Feasibility of Photoplethysmography in Detecting Arterial Stiffness in Hypertension
Abstract
1. Introduction
2. Methods
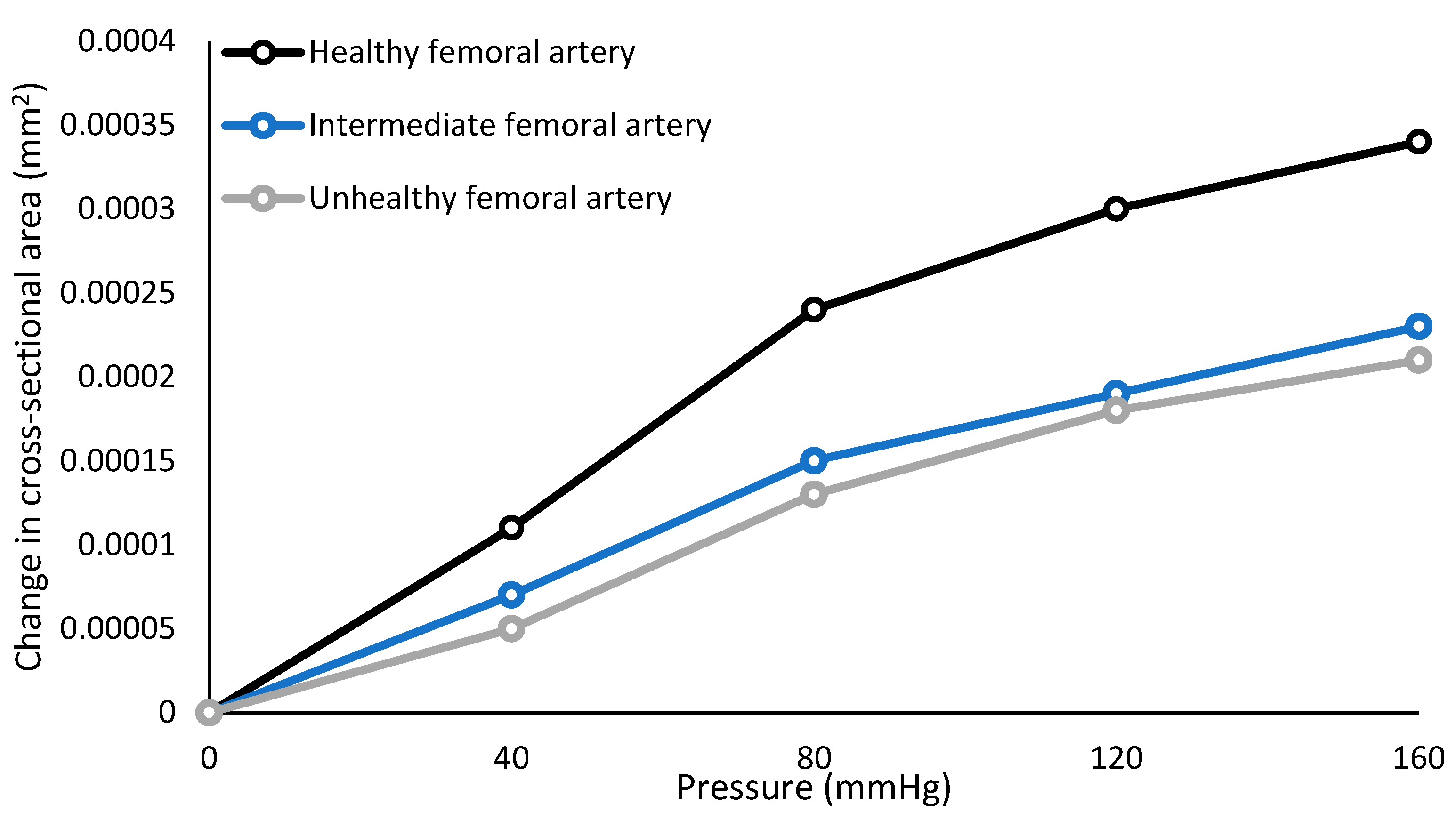
2.1. Fabrication of Femoral Arteries
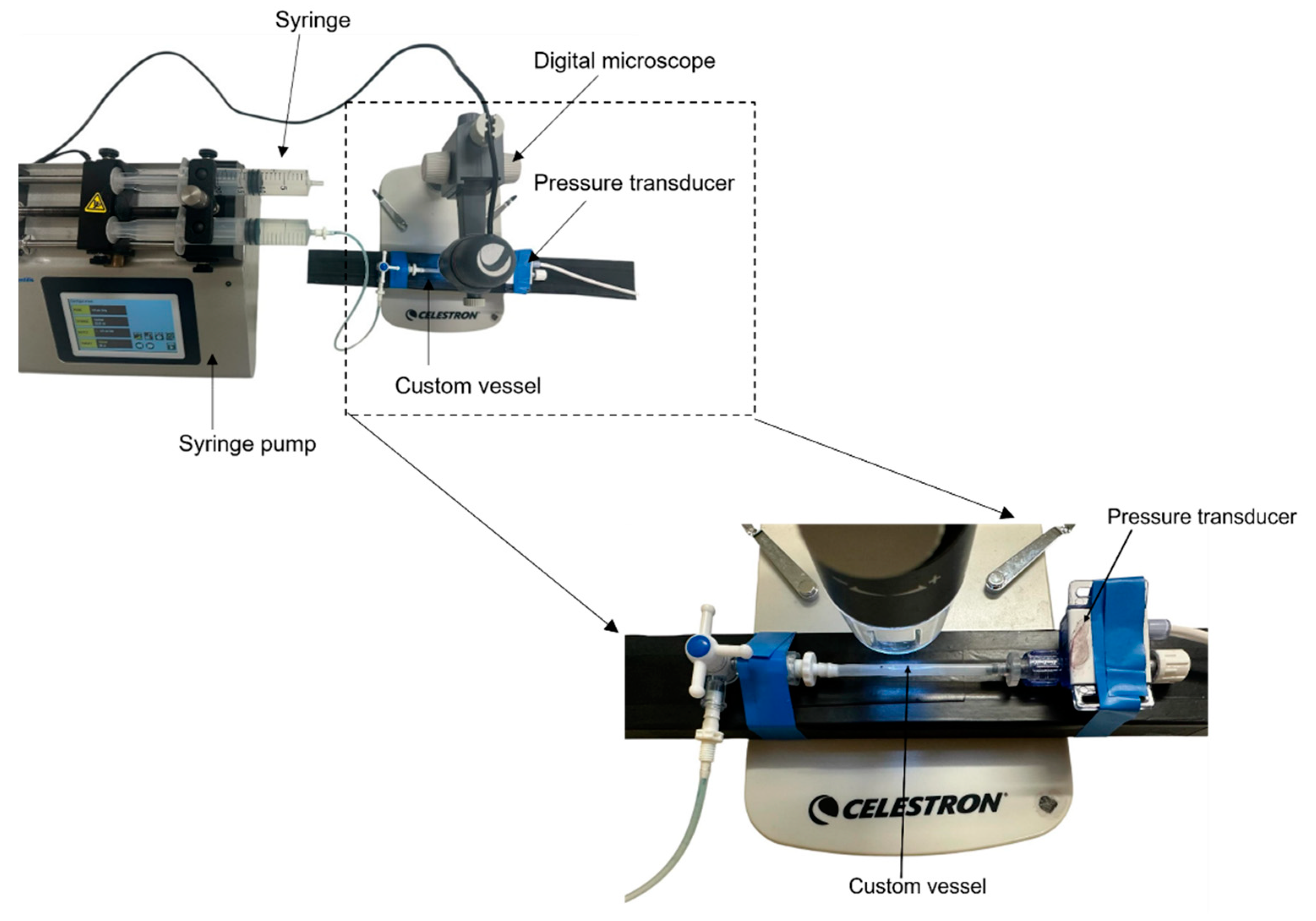
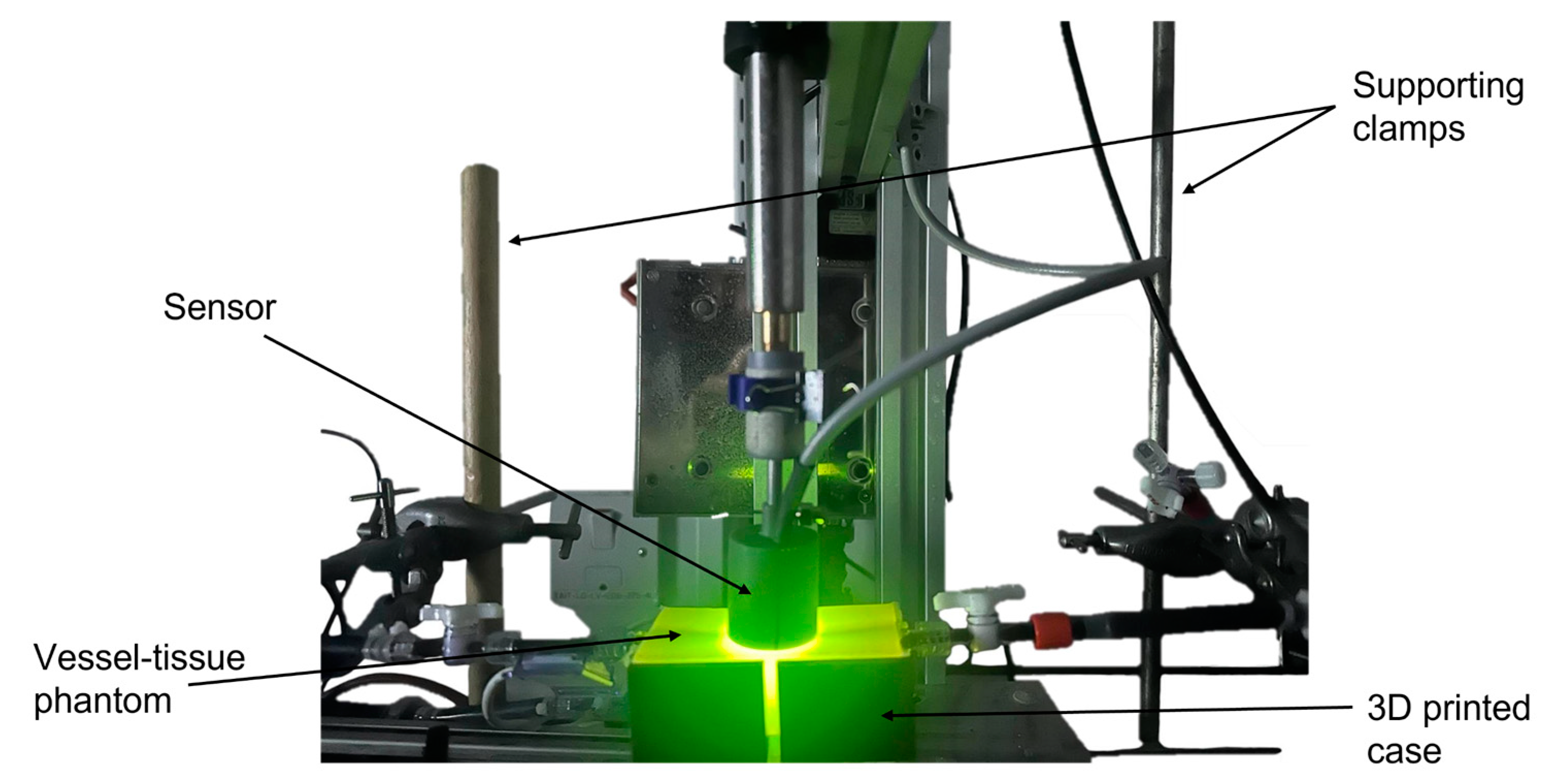
2.2. In Vitro Cardiovascular System Configuration
2.3. Determining Blood Pressure Values
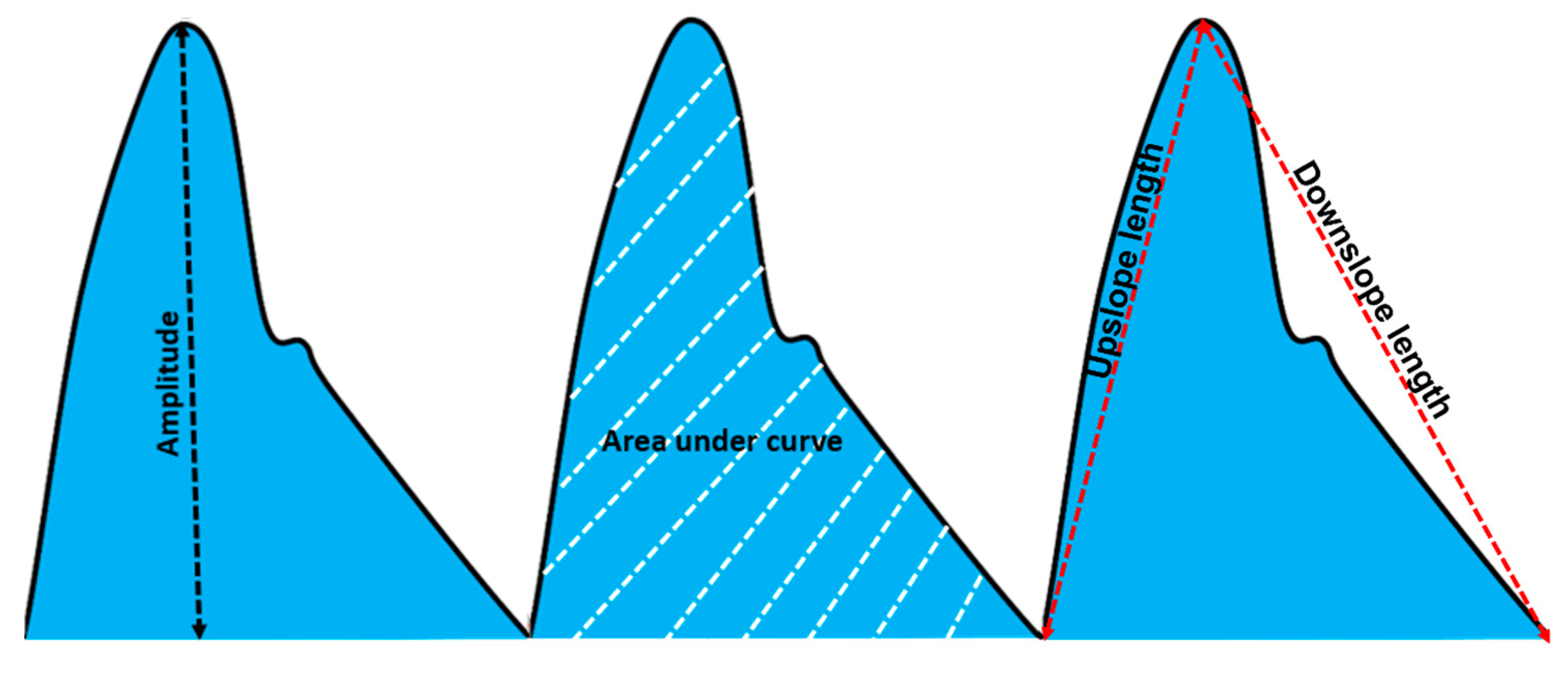
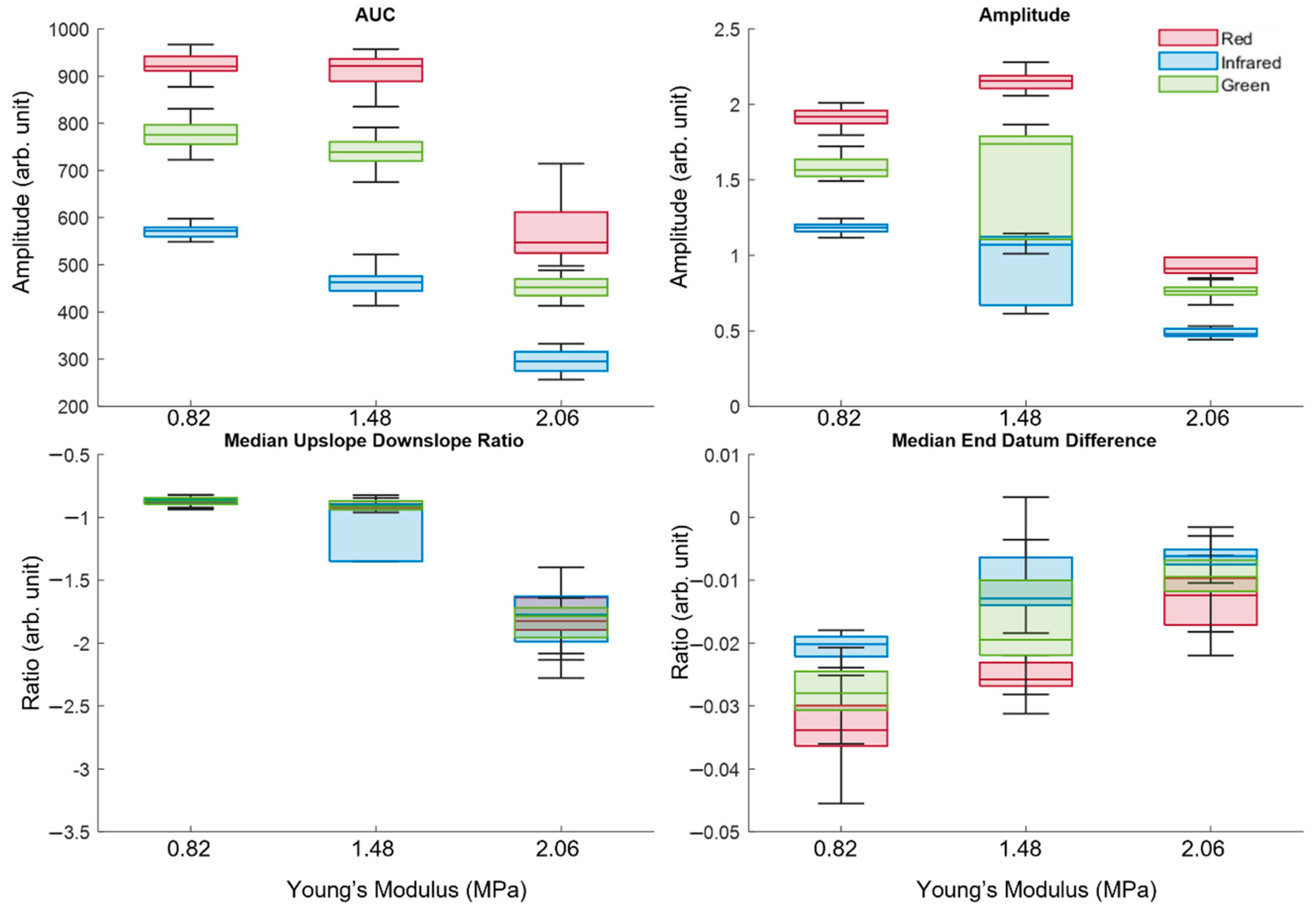
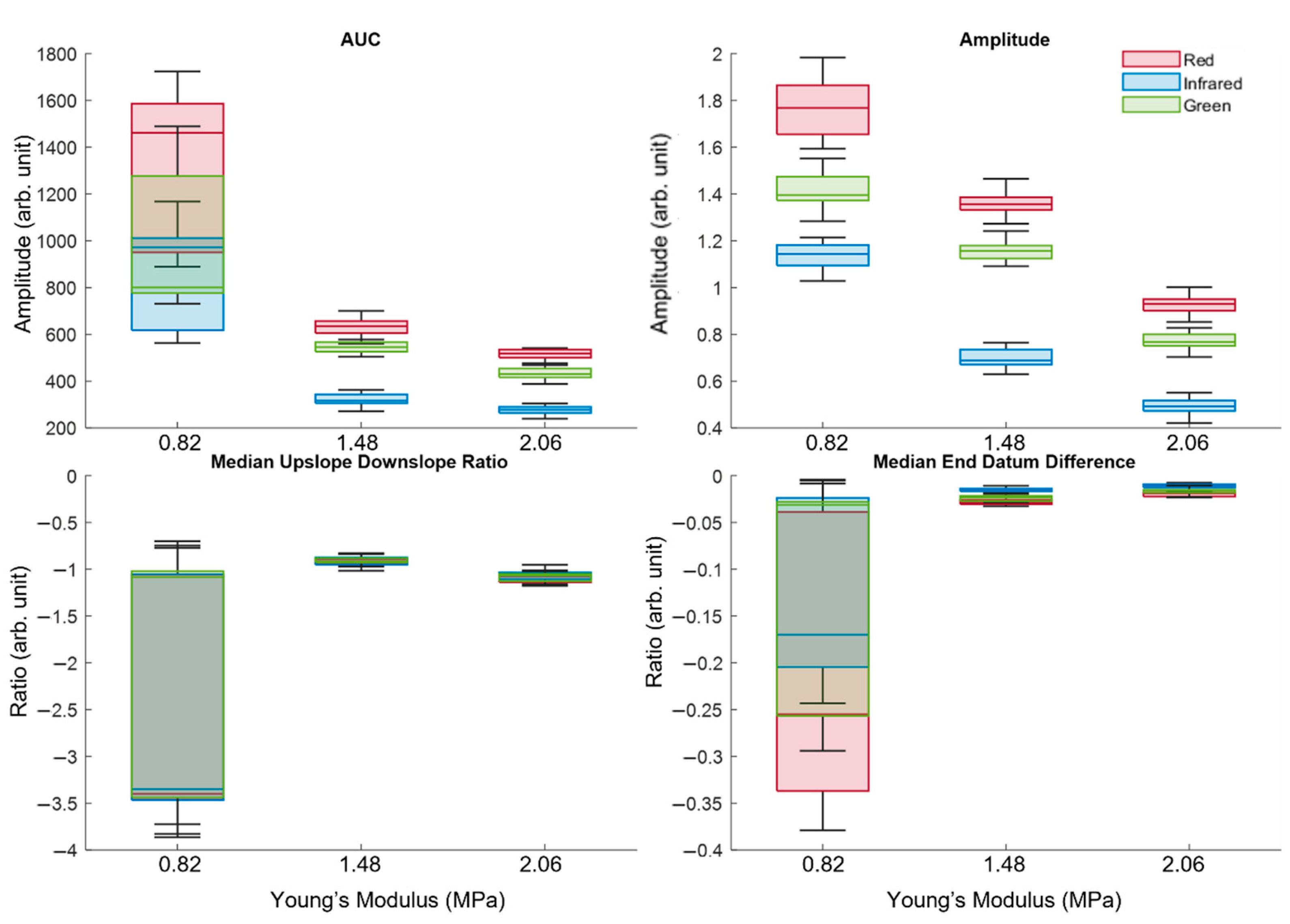
2.4. Feature Extraction
2.5. Statistical Analysis
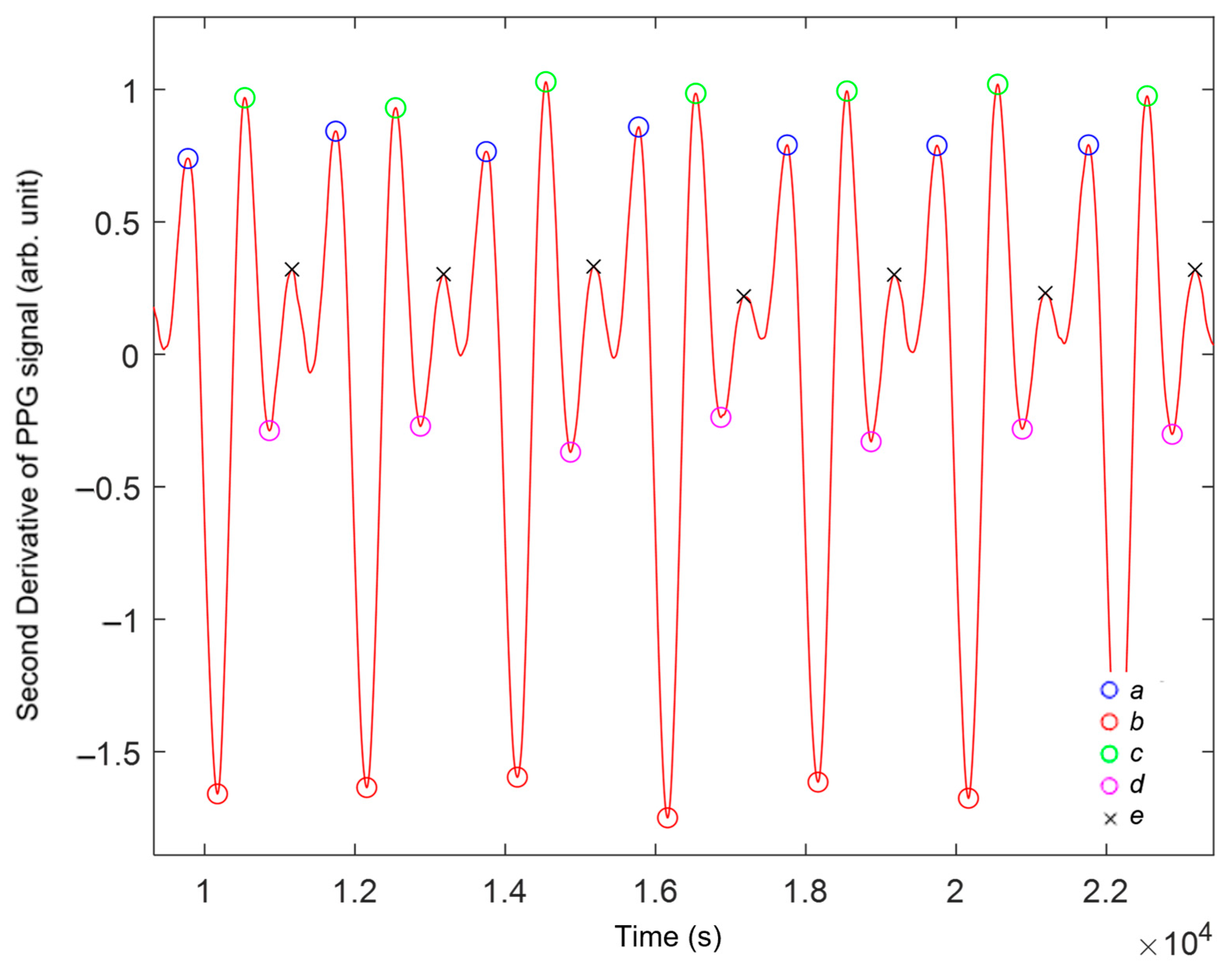
2.6. Second Derivative of Photoplethysmography
3. Results and Discussion
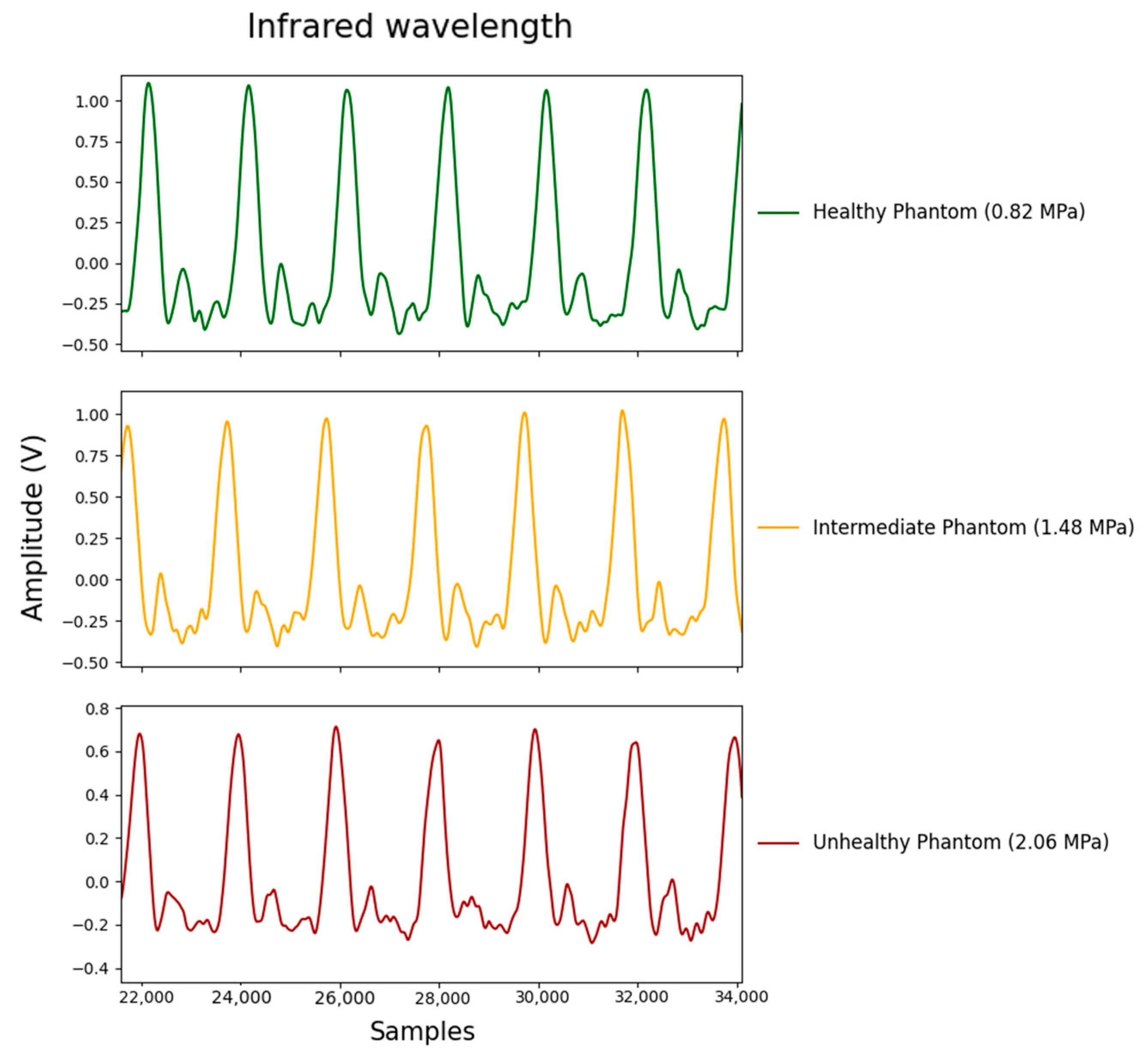
3.1. Mechanical Characterisation
3.2. Analysis of Extracted Features
3.3. Statistical Analysis Using Kruskal–Wallis One-Way Analysis of Variance
3.4. Analysis of the Second Derivative of Photoplethysmography
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L. The Global Burden of Disease Study at 30 years. Nat. Med. 2022, 28, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Karimpour, P.; May, J.M.; Kyriacou, P.A. Photoplethysmography for the Assessment of Arterial Stiffness. Sensors 2023, 23, 9882. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, S.; Agnoletti, D.; Alastruey, J.; Allen, J.; Bianchini, E.; Bikia, V.; Boutouyrie, P.; Bruno, R.M.; Climie, R.; Djeldjli, D.; et al. Developing technologies to assess vascular ageing: A roadmap from VascAgeNet. Physiol. Meas. 2024, 45, 121001. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension: JACC Health Pro-motion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef]
- Lawes, C.M.; Hoorn, S.V.; Rodgers, A.; International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- Karimpour, P.; Ferizoli, R.; May, J.M.; Kyriacou, P.A. Customisable Silicone Vessels and Tissue Phantoms for In Vitro Photoplethysmography Investigations into Cardiovascular Disease. Sensors 2024, 24, 1681. [Google Scholar] [CrossRef]
- Zekavat, S.M.; Aragam, K.; Emdin, C.; Khera, A.V.; Klarin, D.; Zhao, H.; Natarajan, P. Genetic Association of Finger Photoplethysmography-Derived Arterial Stiffness Index With Blood Pressure and Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2019, 39, 1253–1261. [Google Scholar] [CrossRef]
- Bortolotto, L.A.; Blacher, J.; Kondo, T.; Takazawa, K.; Safar, M.E. Assessment of vascular aging and atherosclerosis in hypertensive subjects: Second derivative of photoplethysmogram versus pulse wave velocity. Am. J. Hypertens. 2000, 13, 165–171. [Google Scholar] [CrossRef]
- Korneeva, O.; Drapkina, O. PP.33.08: Arterial stiffness parameters in obese hypertensive patients using different statin-based regimens and fixed antihypertensive combination. J. Hypertens. 2015, 33, e431. [Google Scholar] [CrossRef]
- Brum, J.; Balay, G.; Bia, D.; Benech, N.; Ramos, A.; Armentano, R.; Negreira, C. Improvement of Young modulus estimation by ultrasound using static pressure steps. Phys. Procedia 2010, 3, 1087–1094. [Google Scholar] [CrossRef]
- ASTM D412-16; Test Methods for Vulcanized Rubber and Thermoplastic Elastomers-Tension. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- The Definitive Guide to ASTM D412 Tensile Testing of Elastomers’. Available online: https://www.instron.com/en/testing-solutions/astm-standards/astm-d412 (accessed on 20 February 2024).
- Spector, K.S.; Lawson, W.E. Optimizing safe femoral access during cardiac catheterization. Catheter. Cardiovasc. Interv. 2001, 53, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, A.; Farahmand, M.; Hasan, S.; Vasudevan, S.; Vogt, W.C.; Ibarra, B.; Weininger, S.; Scully, C.G.; Zhang, X.F.; Chen, Y.; et al. Development and characterization of silicone-based tissue phantoms for pulse oximeter performance testing. J. Biomed. Opt. 2024, 29, S33314. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Baker, D.J.; D’uscio, L.V.; Mozammel, G.; Katusic, Z.S.; van Deursen, J.M. Aging-Associated Vascular Phenotype in Mutant Mice with Low Levels of BubR1. Stroke 2007, 38, 1050–1056. [Google Scholar] [CrossRef]
- Ferizoli, R.; Karimpour, P.; May, J.M.; Kyriacou, P.A. Arterial stiffness assessment using PPG feature extraction and significance testing in an in vitro cardiovascular system. Sci. Rep. 2024, 14, 1–10. [Google Scholar] [CrossRef]
- May, J.M. Investigation of Fontanelle Photoplethysmographs and Oxygen Saturations in Intensive Care Neonates and Infants Utilising Miniature Photometric Sensors. Ph.D. Thesis, City University London, London, UK, 2013. [Google Scholar]
- Shahoud, J.S.; Sanvictores, T.; Aeddula, N.R. Physiology, Arterial Pressure Regulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538509/ (accessed on 16 October 2024).
- Camasão, D.; Mantovani, D. The mechanical characterization of blood vessels and their substitutes in the continuous quest for physiological-relevant performances. A critical review. Mater. Today Biol. 2021, 10, 100106. [Google Scholar] [CrossRef]
- Theodorsson-Norheim, E. Kruskal-Wallis test: BASIC computer program to perform nonparametric one-way analysis of variance and multiple comparisons on ranks of several independent samples. Comput. Methods Programs Biomed. 1986, 23, 57–62. [Google Scholar] [CrossRef]
- Whitley, E.; Ball, J. Statistics review 6: Nonparametric methods. Crit. Care 2002, 6, 509–513. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 10: Further nonparametric methods. Crit. Care 2004, 8, 196–199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Concato, J.; Hartigan, J.A. p-Values: From Suggestion to Superstition. J. Investig. Med. 2016, 64, 1166–1171. [Google Scholar] [CrossRef]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [CrossRef]
- Elgendi, M. On the Analysis of Fingertip Photoplethysmogram Signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Bradley, G. Enantiodromis/PPGExtract (16 August 2023). Python. Available online: https://github.com/Enantiodromis/PPGExtract (accessed on 24 August 2023).
- Mejía-Mejía, E.; Allen, J.; Budidha, K.; El-Hajj, C.; Kyriacou, P.A.; Charlton, P.H. 4—Photoplethysmography signal processing and synthesis. In Photoplethysmography; Allen, J., Kyriacou, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 69–146. [Google Scholar] [CrossRef]
- Jadidi, M.; Razian, S.A.; Habibnezhad, M.; Anttila, E.; Kamenskiy, A. Mechanical, structural, and physiologic differences in human elastic and muscular arteries of different ages: Comparison of the descending thoracic aorta to the superficial femoral artery. Acta Biomater. 2021, 119, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Almarshad, M.A.; Islam, S.; Al-Ahmadi, S.; BaHammam, A.S. Diagnostic Features and Potential Applications of PPG Signal in Healthcare: A Systematic Review. Healthcare 2022, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Takazawa, K.; Tanaka, N.; Fujita, M.; Matsuoka, O.; Saiki, T.; Aikawa, M.; Tamura, S.; Ibukiyama, C. Assessment of Vasoactive Agents and Vascular Aging by the Second Derivative of Photoplethysmogram Waveform. Hypertension 1998, 32, 365–370. [Google Scholar] [CrossRef]
- Baek, H.J.; Kim, J.S.; Kim, Y.S.; Lee, H.B.; Park, K.S. Second Derivative of Photoplethysmography for Estimating Vascular Aging. In Proceedings of the 2007 6th International Special Topic Conference on Information Technology Applications in Biomedicine, Tokyo, Japan, 8–11 November 2007; pp. 70–72. [Google Scholar]
- Rozi, R.M.; Usman, S.; Ali, M.M.; Reaz, M. Second derivatives of photoplethysmography (PPG) for estimating vascular aging of atherosclerotic patients. In Proceedings of the 2012 IEEE EMBS Conference on Biomedical Engineering and Sciences (IECBES 2012), Langkawi, Malaysia, 17–19 December 2012; pp. 256–259. [Google Scholar]
- Yousef, K.Q.; Rubins, U.; Mafawez, A. Photoplethysmogram second derivative review: Analysis and applications. Sci. Res. Essays 2015, 10, 633–639. [Google Scholar] [CrossRef]
- Gayapersad, N.; Rocke, S.; Ramsaroop, Z.; Singh, A.; Ramlal, C. Beyond Blood Pressure and Heart Rate Monitoring: Towards a Device for Continuous Sensing and Automatic Feature Extraction of Cardiovascular Data. In Proceedings of the 2016 8th International Conference on Computational Intelligence and Communication Networks (CICN), Tehri, India, 23–25 December 2016; pp. 261–265. [Google Scholar]
- Suboh, M.Z.; Jaafar, R.; Nayan, N.A.; Harun, N.H.; Mohamad, M.S.F. Analysis on Four Derivative Waveforms of Photoplethysmogram (PPG) for Fiducial Point Detection. Front. Public Health 2022, 10, 920946. [Google Scholar] [CrossRef]
- Choi, J.; Park, M.-G. Variations in the Second Derivative of a Photoplethysmogram with Age in Healthy Korean Adults. Int. J. Environ. Res. Public Health 2022, 20, 236. [Google Scholar] [CrossRef]
- Iketani, Y.; Iketani, T.; Takazawa, K.; Murata, M. Second Derivative of Photoplethysmogram in Children and Young People. Jpn. Circ. J. 2000, 64, 110–116. [Google Scholar] [CrossRef][Green Version]

| Healthy–Intermediate | Intermediate–Unhealthy | Healthy–Unhealthy | |
|---|---|---|---|
| Area Under Curve | 0.0662 | p < 0.05 | p < 0.05 |
| Amplitude | 0.6690 | p < 0.05 | p < 0.05 |
| Median Upslope–Downslope-Ratio | p < 0.05 | p < 0.05 | p < 0.05 |
| Median End Datum Difference | p < 0.05 | p < 0.05 | p < 0.05 |
| Healthy–Intermediate | Intermediate–Unhealthy | Healthy–Unhealthy | |
|---|---|---|---|
| Area Under Curve | 0.1540 | p < 0.05 | p < 0.05 |
| Amplitude | p < 0.05 | p < 0.05 | p < 0.05 |
| Median Upslope–Downslope-Ratio | p < 0.05 | p < 0.05 | 0.0986 |
| Median End Datum Difference | p < 0.05 | p < 0.05 | p < 0.05 |
| Healthy–Intermediate | Intermediate–Unhealthy | Healthy–Unhealthy | |
|---|---|---|---|
| Area Under Curve | p < 0.05 | p < 0.05 | p < 0.05 |
| Amplitude | p < 0.05 | p < 0.05 | p < 0.05 |
| Median Upslope–Downslope-Ratio | p < 0.05 | p < 0.05 | p < 0.05 |
| Median End Datum Difference | p < 0.05 | p < 0.05 | p < 0.05 |
| Hypertensive | Normotensive | Hypotensive | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red signal | Healthy | −2.13 | 1.29 | −0.43 | 0.37 | −2.18 | 1.34 | −0.41 | 0.38 | −1.98 | 1.05 | −0.21 | 0.28 |
| Unhealthy | −2.06 | 1.01 | −0.44 | 0.36 | −2.17 | 1.33 | −0.43 | 0.37 | −1.98 | 1.02 | −0.22 | 0.28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimpour, P.; May, J.M.; Kyriacou, P.A. Feasibility of Photoplethysmography in Detecting Arterial Stiffness in Hypertension. Photonics 2025, 12, 430. https://doi.org/10.3390/photonics12050430
Karimpour P, May JM, Kyriacou PA. Feasibility of Photoplethysmography in Detecting Arterial Stiffness in Hypertension. Photonics. 2025; 12(5):430. https://doi.org/10.3390/photonics12050430
Chicago/Turabian StyleKarimpour, Parmis, James M. May, and Panicos A. Kyriacou. 2025. "Feasibility of Photoplethysmography in Detecting Arterial Stiffness in Hypertension" Photonics 12, no. 5: 430. https://doi.org/10.3390/photonics12050430
APA StyleKarimpour, P., May, J. M., & Kyriacou, P. A. (2025). Feasibility of Photoplethysmography in Detecting Arterial Stiffness in Hypertension. Photonics, 12(5), 430. https://doi.org/10.3390/photonics12050430





