Characterizing Normal and Tumour Blood Microcirculatory Systems Using Optical Coherence Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Flow Phantom
2.2. Mice
2.3. Swept Source OCT (SS-OCT)
2.4. Phase-Resolved DOCT
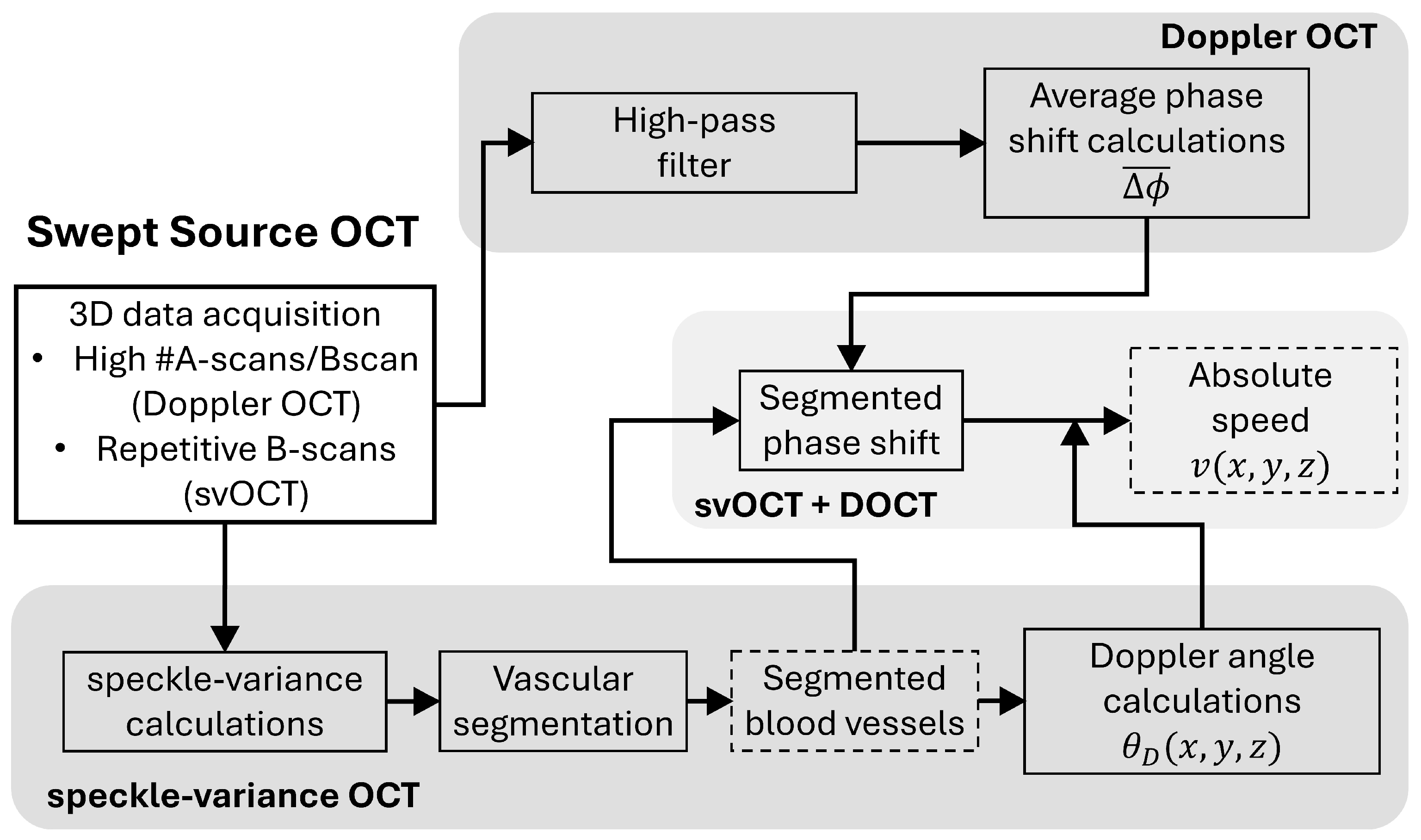
2.5. svOCT Microvascular Segmentation and Doppler Angle Calculations
2.5.1. Microvascular Segmentation Pipeline
2.5.2. Doppler Angle Measurements
2.6. Quantification of the Microcirculatory System
3. Results and Discussion
3.1. Validation of DOCT and Its Sensitivity in Flow Phantom
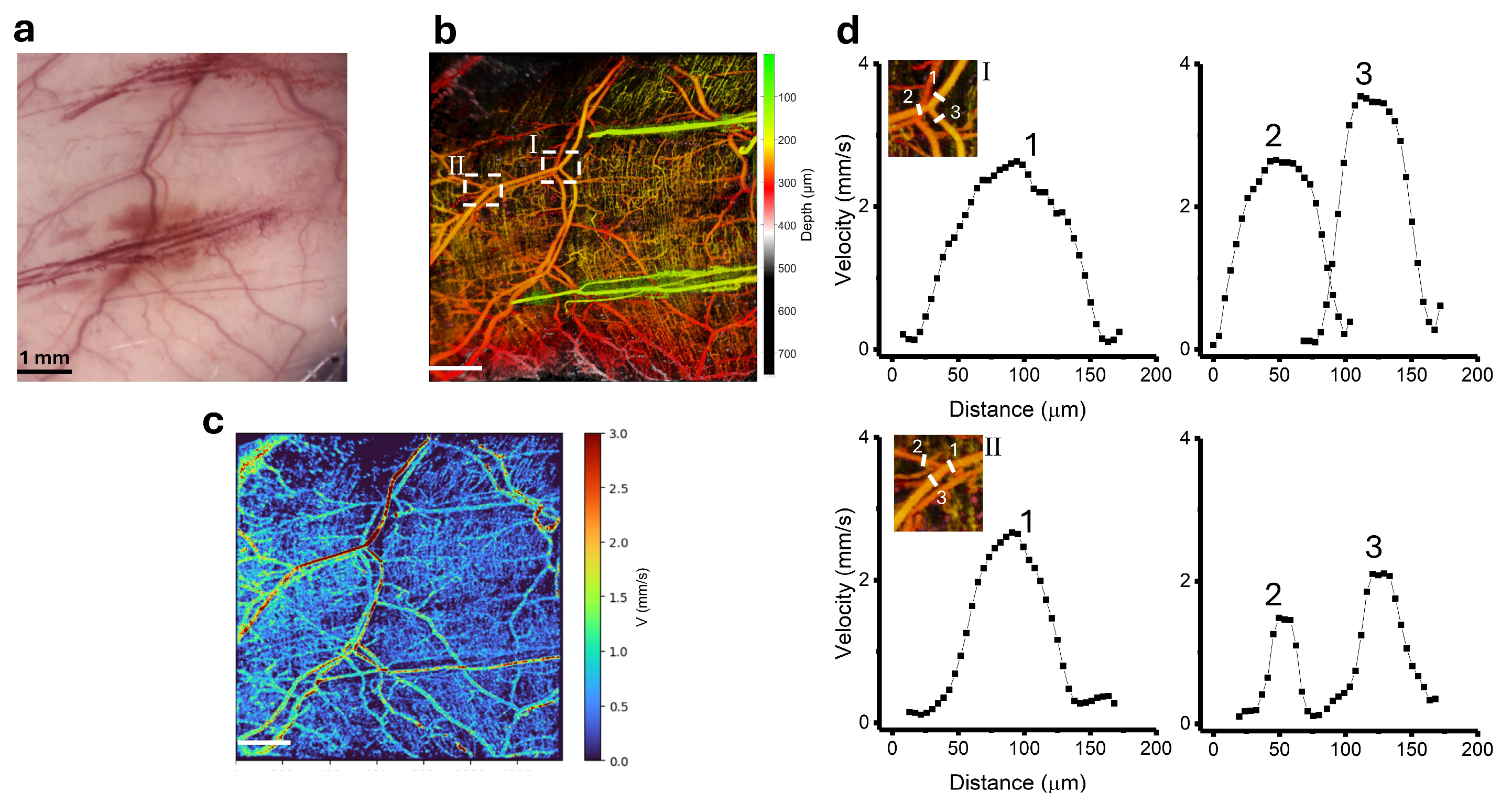
3.2. Blood Velocity Measurements in Healthy Skin
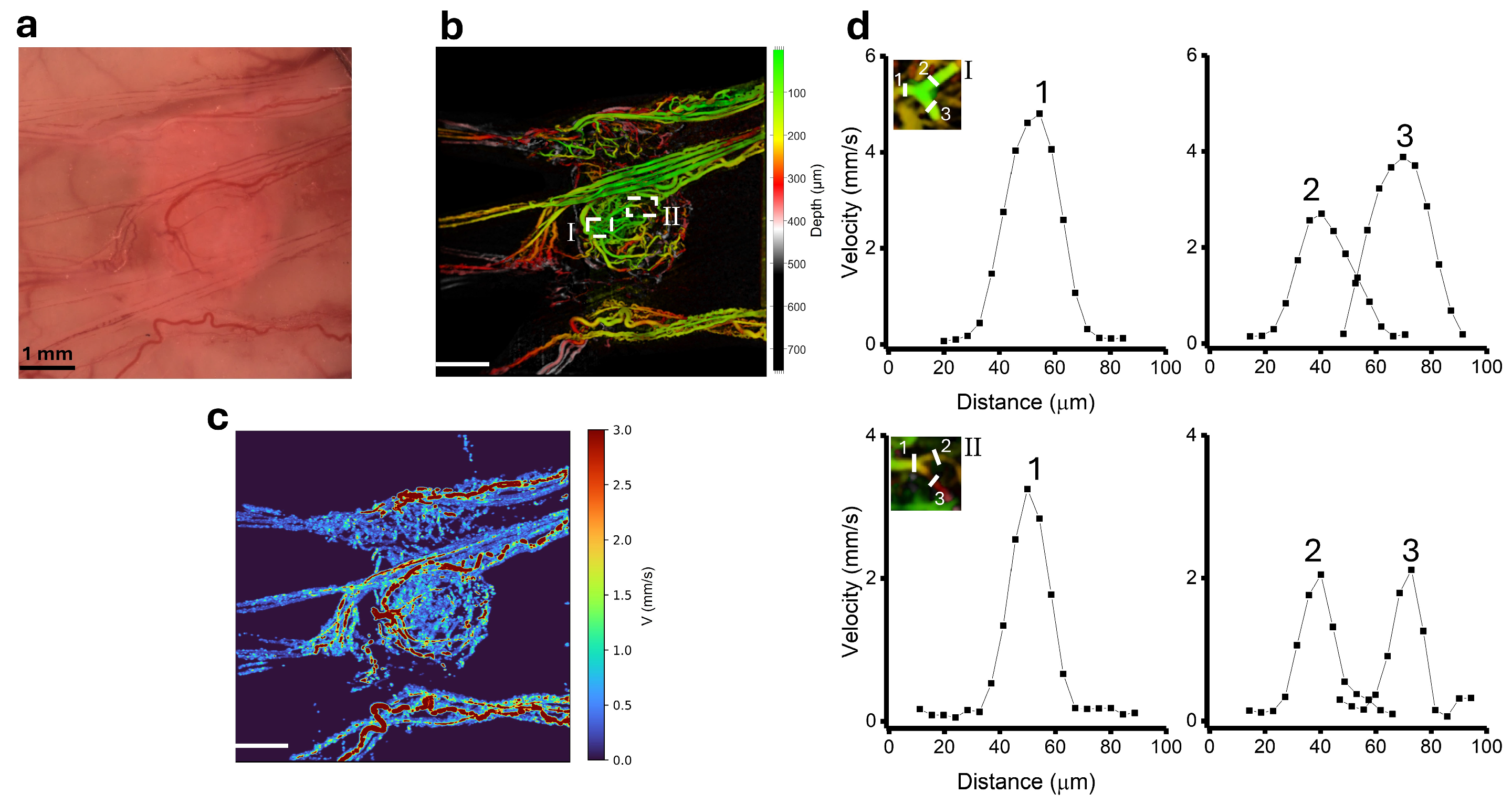
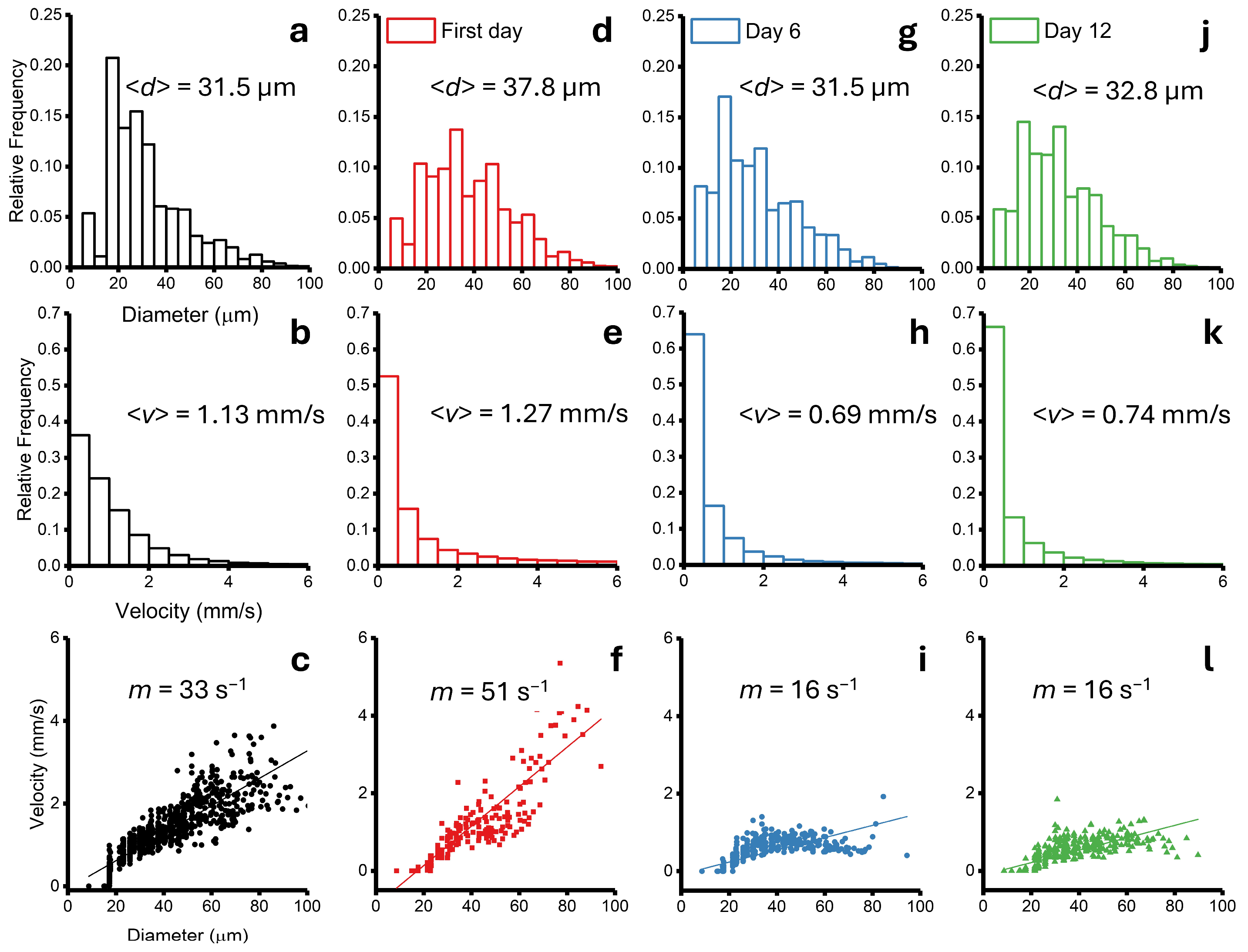
3.3. Blood Velocity Measurements in Tumour
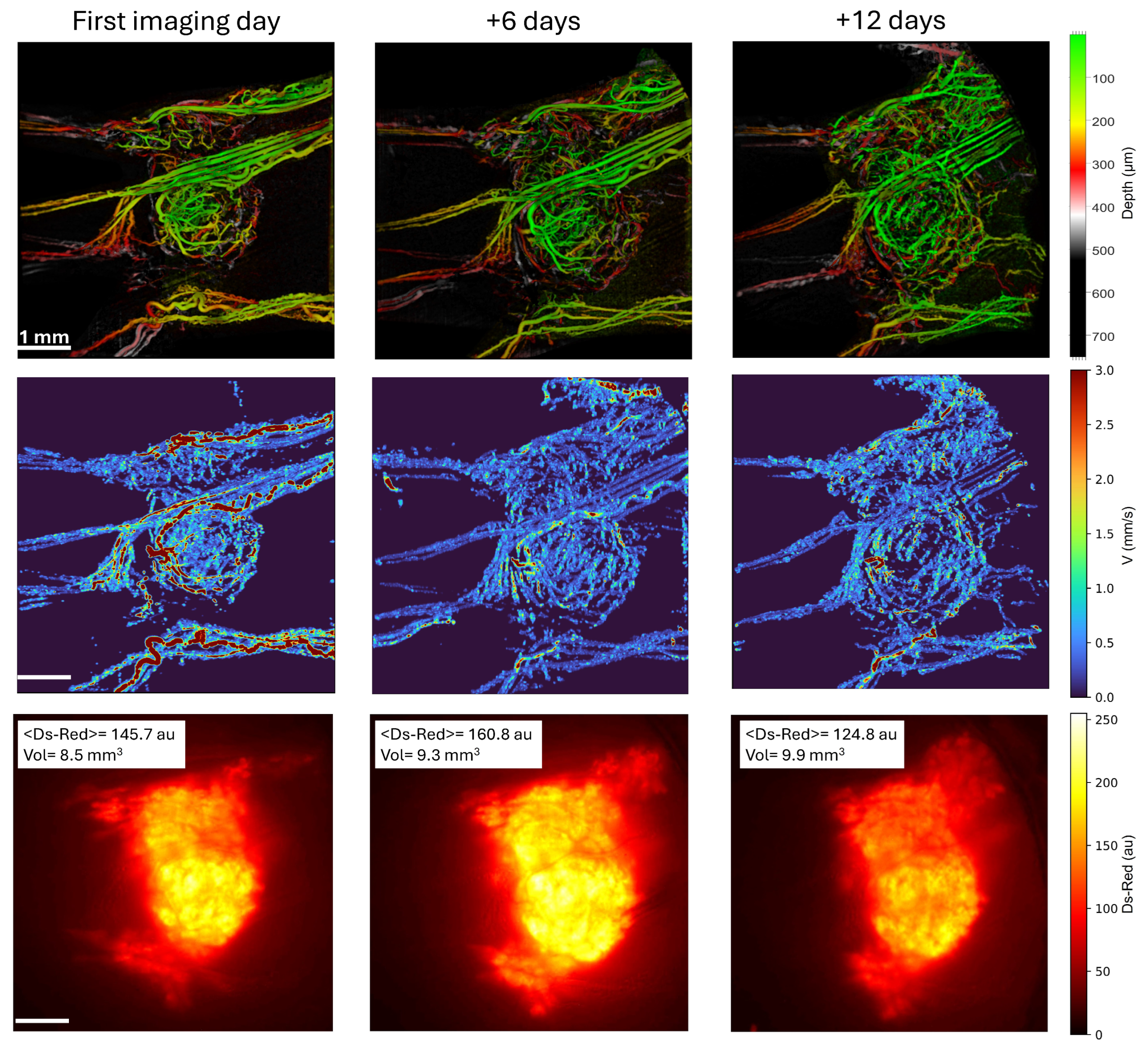
3.4. Combining Microvascular Architecture and Functionality Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munoz, C.J.; Lucas, A.; Williams, A.T.; Cabrales, P. A Review on Microvascular Hemodynamics: The Control of Blood Flow distribution, and Tissue Oxygenation. Crit. Care Clin. 2020, 36, 293–305. [Google Scholar] [CrossRef] [PubMed]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Secomb, T.W. Blood flow in the microcirculation. Annu. Rev. Fluid. Mechanics. 2017, 49, 443–461. [Google Scholar] [CrossRef]
- Pries, A.R.; Secomb, T.W.; Gaehtgens, P. Biophysical aspects of blood flow in microvasculature. Cardiovascular. Res. 1996, 32, 654–667. [Google Scholar] [CrossRef]
- Forster, J.C.; Harriss-Phillips, W.M.; Douglass, M.J.J.; Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 2017, 5, 21–32. [Google Scholar] [CrossRef]
- Fukumura, D.; Duda, D.G.; Munn, L.L.; Jain, R.K. Tumor microvasculature and microenvironment: Novel insights through intravital imaging in pre-clinical models. Microcirculation 2010, 17, 206–225. [Google Scholar] [CrossRef]
- Nagy, J.A.; Chang, S.-H.; Dvorak, A.M.; Dvorak, H.F. Why are tumor blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef]
- Horsman, M.R.; Vaupel, P. Pathophysiological basis for the formation of the tumor microenvironment. Front. Oncol. 2016, 6, 66. [Google Scholar] [CrossRef]
- Komar, G.; Kauhanen, S.; Liukko, K.; Seppanen, M.; Kajander, S.; Ovaska, J.; Nuutila, P.; Minn, H. Decreased blood flow with increased metabolic activity: A novel sign of pancreatic tumor aggressiveness. Clin. Cancer Res. 2009, 15, 5511–5517. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Dunnwald, L.K.; Partridge, S.C.; Specht, J.M. Blood flow-metabolism mismatch: Good for the tumor, bad for the patient. Clin. Cancer Res. 2009, 15, 5294–5296. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Primeau, A.J.; Rendon, A.; Hedley, D.; Lilge, L.; Tannock, I.F. The distribution of the anticancer drug doxorubicin in relation to blood vessels in solid tumors. Clin. Cancer Res. 2005, 11, 8782–8788. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, S.; Dobrucki, I.T.; Kim, E.Y.; Marrison, S.T.; Vu, V.T. Hypoxia and radiation therapy: Past history, ongoing research, and future promise. Curr. Mol. Med. 2009, 9, 442–458. [Google Scholar] [CrossRef]
- Daly, S.M.; Leahy, M.J. ‘Go with the flow’: A review of methods and advancements in blood flow imaging. J. Biophotonics 2013, 6, 217–255. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.; Zabel, W.J.; Demidov, V.; Jones, B.; Flueraru, C.; Taylor, E.; Vitkin, I.A. Longitudinal in-vivo quantification of tumour microvascular heterogeneity by optical coherence angiography in pre-clinical radiation therapy. Sci. Rep. 2022, 12, 6140. [Google Scholar] [CrossRef]
- Demidov, V.; Maeda, A.; Sugita, M.; Madge, V.; Sadanand, S.; Flueraru, C.; Vitkin, I.A. Preclinical longitudinal imaging of tumor microvascular radiobiological response with functional optical coherence tomography. Sci. Rep. 2018, 8, 38. [Google Scholar] [CrossRef]
- Kim, H.; Eom, T.J.; Kim, J.G. Vascular Morphometric Changes during Tumor Growth and Chemotherapy in a Murine Mammary Tumor Model Using OCT Angiography: A Preliminary Study. Curr. Opt. Photonics 2019, 3, 54–65. [Google Scholar]
- Skala, M.C.; Fontanella, A.N.; Lan, L.; Izatt, J.A.; Dewhirst, M.W. Longitudinal optical imaging of tumor metabolism and hemodynamics. J. Biomed. Opt. 2010, 15, 011112. [Google Scholar] [CrossRef]
- Lian, C.-P.; Nakajima, T.; Watanabe, R.; Sato, K.; Choyke, P.L.; Chen, Y.; Kobayashi, H. Real-time monitoring of hemodynamic changes in tumor vessels during photoimmunotherapy using optical coherence tomography. J. Biomed. Opt. 2014, 19, 098004. [Google Scholar]
- Chen, C.-L.; Wang, R.K. Optical coherence tomography based angiography. Biomed. Opt. Express 2017, 8, 1056–1082. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye. Res. 2017, 60, 66–100. [Google Scholar] [CrossRef] [PubMed]
- Mariampillai, A.; Standish, B.A.; Moriyama, E.H.; Khurana, M.; Munce, N.R.; Leung, M.K.K.; Jiang, J.; Cable, A.; Wilson, B.C.; Vitkin, I.A.; et al. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt. Lett. 2008, 33, 1530–1532. [Google Scholar] [CrossRef]
- Mariampillai, A.; Leung, M.K.K.; Jarvi, M.; Standish, B.A.; Lee, K.; Wilson, B.C.; Vitkin, A.; Yang, V.X.D. Optimized speckle variance OCT imaging of microvasculature. Opt. Lett. 2010, 35, 1257–1259. [Google Scholar] [CrossRef]
- Leitgeb, R.A.; Werkmeister, R.M.; Blatter, C.; Schmetterer, L. Doppler optical coherence tomography. Prog. Retin. Eye. Res. 2014, 41, 26–43. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Z. Advances in Doppler OCT. Chin. Opt. Lett. 2013, 11, 011702. [Google Scholar]
- Liu, G.; Lin, A.J.; Tromberg, B.J.; Chen, Z. A comparison of Doppler optical coherence tomography methods. Biomed. Opt. Express 2012, 3, 2669–2680. [Google Scholar] [CrossRef]
- Singh, A.S.G.; Kolbitsch, C.; Schmoll, T.; Leitgeb, R.A. Stable absolute flow estimation with Doppler OCT based on virtual circumpapillary scans. Biomed. Opt. Express 2010, 1, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Michaely, R.; Bachmann, A.H.; Villiger, M.L.; Blatter, C.; Lasser, T.; Leitgeb, R.A. Vectorial reconstruction of retinal blood flow in three dimension measured with high resolution resonant Doppler Fourier domain optical coherence tomography. J. Biomed. Opt. 2007, 12, 041213. [Google Scholar] [CrossRef][Green Version]
- You, J.; Du, C.; Volkow, N.D.; Pan, Y. Optical coherence Doppler tomography for quantitative cerebral blood flow imaging. Biomed. Opt. Express 2014, 5, 3217–3230. [Google Scholar] [CrossRef]
- Qi, L.; Zhu, J.; Hancock, A.M.; Dai, C.; Zhang, X.; Frostig, R.D.; Chen, Z. Fully distributed absolute blood flow velocity measurement for middle cerebral arteries using Doppler optical coherence tomography. Biomed. Opt. Express 2016, 7, 601–615. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Li, A.; Du, C.; Pan, Y. Volumetric Doppler angle correction for ultrahigh-resolution optical coherence Doppler tomography. Appl. Phys. Lett. 2017, 110, 011102. [Google Scholar] [CrossRef] [PubMed]
- Trasischker, W.; Werkmeister, R.M.; Zotter, S.; Baumann, B.; Torzicky, T.; Pircher, M.; Hitzenberger, C.K. In vitro and in vivo three-dimensional velocity vector measurement by three-beam spectral-domain Doppler optical coherence tomography. J. Biomed. Opt. 2013, 18, 116010. [Google Scholar] [CrossRef]
- Wartak, A.; Haindl, R.; Trasischker, W.; Baumann, B.; Pircher, M.; Hitzenberger, C.K. Active-passive path-length encoded (apple) Doppler OCT. Biomed. Opt. Express 2016, 7, 5233–5251. [Google Scholar] [CrossRef]
- Huang, S.; Shen, M.; Zhu, D.; Chen, Q.; Shi, C.; Chen, Z.; Lu, F. In vivo imaging of retinal hemodynamics with OCT angiography and Doppler OCT. Biomed. Opt. Express 2016, 7, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.B. Velocity-diameter relationships of the microcirculation. Med. Inform. 1979, 4, 243–256. [Google Scholar] [CrossRef]
- Fukumura, D.; Yuan, F.; Monsky, W.L.; Chen, Y.; Jain, R.K. Effects of host microenvironment on the microcirculation of human colon adenocarcinoma. Am. J. Pathol. 1997, 151, 679–688. [Google Scholar]
- Hartley, C.J.; Reddy, A.K.; Madala, S.; Entman, M.L.; Michael, L.H.; Taffet, G.E. Doppler velocity measurements from large and small arteries of mice. Am. J. Physiol. Heart. Circ. Physiol. 2011, 301, 269–278. [Google Scholar] [CrossRef]
- Kamoun, W.S.; Chae, S.-S.; Lacorre, D.A.; Tyrrell, J.A.; Mitre, M.; Gillissen, M.A.; Fukumura, D.; Jain, R.K.; Munn, L.L. Simultaneous measurement of RBC velocity, flux, hematocrit and shear rate in vascular networks. Nat. Methods 2010, 7, 655–660. [Google Scholar] [CrossRef]
- Zabel, W.J.; Allam, N.; Contreras Sanchez, H.A.; Foltz, W.; Flueraru, C.; Taylor, E.; Vitkin, A. A Dorsal Skinfold Window Chamber Tumor Mouse Model for Combined Intravital Microscopy and Magnetic Resonance Imaging in Translational Cancer Research. J. Vis. Exp. 2024, 206, e66383. [Google Scholar] [CrossRef]
- Constantinides, C.; Mean, R.; Janssen, B.J. Effects of Isoflurane Anesthesia on the Cardiovascular Function of the C57BL/6 Mouse. ILAR J. 2011, 52, e21–e31. [Google Scholar] [PubMed]
- Mao, Y.; Sherif, S.; Flueraru, C.; Chang, S. 3x3 Mach-Zehnder interferometer with unbalance differential detection for full-range swept-source optical coherence tomography. Appl. Opt. 2008, 47, 2004–2010. [Google Scholar] [CrossRef]
- Mao, Y.; Flueraru, C.; Chang, S.; Popescu, D.P.; Sowa, M.G. High-quality tissue imaging using a catheter-based swept source optical coherence tomography systems with an integrated semiconductor optical amplifier. IEEE Trans. Instrum. Meas. 2011, 60, 3376–3383. [Google Scholar]
- Liu, B. Doppler Optical Coherence Tomography. In Optical Coherence Tomography: Principles and Applications; Brezinski, M., Ed.; Academic Press: Burlington, MA, USA, 2006; pp. 278–300. [Google Scholar]
- Tang, J.; Erdener, S.E.; Fu, B.; Boas, D.A. Capillary red blood cell velocimetry by phase resolved optical coherence tomography. Opt. Lett. 2017, 42, 3976–3979. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.L.; Goyal, K.G.; Worth, B.W.; Makkar, S.S.; Calhoun, W.R.; Bali, L.M.; Bali, S. Accurate in situ measurement of complex refractive index and particle size in intralipid emulsions. J. Biomed. Opt. 2013, 18, 087003. [Google Scholar] [CrossRef][Green Version]
- Gosh, N.; Buddhiwant, P.; Uppal, A.; Majumder, S.K.; Patel, H.S.; Gupta, P.K. Simultaneous determination of size and refractive index of red blood by light scattering measurements. Appl. Phys. Lett. 2006, 88, 084101. [Google Scholar] [CrossRef]
- Casper, M.; Schulz-Hildebrandt, H.; Evers, M.; Birngruber, R.; Manstein, D.; Huttmann, G. Optimization-based vessel segmentation pipeline for robust quantification of capillary networks in skin with optical coherence tomography angiography. J. Biomed. Opt. 2019, 24, 046005. [Google Scholar] [CrossRef]
- Frangi, A.F.; Niessen, W.J.; Vincken, K.L.; Viergever, M.A. Multiscale vessel enhancement filtering. MICCAI’98. 1998, 1, 130–137. [Google Scholar]
- Farrance, I.; Frenkel, R. Uncertainty of Measurement: A Review of the Rules for Calculating Uncertainty Components through Functional Relationships. Clin. Biochem. Rev. 2012, 33, 49–75. [Google Scholar]
- Yang, V.X.D.; Gordon, M.L.; Qi, B.; Pekar, J.; Lo, S.; Seng-Yue, E.; Mok, A.; Wilson, B.C.; Vitkin, I.A. High speed, wide velocity dynamic range Doppler optical coherence tomography (part I): System design, signal processing, and performance. Opt. Express 2003, 11, 794–809. [Google Scholar] [CrossRef]
- Song, S.; Xu, J.; Men, S.; Shen, T.T.; Wang, R.K. Robust numerical phase stabilization for long-range swept-source optical coherence tomography. J. Biophotonics 2017, 10, 1398–1410. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; You, J.; Gu, X.; Du, C.; Pan, Y. High-speed swept source optical Doppler tomography for deep brain microvascular imaging. Sci. Rep. 2016, 6, 38786. [Google Scholar] [CrossRef]
- Chiu, C.-L.; Clack, N.; The Napari Community. Napari: A python multi-dimensional image viewer platform for the research community. Microsc. Microanal. 2022, 28, 1576–1577. [Google Scholar] [CrossRef]
- Werkmeister, R.M.; Dragostinoff, N.; Palkovits, S.; Told, R.; Boltz, A.; Leitgeb, R.A.; Groschl, M.; Garhofer, G.; Schmetterer, L. Measurement of absolute blood flow velocity and blood flow in the human retina by dual-beam bidirectional Doppler Fourier-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6062–6071. [Google Scholar] [CrossRef]
- Everds, N. Hematology of the Mouse. In The Laboratory Mouse; Hedrich, H.J., Bullock, G., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 271–286. [Google Scholar]
- Srinivasan, V.J.; Radhakrishnan, H.; Lo, E.H.; Mandeville, E.T.; Jiang, J.Y.; Barry, S.; Cable, A.E. OCT methods for capillary velocimetry. Biomed. Opt. Express 2012, 3, 612–629. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Wu, W.; Lesage, F.; Boas, D.A. Multiple-capillary measurement of RBC speed, flux, and density with optical coherence tomography. J. Cereb. Blood. Flow. Metab. 2013, 33, 1707–1710. [Google Scholar] [CrossRef]
- Choi, W.J.; Li, Y.; Qin, W.; Wang, R.K. Cerebral capillary velocimetry based on temporal OCT speckle contrast. Biomed. Opt. Express 2016, 7, 4859–4873. [Google Scholar] [CrossRef]
- Verkhusha, V.V.; Kuznetsova, I.M.; Stepaneko, O.V.; Zaraisky, A.G.; Shavlovsky, M.M.; Turoverov, K.K.; Uversky, V.N. High Stability of Discosoma DsRed As Compared to Aequorea EGFP. Biochemistry 2003, 42, 7879–7884. [Google Scholar] [CrossRef]
- Gillies, R.J.; Brown, J.S.; Anderson, A.R.A.; Gatenby, R.A. Eco-evolutionary causes and consequences of temporal changes in intratumoural blood flow. Nat. Rev. Cancer 2018, 18, 576–585. [Google Scholar] [CrossRef]
- Chaplin, D.J.; Olive, P.L.; Durand, R.E. Intermittent Blood Flow in a Murine Tumor: Radiobiological Effects. Cancer Res. 1987, 47, 597–601. [Google Scholar]



| (mm/s) | (mm/s) | A (103 µm2) | (106 µm3/s) | |||
|---|---|---|---|---|---|---|
| Branching point I (Healthy) | 1 | 1.7 ± 0.1 | 3.2 ± 0.2 | 7.7 ± 0.3 | 12.9 ± 1.0 | Q2 + Q3 |
| 2 | 1.7 ± 0.1 | 3.1 ± 0.1 | 4.0 ± 0.7 | 6.9 ± 1.7 | 16.5 ± 5.2 | |
| 3 | 2.1 ± 0.1 | 4.0 ± 0.1 | 4.5 ± 0.9 | 9.6 ± 4.2 | ||
| Branching point II (Healthy) | 1 | 1.2 ± 0.4 | 2.4 ± 0.7 | 3.2 ± 0.3 | 4.0 ± 1.7 | Q2 + Q3 |
| 2 | 0.8 ± 0.2 | 1.7 ± 0.4 | 1.1 ± 0.2 | 0.9 ± 0.4 | 4.4 ± 2.1 | |
| 3 | 1.4 ± 0.3 | 2.9 ± 0.7 | 2.4 ± 0.5 | 3.5 ± 2.0 | ||
| Branching point I (Tumour) | 1 | 2.7 ± 0.3 | 5.1 ± 0.3 | 2.6 ± 0.2 | 7.2 ± 1.4 | Q2 + Q3 |
| 2 | 1.9 ± 0.4 | 3.4 ± 0.8 | 1.2 ± 0.1 | 2.4 ± 0.7 | 5.7 ± 1.8 | |
| 3 | 2.0 ± 0.2 | 4.1 ± 0.4 | 1.6 ± 0.3 | 3.3 ± 1.4 | ||
| Branching point II (Tumour) | 1 | 1.4 ± 0.2 | 3.2 ± 0.3 | 1.4 ± 0.3 | 1.9 ± 0.7 | Q2 + Q3 |
| 2 | 1.1 ± 0.2 | 2.2 ± 0.5 | 0.8 ± 0.1 | 0.9 ± 0.4 | 1.8 ± 0.3 | |
| 3 | 1.1 ± 0.3 | 2.1 ± 0.4 | 0.8 ± 0.1 | 0.9 ± 0.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Sánchez, H.A.; Zabel, W.J.; Flueraru, C.; Lilge, L.; Taylor, E.; Vitkin, A. Characterizing Normal and Tumour Blood Microcirculatory Systems Using Optical Coherence Tomography. Photonics 2024, 11, 891. https://doi.org/10.3390/photonics11090891
Contreras-Sánchez HA, Zabel WJ, Flueraru C, Lilge L, Taylor E, Vitkin A. Characterizing Normal and Tumour Blood Microcirculatory Systems Using Optical Coherence Tomography. Photonics. 2024; 11(9):891. https://doi.org/10.3390/photonics11090891
Chicago/Turabian StyleContreras-Sánchez, Héctor A., William Jeffrey Zabel, Costel Flueraru, Lothar Lilge, Edward Taylor, and Alex Vitkin. 2024. "Characterizing Normal and Tumour Blood Microcirculatory Systems Using Optical Coherence Tomography" Photonics 11, no. 9: 891. https://doi.org/10.3390/photonics11090891
APA StyleContreras-Sánchez, H. A., Zabel, W. J., Flueraru, C., Lilge, L., Taylor, E., & Vitkin, A. (2024). Characterizing Normal and Tumour Blood Microcirculatory Systems Using Optical Coherence Tomography. Photonics, 11(9), 891. https://doi.org/10.3390/photonics11090891




