1. Introduction
The pursuit of compact, efficient light sources has driven innovation in photonics, with hybrid nanomaterials emerging as a frontier for combining synergistic optical properties. Nanoporous anodic alumina (NAA) membranes are unique materials used in a variety of applications, including electronics, catalysis, and sensing [
1,
2]. Nanoporous alumina is a solid matrix obtained from a double anodizing process of thin aluminum wafers. Nanoporous anodized alumina exhibits high chemical and thermal stability, in addition to favorable dielectric properties [
3]. The dielectric permittivity is inversely proportional to the porosity of the NAA and the frequency of the applied alternating current [
4,
5,
6]. The optical properties of NAA are anisotropic, making them suitable for use in light-collecting systems [
7]. Additionally, nanoporous anodic alumina membranes exhibit adjustable wetting properties that vary according to the size, depth, and organization of the pores [
8]. NAA is a promising medium that can be used as a matrix for the introduction of organic dyes to obtain quantum oscillators with scattering feedback, known as random lasers [
9]. Random lasers have primarily been realized using Rhodamine 6G dye as the active medium [
10]. However, such lasers exhibited low energy conversion capability and were not stable over long periods.
The combination of unique optical properties and a wide range of potential applications—such as photodynamic therapy, imaging agents, optoelectronic devices, and biological probes—positions pseudoisocyanine dye as a significant compound in both research and industry, making it a focus of ongoing scientific investigations [
11]. Moreover, these molecules have a unique ability to self-assemble into J-aggregates, which are ordered clusters of molecular dyes that exhibit properties quite different from those of their constituent organic molecules, while the electronic structure of the molecules remains unchanged. J-aggregates exhibit an absorption peak, known as the J peak (about 10–20 nm wide), which is red-shifted (~100 nm) relatively to the absorption of the monomeric form of the dye [
12,
13]. The fluorescence spectra of J-aggregates coincide with the absorption spectra and have the same narrow width. Demonstrating behavior close to resonant, they have very small (∼200 cm
−1 and less) Stokes shifts and insensitivity to the environment [
14]. Additionally, a defining attribute of J-aggregates is enhanced molar absorption (quantified in M
−1 cm
−1 units) compared to isolated monomeric molecules [
15]. It should be noted that the quantum yield of J-aggregates can vary significantly; in some cases, it is much higher than that of the constituent monomers.
NAA, a dielectric matrix with tunable porosity and anisotropic light-scattering capabilities, offers a robust platform for integrating functional organic components such as J-aggregates of cyanine dyes. Unlike conventional random lasers reliant on Rhodamine 6G—a system plagued by low stability and efficiency [
16]—J-aggregates exhibit narrow resonant bands and enhanced quantum yields, enabling coherent feedback through multiple scattering in disordered media [
17]. However, traditional J-aggregate synthesis often requires polar solvents or electrolytes, complicating integration with nanostructured matrices. Here, we present a salt-free, solvent-controlled method to embed pseudoisocyanine J-aggregates into NAA pores via thermostimulated self-assembly. By leveraging NAA’s anisotropic dielectric properties and the resonant behavior of J-aggregates, we demonstrate a hybrid nanomaterial capable of amplified spontaneous emission without external resonators, advancing toward practical lasers for sensing and on-chip photonics.
2. Materials and Methods
The nanoporous anodic aluminum oxide membrane SM75100105 (thickness: 100 μm, pore diameter: 75 nm, interpore distance: 125 nm) was procured from Smart Membranes (Halle, Germany). The pseudoisocyanine dye, 1,1′-diethyl-2,2′-cyanine iodide (C23H23IN2), was purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultra-high purity grade ethanol 99.5% (purchased from LC “Ecohim”) was used as the solvent.
To prepare the hybrid material, a salt-free saturated ethanol solution of pseudoisocyanine dye was heated to ~75 °C (ethanol boiling point is 78.3 °C at standard atmospheric pressure) under ambient conditions using a digitally controlled magnetic stirrer with a hot plate and external temperature control. The NAA membrane was immersed in the heated dye solution for varying time intervals (0.5–8 h) and subsequently dried at room temperature, 23 °C, for reproducibility.
The morphology and nanopore dimensions of the NAA membranes were analyzed using a Merlin scanning electron microscope (SEM) (Zeiss, Jena, Germany). Absorption spectra of the samples were recorded in the range of 300–1100 nm using an SF56 spectrophotometer (LOMO, St. Petersburg, Russia). Luminescence spectra were acquired with an RF5301-PC spectrofluorometer (Shimadzu, Kyoto, Japan). Time-resolved photoluminescence decay kinetics with a temporal resolution of up to 100 ps were studied using a MicroTime 100 laser-scanning confocal microscope (PicoQuant, Berlin, Germany) equipped with a pulsed diode laser (wavelength: 405 nm) and correlated single-photon counting. To record an input–output characteristic of emission, the hybrid composite samples were irradiated with the second-harmonic of a Nd:YAG laser (LQ829 model, 532 nm wavelength, 10 ns pulse duration). The resulting emission spectra were measured using an AvaSpec-2048 Fiber Optic Spectrometer (Avantes BV, Eerbek, The Netherlands).
3. Results
3.1. Properties of the Nanoporous Alumina Membrane
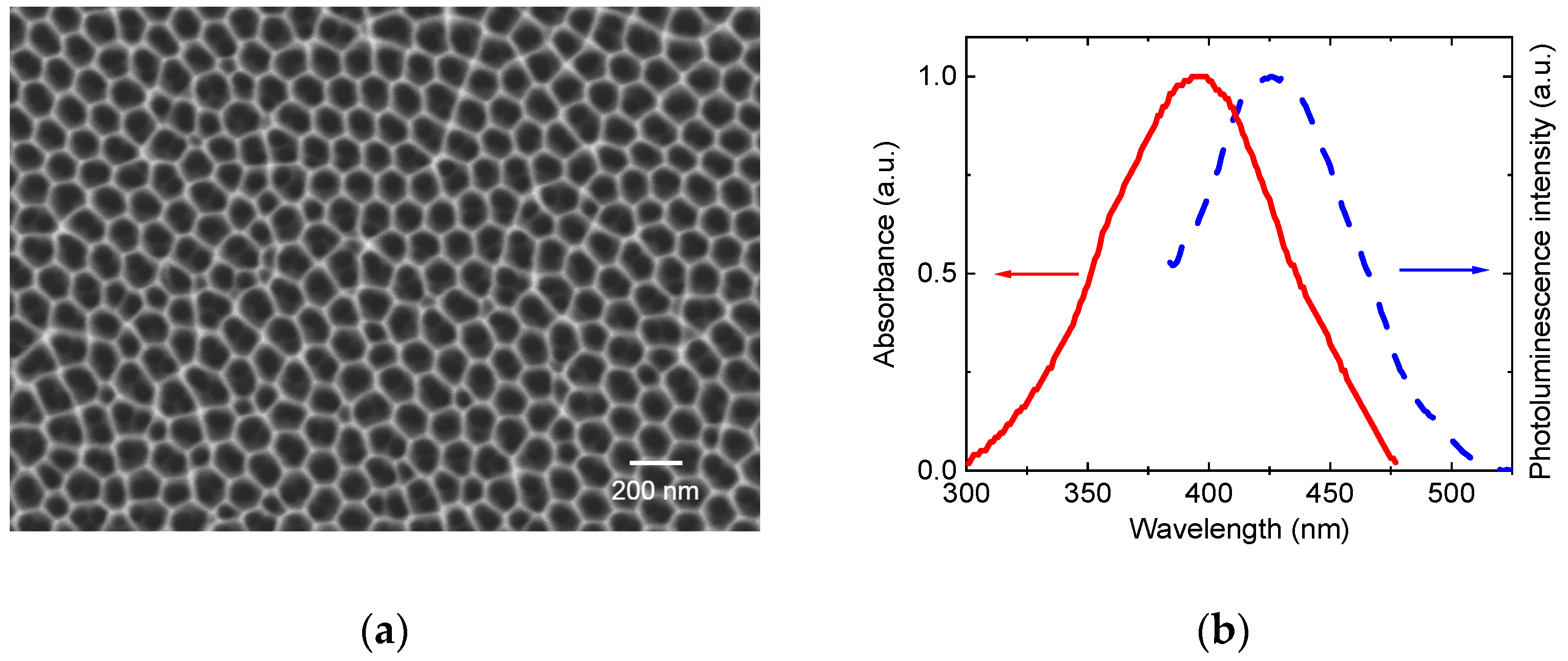
A commercially available SM75100105 anodic alumina membrane transparent in the visible range (thickness of 100 μm, pore diameter of 75 nm, interpore distance of 125 nm, manufactured by Smart membranes) was used as a matrix. The surface morphology (
Figure 1a) was studied using a SEM.
The optical properties of the NAA membrane were investigated using absorption and photoluminescence spectroscopy (
Figure 1b). The photoluminescence spectrum, acquired under 340 nm excitation (selected to align with the absorption edge of alumina), exhibited a broad emission band centered at 440 nm, attributed to F centers (oxygen vacancies) formed during the anodization process [
18]. The absence of significant absorption features in the visible range (450–700 nm) confirms the matrix’s suitability as a host for dye integration.
3.2. Optical Properties of the Hybrid Material
A series of experiments investigated how the optical properties of the hybrid material depend on the dye impregnation time. The normalized absorption spectra (
Figure 2) demonstrated that optimal J-aggregate formation occurred after 4 h of impregnation in heated ethanol (~75 °C), followed by ambient drying (23 °C, 40–60% humidity). The drying process was conducted to remove the solvent and produce dry samples. Cationic polymethine dyes, which include pseudoisocyanine dye, were adsorbed onto the surface through electrostatic interactions with oxygen [
19], forming molecular layers on the inner surfaces of the nanopores. Prolonged immersion (>4 h) reduced aggregation efficiency, likely due to dye leaching or re-dissolution.
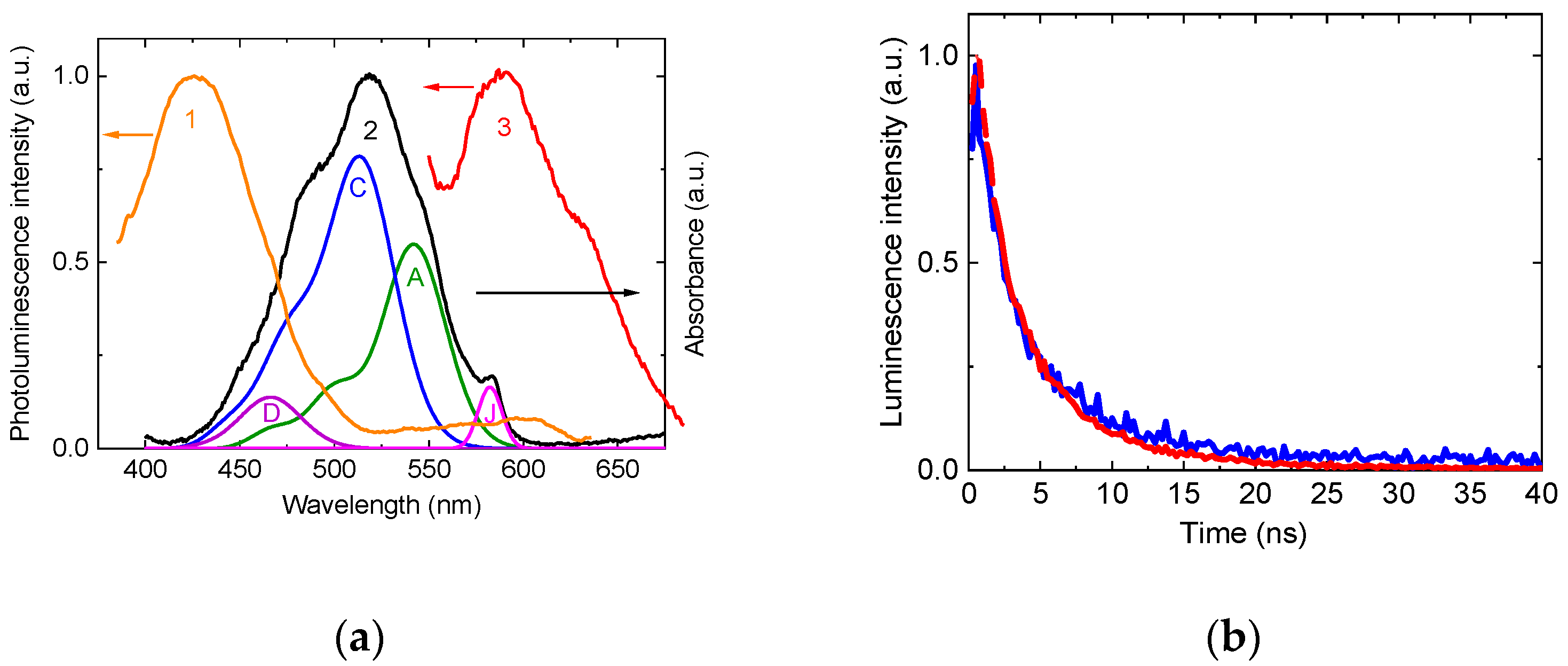
The hybrid material’s absorption spectrum (
Figure 2) exhibited inhomogeneous broadening compared to the dye on an ordinary sapphire slide, attributed to trans-cis-isomerization and aggregation (formation of dimer and J-aggregates) within the nanopores. Deconvolution identified four components: all-trans isomers (A, λ ≈ 542 nm), cis-isomers (C, λ ≈ 512 nm), dimers (D, λ ≈ 466 nm), and J-aggregates (J, λ = 583 nm) [
20]. The J-aggregate absorption peak is redshifted by ~40 nm in relation to the absorption of monomeric forms of the dye, and its narrow bandwidth aligns with characteristic excitonic coupling in ordered aggregates.
Photoluminescence spectra under 340 nm (NAA excitation) and 510 nm (dye excitation) revealed energy transfer from the alumina matrix to J-aggregates (
Figure 3a). Excitation at 510 nm produced a sharp emission band for the J-aggregates at 585 nm [
12], while 340 nm excitation yielded weaker J-aggregate luminescence alongside the NAA’s intrinsic 440 nm F-center emission. The luminescence of J-aggregates in alumina is enhanced due to Förster resonance energy transfer [
6]. This is evidenced by the quenching of luminescence in the luminescent centers of NAA, an increase in the intensity of luminescence from the dye molecules, and a decrease in the decay time of anodic alumina luminescence with the addition of dye.
Time-resolved photoluminescence decay kinetics, measured using 405 nm excitation (
Figure 3b), were fitted to a biexponential model, where each exponent corresponds to a specific type of luminescent center [
21]. τ
1 and τ
2 represent lifetimes of the fast and the slow components, and A
1, A
2 denote their relative contributions (
Table 1). Kinetics reveal competing effects: energy transfer from NAA’s F-centers to J-aggregates reduces τ
2 by 50% (13→6.4 ns), while the 4× rise in A
2 (0.31→1.3) reflects emergent radiative pathways from J-aggregates. This duality enables efficient light amplification in the hybrid material.
3.3. Study of the Process of Laser-Induced Emission of a Hybrid Sample
An experimental setup (
Figure 4) was designed to achieve stimulated emission from the hybrid sample without an external resonator. The sample was pumped using the second harmonic of a Nd:YAG laser with 250 mJ output energy. The beam spot was close to circular with a diameter of 8 mm. Neutral density filters were used to achieve variable pump power densities, and a long pass filter was used to cut off the wavelength of the excitation laser radiation. The emitted signal was recorded with an Avantes fiber spectrometer.
The pump power density on the sample ranged from 0.5 to 4.3 MW/cm
2 after attenuation. Emission spectra (
Figure 5a) revealed a nonlinear increase in intensity with rising pump power density, accompanied by spectral narrowing. The full-width-at-half-maximum (FWHM) of the emission peak decreased from 1060 cm
−1 to 300 cm
−1, confirming the onset of amplification [
22,
23].
Based on the emission spectra obtained during a series of experiments, the dependence of the output emission intensity of the dye-activated NAA membrane on the attenuated pump energy (via neutral density filters) was analyzed (
Figure 5b). As the pump energy increases, the emission intensity exhibits a nonlinear rise, accompanied by a pronounced narrowing of the spectral profile. This contrasts sharply with the linear dependence characteristic of photoluminescence (e.g., under 510 nm excitation), confirming the transition to an amplified emission regime.
The hybrid material exhibits stability, retaining its absorption and luminescence properties under high-power excitation (4.3 MW/cm2). Long-term stability tests (up to 60 days) in ambient laboratory conditions (dark, 23 °C, 40–60% humidity) indicated no significant changes in the absorption spectra, suggesting a stable performance of the dye in this matrix.
4. Discussion
The integration of J-aggregates into nanostructured matrices represents a compelling strategy for advancing photonic materials [
24,
25], yet achieving precise control over aggregation dynamics while maintaining compatibility with the host matrix remains a challenge [
26]. Our work addresses this by demonstrating a salt-free thermal-stimulated synthesis of pseudoisocyanine J-aggregates within NAA, a method that bypasses the need for polar solvents or electrolytes typically required for J-aggregate stabilization. This approach not only simplifies fabrication but also minimizes dye leaching—a common issue in porous systems—by leveraging the interplay between solvent polarity, thermal activation, and pore confinement.
The observed time-dependent decline in J-aggregate luminescence beyond 4 h of impregnation (
Figure 2) suggests a delicate balance between aggregation and dissociation. Prolonged immersion likely disrupts the equilibrium between monomeric dye and J-aggregates, favoring the re-dissolution or reorientation within the pores. This behavior aligns with studies on aggregation kinetics in confined geometries [
3,
27], where pore size and surface interactions critically influence molecular packing. Our results imply that kinetic control, rather than thermodynamic equilibrium, governs J-aggregate formation in NAA, a distinction with profound implications for scalable fabrication [
28].
The inhomogeneous broadening of the hybrid material’s absorption spectrum (
Figure 2) underscores the complexity of dye-matrix interactions. While trans-cis isomerization and dimerization are well documented in cyanine dyes [
29,
30], the coexistence of these species with J-aggregates in NAA pores highlights the matrix’s role as a nanoscale reactor that stabilizes multiple aggregation states. Notably, the J-aggregate absorption at 583 nm exhibits a redshift of ~40 nm compared to monomeric dye, consistent with excitonic coupling in tightly packed molecular stacks [
12]. However, the broader J-band relative to ideal J-aggregates (Δν < 100 cm
−1) suggests partial disorder, possibly arising from pore wall interactions or heterogeneous dye distribution [
31].
The energy transfer from NAA’s oxygen vacancies (F centers) to J-aggregates (
Figure 3a) provides a pathway for indirect excitation of the dye, a phenomenon rarely exploited in random lasers [
23,
32,
33,
34]. Under 340 nm excitation—where the dye itself does not absorb—the weak but detectable J-aggregate emission (585 nm) implies non-radiative Förster resonance energy transfer (FRET) [
6]. This mechanism could enhance device efficiency by utilizing the matrix’s broadband absorption to sensitize the dye, a feature that is absent in conventional Rhodamine 6G-based systems.
5. Conclusions
This study successfully demonstrates the development and characterization of a novel hybrid material composed of nanoporous anodic alumina integrated with J-aggregates of pseudoisocyanine dye. The stable incorporation of J-aggregates into the alumina matrix was achieved through impregnation using a thermo-stimulated self-assembly method in an ethanol solution of dye, without the addition of salts. Optimal impregnation conditions (4 h at ~75 °C) were critical to maximizing J-aggregate formation, as prolonged exposure led to reduced aggregation efficiency, evidenced by absorption and photoluminescence spectral analysis.
Key findings include the observation of energy transfer from oxygen vacancy centers (F centers) in the alumina matrix to the J-aggregates, confirmed by luminescence studies under 340 nm excitation. Furthermore, the material exhibited signs of amplified emission under the pulsed second harmonic of Nd:YAG-laser excitation, characterized by a nonlinear increase in output intensity and spectral narrowing above a threshold pump power density of 0.9 MW/cm2. This behavior, achieved without an external resonator, underscores the role of multiple scattering within the alumina porous structure in providing feedback for amplified spontaneous emission.
The developed hybrid material holds promising potential to fabricate compact and efficient light sources for various applications, including bio-visualization and sensing.
Author Contributions
Conceptualization, A.A.S.; investigation, A.A.S. and E.O.S.; writing—original draft preparation, E.O.S.; writing—review and editing, A.A.S. and N.T.; visualization, A.A.S. and E.O.S.; supervision, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by ITMO University Research Projects in AI Initiative (RPAII), project number 640098.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
The authors would like to thank Resida D. Nabiullina for her technical assistance in conducting the experiments described in
Section 3.3, as well as Mikhail A. Baranov for performing the measurements using the SEM (
Figure 1a).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| NAA | Nanoporous anodized alumina |
| FWHM | Full-width-at-half-maximum |
| FRET | Förster resonance energy transfer |
| SEM | Scanning electron microscopy |
References
- Ruiz-Clavijo, A.; Caballero-Calero, O.; Martín-González, M. Revisiting Anodic Alumina Templates: From Fabrication to Applications. Nanoscale 2021, 13, 2227–2265. [Google Scholar] [CrossRef] [PubMed]
- Domagalski, J.T.; Xifre-Perez, E.; Marsal, L.F. Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications. Nanomaterials 2021, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Santos, A.; Losic, D. Nanoporous Anodic Alumina Platforms: Engineered Surface Chemistry and Structure for Optical Sensing Applications. Sensors 2014, 14, 11878–11918. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Shah, M.A.; Ganai, P.A. Dielectric study of nanoporous alumina fabricated by two-step anodization technique. Chem. Pap. 2021, 75, 503–513. [Google Scholar] [CrossRef]
- Nikitin, I.Y.; Nabiullina, R.D.; Nashyokin, F.V.; Starovoytov, A.A.; Gladskikh, I.A. Anodization Parameters Influence on Anodic Aluminum Oxide Formed above the Silver Island Film. J. Sci. Tech. Inf. Technol. Mech. Opt. 2023, 23, 28–34. [Google Scholar] [CrossRef]
- Nikitin, I.Y.; Borodina, L.N.; Boltenko, A.V.; Baranov, M.A.; Gladskikh, I.A.; Vartanyan, T.A. Förster Resonant Energy Transfer from Alumina Luminescence Centers to Dye Molecules Adsorbed in Anodic Alumina Thin Films Used as Coatings and in Sensors. Opt. Mater. 2025, 160, 116741. [Google Scholar] [CrossRef]
- Lutich, A.A.; Gaponenko, S.V.; Gaponenko, N.V.; Molchan, I.S.; Sokol, V.A.; Parkhutik, V. Anisotropic Light Scattering in Nanoporous Materials: A Photon Density of States Effect. Nano Lett. 2004, 4, 1755–1758. [Google Scholar] [CrossRef]
- Singh, S.K.; Khandekar, S.; Pratap, D.; Ramakrishna, S.A. Wetting Dynamics and Evaporation of Sessile Droplets on Nano-Porous Alumina Surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2013, 432, 71–81. [Google Scholar] [CrossRef]
- Gunenthiran, S.; Wang, J.; Law, C.S.; Abell, A.D.; Alwahabi, Z.T.; Santos, A. Nanoporous Anodic Alumina Photonic Crystals for Solid-State Lasing Systems: State-of-the-Art and Perspectives. J. Mater. Chem. C 2025, 13, 985–1012. [Google Scholar] [CrossRef]
- Ibrayev, N.K.; Zeinidenov, A.K. Plasmon-Enhanced Stimulated Emission of Rhodamine 6 G in Nanoporous Alumina. Laser Phys. Lett. 2014, 11, 115805. [Google Scholar] [CrossRef]
- Ilina, K.; Henary, M. Cyanine Dyes Containing Quinoline Moieties: History, Synthesis, Optical Properties, and Applications. Chem. –A Eur. J. 2021, 27, 4230–4248. [Google Scholar] [CrossRef]
- Bricks, J.L.; Slominskii, Y.L.; Panas, I.D.; Demchenko, A.P. Fluorescent J-Aggregates of Cyanine Dyes: Basic Research and Applications Review. Methods Appl. Fluoresc. 2017, 6, 012001. [Google Scholar] [CrossRef]
- Shindy, H.A. Fundamentals in the Chemistry of Cyanine Dyes: A Review. Dye. Pigment. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef]
- Eisfeld, A.; Briggs, J.S. The Shape of the J-Band of Pseudoisocyanine. Chem. Phys. Lett. 2007, 446, 354–358. [Google Scholar] [CrossRef]
- Padiyakkuth, N.; Thomas, S.; Antoine, R.; Kalarikkal, N. Recent Progress and Prospects of Random Lasers Using Advanced Materials. Mater. Adv. 2022, 3, 6687–6706. [Google Scholar] [CrossRef]
- Marangi, M.; Wang, Y.; Wu, M.; Tjiptoharsono, F.; Kuznetsov, A.I.; Adamo, G.; Soci, C. Enhancing Cooperativity of Molecular J-Aggregates by Resonantly Coupled Dielectric Metasurfaces. Nanophotonics 2024, 13, 3519–3526. [Google Scholar] [CrossRef]
- Cantelli, L.; Santos, J.S.; Silva, T.F.; Tabacniks, M.H.; Delgado-Silva, A.O.; Trivinho-Strixino, F. Unveiling the Origin of Photoluminescence in Nanoporous Anodic Alumina (NAA) Obtained by Constant Current Regime. J. Lumin. 2019, 207, 63–69. [Google Scholar] [CrossRef]
- Kaliteevskaya, E.; Krutyakova, V.; Razumova, T.; Roshal, A.; Starovoytov, A. Optical properties and component composition of layers of cyanine dyes on dielectric supports: Influence of asymmetry of the molecular electron density distribution. Opt. Quantum Electron. 2017, 49, 32. [Google Scholar] [CrossRef]
- Kaliteevskaya, E.N.; Krutyakova, V.P.; Razumova, T.K.; Starovoytov, A.A. Identification of Absorption Bands of Monomers and Aggregates in a Layer of Cyanine Dye and Determination of the Orientation of Molecules. Opt. Spectrosc. 2018, 125, 425–432. [Google Scholar] [CrossRef]
- Sorokin, A.V. Features of J-Aggregates Formation in Pores of Nanostructured Anodic Aluminum Oxide. Funct. Mater. 2014, 21, 42–46. [Google Scholar] [CrossRef]
- Vrublevsky, I.; Chernyakova, K.; Ispas, A.; Bund, A.; Gaponik, N.; Dubavik, A. Photoluminescence properties of heat-treated porous alumina films formed in oxalic acid. J. Lumin. 2011, 131, 938–942. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Shaik, S.; Ramakrishna, S.A. Lasing in Dye-Infiltrated Nanoporous Anodic Alumina Membranes. Appl. Phys. B 2018, 124, 127. [Google Scholar] [CrossRef]
- Sundaresan, A.; Nuchikkat, S.; Alee, K.S. On Chip Random Lasing Performance of the Acceptor Dye in a Specially Designed Linear and Zig Zag Array of Microdroplets with Intrinsic Disorder. Sci. Rep. 2022, 12, 3939. [Google Scholar] [CrossRef]
- Barotov, U.; Thanippuli Arachchi, D.H.; Klein, M.D.; Zhang, J.; Šverko, T.; Bawendi, M.G. Near-Unity Superradiant Emission from Delocalized Frenkel Excitons in a Two-Dimensional Supramolecular Assembly. Adv. Opt. Mater. 2023, 11, 2201471. [Google Scholar] [CrossRef]
- Cacciola, A.; Triolo, C.; Di Stefano, O.; Genco, A.; Mazzeo, M.; Saija, R.; Patanè, S.; Savasta, S. Subdiffraction Light Concentration by J-Aggregate Nanostructures. ACS Photonics 2015, 2, 971–979. [Google Scholar] [CrossRef]
- Kanno, R.; Stolte, M.; Stepanenko, V.; Pöppler, A.-C.; Terashima, T.; Würthner, F. Perylene Bisimide J-Aggregates in a Polymer Matrix: Controlling Self-Assembly and Fluorescence Properties. J. Mater. Chem. C 2025, 13, 5981–5987. [Google Scholar] [CrossRef]
- Pisklova, P.; Ropakova, I.; Bespalova, I.; Kryvonogov, S.; Viagin, O.; Yefimova, S.; Sorokin, A. Features of Cyanine Dyes Aggregation on Differently Charged TiO2 Matrices. Chem. Phys. Impact 2023, 6, 100176. [Google Scholar] [CrossRef]
- Occhiuto, I.G.; Castriciano, M.A.; Trapani, M.; Zagami, R.; Romeo, A.; Pasternack, R.F.; Monsù Scolaro, L. Controlling J-Aggregates Formation and Chirality Induction through Demetallation of a Zinc(II) Water Soluble Porphyrin. Int. J. Mol. Sci. 2020, 21, 4001. [Google Scholar] [CrossRef]
- Espinoza Cangahuala, M.K.; Krishnaswamy, S.R.; Kuevda, A.V.; Pshenichnikov, M.S.; Jansen, T.L.C. The First Step of Cyanine Dye Self-Assembly: Dimerization. J. Chem. Phys. 2025, 162, 054311. [Google Scholar] [CrossRef]
- Pronkin, P.; Tatikolov, A. Isomerization and Properties of Isomers of Carbocyanine Dyes. Sci 2019, 1, 19. [Google Scholar] [CrossRef]
- Eisfeld, A.; Briggs, J.S. The J-Band of Organic Dyes: Lineshape and Coherence Length. Chem. Phys. 2002, 281, 61–70. [Google Scholar] [CrossRef]
- Sakurayama, Y.; Onodera, T.; Araki, Y.; Wada, T.; Oikawa, H. Random Laser Oscillation from an Organic Fluorescent Dye Loaded inside a Porous Zirconia Medium. RSC Adv. 2021, 11, 32030–32037. [Google Scholar] [CrossRef] [PubMed]
- Toropov, N.; Kamalieva, A.; Starovoytov, A.; Zaki, S.; Vartanyan, T. Polarized Stimulated Emission of 2D Ensembles of Plasmonic Nanolasers. Adv. Photonics Res. 2021, 2, 2000083. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).