Review of Artifacts and Related Processing in Ophthalmic Optical Coherence Tomography Angiography (OCTA)
Abstract
1. Introduction
2. OCTA Data Acquisition Process and Artifact Generation
3. Types and Processing of Artifacts
3.1. Light Propagation and Signal Intensity-Related Artifacts
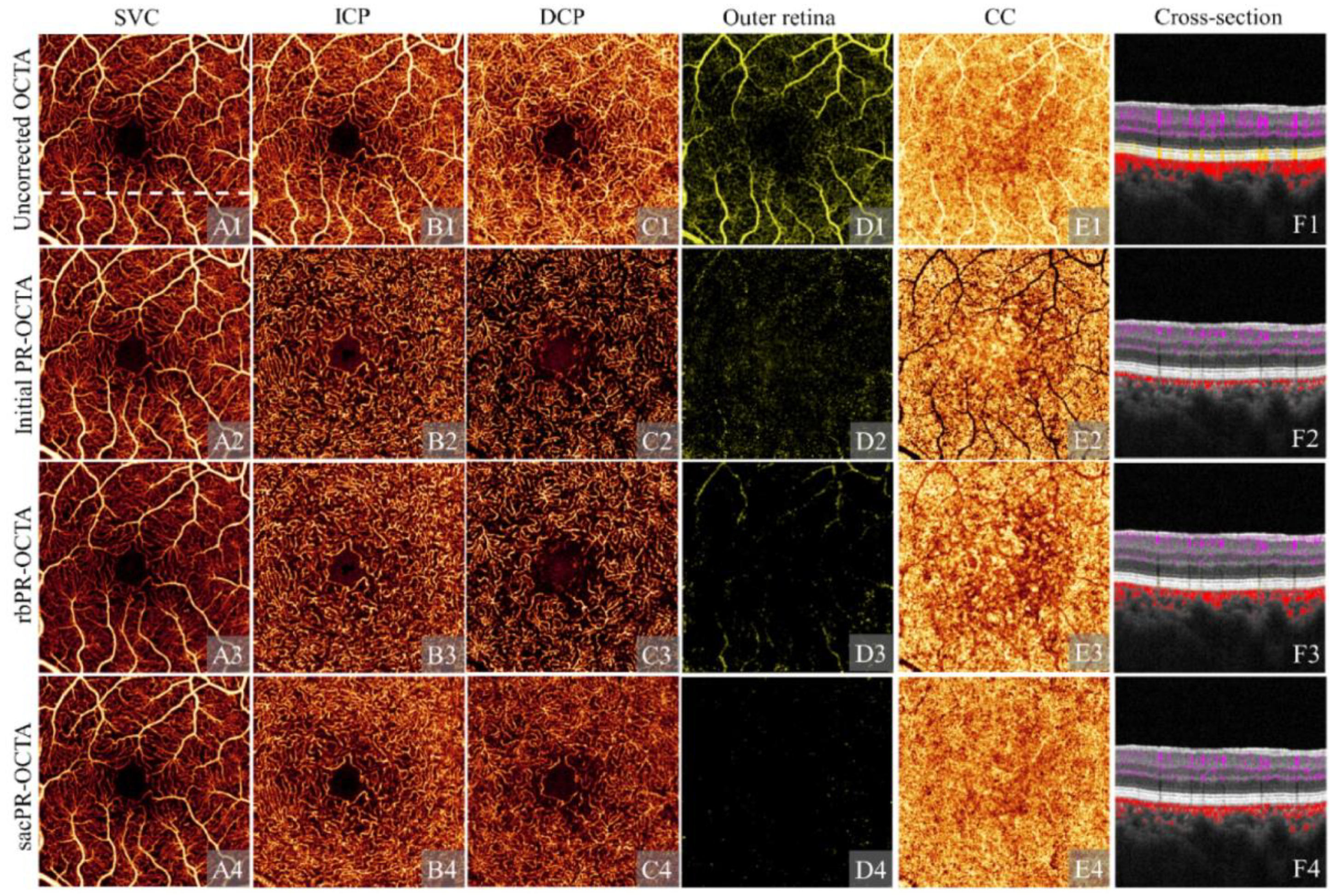
3.1.1. Projection Artifact
3.1.2. Weak Signal Artifact
3.1.3. Unmasking Artifact
3.2. Motion Artifacts
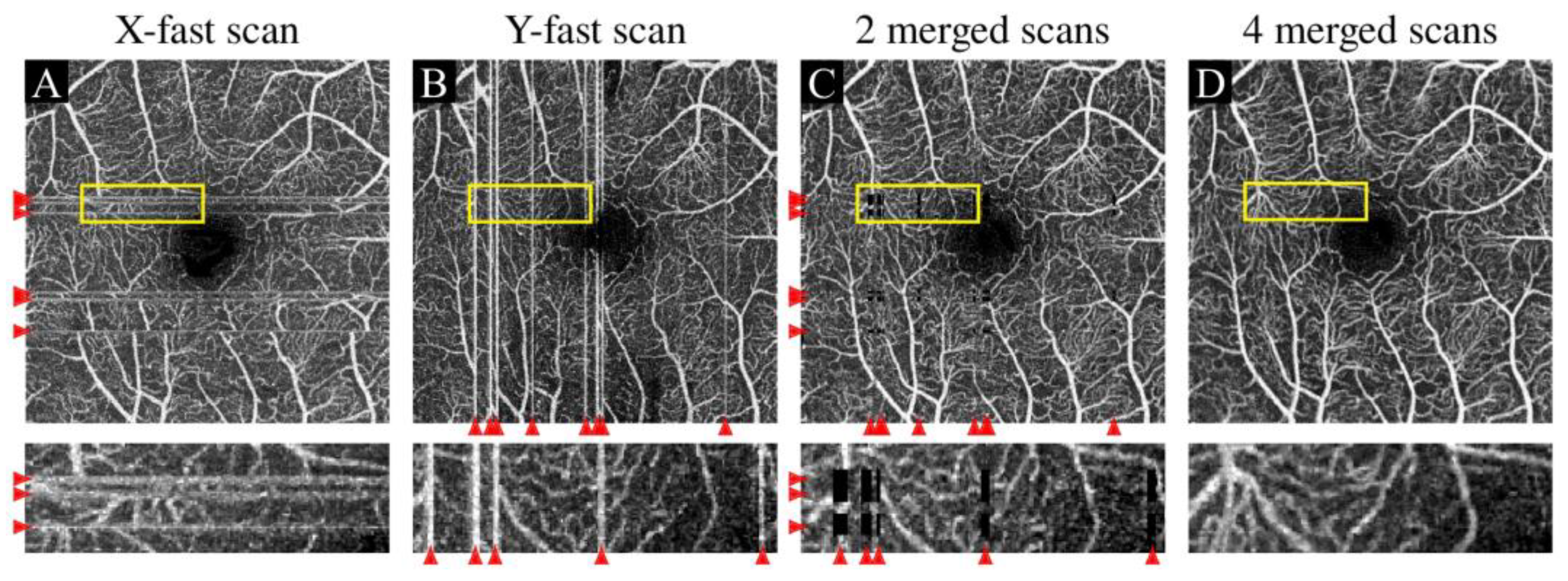
3.2.1. Eye Movement Artifact
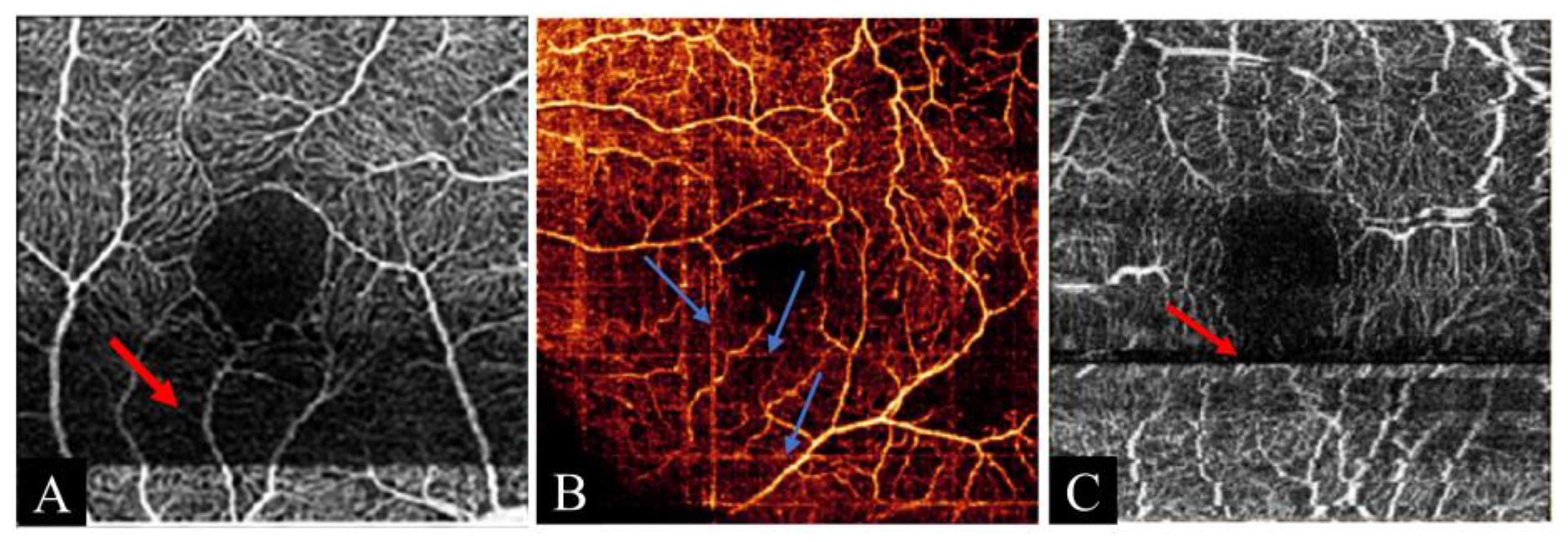
3.2.2. Banding Artifact
3.2.3. Blinking Artifact
3.2.4. Fringe Washout Artifact
3.3. Improper Operation Artifacts
3.3.1. Defocus Artifact
3.3.2. Mirror Artifact
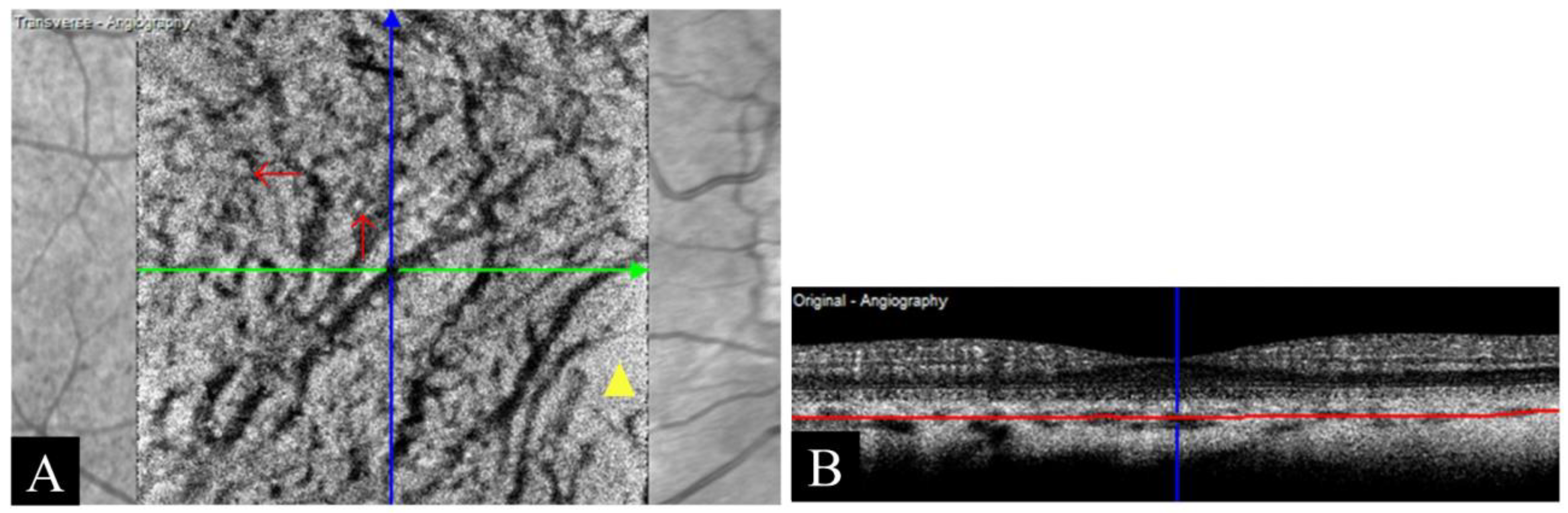
3.3.3. Decentration Artifact
3.3.4. Z-Offset Artifact
3.4. Signal Processing-Related Artifacts
3.4.1. Segmentation Error Artifact
3.4.2. Doubling Artifact
3.4.3. Stretching Artifact
4. Artificial Intelligence for OCTA Artifact Processing
5. Discussion
6. Future Research Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R. Optical coherence tomography today: Speed, contrast, and multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef]
- Cogliati, A.; Canavesi, C.; Hayes, A.; Tankam, P.; Duma, V.-F.; Santhanam, A.; Thompson, K.P.; Rolland, J.P. MEMS-based handheld scanning probe with pre-shaped input signals for distortion-free images in Gabor-domain optical coherence microscopy. Opt. Express 2016, 24, 13365–13374. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Romano, V.; Steger, B.; Vinciguerra, R.; Lawman, S.; Williams, B.; Hicks, N.; Czanner, G.; Zheng, Y.; Willoughby, C.E.; et al. Imaging of corneal neovascularization: Optical coherence tomography angiography and fluorescence angiography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1263–1269. [Google Scholar] [CrossRef]
- Chalam, K.V.; Sambhav, K. Optical coherence tomography angiography in retinal diseases. J. Ophthal Vis. Res. 2016, 11, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Bailey, S.T.; Hwang, T.S.; McClintic, S.M.; Gao, S.S.; Pennesi, M.E.; Flaxel, C.J.; Lauer, A.K.; Wilson, D.J.; Hornegger, J.; et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc. Natl. Acad. Sci. USA 2015, 112, E2395–E2402. [Google Scholar] [CrossRef]
- Chen, C.L.; Wang, R.K. Optical coherence tomography based angiography. Biomed. Opt. Express 2017, 8, 1056–1082. [Google Scholar] [CrossRef]
- Fingler, J.; Schwartz, D.; Yang, C.; Fraser, S.E. Mobility and transverse flow visualization using phase variance contrast with spectral domain optical coherence tomography. Opt. Express 2007, 15, 12636–12653. [Google Scholar] [CrossRef]
- Kim, D.Y.; Fingler, J.; Werner, J.S.; Schwartz, D.M.; Fraser, S.E.; Zawadzki, R.J. In vivo volumetric imaging of human retinal circulation with phase-variance optical coherence tomography. Biomed. Opt. Express 2011, 2, 1504–1513. [Google Scholar] [CrossRef]
- Mariampillai, A.; Standish, B.A.; Moriyama, E.H.; Khurana, M.; Munce, N.R.; Leung, M.K.; Jiang, J.; Cable, A.; Wilson, B.C.; Vitkin, I.A.; et al. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt. Lett. 2008, 33, 1530–1532. [Google Scholar] [CrossRef]
- Blatter, C.; Klein, T.; Grajciar, B.; Schmoll, T.; Wieser, W.; Andre, R.; Huber, R.; Leitgeb, R.A. Ultrahigh-speed non-invasive widefield angiography. J. Biomed. Opt. 2012, 17, 070505. [Google Scholar] [CrossRef]
- Wang, R.K.; Jacques, S.L.; Ma, Z.; Hurst, S.; Hanson, S.R.; Gruber, A. Three dimensional optical angiography. Opt. Express 2007, 15, 4083–4097. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; An, L.; Saunders, S.; Wilson, D.J. Optical microangiography provides depth-resolved images of directional ocular blood perfusion in posterior eye segment. J. Biomed. Opt. 2010, 15, 020502. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K. Optical microangiography: A label-free 3-D imaging technology to visualize and quantify blood circulations within tissue beds in vivo. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 545–554. [Google Scholar] [CrossRef]
- Stanga, P.E.; Lim, J.I.; Hamilton, P. Indocyanine green angiography in choriorefinal diseases: Indications and interpretation-An evidence-based update. Ophthalmology 2003, 110, 15–21. [Google Scholar] [CrossRef]
- Hope-Ross, M.; Yannuzzi, L.A.; Gragoudas, E.S.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Krupsky, S.; Orlock, D.A.; Puliafito, C.A. Adverse reactions due to indocyanine green. Ophthalmology 1994, 101, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Saez, M.P.; Ordoqui, E.; Tornero, P.; Baeza, A.; Sainza, T.; Zubeldia, J.M.; Baeza, M.L. Fluorescein-induced allergic reaction. Ann. Allergy Asthma Immunol. 1998, 81, 428–430. [Google Scholar] [CrossRef]
- Hagag, A.M.; Gao, S.S.; Jia, Y.; Huang, D. Optical coherence tomography angiography: Technical principles and clinical applications in ophthalmology. Taiwan. J. Ophthalmol. 2017, 7, 115–129. [Google Scholar] [CrossRef]
- Guo, Y.; Hormel, T.T.; Xiong, H.; Wang, B.; Camino, A.; Wang, J.; Huang, D.; Hwang, T.S.; Jia, Y. Development and validation of a deep learning algorithm for distinguishing the nonperfusion area from signal reduction artifacts on OCT angiography. Biomed. Opt. Express 2019, 10, 3257–3268. [Google Scholar] [CrossRef]
- Parodi, M.B.; Cicinelli, M.V.; Rabiolo, A.; Pierro, L.; Gagliardi, M.; Bolognesi, G.; Bandello, F. Vessel density analysis in patients with retinitis pigmentosa by means of optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 428–432. [Google Scholar] [CrossRef]
- Chidambara, L.; Gadde, S.G.K.; Yadav, N.K.; Jayadev, C.; Bhanushali, D.; Appaji, A.M.; Akkali, M.; Khurana, A.; Shetty, R. Characteristics and quantification of vascular changes in macular telangiectasia type 2 on optical coherence tomography angiography. Br. J. Ophthalmol. 2016, 100, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Zafar, S.; Gonzalez, N.; Murphy, O.; Kwakyi, M.S.O.; Feldman, B.S.S.; Calabresi, P.A.; Saidha, S.; Channa, R. Image artifacts in optical coherence tomography angiography among patients with multiple sclerosis. Curr. Eye Res. 2019, 44, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Rashad, R.; Sorour, O.; Moult, E.M.; Fujimoto, J.G.; Waheed, N.K. Optical coherence tomography angiography (OCTA) flow speed mapping technology for retinal diseases. Expert. Rev. Med. Devices 2018, 15, 875–882. [Google Scholar] [CrossRef]
- Hormel, T.T.; Huang, D.; Jia, Y. Artifacts and artifact removal in optical coherence tomographic angiography. Quant. Imaging Med. Surg. 2021, 11, 1120–1133. [Google Scholar] [CrossRef]
- Barton, J.K.; Stromski, S. Flow measurement without phase information in optical coherence tomography images. Opt. Express 2005, 13, 5234–5239. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef]
- Bazvand, F.; Ghassemi, F. Artifacts in macular optical coherence tomography. J. Curr. Ophthalmol. 2020, 32, 123–131. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Q.; Wang, R.K. Efficient method to suppress artifacts caused by tissue hyper-reflections in optical microangiography of retina in vivo. Biomed. Opt. Express 2015, 6, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, S.S.; Bailey, S.T.; Huang, D.; Li, D.; Jia, Y. Automated choroidal neovascularization detection algorithm for optical coherence tomography angiography. Biomed. Opt. Express 2015, 6, 3564–3576. [Google Scholar] [CrossRef]
- Liu, Y.; Carass, A.; Filippatou, A.; He, Y.; Solomon, S.D.; Saidha, S.; Calabresi, P.A.; Prince, J.L. Projection artifact suppression for inner retina in OCT angiography. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 592–596. [Google Scholar]
- Wang, J.; Hormel, T.T.; Bailey, S.T.; Hwang, T.S.; Huang, D.; Jia, Y. Signal attenuation-compensated projection-resolved OCT angiography. Biomed. Opt. Express 2023, 14, 2040–2054. [Google Scholar] [CrossRef]
- Wang, R.K. Signal degradation by multiple scattering in optical coherence tomography of dense tissue: A Monte Carlo study towards optical clearing of biotissues. Phys. Med. Biol. 2002, 47, 2281. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, K.V.; Jia, Y.; Wang, J.; Patel, R.C.; Lauer, A.K.; Huang, D.; Bailey, S.T. Projection-resolved optical coherence tomography angiography exhibiting early flow prior to clinically observed retinal angiomatous proliferation. Am. J. Ophthalmol. Case Rep. 2017, 8, 53–57. [Google Scholar] [CrossRef]
- Leahy, C.; Radhakrishnan, H.; Bernucci, M.; Srinivasan, V.J. Imaging and graphing of cortical vasculature using dynamically focused optical coherence microscopy angiography. J. Biomed. Opt. 2016, 21, 020502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Q.; Wang, R.K. Minimizing projection artifacts for accurate presentation of choroidal neovascularization in OCT micro-angiography. Biomed. Opt. Express 2015, 6, 4130–4143. [Google Scholar] [CrossRef]
- Zhang, M.; Hwang, T.S.; Campbell, J.P.; Bailey, S.T.; Wilson, D.J.; Huang, D.; Jia, Y. Projection-resolved optical coherence tomographic angiography. Biomed. Opt. Express 2016, 7, 816–828. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Huang, D.; Wilson, D.J.; Jia, Y. Reflectance-based projection-resolved optical coherence tomography angiography. Biomed. Opt. Express 2017, 8, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Liu, T.; Wang, R.K. Segmentation and quantification of blood vessels for OCT-based micro-angiograms using hybrid shape/intensity compounding. Microvasc. Res. 2015, 97, 37–46. [Google Scholar] [CrossRef]
- Li, P.; Huang, Z.; Yang, S.; Liu, X.; Ren, Q.; Li, P. Adaptive classifier allows enhanced flow contrast in OCT angiography using a histogram-based motion threshold and 3D Hessian analysis-based shape filtering. Opt. Lett. 2017, 42, 4816–4819. [Google Scholar] [CrossRef]
- Tang, J.; Erdener, S.E.; Sunil, S.; Boas, D.A. Normalized field autocorrelation function-based optical coherence tomography three-dimensional angiography. J. Biomed. Opt. 2019, 24, 036005. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Tang, J. Adaptive dynamic analysis-based optical coherence tomography angiography for blood vessel projection artifact suppression. Biomed. Opt. Express 2023, 14, 477–488. [Google Scholar] [CrossRef]
- Stefan, S.; Lee, J. Deep learning toolbox for automated enhancement, segmentation, and graphing of cortical optical coherence tomography microangiograms. Biomed. Opt. Express 2020, 11, 7325–7342. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.E.; Enders, C.; Loidl, M.; Lang, G.K.; Werner, J.U. Accurate OCT-angiography interpretation-detection and exclusion of artifacts. Klin. Monatsblatter Augenheilkd. 2017, 234, 1109–1118. [Google Scholar] [CrossRef]
- Camino, A.; Jia, Y.; Yu, J.; Wang, J.; Liu, L.; Huang, D. Automated detection of shadow artifacts in optical coherence tomography angiography. Biomed. Opt. Express 2019, 10, 1514–1531. [Google Scholar] [CrossRef] [PubMed]
- Hormel, T.T.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Huang, D.; Jia, Y. Artificial intelligence in OCT angiography. Prog. Retin. Eye Res. 2021, 85, 100965. [Google Scholar] [CrossRef]
- Wei, X.; Hormel, T.T.; Guo, Y.; Hwang, T.S.; Jia, Y. High-resolution wide-field OCT angiography with a self-navigation method to correct microsaccades and blinks. Biomed. Opt. Express 2020, 11, 3234–3245. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Reich, M.; Boehringer, D.; Rothaus, K.; Cakir, B.; Bucher, F.; Daniel, M.; Lang, S.J.; Lagreze, W.A.; Agostini, H.; Lange, C. Swept-source optical coherence tomography angiography alleviates shadowing artifacts caused by subretinal fluid. Int. Ophthalmol. 2020, 40, 2007–2016. [Google Scholar] [CrossRef]
- Hormel, T.T.; Jia, Y.; Jian, Y.; Hwang, T.S.; Bailey, S.T.; Pennesi, M.E.; Wilson, D.J.; Morrison, J.C.; Huang, D. Plexus-specific retinal vascular anatomy and pathologies as seen by projection-resolved optical coherence tomographic angiography. Prog. Retin. Eye Res. 2021, 80, 100878. [Google Scholar] [CrossRef]
- Wei, X.; Hormel, T.T.; Guo, Y.; Jia, Y. 75-degree non-mydriatic single-volume optical coherence tomographic angiography. Biomed. Opt. Express 2019, 10, 6286–6295. [Google Scholar] [CrossRef]
- De Pretto, L.R.; Moult, E.M.; Alibhai, A.Y.; Carrasco-Zevallos, O.M.; Chen, S.; Lee, B.; Witkin, A.J.; Baumal, C.R.; Reichel, E.; de Freitas, A.Z.; et al. Controlling for artifacts in widefield optical coherence tomography angiography measurements of non-perfusion area. Sci. Rep. 2019, 9, 9096. [Google Scholar] [CrossRef]
- Yu, J.J.; Camino, A.; Liu, L.; Zhang, X.; Wang, J.; Gao, S.S.; Jia, Y.; Huang, D. Signal strength reduction effects in OCT angiography. Ophthalmol. Retin. 2019, 3, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Jia, Y.; Liu, L.; Zhang, M.; Takusagawa, H.L.; Morrison, J.C.; Huang, D. Compensation for reflectance variation in vessel density quantification by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4485–4492. [Google Scholar] [CrossRef] [PubMed]
- Chlebiej, M.; Gorczynska, I.; Rutkowski, A.; Kluczewski, J.; Grzona, T.; Pijewska, E.; Sikorski, B.L.; Szkulmowska, A.; Szkulmowski, M. Quality improvement of OCT angiograms with elliptical directional filtering. Biomed. Opt. Express 2019, 10, 1013–1031. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhu, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Wu, D.M.; Vavvas, D.G.; Husain, D.; Miller, J.W.; et al. Imaging artifacts and segmentation errors with wide-field swept-source optical coherence tomography angiography in diabetic retinopathy. Transl. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Falavarjani, K.G.; Al-Sheikh, M.; Akil, H.; Sadda, S.R. Image artefacts in swept-source optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 564–568. [Google Scholar] [CrossRef]
- Wang, Y.; Bo, Q.; Jia, H.; Sun, M.; Yu, Y.; Huang, P.; Wang, J.; Xu, N.; Wang, F.; Wang, H.; et al. Small dome-shaped pigment epithelium detachment in polypoidal choroidal vasculopathy: An under-recognized sign of polypoidal lesions on optical coherence tomography? Eye 2021, 36, 733–741. [Google Scholar] [CrossRef]
- Kraus, M.F.; Potsaid, B.; Mayer, M.A.; Bock, R.; Baumann, B.; Liu, J.J.; Hornegger, J.; Fujimoto, J.G. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed. Opt. Express 2012, 3, 1182–1199. [Google Scholar] [CrossRef]
- Ploner, S.B.; Kraus, M.F.; Moult, E.M.; Husvogt, L.; Schottenhamml, J.; Alibhai, A.Y.; Waheed, N.K.; Duker, J.S.; Fujimoto, J.G.; Maier, A.K. Efficient and high accuracy 3-D OCT angiography motion correction in pathology. Biomed. Opt. Express 2021, 12, 125–146. [Google Scholar] [CrossRef]
- Zang, P.; Liu, G.; Zhang, M.; Dongye, C.; Wang, J.; Pechauer, A.D.; Hwang, T.S.; Wilson, D.J.; Huang, D.; Li, D. Automated motion correction using parallel-strip registration for wide-field en face OCT angiogram. Biomed. Opt. Express 2016, 7, 2823–2836. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in optical coherence tomography angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef]
- Vienola, K.V.; Braaf, B.; Sheehy, C.K.; Yang, Q.; Tiruveedhula, P.; Arathorn, D.W.; de Boer, J.F.; Roorda, A. Real-time eye motion compensation for OCT imaging with tracking SLO. Biomed. Opt. Express 2012, 3, 2950–2963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, Y.; Zhang, T.; Kubach, S.; An, L.; Laron, M.; Sharma, U.; Wang, R.K. Wide-field imaging of retinal vasculature using optical coherence tomography-based microangiography provided by motion tracking. J. Biomed. Opt. 2015, 20, 066008. [Google Scholar] [CrossRef] [PubMed]
- Camino, A.; Zhang, M.; Gao, S.S.; Hwang, T.S.; Sharma, U.; Wilson, D.J.; Huang, D.; Jia, Y. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed. Opt. Express 2016, 7, 3905–3915. [Google Scholar] [CrossRef]
- Camino, A.; Zhang, M.; Dongye, C.; Pechauer, A.D.; Hwang, T.S.; Bailey, S.T.; Lujan, B.; Wilson, D.J.; Huang, D.; Jia, Y. Automated registration and enhanced processing of clinical optical coherence tomography angiography. Quant. Imaging Med. Surg. 2016, 6, 391–401. [Google Scholar] [CrossRef]
- Zang, P.; Liu, G.; Zhang, M.; Wang, J.; Hwang, T.S.; Wilson, D.J.; Huang, D.; Li, D.; Jia, Y. Automated three-dimensional registration and volume rebuilding for wide-field angiographic and structural optical coherence tomography. J. Biomed. Opt. 2017, 22, 026001. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.F.; Liu, J.J.; Schottenhamml, J.; Chen, C.-L.; Budai, A.; Branchini, L.; Ko, T.; Ishikawa, H.; Wollstein, G.; Schuman, J.; et al. Quantitative 3D-OCT motion correction with tilt and illumination correction, robust similarity measure and regularization. Biomed. Opt. Express 2014, 5, 2591–2613. [Google Scholar] [CrossRef]
- Camino, A.; Jia, Y.; Liu, G.; Wang, J.; Huang, D. Regression-based algorithm for bulk motion subtraction in optical coherence tomography angiography. Biomed. Opt. Express 2017, 8, 3053–3066. [Google Scholar] [CrossRef]
- Hossbach, J.; Husvogt, L.; Kraus, M.F.; Fujimoto, J.G.; Maier, A.K. Deep OCT angiography image generation for motion artifact suppression. In Bildverarbeitung für die Medizin 2020; Springer: Wiesbaden, Germany, 2020; pp. 248–253. [Google Scholar]
- Lauermann, J.L.; Treder, M.; Heiduschka, P.; Clemens, C.R.; Eter, N.; Alten, F. Impact of eye-tracking technology on OCT-angiography imaging quality in age-related macular degeneration. Graef Arch. Clin. Exp. 2017, 255, 1535–1542. [Google Scholar] [CrossRef]
- Cheng, W.; Song, Y.; Lin, F.; Xiong, J.; Li, F.; Jin, L.; Wang, Z.; Yang, C.; Yang, B.; Wang, F.; et al. Assessment of artifacts in swept-source optical coherence tomography angiography for glaucomatous and bormal eyes. Transl. Vis. Sci. Technol. 2022, 11, 23. [Google Scholar] [CrossRef]
- Enders, C.; Lang, G.E.; Dreyhaupt, J.; Loidl, M.; Lang, G.K.; Werner, J.U. Quantity and quality of image artifacts in optical coherence tomography angiography. PLoS ONE 2019, 14, e0210505. [Google Scholar] [CrossRef]
- Murphy, O.C.; Kwakyi, O.; Iftikhar, M.; Zafar, S.; Lambe, J.; Pellegrini, N.; Sotirchos, E.S.; Gonzalez-Caldito, N.; Ogbuokiri, E.; Filippatou, A.; et al. Alterations in the retinal vasculature occur in multiple sclerosis and exhibit novel correlations with disability and visual function measures. Ophthalmol. Retin. 2020, 26, 815–828. [Google Scholar] [CrossRef]
- Abu-Yaghi, N.E.; Obiedat, A.F.; AlNawaiseh, T.I.; Hamad, A.M.; Ata, B.A.B.; Quzli, A.A.; AlRyalat, S.A. Optical coherence tomography angiography in healthy adult subjects: Bormative values, frequency, and impact of artifacts. Biomed. Res. Int. 2022, 2022, 7286252. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.K.; Viljoen, R.D.; Bukowska, D.M. Classification of image artefacts in optical coherence tomography angiography of the choroid in macular diseases. Clin. Exp. Ophthalmol. 2016, 44, 388–399. [Google Scholar] [CrossRef]
- Walther, J.; Mueller, G.; Morawietz, H.; Koch, E. Signal power decrease due to fringe washout as an extension of the limited Doppler flow measurement range in spectral domain optical coherence tomography. J. Biomed. Opt. 2010, 15, 041511. [Google Scholar] [CrossRef]
- Brown, T.G.; Cogswell, C.J.; Wilson, T.; Meemon, P.; Palawong, K.; Pongchalee, P. Simplified methods of design, implementation, and characterization of a spectrometer-based FD-OCT. In Proceedings of the Three-Dimensional and Multidimensional Microscopy: Image Acquisition and Processing XXI, San Francisco, CA, USA, 3–6 February 2014; pp. 232–237. [Google Scholar]
- Yun, S.; Tearney, G.; De Boer, J.; Bouma, B. Motion artifacts in optical coherence tomography with frequency-domain ranging. Opt. Express 2004, 12, 2977–2998. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Wang, H. Dark signals in the choroidal vasculature on optical coherence tomography angiography: An artefact or not? J. Ophthalmol. 2017, 2017, 5498125. [Google Scholar] [CrossRef] [PubMed]
- Kamalipour, A.; Moghimi, S.; Hou, H.; Penteado, R.C.; Hyuk, W.; Proudfoot, J.A.; El-Nimri, N.; Ekici, E.; Rezapour, J.; Zangwill, L.M.; et al. OCT angiography artifacts in glaucoma. Ophthalmology 2021, 128, 1426–1437. [Google Scholar] [CrossRef]
- Chhablani, J.; Krishnan, T.; Sethi, V.; Kozak, I. Artifacts in optical coherence tomography. Saudi J. Ophthalmol. 2014, 28, 81–87. [Google Scholar] [CrossRef]
- Czako, C.; Istvan, L.; Benyo, F.; Elo, A.; Erdei, G.; Horvath, H.; Nagy, Z.Z.; Kovacs, I. The impact of deterministic signal loss on OCT angiography measurements. Transl. Vis. Sci. Technol. 2020, 9, 10. [Google Scholar] [CrossRef]
- Raevis, J.; Etheridge, T.; Cleland, S.; Mititelu, M. Autoimmune retinopathy: Findings and limitations from optical coherence tomography angiography. Int. J. Retin. Vitr. 2020, 6, 64. [Google Scholar] [CrossRef]
- Holmen, I.C.; Konda, S.M.; Pak, J.W.; McDaniel, K.W.; Blodi, B.; Stepien, K.E.; Domalpally, A. Prevalence and severity of artifacts in optical coherence tomographic angiograms. JAMA Ophthalmol. 2020, 138, 119–126. [Google Scholar] [CrossRef]
- Kim, M.; Zhou, X.; Wuppukondur, S.N.; Jiang, X.; Wang, R.K.; Kashani, A.H. Characterizing and quantifying image artifacts in optical coherence tomography angiograms of normal retinal vasculature. Investig. Ophthalmol. Vis. Sci. 2022, 63, 2939–F0092. [Google Scholar]
- Pierro, L.; Aragona, E.; Arrigo, A.; Gagliardi, M.; Bandello, F. The mirror artifact effect on OCTA reconstructions of patients with high myopia. Spektrum Augenheilkd. 2017, 31, 257–261. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Khadamy, J.; Safi, H.; Karimi, N.; Amirkourjani, F. Effect of grid decentration on macular thickness measurements in normal subjects and patients with diabetic macular edema. Eur. J. Ophthalmol. 2015, 25, 218–221. [Google Scholar] [CrossRef]
- Li, M.C.; Chen, Y.R.; Ji, Z.X.; Xie, K.R.; Yuan, S.T.; Chen, Q.; Li, S. Image projection network: 3D to 2D image segmentation in OCTA images. IEEE Trans. Med. Imaging 2020, 39, 3343–3354. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Sull, A.C.; Vuong, L.N.; Chen, Y.; Liu, J.; Fujimoto, J.G.; Schuman, J.S.; Duker, J.S. Assessment of artifacts and reproducibility across spectral- and time-domain optical coherence tomography devices. Ophthalmology 2009, 116, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Lentzsch, A.; Schoellhorn, L.; Schnorr, C.; Siggel, R.; Liakopoulos, S. Comparison of swept-source versus spectral-domain optical coherence tomography angiography for detection of macular neovascularization. Graef Arch. Clin. Exp. 2022, 260, 113–119. [Google Scholar] [CrossRef]
- Han, I.C.; Jaffe, G.J. Evaluation of artifacts associated with macular spectral-domain optical coherence tomography. Ophthalmology 2010, 117, 1177–1189.e1174. [Google Scholar] [CrossRef]
- Spaide, R.F.; Curcio, C.A. Evaluation of segmentation of the superficial and deep vascular layers of the retina by optical coherence tomography angiography instruments in normal eyes. JAMA Ophthalmol. 2017, 135, 259–262. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, M.K.; Kim, D.W. Segmentation errors in macular ganglion cell analysis as determined by optical coherence tomography. Ophthalmology 2016, 123, 950–958. [Google Scholar] [CrossRef]
- Domalpally, A.; Danis, R.P.; Zhang, B.; Myers, D.; Kruse, C.N. Quality issues in interpretation of optical coherence tomograms in macular diseases. Retina 2009, 29, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Fabritius, T.; Makita, S.; Miura, M.; Myllyla, R.; Yasuno, Y. Automated segmentation of the macula by optical coherence tomography. Opt. Express 2009, 17, 15659–15669. [Google Scholar] [CrossRef]
- Ahlers, C.; Simader, C.; Geitzenauer, W.; Stock, G.; Stetson, P.; Dastmalchi, S.; Schmidt-Erfurth, U. Automatic segmentation in three-dimensional analysis of fibrovascular pigmentepithelial detachment using high-definition optical coherence tomography. Br. J. Ophthalmol. 2008, 92, 197–203. [Google Scholar] [CrossRef]
- Pekala, M.; Joshi, N.; Liu, T.Y.A.; Bressler, N.M.; DeBuc, D.C.; Burlina, P. Deep learning based retinal OCT segmentation. Comput. Biol. Med. 2019, 114, 103445. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, S.; He, Z.; Guan, H.; Chen, R.; Xu, Y.; Wang, T.; Qi, S.; Mei, J.; Wang, W. DeepRetina: Layer Segmentation of Retina in OCT Images Using Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 61. [Google Scholar] [CrossRef]
- Shah, A.; Zhou, L.; Abramoff, M.D.; Wu, X. Multiple surface segmentation using convolution neural nets: Application to retinal layer segmentation in OCT images. Biomed. Opt. Express 2018, 9, 4509–4526. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.J.; Li, X.T.; Nicholas, P.; Toth, C.A.; Izatt, J.A.; Farsiu, S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt. Express 2010, 18, 19413–19428. [Google Scholar] [CrossRef]
- Zang, P.; Gao, S.S.; Hwang, T.S.; Flaxel, C.J.; Wilson, D.J.; Morrison, J.C.; Huang, D.; Li, D.; Jia, Y. Automated boundary detection of the optic disc and layer segmentation of the peripapillary retina in volumetric structural and angiographic optical coherence tomography. Biomed. Opt. Express 2017, 8, 1306–1318. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Pechauer, A.D.; Hwang, T.S.; Gao, S.S.; Liu, L.; Liu, L.; Bailey, S.T.; Wilson, D.J.; Huang, D.; et al. Advanced image processing for optical coherence tomographic angiography of macular diseases. Biomed. Opt. Express 2015, 6, 4661–4675. [Google Scholar] [CrossRef]
- Kugelman, J.; Alonso-Caneiro, D.; Read, S.A.; Vincent, S.J.; Collins, M.J. Automatic segmentation of OCT retinal boundaries using recurrent neural networks and graph search. Biomed. Opt. Express 2018, 9, 5759–5777. [Google Scholar] [CrossRef]
- Guo, Y.; Camino, A.; Zhang, M.; Wang, J.; Huang, D.; Hwang, T.; Jia, Y. Automated segmentation of retinal layer boundaries and capillary plexuses in wide-field optical coherence tomographic angiography. Biomed. Opt. Express 2018, 9, 4429–4442. [Google Scholar] [CrossRef] [PubMed]
- Zang, P.; Wang, J.; Hormel, T.T.; Liu, L.; Huang, D.; Jia, Y. Automated segmentation of peripapillary retinal boundaries in OCT combining a convolutional neural network and a multi-weights graph search. Biomed. Opt. Express 2019, 10, 4340–4352. [Google Scholar] [CrossRef]
- Sui, X.; Zheng, Y.; Wei, B.; Bi, H.; Wu, J.; Pan, X.; Yin, Y.; Zhang, S. Choroid segmentation from optical coherence tomography with graph edge weights learned from deep convolutional neural networks. Neurocomputing 2017, 237, 332–341. [Google Scholar] [CrossRef]
- de Sisternes, L.; Jonna, G.; Moss, J.; Marmor, M.F.; Leng, T.; Rubin, D.L. Automated intraretinal segmentation of SD-OCT images in normal and age-related macular degeneration eyes. Biomed. Opt. Express 2017, 8, 1926–1949. [Google Scholar] [CrossRef]
- Treder, M.; Lauermann, J.L.; Eter, N. Automated detection of exudative age-related macular degeneration in spectral domain optical coherence tomography using deep learning. Graef Arch. Clin. Exp. 2018, 256, 259–265. [Google Scholar] [CrossRef]
- Bogunovic, H.; Waldstein, S.M.; Schlegl, T.; Langs, G.; Sadeghipour, A.; Liu, X.; Gerendas, B.S.; Osborne, A.; Schmidt-Erfurth, U. Prediction of anti-VEGF treatment requirements in neovascular AMD using a machine learning approach. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, D.; Berlin, M.; Reidl, F.; Kuenzel, S.E.; Pleyer, U.; Joussen, A.M.; Winterhalter, S. Longitudinal comparison of constant artifacts in optical coherence tomography angiography in patients with posterior uveitis compared to healthy subjects. J. Clin. Med. 2022, 11, 5376. [Google Scholar] [CrossRef] [PubMed]
- Eastline, M.; Munk, M.R.; Wolf, S.; Schaal, K.B.; Ebneter, A.; Tian, M.; Giannakaki-Zimmermann, H.; Zinkernagel, M.S. Repeatability of wide-field optical coherence tomography angiography in normal retina. Transl. Vis. Sci. Technol. 2019, 8, 6. [Google Scholar] [CrossRef]
- Ho, J.; Dans, K.; You, Q.; Nudleman, E.N.; Freeman, W.R. Comparison of 3 × 3 mm versus 6 × 6 mm optical coherence tomography angiography scan sizes in the evaluation of non-proliferative diabetic retinopathy. Retina 2019, 39, 259–264. [Google Scholar] [CrossRef]
- Bontzos, G.; Kabanarou, S.A.; Garnavou-Xirou, C.; Gkizis, I.; Kontou, E.; Triantafyllou, D.; Xirou, T. Segmentation errors and motion artifacts in OCTA associated with epiretinal membranes. Can. J. Ophthalmol. 2020, 55, 293–300. [Google Scholar] [CrossRef]
- Tan, A.C.; Tan, G.S.; Denniston, A.K.; Keane, P.A.; Ang, M.; Milea, D.; Chakravarthy, U.; Cheung, C.M.G. An overview of the clinical applications of optical coherence tomography angiography. Eye 2018, 32, 262–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ran, A.R.; Nguyen, T.X.X.; Lin, T.P.H.; Chen, H.; Lai, T.Y.Y.; Tham, C.C.C.; Cheung, C.Y.Y. Deep learning in optical coherence tomography angiography: Current progress, challenges, and future directions. Diagnostics 2023, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Choi, J.Y.; Kim, H.K. Feasibility study to improve deep learning in OCT diagnosis of rare retinal diseases with few-shot classification. Med. Biol. Eng. Comput. 2021, 59, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Tang, Z.; Ran, A.; Nguyen, T.X.; Szeto, S.; Chan, J.; Wong, C.Y.; Hui, V.; Tsang, K.; Chan, C.K. Assessment of parafoveal diabetic macular ischemia on optical coherence tomography angiography images to predict diabetic retinal disease progression and visual acuity deterioration. JAMA Ophthalmol. 2023, 141, 641–649. [Google Scholar] [CrossRef]
- Jia, H.; Lu, B.; Zhao, Z.; Yu, Y.; Wang, F.; Zhou, M.; Sun, X. Prediction of the short-term efficacy of anti-VEGF therapy for neovascular age-related macular degeneration using optical coherence tomography angiography. Eye Vis. 2022, 9, 16. [Google Scholar] [CrossRef]
- Alryalat, S.A.; Al-Antary, M.; Arafa, Y.; Azad, B.; Boldyreff, C.; Ghnaimat, T.; Al-Antary, N.; Alfegi, S.; Elfalah, M.; Abu-Ameerh, M. Deep learning prediction of response to anti-VEGF among diabetic macular edema patients: Treatment response analyzer system (TRAS). Diagnostics 2022, 12, 312. [Google Scholar] [CrossRef]
- Kashani, A.H.; Liu, T.A.; Jones, C. Optical Coherence Tomography Angiography, Artificial Intelligence, and the Missing Capillaries. JAMA Ophthalmol. 2023, 141, 649–650. [Google Scholar] [CrossRef]
- Mei, S.; Mao, Z.; Wang, Z.; Chan, K. Deep-learning-based projection artifact removal in optical coherence tomography angiography volumes. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4577. [Google Scholar]
- Li, A.; Du, C.; Pan, Y. Deep-learning-based motion correction in optical coherence tomography angiography. J. Biophotonics 2021, 14, e202100097. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Q.; Lan, G.; Xu, J.; Qin, J.; An, L.; Huang, Y. Deep learning for motion artifact-suppressed OCTA image generation from both repeated and adjacent OCT scans. Mathematics 2024, 12, 446. [Google Scholar] [CrossRef]
- Wang, J.; Hormel, T.T.; Bailey, S.T.; Hwang, T.S.; Jia, Y. Artificial intelligence-assisted projection-resolved optical coherence tomographic angiography (aiPR-OCTA). Opt. Express 2025, 33, 16658–16670. [Google Scholar] [CrossRef]
- Xie, H.; Xu, W.; Xing, Y.X.; Wu, X. Deep learning network with differentiable dynamic programming for retina OCT surface segmentation. Biomed. Opt. Express 2023, 14, 3190–3202. [Google Scholar] [CrossRef]
- Dhodapkar, R.M.; Li, E.; Nwanyanwu, K.; Adelman, R.; Krishnaswamy, S.; Wang, J.C. Deep learning for quality assessment of optical coherence tomography angiography images. Sci. Rep. 2022, 12, 13775. [Google Scholar] [CrossRef] [PubMed]
- Lauermann, J.L.; Treder, M.; Alnawaiseh, M.; Clemens, C.R.; Eter, N.; Alten, F. Automated OCT angiography image quality assessment using a deep learning algorithm. Graef Arch. Clin. Exp. 2019, 257, 1641–1648. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Habibi, A.; Anvari, P.; Ghasemizadeh, S.; Khorasani, M.A.; Shenazandi, H.; Sarraf, D. Effect of segmentation error correction on optical coherence tomography angiography measurements in healthy subjects and diabetic macular oedema. Br. J. Ophthalmol. 2020, 104, 162–166. [Google Scholar] [CrossRef]
- Li, X.-X.; Wu, W.; Zhou, H.; Deng, J.-J.; Zhao, M.-Y.; Qian, T.-W.; Yan, C.; Xu, X.; Yu, S.-Q. A quantitative comparison of five optical coherence tomography angiography systems in clinical performance. Int. J. Ophthalmol. 2018, 11, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Sampson, D.M.; Yong, A.A.; Jeewa, R.; Rajgopal, S.; Dutt, D.D.C.S.; Mohamed, S.; Mohamed, S.; Menghini, M.; Hansen, A.; et al. Clinical validation of the RTVue optical coherence tomography angiography image quality indicators. Clin. Exp. Ophthalmol. 2020, 48, 192–203. [Google Scholar] [CrossRef]
- Lauermann, J.L.; Woetzel, A.K.; Treder, M.; Alnawaiseh, M.; Clemens, C.R.; Eter, N.; Alten, F. Prevalences of segmentation errors and motion artifacts in OCT-angiography differ among retinal diseases. Graef Arch. Clin. Exp. 2018, 256, 1807–1816. [Google Scholar] [CrossRef]
- Scuderi, L.; Fragiotta, S.; Ciancimino, C.; Mafrici, M.; Mazzola, M.; Varano, M.; Rossi, T.; Parravano, M. Leveraging optical coherence tomography and angiography artifacts to identify clinicopathological correlates in macular disorders. Photonics 2024, 11, 991. [Google Scholar] [CrossRef]
- Greig, E.C.; Duker, J.S.; Waheed, N.K. A practical guide to optical coherence tomography angiography interpretation. Int. J. Retin. Vitr. 2020, 6, 55. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Ghasemi Falavarjani, K.; Akil, H.; Sadda, S.R. Impact of image quality on OCT angiography based quantitative measurements. Int. J. Retin. Vitr. 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, X.; Na, J.; Wang, W. Denoising, segmentation and volumetric rendering of optical coherence tomography angiography (OCTA) image using deep learning techniques: A review. arXiv 2025, arXiv:2502.14935. [Google Scholar]
- Deussen, D.N.; Heinke, A.; Elsner, W.; Galang, C.M.B.; Kalaw, F.G.P.; Warter, A.; Bartsch, D.-U.; Cheng, L.; Freeman, W.R. Effect of manual OCTA segmentation correction to improve image quality and visibility of choroidal neovascularization in AMD. Sci. Rep. 2024, 14, 13990. [Google Scholar] [CrossRef] [PubMed]
- Binotti, W.W.; Romano, A.C. Investigative ophthalmology visual science. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, A.; Lee, C.S.; Lee, A.Y.; Rezaei, K.A.; Roisman, L.; Miller, A.; Zheng, F.; Gregori, G.; Durbin, M.K.; et al. Projection artifact removal improves visualization and quantitation of macular neovascularization imaged by optical coherence tomography angiography. Ophthalmol. Retin. 2017, 1, 124–136. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Mirshahi, R.; Ghasemizadeh, S.; Sardarinia, M. Stepwise segmentation error correction in optical coherence tomography angiography images of patients with diabetic macular edema. Ther. Adv. Ophthalmol. 2020, 12, 2515841420947931. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, L.; Pan, C.; Lu, T.; Hong, T.; Ding, Z.; Li, P. Statistical analysis of motion contrast in optical coherence tomography angiography. J. Biomed. Opt. 2015, 20, 116004. [Google Scholar] [CrossRef]
- Kolb, J.P.; Klein, T.; Kufner, C.L.; Wieser, W.; Neubauer, A.S.; Huber, R. Ultra-widefield retinal MHz-OCT imaging with up to 100 degrees viewing angle. Biomed. Opt. Express 2015, 6, 1534–1552. [Google Scholar] [CrossRef]











| Artifact Category | Artifact Type | Artifact Description |
|---|---|---|
| Light propagation and signal intensity-related artifacts | Projection artifact | Blood flow tail phenomenon in depth inherently caused by mechanism of optical propagation |
| Masking artifact | Blood flow shadowing below light-blocked area | |
| Attenuation artifact | Loss of real blood flow or emergence of noise-induced false blood flow caused by thresholding of weak OCT signal | |
| Unmasking artifact | Abruptly enhanced blood flow caused by strong incident optical intensity resulting from degradation of superficial tissues | |
| Motion artifacts | Eye movement artifact | Horizontal or vertical white lines caused by transient eye movement |
| Banding artifact | Abnormal wide stripes arising from eye movement lasting for a certain long period | |
| Fringe washout artifact | Dark choroidal large vessels caused by interference fringe washout effect | |
| Blinking artifact | Vertical or horizontal dark line in image due to subject blinking | |
| Improper operation artifacts | Defocus artifact | Loss of small vessels caused by light defocusing of scan region |
| Mirror artifact | Image folding around the zero-delay reference line leading to inverted tissues in part of the image | |
| Decentration artifact | Fovea is off-center in planned fovea-centered scanning | |
| Z-offset artifact | Abnormal image area created by a portion of the scanned tissue vertically shifted beyond the imaging area | |
| Signal processing-related artifacts | Segmentation error artifact | Partial incorrect layer segmentation leading to false blood flow information |
| Doubling artifact | Doubling of the same vessel caused by improper processing of eye movement correction | |
| Stretching artifact | Short stripes with varying brightness at the edge of OCTA images caused by incorrect vessel registration |
| Authors | Artifact Type and Issues Addressed | Method/Network Structure | Input and Label (Ground Truth) | Function and Advantages |
|---|---|---|---|---|
| Stefan and Lee (2020) [42] | Projection artifact; Enhancement of low-SNR images; Graph extraction | CNN-based toolbox with enhancement, segmentation, and graphing modules | Input: Raw OCTA images with tail artifact Label: Manually annotated vascular structures | Suppresses tail artifact; Enhances blood flow continuity; Automates multiple stages including enhancement, segmentation, and graph extraction |
| Mei et al. (2020) [121] | Projection artifact | U-Net | Input: B-scan OCTA (flow signal overlay on structural OCT) with projection artifact Label: Corrected B-scan OCTA (3D-PAR output) | Effectively removes projection artifacts in OCTA data |
| Guo et al. (2019) [19] | Shadowing artifact; Non-perfusion area identification | Multi-scaled encoder–decoder network (MEDnet-V2) | Input: En-face OCTA images Label: Segmented non-perfusion areas (NPAs) vs. shadow artifacts | Accurately distinguishes NPA from shadow artifacts; Enhances segmentation accuracy |
| Hossbach et al. (2020) [69] | Motion artifact | DL model for translating structural OCT B-scans to motion-corrected OCTA B-scans | Input: Single B-scan OCT images Label: Corrected B-scan OCTA images | Generates artifact-free OCTA images to replace motion-degraded scans, thereby reducing motion artifacts |
| Li et al. (2021) [122] | Motion artifact; Artifact detection and vessel reconstruction | Two-stage DL model: ① CLNet for B-scan artifact classification (residual CNN) ② SegNet (dense U-Net) + ComNet (gated encoder–decoder) for vessel structure recovery | CLNet: Input: B-scan OCTA images Label: Clean vs. B-scan OCTA image with motion artifact (manually labeled) SegNet and ComNet: Input: Broken OCTA images with vessel masks Label: Ground truth vessel masks without motion artifact | CLNet accurately detects motion-corrupted B-scans (98.5% accuracy); SegNet + ComNet restores vascular continuity by learning topological features; Robust against various artifact patterns |
| Lin et al. (2024) [123] | Motion artifact | Fusion of adjacent and repeated B-scans + DL generation model | Input: Repeated and adjacent OCT scans Label: Fused high-quality OCTA images | Leverages multiple scans to generate motion-suppressed OCTA images |
| Wang et al. (2025) [124] | Projection artifact | CNN trained with sacPR-OCTA labels | Input: OCT and OCTA data Label: Expert-optimized sacPR-OCTA images | Effectively reduces projection artifacts, enhances SNR, and preserves clinically relevant pathological features |
| Shah et al. (2018) [99] | Segmentation error artifact; Precise retinal layer segmentation | CNN based framework for simultaneous multiple surface segmentation | Input: B-scan OCT images Label: Manual annotations of retinal layer boundaries | Performs direct segmentation of each B-scan in a single pass |
| Xie et al. (2023) [125] | Segmentation error artifact; Precise retinal layer segmentation | U-Net with constrained differentiable dynamic programming (DDP) module | Input: B-scan OCT images Label: Correct retinal layer segmentations | Achieves end-to-end learning for retinal OCT surface segmentation while explicitly enforcing surface smoothness |
| Dhodapkar et al. (2022) [126] | Comprehensive quality-related artifacts; OCTA image quality assessment | ResNet152 | Input: Superficial capillary plexus OCTA images Label: Manual gradings by two independent graders | Achieves high AUCs: 0.99 for low-quality and 0.97 for high-quality image identification |
| Lauermann et al. (2019) [127] | Comprehensive quality-related artifacts; OCTA image quality assessment | DCNN, DL-based image quality grading | Input: En-face OCTA images Label: Images defined as sufficient or insufficient image quality based on MAS and SAS | Grades artifacts like motion, segmentation, foveal centration; Guides re-acquisition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Hu, Y.; Lan, G.; Xu, J.; Qin, J.; An, L.; Huang, Y. Review of Artifacts and Related Processing in Ophthalmic Optical Coherence Tomography Angiography (OCTA). Photonics 2025, 12, 536. https://doi.org/10.3390/photonics12060536
Lin Z, Hu Y, Lan G, Xu J, Qin J, An L, Huang Y. Review of Artifacts and Related Processing in Ophthalmic Optical Coherence Tomography Angiography (OCTA). Photonics. 2025; 12(6):536. https://doi.org/10.3390/photonics12060536
Chicago/Turabian StyleLin, Zhefan, Yitao Hu, Gongpu Lan, Jingjiang Xu, Jia Qin, Lin An, and Yanping Huang. 2025. "Review of Artifacts and Related Processing in Ophthalmic Optical Coherence Tomography Angiography (OCTA)" Photonics 12, no. 6: 536. https://doi.org/10.3390/photonics12060536
APA StyleLin, Z., Hu, Y., Lan, G., Xu, J., Qin, J., An, L., & Huang, Y. (2025). Review of Artifacts and Related Processing in Ophthalmic Optical Coherence Tomography Angiography (OCTA). Photonics, 12(6), 536. https://doi.org/10.3390/photonics12060536






