1. Introduction
With light-emitting diodes (LEDs) having become ubiquitous, their technology has seen rapid developments on many fronts [
1,
2,
3]. The semiconductor chips powering LED lighting have, more or less, matured over the past decades [
4]. However, the luminescent wavelength conversion materials (phosphors) as well as the optical design and packaging of LEDs are still undergoing rapid developments. These are driven by the desire to have superior products that could be differentiated in a crowded marketplace. While the very first white-emitting LEDs were based on cerium-doped yttrium aluminum garnet (YAG) phosphor applied to blue-emitting InGaN/GaN LED chips, nowadays LEDs incorporating multiple phosphor components are readily available [
5,
6,
7]. These can emit white light of different chromaticities (color point), with their visual appearance ranging from cool white to warm white. Such phosphors are based on the traditional YAG:Ce phosphor mixed with a small amount of red- or orange-emitting phosphor. Their exact mixing ratio determines the spectral content of LED emission and, thus, the correlated color temperature (CCT). This way of making white LEDs of different CCTs has remained the prevailing technology for well over two decades. Although this technique works well and is easy to use, it only allows adjusting LED chromaticity within a limited range. Furthermore, manufacturers have to resort to different red- or orange-emitting phosphors to obtain a range of chromaticity values and CCTs. Here, we describe our recent work on a much more flexible technique for adjusting LED chromaticity that allows adjustment within a large emission color space and makes use of only one material for this purpose. Our technology makes use of solvatochromism [
8] to obtain a virtually unlimited palette of emission colors that can be used to adjust the chromaticity point of LEDs based on YAG:Ce or similar phosphors.
2. Principles of Solvatochromism
Luminescent materials can emit radiation at one wavelength and emit the absorbed energy as radiation at another (usually longer) wavelength. While most luminescent materials are used in their solid state, some are liquids or can be dissolved in appropriate solvents to make luminescent liquids. Some of these solute–solvent systems can be used as liquid phosphors for wavelength conversion. The use of traditional powder phosphors is obviously more convenient than the use of liquid materials for wavelength conversion. However, a certain class of liquid phosphors offers the advantage of easy emission wavelength tunability, which makes them very interesting. They owe their color-adjusting properties to the phenomenon of solvatochromism, where changes in a solvent affect the absorption and emission spectrum of a dissolved solute. Solvatochromic dyes have been well-known for many years. These dyes exhibit colors that depend on the identity of the solvent they are dissolved in. The most prominent example is Reichardt’s dye, which can show a very wide range of colors when dissolved in different solvents. However, to be useful for LED wavelength conversion applications, dyes need to show both solvatochromism and luminescence (fluorescence), which limits the number of potentially useful compounds.
Solvatochromism is the liquid-state analog of the crystal field effect that is well-known in solid-state systems. An ion substitutionally doped inside a crystal host gets its energy levels altered by the electrostatic effect of the surrounding host matrix. Thus, the same ion doped in different crystals can exhibit different absorption and emission spectra. This crystal field effect is widely used to tune the spectral characteristics of various ions in order to suit application requirements. In situations where solvatochromism is observed, a chromophore-bearing solute molecule is dissolved in a suitable solvent. The surrounding solvent molecules can perturb the atomic or molecular energy levels of the solute molecules, producing a solvent-dependent shift in the absorption and emission spectra of the solute [
9]. The polarity of the solvent plays the main role here because it is the dipolar electrostatic field of the ambient solvent that affects the energy levels of the solute. Thus, a change in polarity of the solvent changes the spectrum of light transmitted out of a solution [
10,
11]. The change in solvent polarity can be affected in one of several different ways. These include use of different solvents having different polarities, mixing a solvent of a different polarity in a main solvent, etc. Because of the fine control possible over solvent polarity, it is possible to finely tune the spectral response of a given solvatochromic solute. Thus, a single active luminescent compound can be used with a customized solvent system to achieve the desired spectral conversion. Obviously, this is a powerful technique for achieving selected emission color points within the space of possible chromaticities, given a specific solvatochromic compound. The use of solvatochromic compounds as wavelength up-converters in LEDs has been now been recognized for a few years [
12,
13] and several researcher groups, including ours, have been working on exploring various possibilities, including those described here.
We used the solvatochromic compound Tris(bipyridine)ruthenium(II) chloride, abbreviated hereafter as TBPR chloride, in the work reported here. This organo-metallic coordination compound has a ruthenium atom core surrounded by three bipyridine ligands. It forms transparent red crystals that are soluble in a variety of solvents. In our previous work, we have demonstrated the utility of this compound for making red LEDs capable of emitting different shades of red light through the solvatochromic tuning of down-converted emission wavelengths [
14]. Our previous paper should be consulted for further in-depth information on various aspects of the science and operation of solvatochromic LEDs.
3. Device Construction
Our approach, described here, toward making white light LEDs with specific chromaticities or CCTs was based on combining red, green, and blue (RGB) light components. The blue color was generated as the ‘bleed’ radiation from a blue pump LED. The green color was produced by a blue-to-green color-converting powder phosphor. The red color was solvatochromically synthesized from the absorption of some of the blue light from the LED. In accordance with this description, our device consisted of several distinct components: a blue-emitting packaged LED device, a layer of green-emitting phosphor, and a cell containing a liquid solvatochromic medium. By appropriately combining these components we were able to generate white light and by changing the solvatochromic component we were able to obtain different white light chromaticities.
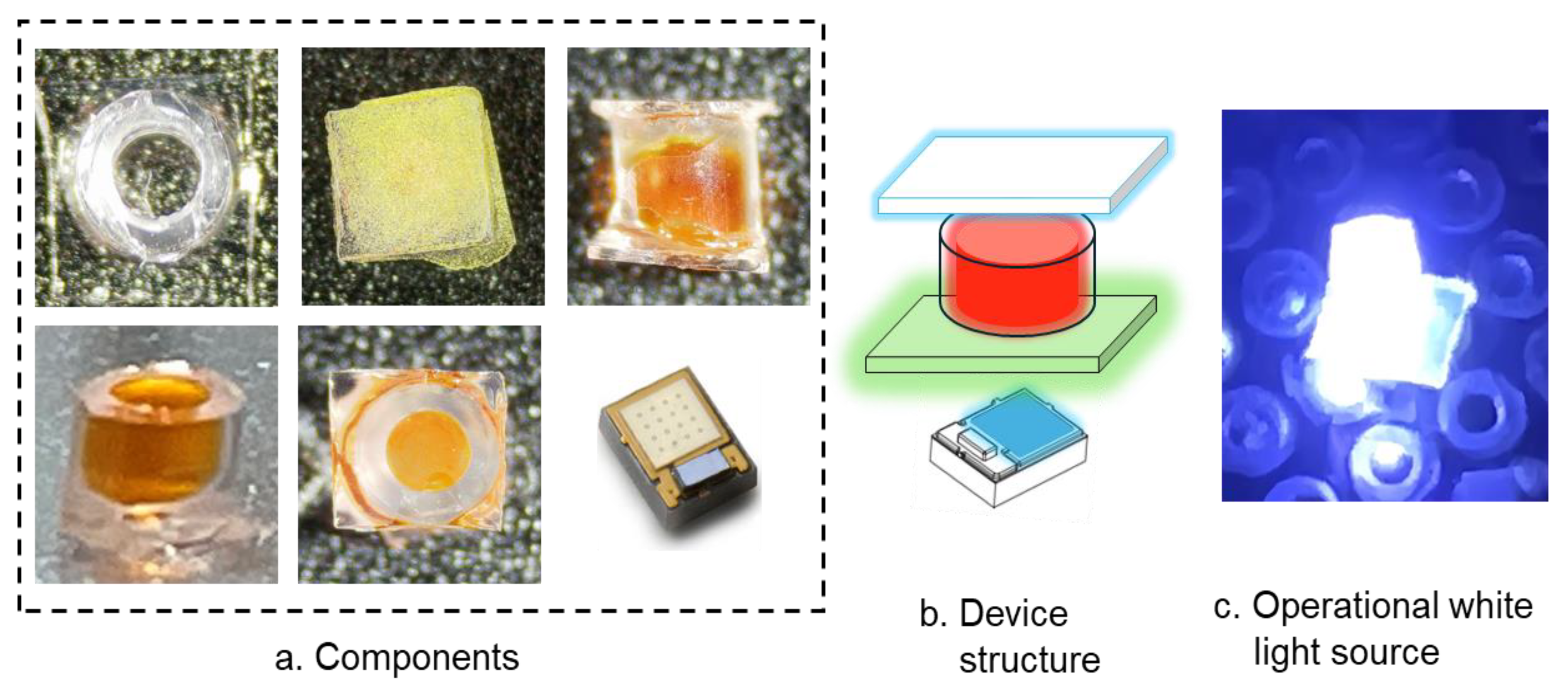
In
Figure 1, an operational white light LED is shown along with the structure of the device and its component parts. The compilation of six images in
Figure 1a depicts the various sub-components: the cylindrical casing for the liquid red phosphor (both side and top views provided), the green phosphor film, and the pump LED. The cool white light emission shown is hard to assess in
Figure 1c because of the brightness of the light tending to saturate the camera used to take the images, despite the use of an optical neutral density filter. The six major components depicted in
Figure 1a were as follows: The blue pump LED was a commercial InGaN/GaN (DS’105 Luxeon Z) from LumiLeds (San Jose, CA, USA), with peak emission at 450 nm. It was driven at a constant current of 500 mA. The choice of the LED was made to align the peak emission with the peak absorption band of the liquid phosphor and the green phosphor used. These tiny SMD LEDs with a footprint of 2.2 mm
2 formed the base of the white LED structure. The second component was a small flat glass plate (2.0 mm × 2.0 mm × 0.15 mm), coated with a green-emitting cerium-doped YAG phosphor (YAG:Ce) from Sigma Aldrich Corporation (St. Louis, MO, USA) (SGA 545 100 isiphor™). Polymethyl methacrylate (PMMA 996 K molecular weight) 5.0 wt. % dissolved in ethyl acetate was used as the binder for the green phosphor. The PMMA-bound phosphor film thickness was 120 µm. We tested other commonly used binders like KASIL™ and Dow Corning gel but decided to use PMMA as a low-cost, versatile, and easy-to-process option. Fixed to the top of the phosphor-coated plate was a hollow cylindrical plastic cylinder (2.0 mm height × 0.45 mm radius, with an outer radius of 1.05 mm) filled with a liquid, red-emitting, solvatochromic phosphor. The cylinder was hermetically sealed with another thin glass plate.
For the purpose of making our red solvatochromic luminescent medium, we dissolved TBPR chloride crystals in four separate solvents with different polarities: deionized (DI) water, 1-butanol, dimethyl sulfoxide (DMSO), and acetonitrile. Additionally, 16 mg of TBPR chloride was dissolved in 6 mL of each solvent.
Figure 1b provides a schematic diagram of our LEDs. The proper arrangement during assembling the device is crucial, as shorter wavelength photons from the optical pump source need to be partially converted to longer wavelength photons in the green and red luminescent materials, such that the final output is a balanced spectrum of all the three colors.
At room temperature, the photoluminescence quantum yield of TBPR chloride solutions in ordinary solvents is around 28% [
15]. While this appears to be low, it does not matter in applications where this material is used for tuning the red point of an LED because only a small amount of light is used for this purpose, with most light being in the blue-green region.
In our experiments, the demonstrated spectral tunability can be directly attributed to the characteristic solvatochromic shifts of the down-converted red light due to the different TBPR-solvent medium interactions in the liquid phosphor.
4. LED Operation
LEDs, constructed as described above, were tested by operating them with 100 mA of DC current. Their spectra and other relevant parameters were measured. The results thus obtained are described in the two sub-sections below.
It should also be pointed out here that the reliability of LEDs is also a prime consideration in any device development work [
16]. Devices utilizing properly sealed solvatochromic luminescent materials are very stable over hours and days of continuous operation. However, over periods of many days, they do exhibit a gradual shift in chromaticity point. This is believed to be due to the thermal degradation of solvatochromic compounds. Further details can be obtained from [
14]. To address this issue, the heat dissipation design of these LEDs must be improved, such as by using fin-like structures. In addition, designing new solvatochromic molecules that are more robust toward heat will also improve the long-time performance of solvatochromic LEDs.
4.1. LED Emission Spectrum
The optical spectra of the excitation blue light, the partially converted green light, and down-converted red light depicted sequentially in
Figure 2 provide a better understanding of the two-step light conversion leading to the white light emission. The vertical axes in the four plots represent normalized arbitrary light intensities. A color bar has also been provided to serve as a visual reference. All spectra and related information were obtained by an Ocean Optics spectrometer.
From the top of the figure, the first plot (
Figure 2a) shows a relatively narrow but sharp peak (full width at half maximum ~20 nm) from just the blue LED. The second plot (
Figure 2b) shows the spectrum of emission from the green phosphor, excited by light from the blue LED. An additional wide hump around 550 nm is seen, as some of the high energy photons from the pump light are converted to photons of longer wavelengths in the green region. Note that a significant blue “bleed” from non-converted blue photons is present around 450 nm. The third plot (
Figure 2c) depicts the spectrum of blue light directly pumping the liquid red phosphor, in this case, TBPR dissolved in 1-butanol. The down-converted second asymmetric peak is at 606 nm and spans a broad wavelength region from 560 nm to 750 nm. The fourth plot (
Figure 2d) shows the spectrum of emission from the fully assembled device, i.e., of the light that is seen in
Figure 1c. The separate green and blue spectral emissions have smoothly merged together to give a broadband emission from the green to the red region of visible spectrum. The smooth broadband spectral characteristics of solvatochromic LEDs makes them a good choice for illuminating colorful objects because the light from such LEDs contains a wide distribution of wavelengths [
17,
18].
4.2. LED Chromaticity
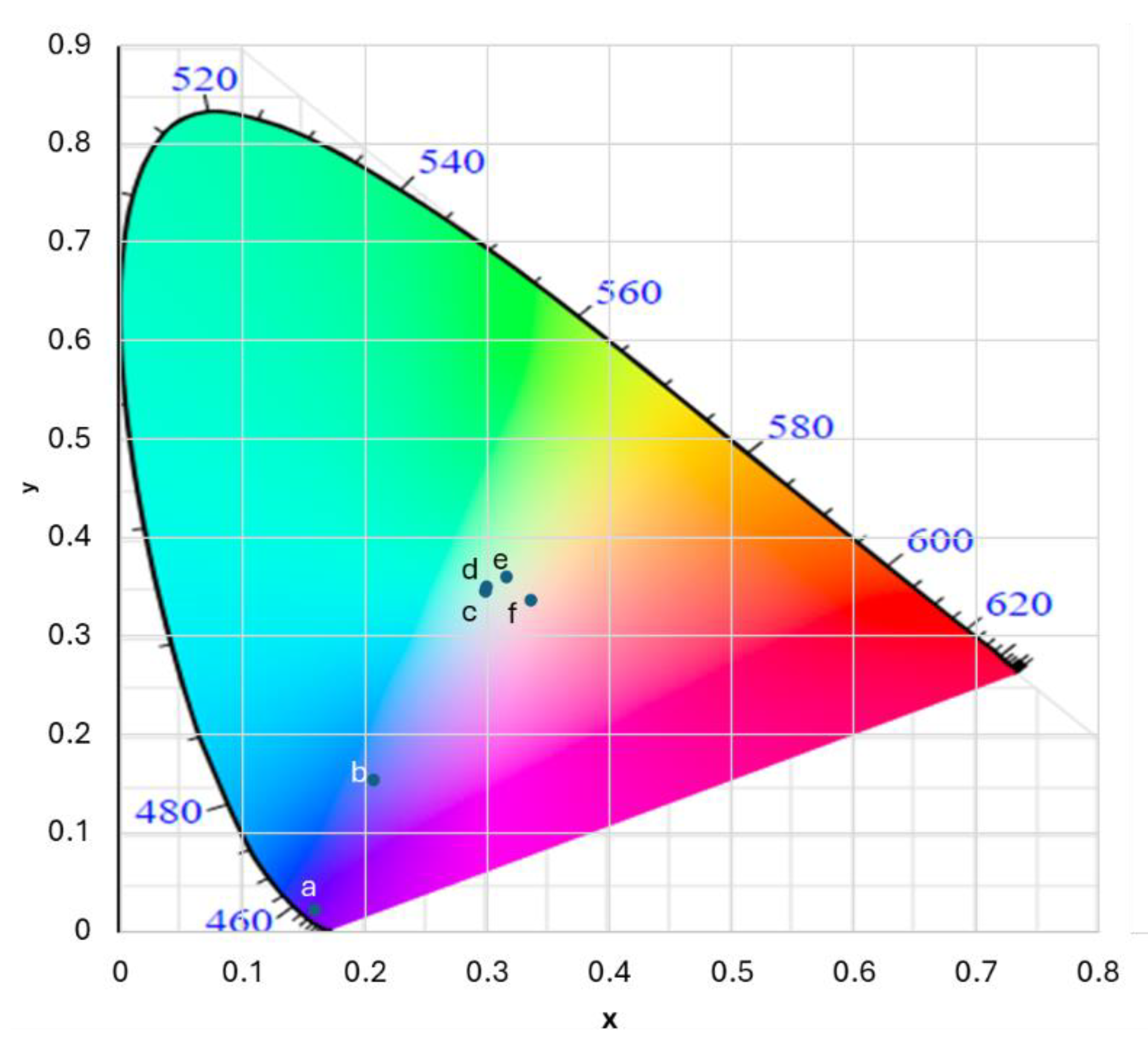
In the CIE diagram shown in
Figure 3, the six chromaticity points belong to different types of emitted light. The point marked ‘a’ is the extreme blue from the pump source (corresponding to the spectrum shown in
Figure 2a). This point is close to the boundary of the diagram because of the high saturation of this emission as a result of narrowband emission from the bare LED. The point marked ‘b’ pertains to the combination of solid phosphor converted green light and the blue bleed light (corresponding to the spectrum shown in
Figure 2b). The four points marked c, d, e, and f are the near-white emissions from fully constructed LEDs utilizing different solvents for TBPR chloride. As can be observed, all white emissions lie in the white region but in such a way that the emissions from the LEDs containing 1-butanol and DMSO appear to be a cooler shade of white, whereas that from LEDS containing acetonitrile and water appear to be a warmer shade of white. It must be noted that multiple other factors, like the amount of blue pump light (drive current of the blue LED), the concentration and thickness of the green phosphor in PMMA, the concentrations of the TBPR dissolved in the polar solvent mediums, etc., need to be balanced in the right proportions in order to generate visibly white light.
Table 1 provides the values of the chromaticity coordinates and correlated color temperatures (CCTs) in Kelvin for all white chromaticity points that appear in
Figure 3.
5. Umbelliferone for Wider Solvatochromic Control
The solvatochromic shift resulting from the change of four different solvents with TBPR chloride offers a limited range of luminescence tunability in the red-orange region. This, however, is completely sufficient to generate various distinct shades of cool or warm white light. By using a different solvatochromic compound—umbelliferone (7-hydroxy-coumarin)—it is possible to bring about even greater tuning of chromaticity points. This is because of the much larger solvation dependence of fluorescence exhibited by this bi-cyclic phenolic compound [
19]. Even varying the pH of aqueous umbelliferone solutions can shift the absorption and emission spectral profiles [
20]. Furthermore, just varying the concentration of water (a highly polar solvent) is enough to cause prominent spectral changes. This can be seen in
Figure 4, where the emission spectra of acetonitrile solutions of umbelliferone containing different amounts (w/w %) of water are shown. Umbelliferone for our experiments was obtained from Sigma-Aldrich Corporation. It should be noted that umbelliferone absorbs strongly in the UV region and converts absorbed radiation to blue-green light in the visible region. Our experiments with this compound were done with a 365 nm UV-emitting LED.
When excited at this wavelength, the emission spectra show two distinct peaks, one around 405 nm and the other around 480 nm. As increasing amounts of de-ionized (DI) water were added to the acetonitrile solution of umbelliferone, the first peak (405 nm) gradually lost strength while the second peak (480 nm) gradually gained strength. These changes are summarized in
Figure 4b. Because of simultaneous changes in two different spectral peaks, umbelliferone solutions show a very distinct emission color change in the blue-green region. This can be, potentially, very useful for making LEDs whose light can be spectrally adjusted over a wide range of wavelengths, yielding well-separated chromaticity points spanning a large area on the chromaticity diagram. In this case, the green conversion component will be based on an umbelliferone solution while the red conversion component can be either a fixed solid red phosphor or a TBPR chloride solution. For even better performance, one can use 6,7-dihydroxycoumarin instead of umbelliferone. This compound absorbs even more strongly at 365 nm [
21] and, thus, can be a better choice for this application. While other approaches to wide chromaticity and CCT color tuning have been described in the recent literature, such as through the use of organolead halide perovskite nanocrystals [
22], our approach, described in this paper, is notable for its simplicity and, thus, its ease of implementation.
6. Conclusions
It is clear from our work that by employing solvatochromism in appropriate configurations, it is possible to tailor the emission spectrum of LEDs and produce white light LEDs with desired chromaticity points. The work described here paired a solvatochromic medium (TBPR chloride) with a traditional green phosphor to produce white light with different chromaticity points and CCTs. We also showed that suitable blue-green solvatochromic converters also exist. Both red and green conversion can be performed, if desired, by using solvatochromic wavelength converters. Furthermore, by incorporating solvatochromic chromophores with liquid crystal molecules it may be possible in the future to dynamically (electrically) change the wavelength of fluorescent emissions. This is a tantalizing possibility which is worth looking into.
Author Contributions
Conceptualization, F.R.; Methodology, F.R., M.M.H. and G.S.II; Validation, M.M.H. and G.S.II; Formal Analysis, F.R., M.M.H., and G.S.II; Investigation, M.M.H. and G.S.II; Resources, F.R.; Writing—Original Draft Preparation, F.R.; Writing—Review and Editing, F.R., M.M.H. and G.S.II; Visualization, M.M.H. and G.S.II; Supervision, F.R.; Project Administration, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All experimental data and associated information are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pryde, J.R.; Whalley, D.C.; Malalasekera, W. A review of LED technology trends and relevant thermal management strategies. In Proceedings of the Fourteenth Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), Orlando, FL, USA, 27–30 May 2014; pp. 31–38. [Google Scholar]
- Rahman, F. Solid-state lighting with wide band gap semiconductors. MRS Energy Sustain. 2014, 1, 6. [Google Scholar] [CrossRef]
- Taki, T.; Strassburg, M. Review—Visible LEDs: More than Efficient Light. ECS J. Solid State Sci. Technol. 2020, 9, 015017. [Google Scholar] [CrossRef]
- Schubert, E.F.; Kim, J.K.; Luo, H.; Xi, J.-Q. Solid-state lighting—A benevolent technology. Rep. Prog. Phys. 2006, 69, 3069–3099. [Google Scholar] [CrossRef]
- Rahman, F.; Jadwisienczak, W. Phosphors—The Driving Force Behind LEDs. Compd. Semicond. 2014, 20, 56–59. [Google Scholar]
- Nair, G.B.; Swart, H.C.; Dhoble, S.J. A review on the advancements in phosphor-converted light emitting diodes (pc-LEDs): Phosphor synthesis, device fabrication and characterization. Prog. Mater. Sci. 2020, 109, 100622. [Google Scholar] [CrossRef]
- Fang, M.-H.; Bao, Z.; Huang, W.-T.; Liu, R.-S. Evolutionary Generation of Phosphor Materials and Their Progress in Future Applications for Light-Emitting Diodes. Chem. Rev. 2022, 122, 11474–11513. [Google Scholar] [CrossRef]
- Marini, A.; Muñoz-Losa, A.; Biancardi, A.; Mennucci, B. What is Solvatochromism? J. Phys. Chem. B 2010, 114, 17128–17135. [Google Scholar] [CrossRef]
- Nigam, S.; Rutan, S. Principles and Applications of Solvatochromism. Appl. Spectrosc. 2001, 55, 362A–370A. [Google Scholar] [CrossRef]
- Bayliss, N.S. The Effect of the Electrostatic Polarization of the Solvent on Electronic Absorption Spectra in Solution. J. Chem. Phys. 1950, 18, 292–296. [Google Scholar] [CrossRef]
- Kuhn, H.; Schweig, A. Theoretical treatment of solvent effects on the electronic spectra of organic dye molecules. Chem. Phys. Lett. 1967, 1, 255–258. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chang, C.-W. Solvatochromism Study of DCM Encapsulated in ZIF-90 and the Potential Application of DCM/ZIF-90 as the Fluorescence Down-Conversion Layer for an LED Chip. J. Phys. Chem. C 2020, 124, 8854–8860. [Google Scholar] [CrossRef]
- Olutas, M.; Sagırlı, A. Solvatochromic and solid-state emissive azlactone-based AIEE-active organic dye: Synthesis, photophysical properties and color-conversion LED application. J. Mol. Liq. 2020, 313, 113482. [Google Scholar] [CrossRef]
- Hasan, M.M.; Jones, E.; Rahman, F. Stable solvatochromic light-emitting diodes and their potential for color temperature adjustment of white LEDs. Opt. Mater. 2024, 152, 115490. [Google Scholar] [CrossRef]
- Lytle, F.E.; Hercules, D.M. The Luminescence of Tris(2,2′-bipyridine)ruthenium(II) Dichloride. J. Am. Chem. Soc. 1969, 91, 253–257. [Google Scholar] [CrossRef]
- Chang, M.-H.; Das, D.; Varde, P.V.; Pecht, M. Light emitting diodes reliability review. Microelectron. Reliab. 2012, 52, 762–782. [Google Scholar] [CrossRef]
- Rahman, F. Broadband LEDs Enhance Colour Fidelity. Compd. Semicond. 2012, 18, 49–52. [Google Scholar]
- Lu, Z.; Cheng, Y.; Fan, W.; Yang, S.; Liu, X.; Qin, Y.; Zhao, R.; Zheng, L.; Zhang, H. A stable silver metallacage with solvatochromic and mechanochromic behavior for white LED fabrication. Chem. Commun. 2019, 55, 8474–8477. [Google Scholar] [CrossRef]
- Nizomov, N.; Kholov, A.U.; Ishchenko, A.A.; Ishchenko, V.V.; Khilya, V.P. Electronic structure and spectral fluorescence properties of umbelliferone and herniarin. J. Appl. Spectrosc. 2007, 74, 626–634. [Google Scholar] [CrossRef]
- Fink, D.W.; Koehler, W.R. pH Effects on Fluorescence of Umbelliferone. Anal. Chem. 1970, 42, 990–993. [Google Scholar] [CrossRef]
- Grzywacz, J.; Taszner, S. Influence of pH on the Absorption and Fluorescence Spectra of 6,7-Dihydroxycoumarin in Aqueous Solution. Z. Naturforsch. 1982, 37, 262–265. [Google Scholar] [CrossRef]
- Adhikari, G.C.; Zhu, P.; Vargas, P.A.; Zhu, H.; Grigoriev, A. Tetradic phosphor white light with variable CCT and superlative CRI through organolead halide perovskite nanocrystals. Nanoscale Adv. 2019, 1, 1791–1798. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).