Impact of Mild Acid and Alkali Treatments on Cotton Fibers with Nonlinear Optical Imaging and SEM Analysis
Abstract
1. Introduction
2. Methods and Materials
2.1. Experimental Instruments and Tools
2.2. Sample Preparation
3. Results and Discussions
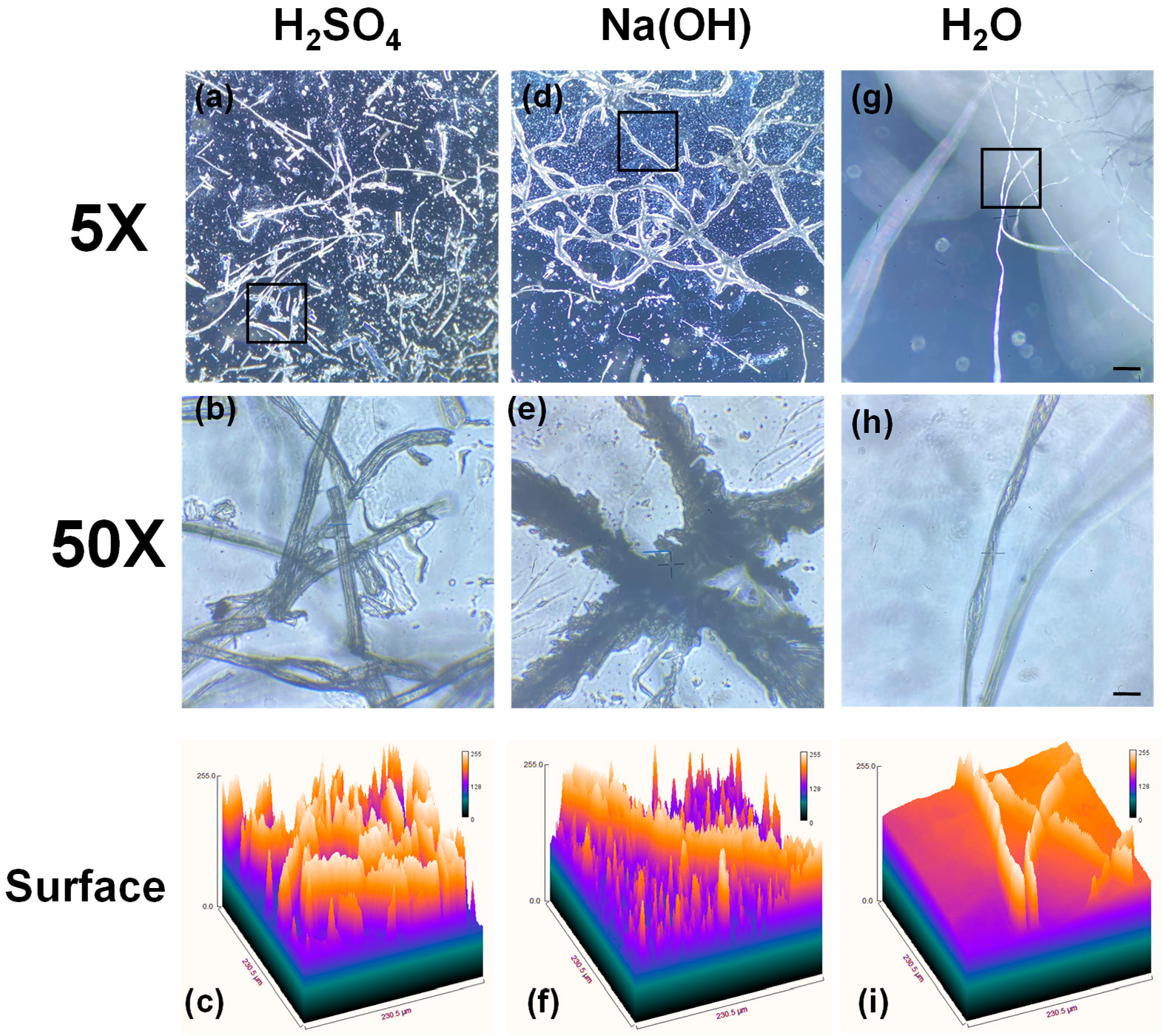
3.1. The Morphological Analysis
3.2. Nonlinear Optical Microscopic Imaging
3.3. The Analysis with SEM Images
3.4. Summary and Comparison of Other Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polko, J.K.; Kieber, J.J. The Regulation of Cellulose Biosynthesis in Plants. Plant Cell 2019, 31, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2015, 23, 93–123. [Google Scholar] [CrossRef]
- Costa, S.M.; Mazzola, P.G.; Silva, J.C.A.R.; Pahl, R.; Pessoa, A.; Costa, S.A. Use of sugar cane straw as a source of cellulose for textile fiber production. Ind. Crops Prod. 2013, 42, 189–194. [Google Scholar] [CrossRef]
- Robertson, G.P.; Hamilton, S.K.; Barham, B.L.; Dale, B.E.; Izaurralde, R.C.; Jackson, R.D.; Landis, D.A.; Swinton, S.M.; Thelen, K.D.; Tiedje, J.M. Cellulosic biofuel contributions to a sustainable energy future: Choices and outcomes. Science 2017, 356, eaal2324. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, G.; Arya, S.K. Biofuel from rice straw. J. Clean. Prod. 2020, 277, 124101. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and Properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Ed.; Advances in Polymer Science; Springer: Berlin, Germany, 2016; Volume 271, pp. 1–52. [Google Scholar]
- Passoth, V.; Sandgren, M. Biofuel production from straw hydrolysates: Current achievements and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5105–5116. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Binod, P.; Pandey, A. Chapter 1—Introduction. In Pretreatment of Biomass; Pandey, A., Negi, S., Binod, P., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–6. [Google Scholar]
- Ioelovich, M. Study of Cellulose Interaction with Concentrated Solutions of Sulfuric Acid. ISRN Chem. Eng. 2012, 2012, 428974. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Deng, Y. Effect of enzymatic treatment on cotton fiber dissolution in NaOH/urea solution at cold temperature. Carbohydr. Polym. 2008, 72, 178–184. [Google Scholar] [CrossRef]
- Ranjithkumar, M.; Ravikumar, R.; Sankar, M.K.; Kumar, M.N.; Thanabal, V. An Effective Conversion of Cotton Waste Biomass to Ethanol: A Critical Review on Pretreatment Processes. Waste Biomass Valoriz. 2016, 8, 57–68. [Google Scholar] [CrossRef]
- Naik, S.; Goud, V.V.; Rout, P.K.; Jacobson, K.; Dalai, A.K. Characterization of Canadian biomass for alternative renewable biofuel. Renew. Energy 2010, 35, 1624–1631. [Google Scholar] [CrossRef]
- Baccile, N.; Falco, C.; Titirici, M.-M. Characterization of biomass and its derived char using13C-solid state nuclear magnetic resonance. Green Chem. 2014, 16, 4839–4869. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Thygesen, L.G.; Felby, C.; Jorgensen, H.; Elder, T. Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 2008, 1, 5. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues: Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Li, X.; Sha, J.; Xia, Y.; Sheng, K.; Liu, Y.; He, Y. Quantitative visualization of subcellular lignocellulose revealing the mechanism of alkali pretreatment to promote methane production of rice straw. Biotechnol. Biofuels 2020, 13, 8. [Google Scholar] [CrossRef]
- Cheng, J.X.; Volkmer, A.; Xie, X.S. Theoretical and experimental characterization of coherent anti-Stokes Raman scattering microscopy. J. Opt. Soc. Am. B-Opt. Phys. 2002, 19, 1363–1375. [Google Scholar] [CrossRef]
- Vilms Pedersen, S.; Brewer, J.R.; Hedegaard, M.A.B.; Arnspang Christensen, E. Spectral Unmixing for Label-Free, In-Liquid Characterization of Biomass Microstructure and Biopolymer Content by Coherent Raman Imaging. Anal. Chem. 2023, 95, 2168–2175. [Google Scholar] [CrossRef]
- Zeng, Y.; Saar, B.G.; Friedrich, M.G.; Chen, F.; Liu, Y.-S.; Dixon, R.A.; Himmel, M.E.; Xie, X.S.; Ding, S.-Y. Imaging Lignin-Downregulated Alfalfa Using Coherent Anti-Stokes Raman Scattering Microscopy. BioEnergy Res. 2010, 3, 272–277. [Google Scholar] [CrossRef]
- Gannaway, J.N.; Sheppard, C.J.R. Second-harmonic imaging in the scanning optical microscope. Opt. Quantum Electron. 1978, 10, 435–439. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Xu, X.; Zhou, Y.; Chen, M.; Sun, M. Optical characterizations of two-dimensional materials using nonlinear optical microscopies of CARS, TPEF, and SHG. Nanophotonics 2018, 7, 873–881. [Google Scholar] [CrossRef]
- Richely, E.; Zarei, A.; Melelli, A.; Rajan, D.K.; Govilas, J.; Gabrion, X.; Clévy, C.; Legland, D.; Perez, J.; Guessasma, S.; et al. Measurement of microfibril angle in plant fibres: Comparison between X-ray diffraction, second harmonic generation and transmission ellipsometry microscopies. Compos. Part C Open Access 2023, 11, 100355. [Google Scholar] [CrossRef]
- Janko, M.; Jocher, M.; Boehm, A.; Babel, L.; Bump, S.; Biesalski, M.; Meckel, T.; Stark, R.W. Cross-Linking Cellulosic Fibers with Photoreactive Polymers: Visualization with Confocal Raman and Fluorescence Microscopy. Biomacromolecules 2015, 16, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Legat, T.; Grachev, V.; Kabus, D.; Lettinga, M.P.; Clays, K.; Verbiest, T.; de Coene, Y.; Thielemans, W.; Van Cleuvenbergen, S. Imaging with a twist: Three-dimensional insights of the chiral nematic phase of cellulose nanocrystals via SHG microscopy. Sci. Adv. 2024, 10, eadp2384. [Google Scholar] [CrossRef]
- Krishnamachari, P.; Hashaikeh, R.; Tiner, M. Modified cellulose morphologies and its composites; SEM and TEM analysis. Micron 2011, 42, 751–761. [Google Scholar] [CrossRef]
- Richely, E.; Nuez, L.; Pérez, J.; Rivard, C.; Baley, C.; Bourmaud, A.; Guessasma, S.; Beaugrand, J. Influence of defects on the tensile behaviour of flax fibres: Cellulose microfibrils evolution by synchrotron X-ray diffraction and finite element modelling. Compos. Part C Open Access 2022, 9, 100300. [Google Scholar] [CrossRef]
- Ji, Z.; Ma, J.-F.; Zhang, Z.-H.; Xu, F.; Sun, R.-C. Distribution of lignin and cellulose in compression wood tracheids of Pinus yunnanensis determined by fluorescence microscopy and confocal Raman microscopy. Ind. Crops Prod. 2013, 47, 212–217. [Google Scholar] [CrossRef]
- Moran-Mirabal, J.M. The study of cell wall structure and cellulose–cellulase interactions through fluorescence microscopy. Cellulose 2013, 20, 2291–2309. [Google Scholar] [CrossRef]
- Agarwal, U.P. Analysis of Cellulose and Lignocellulose Materials by Raman Spectroscopy: A Review of the Current Status. Molecules 2019, 24, 1659. [Google Scholar] [CrossRef] [PubMed]




| Methods | Advantage | Limit |
|---|---|---|
| OM [27] | Simple imaging without complex operations | Macroscopic imaging |
| SHG [28] | Rapid imaging of non-centrosymmetric materials | Requires image processing |
| CARS [25] | Rapid imaging of molecular vibrations | Non-resonant background interference, high light source requirements |
| SEM [29] | Microscopic structural imaging | Sample preparation required |
| X-ray diffraction [30] | Rapid large-area imaging | Requires a synchrotron source to achieve single-fiber scale, a strict geometric arrangement of fibers |
| Confocal fluorescence microscopy [31,32] | Rapid high-resolution imaging | Requires fluorescence dye labeling for sample preparation |
| Raman spectroscopy microscopy [33] | Provides rich spectral information | Only reflects local information |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Li, X.; Li, R.; Wang, C.; Chui, H.-C.; Zhang, Q. Impact of Mild Acid and Alkali Treatments on Cotton Fibers with Nonlinear Optical Imaging and SEM Analysis. Photonics 2025, 12, 688. https://doi.org/10.3390/photonics12070688
Gao H, Li X, Li R, Wang C, Chui H-C, Zhang Q. Impact of Mild Acid and Alkali Treatments on Cotton Fibers with Nonlinear Optical Imaging and SEM Analysis. Photonics. 2025; 12(7):688. https://doi.org/10.3390/photonics12070688
Chicago/Turabian StyleGao, Huipeng, Xiaoxiao Li, Rui Li, Chao Wang, Hsiang-Chen Chui, and Quan Zhang. 2025. "Impact of Mild Acid and Alkali Treatments on Cotton Fibers with Nonlinear Optical Imaging and SEM Analysis" Photonics 12, no. 7: 688. https://doi.org/10.3390/photonics12070688
APA StyleGao, H., Li, X., Li, R., Wang, C., Chui, H.-C., & Zhang, Q. (2025). Impact of Mild Acid and Alkali Treatments on Cotton Fibers with Nonlinear Optical Imaging and SEM Analysis. Photonics, 12(7), 688. https://doi.org/10.3390/photonics12070688





