A Dengue Virus Detection Using Biosensor-Based Two-Dimensional Photonic Crystal Ring Resonator
Abstract
1. Introduction
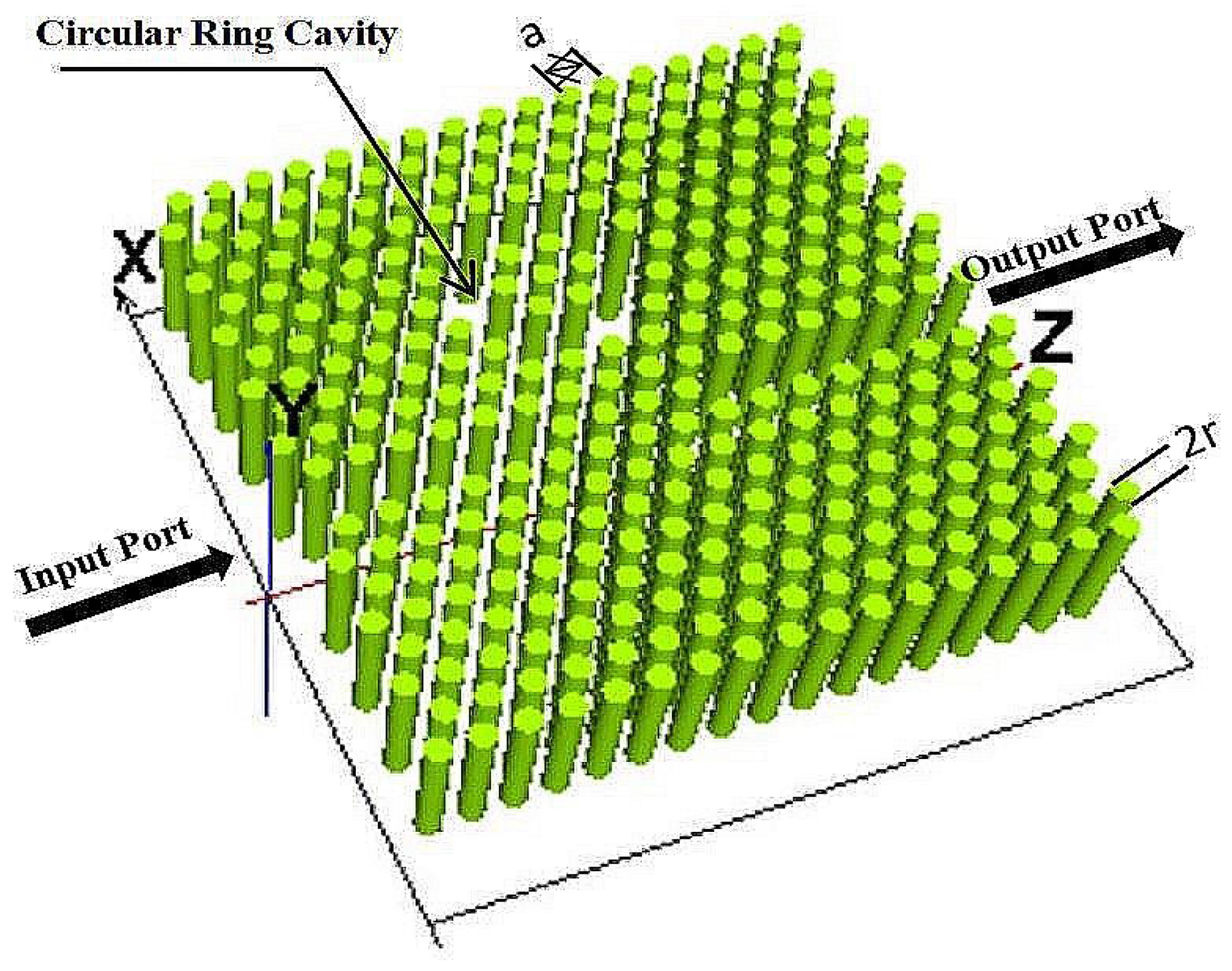
2. Structure and Design
3. Band Gap Calculation
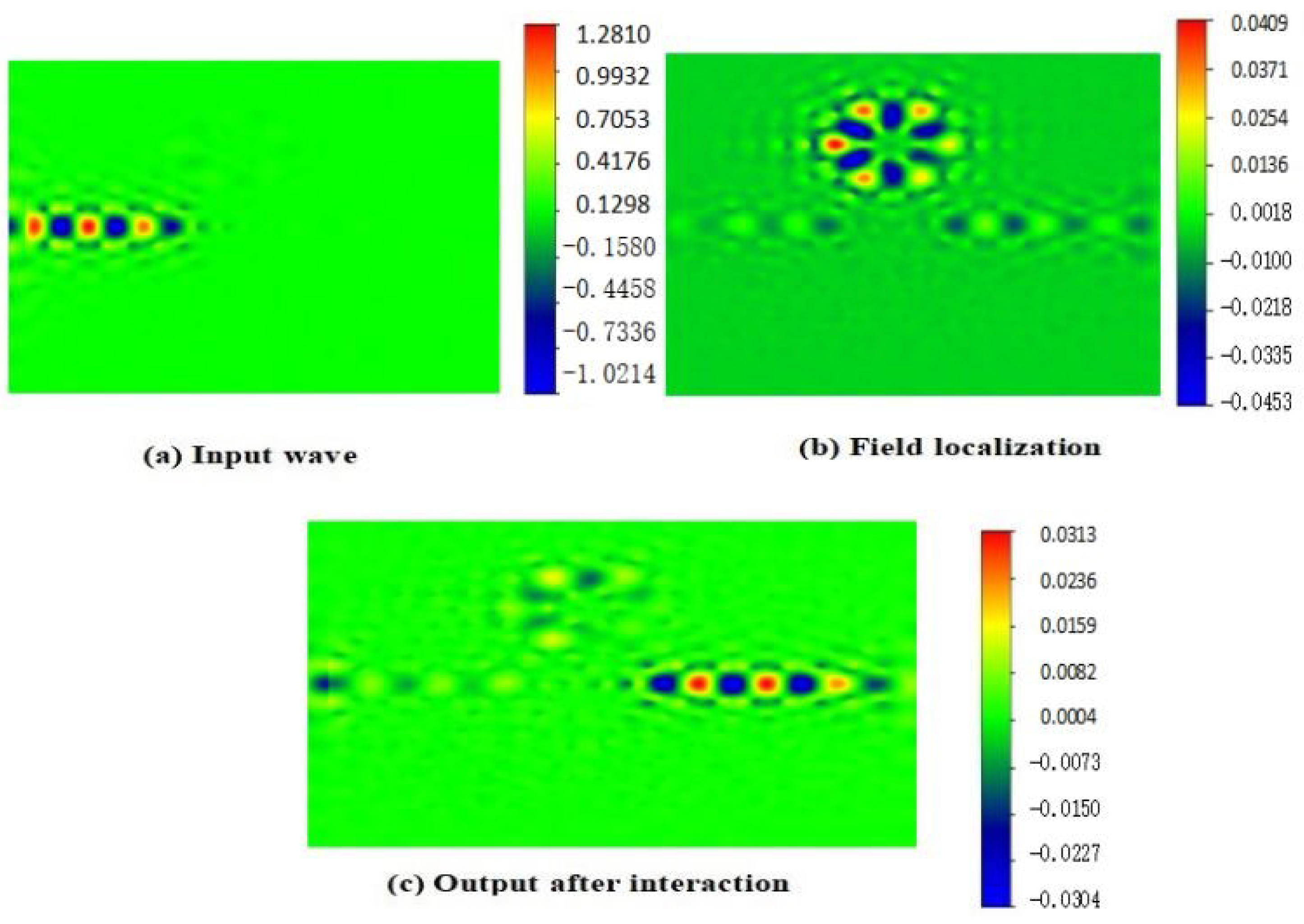
4. Distribution Field
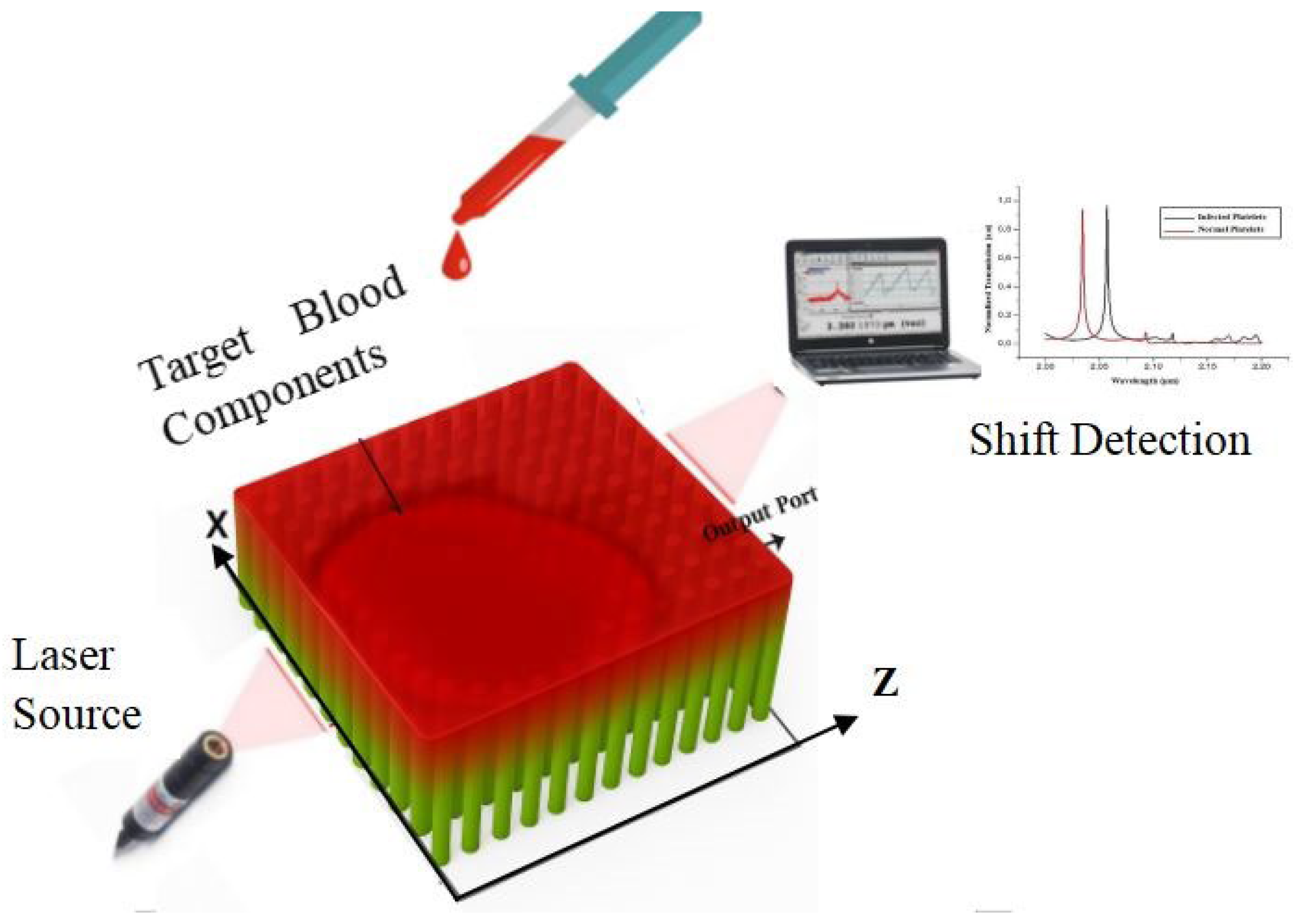
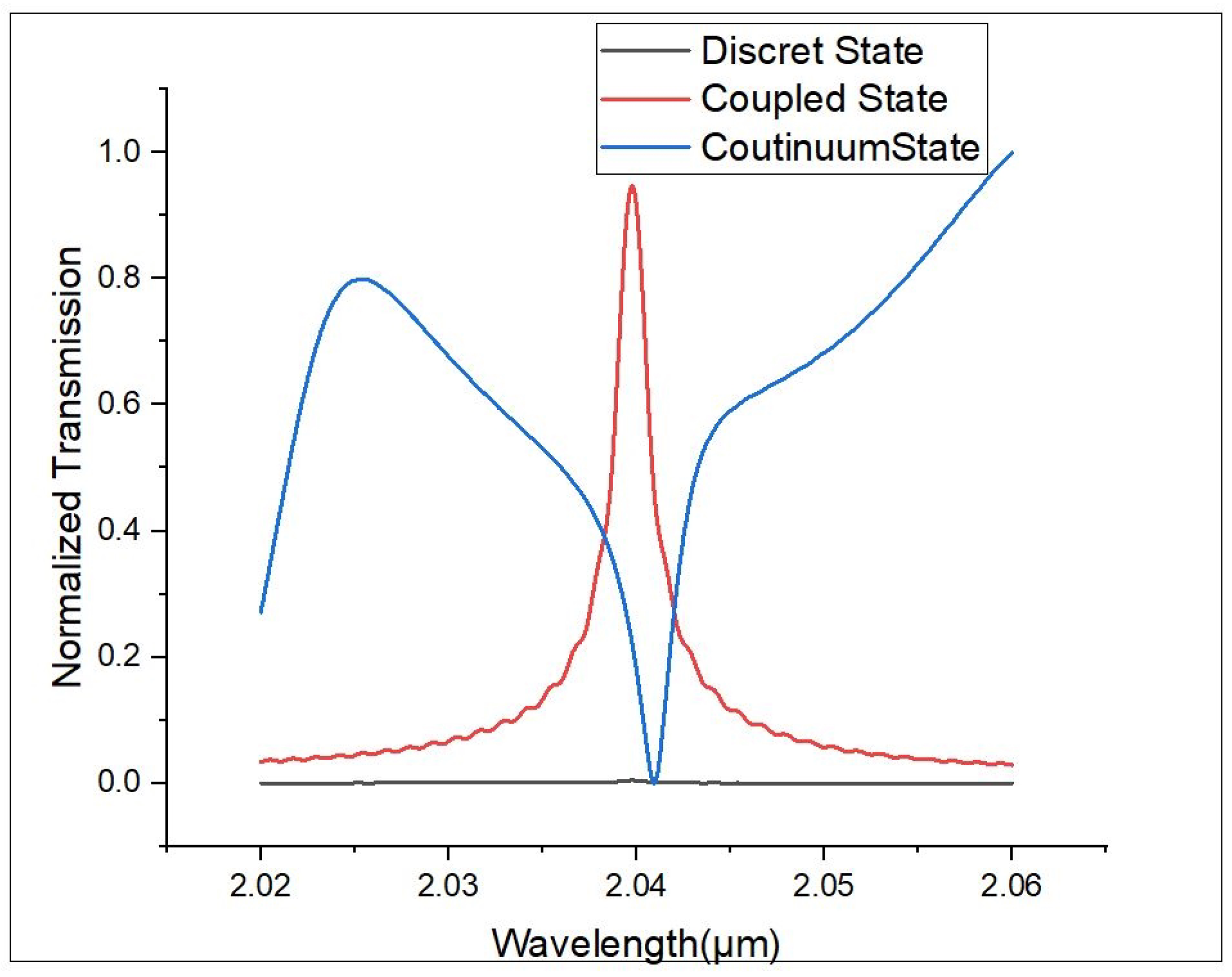
5. Detection Mechanism
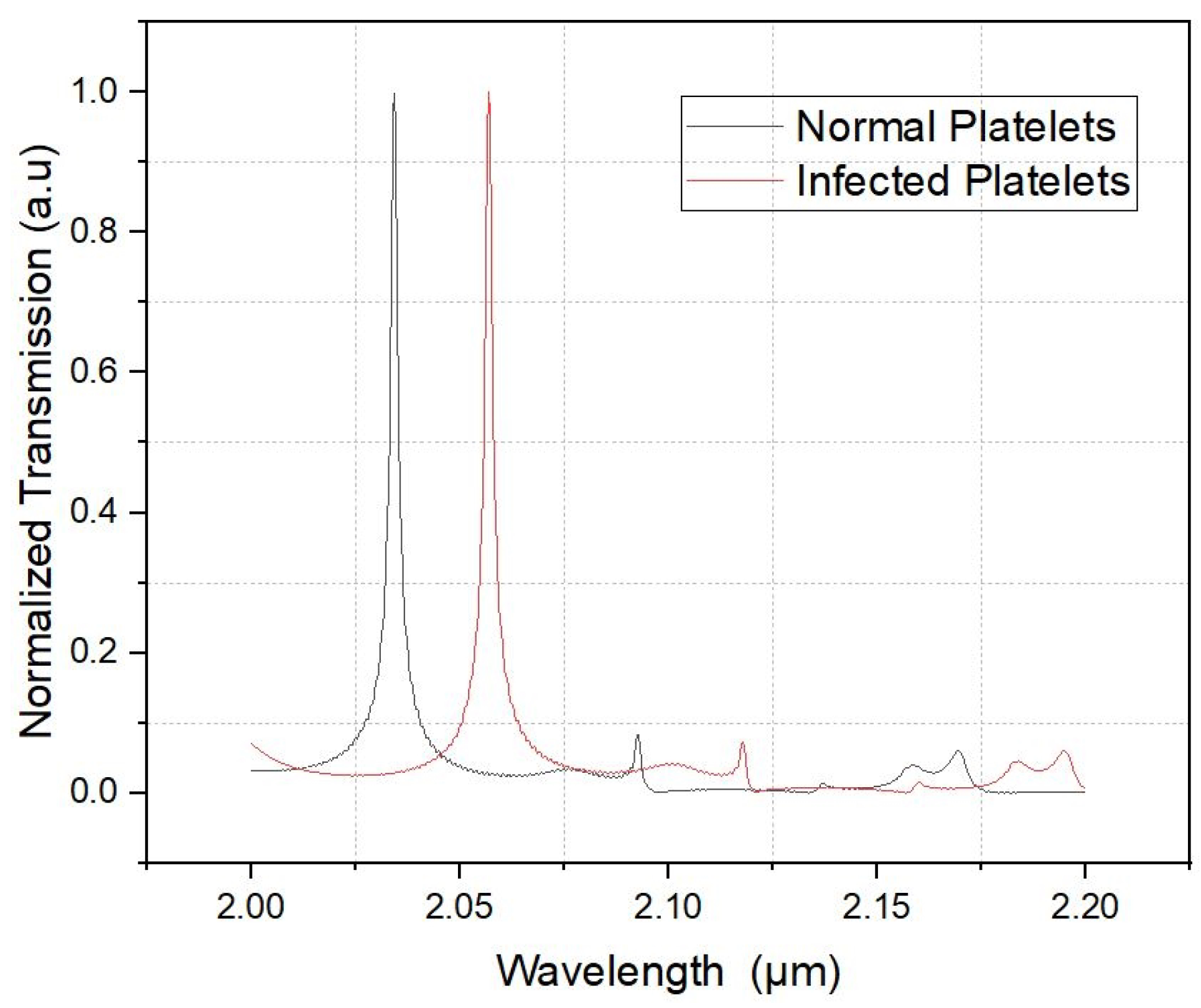
6. Results and Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, G.; He, X.; Shi, F.; Liu, J.; Chen, X.; Dong, J. Topological Photonic Crystals: Physics, Designs, and Applications. Laser Photonics Rev. 2022, 16, 2100300. [Google Scholar] [CrossRef]
- Butt, M.; Khonina, S.; Kazanskiy, N. Recent advances in photonic crystal optical devices: A review. Opt. Laser Technol. 2021, 142, 107265. [Google Scholar] [CrossRef]
- Maleki, M.J.; Soroosh, M.; Akbarizadeh, G. Photonic Crystal Resonators in Designing Optical Decoders. J. Optoelectron. Nanostructures 2023, 8, 1–24. [Google Scholar] [CrossRef]
- Bouaouina, M.S.; Lebbal, M.R. All optical photonic crystal encoder based on nonlinear Kerr effect with ultrahigh contrast ratio and low threshold power. Opt. Quantum Electron. 2023, 55, 867. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Prim. 2016, 2, 16055. [Google Scholar] [CrossRef]
- Frazer, J.L.; Norton, R. Dengue: A review of laboratory diagnostics in the vaccine age. J. Med Microbiol. 2024, 73, 001833. [Google Scholar] [CrossRef]
- Gan, S.D.; Patel, K.R. Enzyme Immunoassay and Enzyme-Linked Immunosorbent Assay. J. Investig. Dermatol. 2013, 133, 1–3. [Google Scholar] [CrossRef]
- Kanagawa, T. Bias and artifacts in multitemplate polymerase chain reactions (PCR). J. Biosci. Bioeng. 2003, 96, 317–323. [Google Scholar] [CrossRef]
- Peeling, R.W.; Artsob, H.; Pelegrino, J.L.; Buchy, P.; Cardosa, M.J.; Devi, S.; Enria, D.A.; Farrar, J.; Gubler, D.J.; Guzman, M.G.; et al. Evaluation of diagnostic tests: Dengue. Nat. Rev. Microbiol. 2010, 8, S30–S37. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Huertas, C.S.; Lechuga, L.M. Label-free plasmonic biosensors for point-of-care diagnostics: A review. Expert Rev. Mol. Diagn. 2018, 19, 71–81. [Google Scholar] [CrossRef]
- Bendib, S.; Zegadi, A.; Djeffal, N. Improved sensitivity of 2D annular photonic crystal biosensor working at THz frequency range. Opt. Quantum Electron. 2016, 48, 528. [Google Scholar] [CrossRef]
- Hegde, S.S.; Bhat, B.R. Dengue detection: Advances and challenges in diagnostic technology. Biosens. Bioelectron. X 2022, 10, 100100. [Google Scholar] [CrossRef]
- Gürel, Ç.S.; Ceylan, N.E. High sensitivity photonic crystal biosensor design for real time detection of viruses in infected blood sample. J. Opt. 2024. [Google Scholar] [CrossRef]
- Ghezal, M.A.; Lidjici, H.; Zoukel, A.; Benchiheb, A.; Hocini, A. High Sensitivity Biosensor Photonic Crystal Focused on Detecting the Concentration of the Biological Analytes. Prog. Electromagn. Res. C 2024, 146, 111–117. [Google Scholar] [CrossRef]
- Meade, R.D.; Rappe, A.M.; Brommer, K.D.; Joannopoulos, J.D.; Alherhand, O.L. Erratum: Accurate theoretical analysis of photonic band-gap materials [Phys. Rev. B 48, 8434 (1993)]. Phys. Rev. B 1997, 55, 15942. [Google Scholar] [CrossRef]
- Chutinan, A.; Noda, S. Waveguides and waveguide bends in two-dimensional photonic crystal slabs. Phys. Rev. B 2000, 62, 4488–4492. [Google Scholar] [CrossRef]
- LShen, F.; He, S.-L.; Wu, L. The application of effective-medium theory in the plane-wave expansion method for analyzing photonic crystals. Acta Phys. Sin. 2002, 51, 1137–1138. [Google Scholar]
- Xie, Z.; Sun, S.; Wang, W.; Qin, L.; Yan, Y.; Hou, R.; Qin, G. Simulation study on improving efficiencies of perovskite solar cell: Introducing nano textures on it. Opt. Commun. 2018, 410, 117–122. [Google Scholar] [CrossRef]
- Lipson, R.H.; Lu, C. Photonic crystals: A unique partnership between light and matter. Eur. J. Phys. 2009, 30, S33–S48. [Google Scholar] [CrossRef]
- Robinson, S.; Nakkeer, R. Photonic Crystal Ring Resonator Based Optical Filters. In Advances in Photonic Crystals; InTech: Rijeka, Croatia, 2013; Volume 13. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A. Design of a Biosensor for the Detection of Dengue Virus Using 1D Photonic Crystals. Plasmonics 2022, 17, 675–680. [Google Scholar] [CrossRef]
- Yariv, A. Critical coupling and its control in optical waveguide-ring resonator systems. IEEE Photonics Technol. Lett. 2002, 14, 483–485. [Google Scholar] [CrossRef]
- Mohapatra, B.; Khan, A.S.; Dash, D.; Ansari, R.; Indu. Design of Biosensor using 1D Photonic Crystal for Dengue Virus Detection. In Proceedings of the 2022 4th International Conference on Advances in Computing, Communication Control and Networking (ICAC3N), Greater Noida, India, 16–17 December 2022; pp. 1667–1670. [Google Scholar]
- Basak, C.; Hosain, K.; Islam, S.; Kouzani, A.Z. Design and modeling of an angular interrogation based surface plasmon resonance biosensor for dengue virus detection. Opt. Quantum Electron. 2023, 55, 438. [Google Scholar] [CrossRef]




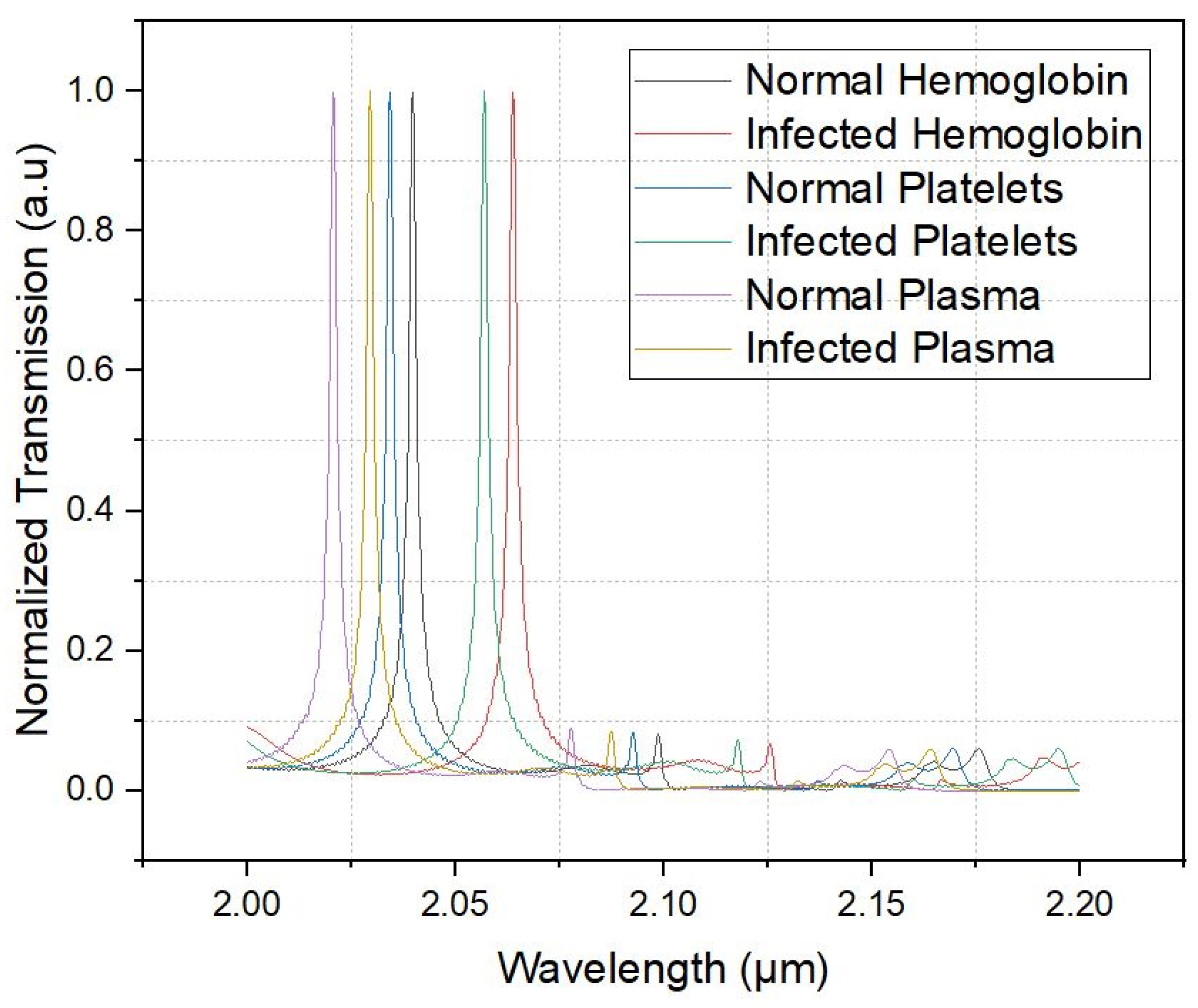


| Refractive Index Central Wavelength | Q | Sensitivity | |
|---|---|---|---|
| µ | nm/RIU | ||
| 1.337 | 2.02083 | 902.004 | |
| 1.35 | 2.0297 | 905.229 | 682.307 |
| 1.357 | 2.03437 | 943.309 | 667.142 |
| 1.36 | 2.03998 | 938.226 | 1870 |
| 1.39 | 2.05679 | 863.469 | 560.333 |
| 1.4 | 2.06427 | 866.369 | 748 |
| Reference Number/Year | Proposed Biosensor | Coupling Resonance | Detection Range (µm) | Quality Factor | Sensitivity (nm/RIU) |
|---|---|---|---|---|---|
| Sharma et al. (2022) [23] | Dengue virus detection | 1D photonic crystals | 1.5–1.8 | 10,000 | 1200 |
| Mohapatra et al. (2022) [25] | Dengue virus detection | 1D photonic crystals | 1.55–1.75 | 8500 | 950 |
| Basak et al. (2023) [26] | Dengue virus detection | surface plasmon resonance | Visible-NIR | 2500 | 680 |
| The proposed work | Dengue virus detection | 2D PhC Ring Resonator | 2.0–2.2 | 943,309 | 1870 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djeffal, N.; Bendib, S.; Hedir, A. A Dengue Virus Detection Using Biosensor-Based Two-Dimensional Photonic Crystal Ring Resonator. Photonics 2025, 12, 514. https://doi.org/10.3390/photonics12050514
Djeffal N, Bendib S, Hedir A. A Dengue Virus Detection Using Biosensor-Based Two-Dimensional Photonic Crystal Ring Resonator. Photonics. 2025; 12(5):514. https://doi.org/10.3390/photonics12050514
Chicago/Turabian StyleDjeffal, Nadhir, Sarra Bendib, and Abdallah Hedir. 2025. "A Dengue Virus Detection Using Biosensor-Based Two-Dimensional Photonic Crystal Ring Resonator" Photonics 12, no. 5: 514. https://doi.org/10.3390/photonics12050514
APA StyleDjeffal, N., Bendib, S., & Hedir, A. (2025). A Dengue Virus Detection Using Biosensor-Based Two-Dimensional Photonic Crystal Ring Resonator. Photonics, 12(5), 514. https://doi.org/10.3390/photonics12050514





