 2022, 396, 133707. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, L.; Jiang, S.; El-Seedi, H.; El-Garawani, I.; Zou, X. Sensitive determination of Patulin by aptamer functionalized magnetic surface enhanced Raman spectroscopy (SERS) sensor. J. Food Compos. Anal. 2022, 115, 104985. [Google Scholar] [CrossRef]
- Yin, L.; Cai, J.; Ma, L.; You, T.; Arslan, M.; Jayan, H.; Zou, X.; Gong, Y. Dual function of magnetic nanocomposites-based SERS lateral flow strip for simultaneous detection of aflatoxin B1 and zearalenone. Food Chem. 2024, 446, 138817. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Picchetti, P.; Zheng, K.; Zhang, X.; Wu, Y.; Shen, Y.; De Cola, L.; Shi, J.; Guo, Z.; et al. Quantitative SERS sensor for mycotoxins with extraction and identification function. Food Chem. 2024, 456, 140040. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, L.; Yin, L.; Arslan, M.; El-Seedi, H.; Zou, X. Novel mesoporous silica surface loaded gold nanocomposites SERS aptasensor for sensitive detection of zearalenone. Food Chem. 2022, 403, 134384. [Google Scholar] [CrossRef]
- Xue, S.; Yin, L.; Gao, S.; Zhou, R.; Zhang, Y.; Jayan, H.; El-Seedi, H.; Zou, X.; Guo, Z. A film-like SERS aptasensor for sensitive detection of patulin based on GO@Au nanosheets. Food Chem. 2024, 441, 138364. [Google Scholar] [CrossRef]
- Wu, X.; Yin, L.; Gao, S.; Zhou, R.; Zhang, Y.; Xue, S.; Jayan, H.; El-Seedi, H.; Zou, X.; Guo, Z. Core-satellite nanoassembly system with aptamer-conjugated Au@Ag nanoparticles for SERS detection of patulin in apples. Food Control 2024, 159, 110293. [Google Scholar] [CrossRef]
- He, P.; Hassan, M.; Yang, W.; Shi, Z.; Zhou, X.; Xu, Y.; Ouyang, Q.; Chen, Q. Rapid and stable detection of three main mycotoxins in rice using SERS optimized AgNPs@K30 coupled multivariate calibration. Food Chem. 2022, 398, 133883. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Q.; Xu, X.; Jin, Y.; Wang, Y.; Zhang, L.; Yang, W.; He, L.; Feng, X.; Chen, Y. Microarray surface enhanced Raman scattering based immunosensor for multiplexing detection of mycotoxin in foodstuff. Sens. Actuators B Chem. 2018, 266, 115–123. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, X.; Rong, Y.; Wei, W.; Wu, S.; Jiao, T.; Chen, Q. Label-free detection of trace level zearalenone in corn oil by surface-enhanced Raman spectroscopy (SERS) coupled with deep learning models. Food Chem. 2023, 414, 135705. [Google Scholar] [CrossRef]
- Fu, X.; Yin, L.; Zhang, Y.; Zhou, R.; El-Seedi, H.; Zou, X.; Gong, Y.; Guo, Z. SERS aptasensor detection of aflatoxin B1 based on silicon-au-ag Janus nanocomposites. Food Chem. 2024, 467, 142325. [Google Scholar] [CrossRef]
- Yin, L.; You, T.; Arslan, M.; El-Seedi, H.; Guo, Z.; Zou, X.; Cai, J. Dual-layers Raman reporter-tagged Au@Ag combined with core-satellite assemblies for SERS detection of Zearalenone. Food Chem. 2023, 429, 136834. [Google Scholar] [CrossRef] [PubMed]
- Kutsanedzie, F.; Agyekum, A.; Annavaram, V.; Chen, Q. Signal-enhanced SERS-sensors of CAR-PLS and GA-PLS coupled AgNPs for ochratoxin A and aflatoxin B1 detection. Food Chem. 2020, 315, 126231. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Yin, L.; Picchetti, P.; Yang, T.; Zhou, R.; Zhao, C.; Xue, S.; Zhang, Z.; Zou, X.; et al. Competitive binding strategy for reliable signal-off surface enhanced Raman scattering sensing in protecting apples from patulin without external interference. J. Food Compos. Anal. 2024, 139, 107052. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Wang, M.; Zuo, M.; El-Seedi, H.; Chen, Q.; Shi, J.; Zou, X. Rapid enrichment detection of patulin and alternariol in apple using surface enhanced Raman spectroscopy with coffee-ring effect. LWT—Food Sci. Technol. 2021, 152, 122333. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Song, P.; Xu, Q.; Long, N.; Li, P.; Zhou, L.; Fu, B.; Wang, J.; Kong, W. Metal-organic framework (MOF)-based sensors for exogenous contaminants in food: Mechanisms, advances, and prospects. Trends Food Sci. Technol. 2023, 138, 238–271. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, D.; Pu, H. Porous materials nanohybridized with metal nanoparticles as substrates for enhancing SERS detection in food safety applications. Trends Food Sci. Technol. 2023, 141, 104202. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Zhang, L.; Chen, Z.; Zhang, W.; Ren, M.; Shi, J.; Xu, X.; Yang, Y. Surface-enhanced Raman scattering based on noble metal nanoassemblies for detecting harmful substances in food. Crit. Rev. Food Sci. Nutr. 2024, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Terry, L.; Sanders, S.; Potoff, R.; Kruel, J.; Jain, M.; Guo, H. Applications of surface-enhanced Raman spectroscopy in environmental detection. Anal. Sci. Adv. 2022, 3, 113–145. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z.; Si, S.; Zhang, X.; Wu, W.; Deng, H.; Wang, F.; Xiao, X.; Jiang, C. Ultrasensitive SERS Substrate Integrated with Uniform Subnanometer Scale “Hot Spots” Created by a Graphene Spacer for the Detection of Mercury Ions. Small 2016, 13, 1603347. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.; Zou, X.; Yang, H. Detection of Heavy Metals in Food and Agricultural Products by Surface-enhanced Raman Spectroscopy. Food Rev. Int. 2021, 39, 1440–1461. [Google Scholar] [CrossRef]
- Xu, G.; Song, P.; Xia, L. Examples in the detection of heavy metal ions based on surface-enhanced Raman scattering spectroscopy. Nanophotonics 2021, 10, 4419–4445. [Google Scholar] [CrossRef]
- Mukherjee, A.; Renu, K.; Gopalakrishnan, A.; Veeraraghavan, V.; Vinayagam, S.; Paz-Montelongo, S.; Dey, A.; Vellingiri, B.; George, A.; Madhyastha, H.; et al. Heavy Metal and Metalloid Contamination in Food and Emerging Technologies for Its Detection. Sustainability 2023, 15, 1195. [Google Scholar] [CrossRef]
- Pu, H.; Fang, T.; Wu, Z.; Sun, D. Advancements in recyclable photocatalytic semiconductor substrates for SERS detection in food safety applications. Trends Food Sci. Technol. 2023, 138, 697–707. [Google Scholar] [CrossRef]
- Wang, C.; Sun, S.; Wang, P.; Zhao, H.; Li, W. Nanotechnology-based analytical techniques for the detection of contaminants in aquatic products. Talanta 2024, 269, 125462. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Ahmad, W.; Zareef, M.; Rong, Y.; Xu, Y.; Jiao, T.; He, P.; Li, H.; Chen, Q. Rapid detection of mercury in food via rhodamine 6G signal using surface-enhanced Raman scattering coupled multivariate calibration. Food Chem. 2021, 358, 129844. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, M.; Lu, Y.; Wu, C.; Xu, Y.; Lin, D.; Lu, D.; Zhou, T.; Feng, S. Facile Ag-Film Based Surface Enhanced Raman Spectroscopy Using DNA Molecular Switch for Ultra-Sensitive Mercury Ions Detection. Nanomaterials 2018, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yao, L.; Yao, B.; Meng, X.; Wu, Q.; Chen, Z.; Chen, W. Low-cost signal enhanced colorimetric and SERS dual-mode paper sensor for rapid and ultrasensitive screening of mercury ions in tea. Food Chem. 2024, 463, 141375. [Google Scholar] [CrossRef]
- Park, J.; Chai, K.; Kim, W.; Yoon, T.; Park, H.; Kim, W.; You, J.; Na, S.; Park, J. Highly enhanced Hg2+2+detection using optimized DNA and a double coffee ring effect-based SERS map. Iosensors Bioelectron. 2024, 264, 116646. [Google Scholar] [CrossRef]
- Yao, L.; Chen, Y.; Wang, R.; Yan, C.; Xu, J.; Yao, B.; Cheng, J.; Chen, W. Rapid and sensitive detection of Hg2+ with a SERS-enhanced lateral flow strip. Analyst 2022, 147, 4337–4347. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Duan, J.; Hou, J.; Hou, Q.; Yang, Y.; Li, H.; Ai, S. Rapid and ultrasensitive detection of mercury ion (II) by colorimetric and SERS method based on silver nanocrystals. Microchem. J. 2020, 161, 105790. [Google Scholar] [CrossRef]
- Wan, Y.; Li, J.; Jiang, G.; Qi, J.; Pi, F. Dual-Probe SERS Chip: A Rapid and Ratiometric Detection of Trace Hg2+ in Food Samples. ACS Appl. Nano Mater. 2025, 8, 6179–6187. [Google Scholar] [CrossRef]
- Barimah, A.; Chen, P.; Yin, L.; El-Seedi, H.; Zou, X.; Guo, Z. SERS nanosensor of 3-aminobenzeneboronic acid labeled Ag for detecting total arsenic in black tea combined with chemometric algorithms. J. Food Compos. Anal. 2022, 110, 104588. [Google Scholar] [CrossRef]
- Barimah, A.; Guo, Z.; Agyekum, A.; Guo, C.; Chen, P.; El-Seedi, H.; Zou, X.; Chen, Q. Sensitive label-free Cu2O/Ag fused chemometrics SERS sensor for rapid detection of total arsenic in tea. Food Control 2021, 130, 108341. [Google Scholar] [CrossRef]
- Liang, Y.; Li, H.; Xu, N.; Zhu, J.; Wu, X.; Wang, Y. Preparation of arsenic (iii) monoclonal antibodies and preliminary evaluation of a novel silver-coated gold nanorod SERS immunoassay strip construction. Anal. Methods 2023, 15, 5823–5836. [Google Scholar] [CrossRef]
- Chen, P.; Yin, L.; El-Seedi, H.; Zou, X.; Guo, Z. Green reduction of silver nanoparticles for cadmium detection in food using surface-enhanced Raman spectroscopy coupled multivariate calibration. Food Chem. 2022, 394, 133481. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yin, L.; Zuo, M.; Chen, Q.; El-Seedi, H.; Zou, X. Determination of lead in food by surface-enhanced Raman spectroscopy with aptamer regulating gold nanoparticles reduction. Food Control 2021, 132, 108498. [Google Scholar] [CrossRef]
- Liu, M.; Zareef, M.; Zhu, A.; Wei, W.; Li, H.; Chen, Q. SERS-based Au@Ag core-shell nanoprobe aggregates for rapid and facile detection of lead ions. Food Control 2023, 155, 110078. [Google Scholar] [CrossRef]
- Song, Y.; Ma, Z.; Fang, H.; Zhang, Q.; Zhou, Q.; Chen, Z.; Yang, H.; Wang, F. Au Sputtered Paper Chromatography Tandem Raman Platform for Sensitive Detection of Heavy Metal Ions. ACS Sens. 2020, 5, 1455–1464. [Google Scholar] [CrossRef]
- Yin, L.; Jayan, H.; Cai, J.; El-Seedi, H.; Guo, Z.; Zou, X. Development of a Sensitive SERS Method for Label-Free Detection of Hexavalent Chromium in Tea Using Carbimazole Redox Reaction. Foods 2023, 12, 2673. [Google Scholar] [CrossRef]
- Liang, J.; Liu, H.; Lan, C.; Fu, Q.; Huang, C.; Luo, Z.; Jiang, T.; Tang, Y. Silver nanoparticle enhanced Raman scattering-based lateral flow immunoassays for ultra-sensitive detection of the heavy metal chromium. Nanotechnology 2014, 25, 495501. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, N.; Su, Y.; Wang, H.; He, Y. Silicon nanohybrid-based SERS chips armed with an internal standard for broad-range, sensitive and reproducible simultaneous quantification of lead (II) and mercury (II) in real systems. Nanoscale 2018, 10, 4010–4018. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, J.; Huang, Z.; Li, H.; Chen, Z.; Xiang, L. SERS scaffold based on silver nanoparticles with multi-ingredient heavy metal ligands for the determination of Mn (II). Colloid Polym. Sci. 2023, 301, 949–956. [Google Scholar] [CrossRef]
- Jin, Y.; Li, C.; Huang, Z.; Jiang, L. Simultaneous Quantitative Determination of Low-Concentration Preservatives and Heavy Metals in Tricholoma Matsutakes Based on SERS and FLU Spectral Data Fusion. Foods 2023, 12, 4267. [Google Scholar] [CrossRef]
- Li, J.; Guan, H.; Zhou, Y.; Pei, S.; Yu, K.; Zhao, Y. Detection of Heavy Metal Copper Stress in Apple Rootstocks Using Surface-Enhanced Raman Spectroscopy. J. Agric. Food Chem. 2025, 73, 10787–10798. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Zuo, L.; Zhao, Y.; Yu, K. Collaborative estimation of heavy metal stress in wheat seedlings based on LIBS-Raman spectroscopy coupled with machine learning. J. Anal. At. Spectrom. 2023, 38, 2059–2072. [Google Scholar] [CrossRef]
- Hassan, M.; He, P.; Zareef, M.; Li, H.; Chen, Q. Rapid detection and prediction of chloramphenicol in food employing label-free HAu/Ag NFs-SERS sensor coupled multivariate calibration. Food Chem. 2021, 374, 131765. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Geng, W.; Zheng, Z.; Haruna, S.; Chen, Q. Flexible SERS sensor using AuNTs-assembled PDMS film coupled chemometric algorithms for rapid detection of chloramphenicol in food. Food Chem. 2023, 418, 135998. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhu, A.; Adade, S.; Ali, S.; Chen, Q.; Wei, J.; Chen, X.; Jiao, T.; Chen, Q. Ag@Au core-shell nanoparticle-based surface-enhanced Raman scattering coupled with chemometrics for rapid determination of chloramphenicol residue in fish. Food Chem. 2023, 438, 138026. [Google Scholar] [CrossRef]
- Hassan, M.; Wei, S.; Xu, Y.; Zareef, M.; Li, H.; Sayada, J.; Chen, Q. Ascorbate functionalized Au@AgNPs SERS sensor combined random frog-partial least squares for the prediction of chloramphenicol in milk. J. Food Compos. Anal. 2024, 129, 106106. [Google Scholar] [CrossRef]
- Michalowska, A.; Krajczewski, J.; Kudelski, A. Magnetic iron oxide cores with attached gold nanostructures coated with a layer of silica: An easily, homogeneously deposited new nanomaterial for surface-enhanced Raman scattering measurements. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 277, 121266. [Google Scholar] [CrossRef]
- Li, J.; Zuo, M.; Zhang, W.; Zou, X.; Sun, Z. Diazo Coupling-Based Ultrasensitive SERS Detection of Capsaicin and Its Application in Identifying Gutter Oil. Food Anal. Methods 2022, 15, 3468–3478. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, W.; Liu, S.; Haruna, S.; Zareef, M.; Ahmad, W.; Hassan, M.; Li, H.; Chen, Q. A tailorable and recyclable TiO2 NFSF/Ti@Ag NPs SERS substrate fabricated by a facile method and its applications in prohibited fish drugs detection. J. Food Meas. Charact. 2022, 16, 2890–2898. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, A.; Ahmad, W.; Adade, S.; Chen, Q.; Wei, W.; Chen, X.; Wei, J. A three-channel biosensor based on stimuli-responsive catalytic activity of the Fe3O4@Cu for on-site detection of tetrodotoxin in fish. Food Chem. 2024, 460, 140566. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, D.; Zhao, L.; Shi, B.; Sun, Y.; Shi, J.; Battino, M.; Wang, G.; Wang, W.; Zou, X. A novel strategy based on dynamic surface-enhanced Raman scattering spectroscopy (D-SERS) for the discrimination and quantification of hydroxyl-sanshools in the pericarps of genus Zanthoxylum. Ind. Crops Prod. 2022, 183, 114940. [Google Scholar] [CrossRef]
- Wei, W.; Hassan, M.; Wu, J.; Mu, X.; Li, H.; Chen, Q. Competitive Ratiometric Aptasensing with Core-Internal Standard-Shell Structure Based on Surface-Enhanced Raman Scattering. J. Agric. Food Chem. 2023, 71, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Adade, S.; Lin, H.; Johnson, N.; Sun, Q.; Nunekpeku, X.; Ahmad, W.; Kwadzokpui, B.; Ekumah, J.; Chen, Q. Rapid qualitative and quantitative analysis of benzo (b) fluoranthene (BbF) in shrimp using SERS-based sensor coupled with chemometric models. Food Chem. 2024, 454, 139836. [Google Scholar] [CrossRef]
- Adade, S.Y.S.S.; Lin, H.; Nunekpeku, X.; Johnson, N.A.N.; Agyekum, A.A.; Zhao, S.; Teye, E.; Qianqian, S.; Kwadzokpui, B.A.; Ekumah, J.N.; et al. Flexible paper-based AuNP sensor for rapid detection of diabenz (a, h) anthracene (DbA) and benzo (b) fluoranthene (BbF) in mussels coupled with deep learning algorithms. Food Control 2024, 168, 110966. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Hu, X.; Han, F.; Zhu, C. Silver-nanoparticles/graphene hybrids for effective enrichment and sensitive SERS detection of polycyclic aromatic hydrocarbons. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117783. [Google Scholar] [CrossRef]
- Rong, Y.; Ali, S.; Ouyang, Q.; Wang, L.; Li, H.; Chen, Q. Development of a bimodal sensor based on upconversion nanoparticles and surface-enhanced Raman for the sensitive determination of dibutyl phthalate in food. J. Food Compos. Anal. 2021, 100, 103929. [Google Scholar] [CrossRef]
- Zhu, A.; Ali, S.; Jiao, T.; Wang, Z.; Xu, Y.; Ouyang, Q.; Chen, Q. Facile synthesis of fluorescence-SERS dual-probe nanocomposites for ultrasensitive detection of sulfur-containing gases in water and beer samples. Food Chem. 2023, 420, 136095. [Google Scholar] [CrossRef]
- Xu, H.; Sun, Y.; Pei, J.; Yu, X.; Zhang, J. Mesoporous Au-CuO substrate with dual super-hydrophilicity and efficient chemical enhancement for 4-aminothiophenol SERS detection. J. Alloys Compd. 2024, 1002, 175198. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Xu, S. Recent advances in surface-enhanced Raman spectroscopy (SERS) combined with machine learning algorithms in biomedical fields. J. Light Scatt. 2024, 36, 1–15. [Google Scholar]
- Li, W.; Guo, L.; Ding, X.; Ding, Y.; Ji, L.; Xia, X.; Wang, K. High-Throughput Single-Molecule Surface-Enhanced Raman Spectroscopic Profiling of Single-Amino Acid Substitutions in Peptides by a Gold Plasmonic Nanopore. ACS Nano 2024, 18, 19200–19207. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, J.; Nicolò, M.; Roman, K.; Wang, K.; Denis, G. Enhanced Optical Spectroscopy for Multiplexed DNA and Protein-Sequencing with Plasmonic Nanopores: Challenges and Prospects. Anal. Chem. 2022, 94, 503–514. [Google Scholar] [CrossRef]
- Zhou, J.; Lan, Q.; Li, W.; Ji, L.; Wang, K.; Xia, X. Single Molecule Protein Segments Sequencing by a Plasmonic Nanopore. Nano Lett. 2023, 23, 2800–2807. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Lan, Q.; Ding, X.; Pan, X.; Saud, A.; Ji, L.; Wang, K.; Xia, X. Single-Molecule Electrical and Spectroscopic Profiling Protein Allostery Using a Gold Plasmonic Nanopore. Nano Lett. 2023, 23, 2586–2592. [Google Scholar] [CrossRef]
- Lenong, S.; Lenong, Y.; Tan, E.; Sim, H.Y.F.; Koh, C.S.L.; Lee, Y.H.; Chong, C.; Ng, L.S.; Chen, J.R.T.; Pang, D.W.C.; et al. Noninvasive and Point-of-Care Surface-Enhanced Raman Scattering (SERS)-Based Breathalyzer for Mass Screening of Coronavirus Disease 2019 (COVID-19) under 5 min. ACS Nano 2022, 16, 2629–2639. [Google Scholar] [CrossRef]
- Cai, J.; Lin, Y.; Yu, X.; Yang, Y.; Hu, Y.; Gao, L.; Xiao, H.; Du, J.; Wang, H.; Zhong, X.; et al. Multifunctional AuAg-doping Prussian Blue-based MOF: Enhanced colorimetric catalytic activities and amplified SERS signals for bacteria discrimination and detection. Sens. Actuators B Chem. 2023, 394, 134279. [Google Scholar] [CrossRef]
- Duan, N.; Chang, B.; Zhang, H.; Wang, Z.; Wu, S. typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Ouyang, Q.; Zhao, J. Fabricating a Novel Raman Spectroscopy-Based Aptasensor for Rapidly Sensing Salmonella typhimurium. Food Anal. Methods 2017, 10, 3032–3041. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.; Barimah, A.; Chen, Q.; Li, H.; Shi, J.; El-Seedi, H.; Zou, X. Label-free surface enhanced Raman scattering spectroscopy for discrimination and detection of dominant apple spoilage fungus. Int. J. Food Microbiol. 2021, 338, 108990. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Hassan, M.; Zhu, J.; Wang, A.; Ouyang, Q.; Zareef, M.; Chen, Q. Amplification of Raman spectra by gold nanorods combined with chemometrics for rapid classification of four Pseudomonas. Int. J. Food Microbiol. 2019, 304, 58–67. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.; Sharma, A.; Li, H.; Chen, Q. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. Nutr. 2021, 63, 486–504. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, Y.; Wang, P.; Pi, J.; Qiu, X.; Guo, Z.; Huang, Y.; Zhao, Y.; Li, S.; Xu, J. Rapid identification of the resistance of urinary tract pathogenic bacteria using deep learning-based spectroscopic analysis. Anal. Bioanal. Chem. 2021, 413, 7401–7410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, B.; Yang, B.; Zhang, X.; Yu, G.; Wang, Z.; Qin, H.; Ma, Y. Precision Fabrication and Optimization of Nanostructures for Exosome Detection via Surface-Enhanced Raman Spectroscopy. Nanomaterials 2025, 15, 266. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, B.; Li, Z.; Shan, Y.; Jian, M.; Meng, X.; Wang, Z. Accurate diagnosis of thyroid cancer using a combination of surface-enhanced Raman spectroscopy of exosome on MXene-coated gold@silver core@shell nanoparticle substrate and deep learning. Chem. Eng. J. 2024, 488, 150835. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Zhao, J.; Weng, G.; Li, J.; Zhao, J. Growth of Spherical Gold Satellites on the Surface of Au@Ag@SiO2 Core Shell Nanostructures Used for an Ultrasensitive SERS Immunoassay of Alpha-Fetoprotein. Acs Appl. Mater. Interfaces 2019, 11, 3617–3626. [Google Scholar] [CrossRef]
- Lu, T.; Wang, L.; Xia, Y.; Jin, Y.; Zhang, L.; Du, S. A multimer-based SERS aptasensor for highly sensitive and homogeneous assay of carcinoembryonic antigens. Analyst 2021, 146, 3016–3024. [Google Scholar] [CrossRef]
- Tong, L.; Fu, H.; Peng, L.; Li, Q.; Shi, Q.; Zhou, J. A Highly Sensitive Label-Free Quantitative Detection Method for Tumor Marker Based on Au NRs/PMMA Substrate. Spectrosc. Spectr. Anal. 2019, 39, 784–790. [Google Scholar]
- Das, A.; Kim, K.; Park, S.; Choi, N.; Choo, J. SERS-based serodiagnosis of acute febrile diseases using plasmonic nanopopcorn microarray platforms. Biosens. Bioelectron. 2021, 192, 113525. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dang, H.; Park, S.; Chen, L.; Choo, J. SERS-PCR assays of SARS-CoV-2 target genes using Au nanoparticles-internalized Au nanodimple substrates. Biosens. Bioelectron. 2022, 197, 113736. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Liao, J.; Tsai, H.; Wang, H.; Sitjar, J. Focused ion beam-fabricated nanorod substrate for label-free surface-enhanced Raman spectroscopy and enabling dual virus detection. Talanta 2024, 278, 126466. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, J.; Xiao, R.; Wang, S. SERS molecular sentinel for the RNA genetic marker of PB1-F2 protein in highly pathogenic avian influenza (HPAI) virus. Biosens. Bioelectron. 2014, 61, 460–465. [Google Scholar] [CrossRef]
- Eremina, O.; Yarenkov, N.; Bikbaeva, G.; Kapitanova, O.; Samodelova, M.; Shekhovtsova, T.; Kolesnikov, I.; Syuy, A.; Arsenin, A.; Volkov, V.; et al. Silver nanoparticle-based SERS sensors for sensitive detection of amyloid-β aggregates in biological fluids. Talanta 2023, 266, 124970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Ali, S.; Jiao, T.; Wang, Z.; Ouyang, Q.; Chen, Q. Advances in surface-enhanced Raman spectroscopy technology for detection of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1466–1494. [Google Scholar] [CrossRef]
- Bodelón, G.; Montes-García, V.; Pérez-Juste, J.; Pastoriza-Santos, I. Surface-Enhanced Raman Scattering Spectroscopy for Label-Free Analysis of P. aeruginosa Quorum Sensing. Front. Cell. Infect. Microbiol. 2018, 8, 143. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, Y.; Zhou, L.; Petti, L.; Wang, Z.; Zhou, J.; Xie, S.; Chen, J.; Qing, Y. Ultrasensitive SERS-Based Immunoassay of Tumor Marker in Serum Using Au-Ag Alloy Nanoparticals and Ag/AgBr Hybrid Nanostructure. Nano 2018, 13, 3–14. [Google Scholar] [CrossRef]
- Wang, H.; Vo-Dinh, T. Multiplex detection of breast cancer biomarkers using plasmonic molecular sentinel nanoprobes. Nanotechnology 2009, 20, 065101. [Google Scholar] [CrossRef]
- Lu, X.; Yao, C.; Sun, L.; Li, Z. Plasmon-enhanced biosensors for microRNA analysis and cancer diagnosis. Biosens. Bioelectron. 2022, 203, 114041. [Google Scholar] [CrossRef] [PubMed]
- Abalde-Cela, S.; Aldeanueva-Potel, P.; Mateo-Mateo, C.; Rodríguez-Lorenzo, L.; Alvarez-Puebla, R.; Liz-Marzán, L. Surface-enhanced Raman scattering biomedical applications of plasmonic colloidal particles. J. R. Soc. Interface 2010, 7, S435–S450. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Jurado-Sánchez, B.; Escarpa, A. Nanomaterials meet surface-enhanced Raman scattering towards enhanced clinical diagnosis: A review. J. Nanobiotechnolog 2022, 20, s12951. [Google Scholar] [CrossRef]
- Chen, Z.; Du, J.; Shi, J. Advances in Surface Enhanced Raman Spectroscopy for Detection of Enviromentental Pollutants. Chemistry 2024, 87, 1045–1054. (In Chinese) [Google Scholar]
- Zhao, Y.; Yamaguchi, Y.; Ni, Y.; Li, M.; Dou, X. A SERS-based capillary sensor for the detection of mercury ions in environmental water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 233, 118193. [Google Scholar] [CrossRef]
- Han, K.; Yan, Z.; Ding, Z.; Zhou, P.; Ye, C.; Qin, L.; Bao, Z.; Zhang, M.; Zhang, W. High-sensitivity SERS sensor leveraging three-dimensional Ti3C2Tx/TiO2/W18O49 semiconductor heterostructures for reliable detection of trace micro/nanoplastics in environmental matrices. Talanta 2025, 286, 127474. [Google Scholar] [CrossRef]
- Vafakish, B.; Wilson, L. A Highly Sensitive Chitosan-Based SERS Sensor for the Trace Detection of a Model Cationic Dye. Int. J. Mol. Sci. 2024, 25, 9327. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, X.; Wang, X.; Yang, J.; Shi, X.; Qu, L.; Gu, Y. Au@Fe2O3 nanoflowers as highly sensitive SERS substrates to detect organic pollutants in water. Mater. Sci. Eng. B Adv. Funct. Solid-State Mater. 2025, 320, 118396. [Google Scholar] [CrossRef]
- Tran, H.; Nguyen, N.; Ly, N.; Joo, S.; Vasseghian, Y. Core-shell Au@ZIF-67-based pollutant monitoring of thiram and carbendazim pesticides. Environ. Pollut. 2023, 317, 120775. [Google Scholar] [CrossRef]
- Wang, A.; Liu, G.; Zhao, Y.; Tan, X.; Rogachev, A.; Ding, Q.; Jiang, X. Highly sensitive SERS platform with analyte enrichment for multiplex organic pollutants detection in river water. Appl. Surf. Sci. 2025, 697, 163016. [Google Scholar] [CrossRef]
- Tira, D.; Potara, M.; Astilean, S. Fabrication of stable network-like gold nanostructures in solution and their assessment as efficient NIR-SERS platforms for organic pollutants detection. Mater. Res. Bull. 2014, 64, 267–273. [Google Scholar] [CrossRef]
- Vassalini, I.; Ribaudo, G.; Gianoncelli, A.; Casula, M.; Alessandri, I. Plasmonic hydrogels for capture, detection and removal of organic pollutants. Environ. Sci. Nano 2020, 7, 3888–3900. [Google Scholar] [CrossRef]
- Li, J.; Peng, W.; Wang, A.; Wan, M.; Zhou, Y.; Zhang, X.; Jin, S.; Zhang, F. Highly sensitive and selective SERS substrates with 3D hot spot buildings for rapid mercury ion detection. Analyst 2023, 148, 4044–4052. [Google Scholar] [CrossRef]
- Song, D.; Yang, R.; Long, F.; Zhu, A. Applications of magnetic nanoparticles in surface-enhanced Raman scattering (SERS) detection of environmental pollutants. J. Environ. Sci. 2019, 80, 14–34. [Google Scholar] [CrossRef]
- Fateixa, S.; Landauer, M.; Schneider, J.; Kumar, S.; Böhm, R. Additive Manufacturing-Enabled Architected Nanocomposite Lattices Coated with Plasmonic Nanoparticles for Water Pollutants Detection. Macromol. Mater. Eng. 2023, 308, 2300060. [Google Scholar] [CrossRef]
- Zhao, K.; Lin, J.; Guo, L. ZnO/Ag porous nanosheets used as substrate for surface-enhanced Raman scattering to detect organic pollutant. RSC Adv. 2015, 5, 53524–53528. [Google Scholar] [CrossRef]
- Xu, Y.; Su, J.; Jia, Z.; Wang, Y. Convenient Au@Ag Double-Layer Nanoarray Fabricated by Rapid Thermal Annealing and Chemical Replacement Method for Surface-Enhanced Raman Spectroscopy Sensing. Adv. Eng. Mater. 2024, 26, 2401177. [Google Scholar] [CrossRef]
- Jayaprakash, V.; You, J.; Kanike, C.; Liu, J.; McCallum, C.; Zhang, X. Determination of Trace Organic Contaminant Concentration via Machine Classification of Surface-Enhanced Raman Spectra. Environ. Sci. Technol. 2024, 58, 15619–15628. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, H.; Park, J. Carboxylate surfactants capped Ag-nanospheres as SERS substrate for detecting water-soluble pollutants. Chem. Phys. Lett. 2023, 832, 140863. [Google Scholar] [CrossRef]
- Lu, Y.; Li, C.; Wang, Y.; Liu, C.; Wang, Z.; Liu, J.; Fan, H.; Feng, Z.; Sun, T. A recyclable SERS-DGT device for in-situ sensing of sulfamethazine by Au@g-C3N4NS in water. Water Res. 2024, 253, 121307. [Google Scholar] [CrossRef]
- La, C.; Piazza, S.; Freni, G. Pollutant Monitoring Solutions in Water and Sewerage Networks: A Scoping Review. Water 2025, 17, 1423. [Google Scholar] [CrossRef]
- Gu, Y.; Fang, P.; Chen, Y.; Xie, T.; Yang, G.; Qu, L. Multi-channel surface-enhanced Raman spectroscopy (SERS) platform for pollutant detection in water fabricated on polydimethylsiloxane. Microchim. Acta 2024, 191, 595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, Z.; Jin, C.; Zhang, J. Machine learning assisted dual-functional nanophotonic sensor for organic pollutant detection and degradation in water. npj Clean Water 2024, 7, 3. [Google Scholar] [CrossRef]
- Raj, D.; Tayyaba, N.; De, V.; Scaglione, F.; Rizzi, P. Ultrasensitive Detection of Malachite Green Isothiocyanate Using Nanoporous Gold as SERS Substrate. Materials 2023, 16, 4620. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Yang, S.; Jiao, Z.; Zhang, M.; Yang, Y.; Gao, Y. Advances in environmental pollutant detection techniques: Enhancing public health monitoring and risk assessment. Environmental 2025, 197, 109365. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Song, H. Synergistic effects of photonic crystal and gold nanostars for quantitative SERS detection of 3-Phenoxybenzoic acid. Appl. Surf. Sci. 2019, 476, 587–593. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).