Iron-Modified Nano-TiO2: Comprehensive Characterization for Enhanced Photocatalytic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Modification Process
2.2.2. Diffuse Reflectance Spectroscopy (DRS)
2.2.3. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
2.2.4. X-ray Diffraction (XRD)
2.2.5. Photocatalytic Efficiency Assessment
3. Results
3.1. Diffuse Reflectance Spectroscopy (DRS)
3.2. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
3.3. X-ray Diffraction (XRD)
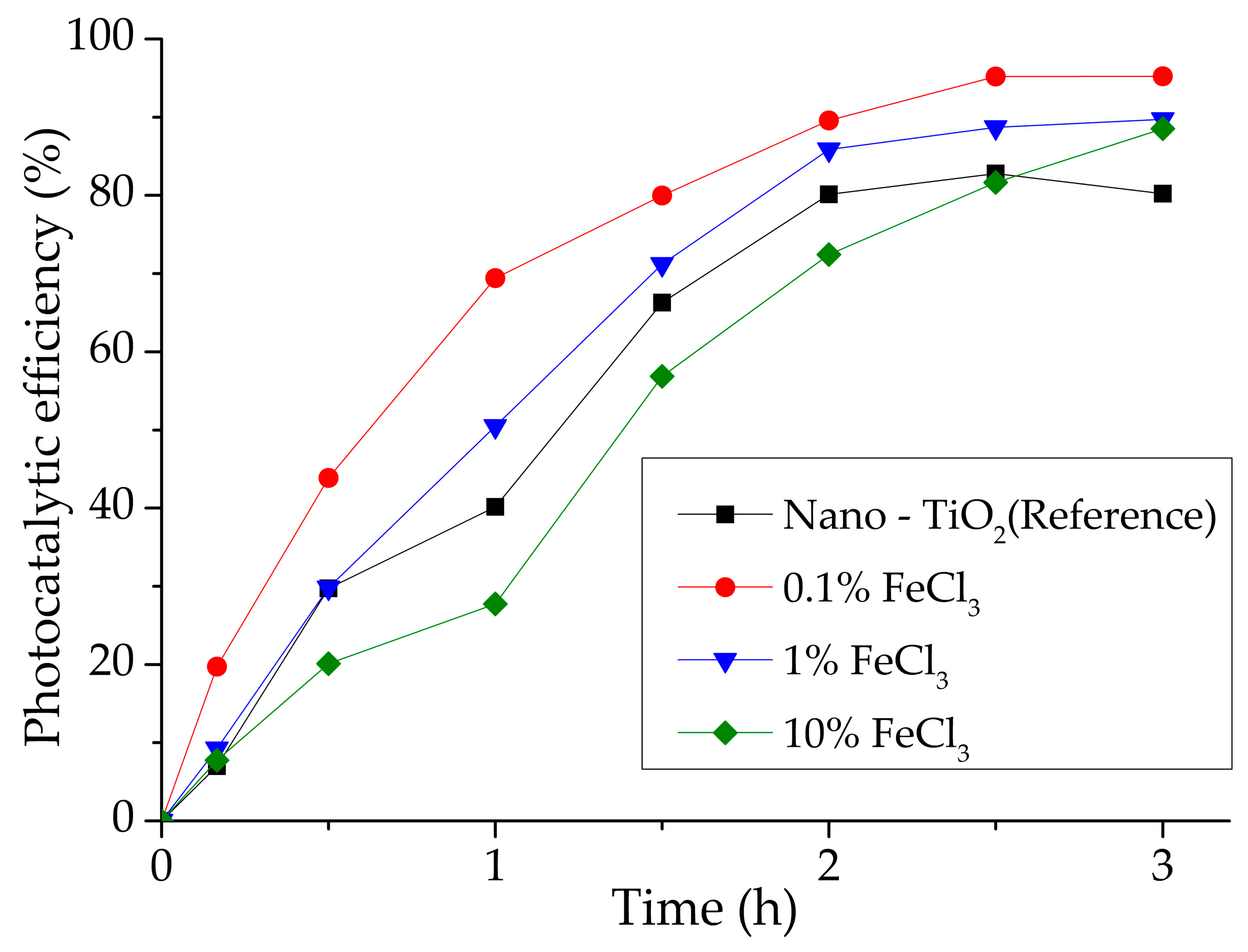
3.4. Photocatalytic Efficiency Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lale, E.; Uyguner-Demirel, C.S.; Bekbolet, M. Visible Light Photocatalytic Response of Fe Doped TiO2: Inactivation of Escherichia Coli. J. Photochem. Photobiol. A Chem. 2024, 456, 115836. [Google Scholar] [CrossRef]
- Segundo, I.R.; Freitas, E.; Branco, V.T.F.C.; Landi, S.; Costa, M.F.; Carneiro, J.O. Review and Analysis of Advances in Functionalized, Smart, and Multifunctional Asphalt Mixtures. Renew. Sustain. Energy Rev. 2021, 151, 111552. [Google Scholar] [CrossRef]
- Fang, M.; Peng, L.; Li, Y.; Cheng, Y.; Zhan, L. Evaluation Test of NO Degradation by Nano-TiO2 Coatings on Road Pavements under Natural Light. Coatings 2022, 12, 1200. [Google Scholar] [CrossRef]
- Bersch, J.D.; Picanço Casarin, R.; Maia, J.; Masuero, A.B.; Dal Molin, D.C.C. TiO2-Based Mortars for Rendering Building Envelopes: A Review of the Surface Finishing for Sustainability. Sustainability 2023, 15, 16920. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S. Synthetic Methods for Titanium Dioxide Nanoparticles: A Review. In Titanium Dioxide—Material for a Sustainable Environment; InTech: Houston, TX, USA, 2018. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, M.; Senthil, T.S.; Senthilkumar, N.; Kang, M. Solar-Light-Induced Photocatalyst Based on Bi–B Co-Doped TiO2 Prepared via Co-Precipitation Method. J. Mater. Sci. Mater. Electron. 2022, 33, 16550–16563. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Azevedo, S.; Fernandes, F.; Freitas, E.; Pereira, M.; Tavares, C.J.; Lanceros-Méndez, S.; Teixeira, V. Synthesis of Iron-Doped TiO2 Nanoparticles by Ball-Milling Process: The Influence of Process Parameters on the Structural, Optical, Magnetic, and Photocatalytic Properties. J. Mater. Sci. 2014, 49, 7476–7488. [Google Scholar] [CrossRef]
- Crişan, M.; Drăgan, N.; Crişan, D.; Ianculescu, A.; Niţoi, I.; Oancea, P.; Todan, L.; Stan, C.; Stănică, N. The Effects of Fe, Co and Ni Dopants on TiO2 Structure of Sol–Gel Nanopowders Used as Photocatalysts for Environmental Protection: A Comparative Study. Ceram. Int. 2016, 42, 3088–3095. [Google Scholar] [CrossRef]
- Matias, M.L.; Pimentel, A.; Reis-Machado, A.S.; Rodrigues, J.; Deuermeier, J.; Fortunato, E.; Martins, R.; Nunes, D. Enhanced Fe-TiO2 Solar Photocatalysts on Porous Platforms for Water Purification. Nanomaterials 2022, 12, 1005. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic Performance of Fe-Doped TiO2 Nanoparticles under Visible-Light Irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Pongwan, P.; Inceesungvorn, B.; Wetchakun, K.; Phanichphant, S.; Wetchakun, N. Highly Efficient Visible-Light-Induced Photocatalytic Activity of Fe-Doped TiO2 Nanoparticles. Eng. J. 2012, 16, 143–152. [Google Scholar] [CrossRef]
- Ambrus, Z.; Balázs, N.; Alapi, T.; Wittmann, G.; Sipos, P.; Dombi, A.; Mogyorósi, K. Synthesis, Structure and Photocatalytic Properties of Fe(III)-Doped TiO2 Prepared from TiCl3. Appl. Catal. B Environ. 2008, 81, 27–37. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Lestari, N.D.; Cinjana, I.R.; Annur, S.; Natsir, T.A.; Mudasir, M. Doping TiO2 with Fe from Iron Rusty Waste for Enhancing Its Activity under Visible Light in the Congo Red Dye Photodegradation. J. Eng. Appl. Sci. 2023, 70, 9. [Google Scholar] [CrossRef]
- Afonso, C.; Lima, O.; Segundo, I.R.; Landi, S.; Margalho, É.; Homem, N.; Pereira, M.; Costa, M.F.M.; Freitas, E.; Carneiro, J. Effect of Iron-Doping on the Structure and Photocatalytic Activity of TiO2 Nanoparticles. Catalysts 2022, 13, 58. [Google Scholar] [CrossRef]
- Li, Z.; Shen, W.; He, W.; Zu, X. Effect of Fe-Doped TiO2 Nanoparticle Derived from Modified Hydrothermal Process on the Photocatalytic Degradation Performance on Methylene Blue. J. Hazard. Mater. 2008, 155, 590–594. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. The Advancements in Sol–Gel Method of Doped-TiO2 Photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Letifi, H.; Dridi, D.; Litaiem, Y.; Ammar, S.; Dimassi, W.; Chtourou, R. High Efficient and Cost Effective Titanium Doped Tin Dioxide Based Photocatalysts Synthesized via Co-Precipitation Approach. Catalysts 2021, 11, 803. [Google Scholar] [CrossRef]
- Mahendran, V.; Gogate, P.R. Ultrasound-Assisted Synthesis of Fe-Doped TiO2 Catalyst for Photocatalytic Oxidation Application. Int. J. Environ. Res. 2021, 15, 1071–1084. [Google Scholar] [CrossRef]
- Ma, J.; He, H.; Liu, F. Effect of Fe on the Photocatalytic Removal of NO over Visible Light Responsive Fe/TiO2 Catalysts. Appl. Catal. B Environ. 2015, 179, 21–28. [Google Scholar] [CrossRef]
- Lucas, S.S.; Ferreira, V.M.; de Aguiar, J.L.B. Incorporation of Titanium Dioxide Nanoparticles in Mortars—Influence of Microstructure in the Hardened State Properties and Photocatalytic Activity. Cem. Concr. Res. 2013, 43, 112–120. [Google Scholar] [CrossRef]
- Díaz, D.D.; Miranda, P.O.; Padrón, J.I.; Martín, V.S. Recent Uses of Iron (III) Chloride in Organic Synthesis. Curr. Org. Chem. 2006, 10, 457–476. [Google Scholar] [CrossRef]
- Jeyachitra, R.; Senthilnathan, V.; Senthil, T.S. Studies on Electrical Behavior of Fe Doped ZnO Nanoparticles Prepared via Co-Precipitation Approach for Photo-Catalytic Application. J. Mater. Sci. Mater. Electron. 2018, 29, 1189–1197. [Google Scholar] [CrossRef]
- Ellouzi, I.; El Hajjaji, S.; Harir, M.; Schmitt-Kopplin, P.; Laânab, L. Coprecipitation Synthesis of Fe-Doped TiO2 from Various Commercial TiO2 for Photocatalytic Reaction. Int. J. Environ. Res. 2020, 14, 605–613. [Google Scholar] [CrossRef]
- Yang, X.; Li, L. Controlled Synthesis of Single-Crystalline α-Fe2O3 Micro/Nanoparticles from the Complex Precursor of FeCl3 and Methyl Orange. Nanotechnology 2010, 21, 355602. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and Misuse of the Kubelka-Munk Function to Obtain the Band Gap Energy from Diffuse Reflectance Measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-Ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Medina-Ramírez, I.; Liu, J.L.; Hernández-Ramírez, A.; Romo-Bernal, C.; Pedroza-Herrera, G.; Jáuregui-Rincón, J.; Gracia-Pinilla, M.A. Synthesis, Characterization, Photocatalytic Evaluation, and Toxicity Studies of TiO2–Fe3+ Nanocatalyst. J. Mater. Sci. 2014, 49, 5309–5323. [Google Scholar] [CrossRef]
- Hung, W.-C.; Chen, Y.-C.; Chu, H.; Tseng, T.-K. Synthesis and Characterization of TiO2 and Fe/TiO2 Nanoparticles and Their Performance for Photocatalytic Degradation of 1,2-Dichloroethane. Appl. Surf. Sci. 2008, 255, 2205–2213. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.-C.; Zakaria, R.; Ying, J.Y. Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
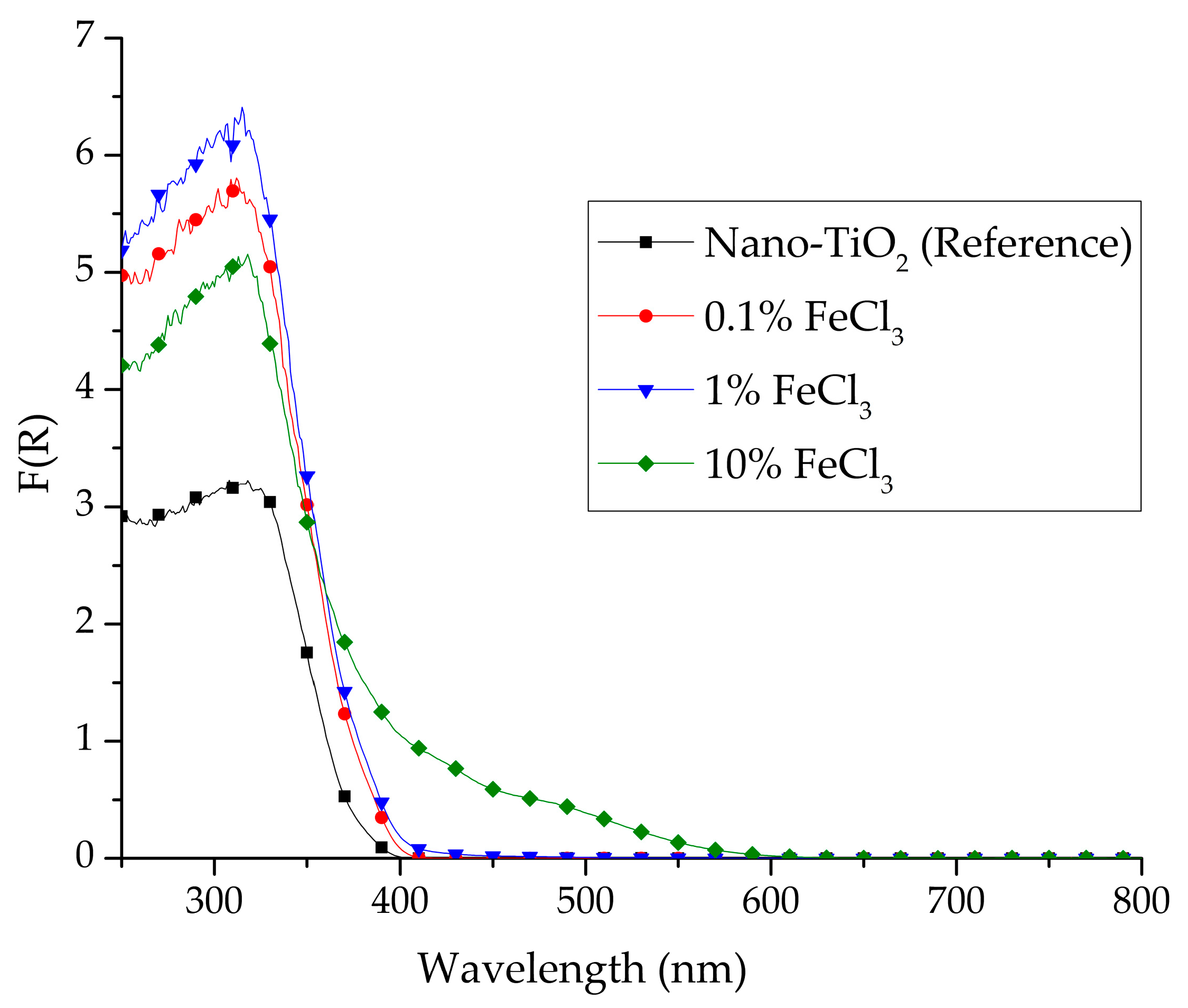
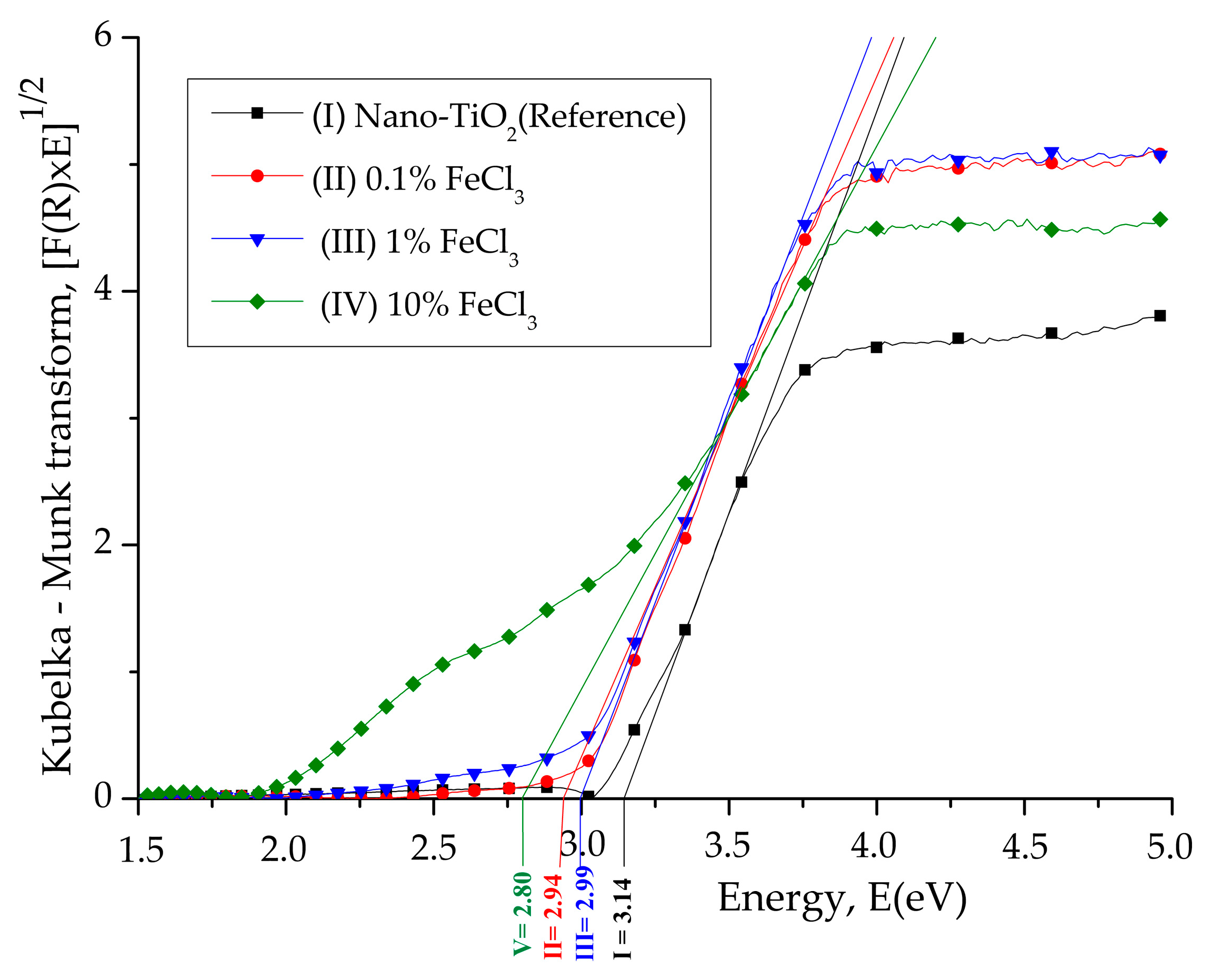
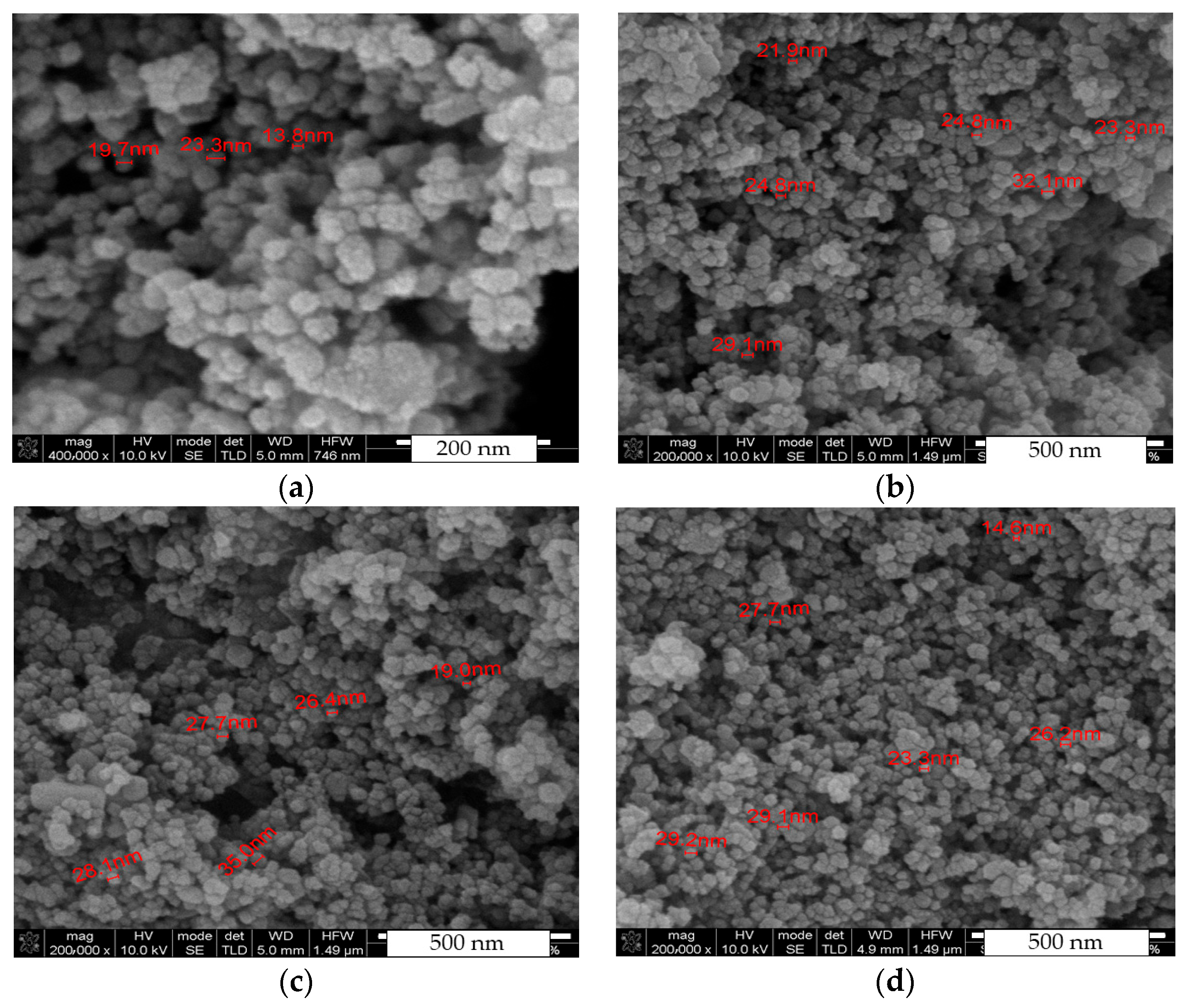
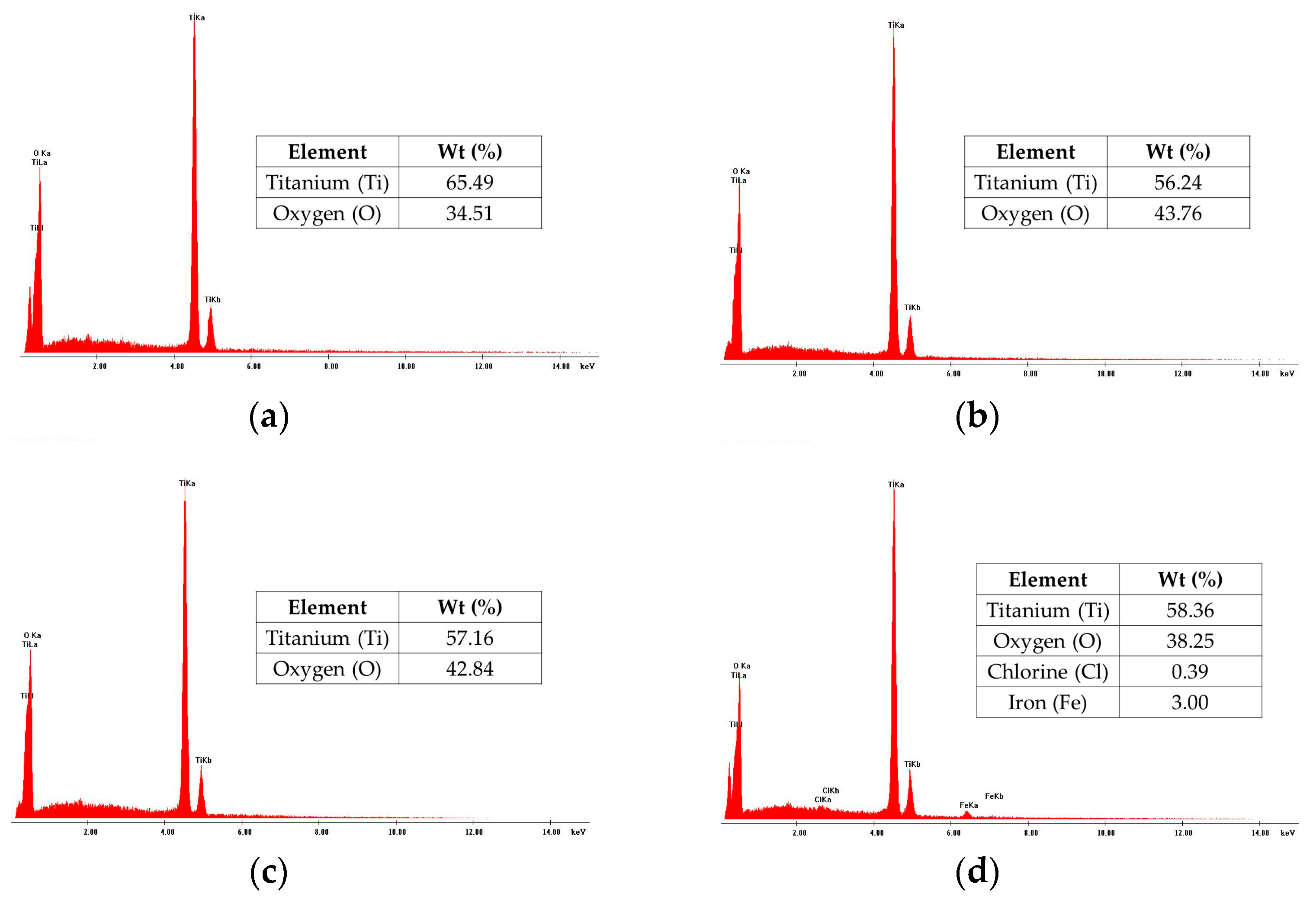
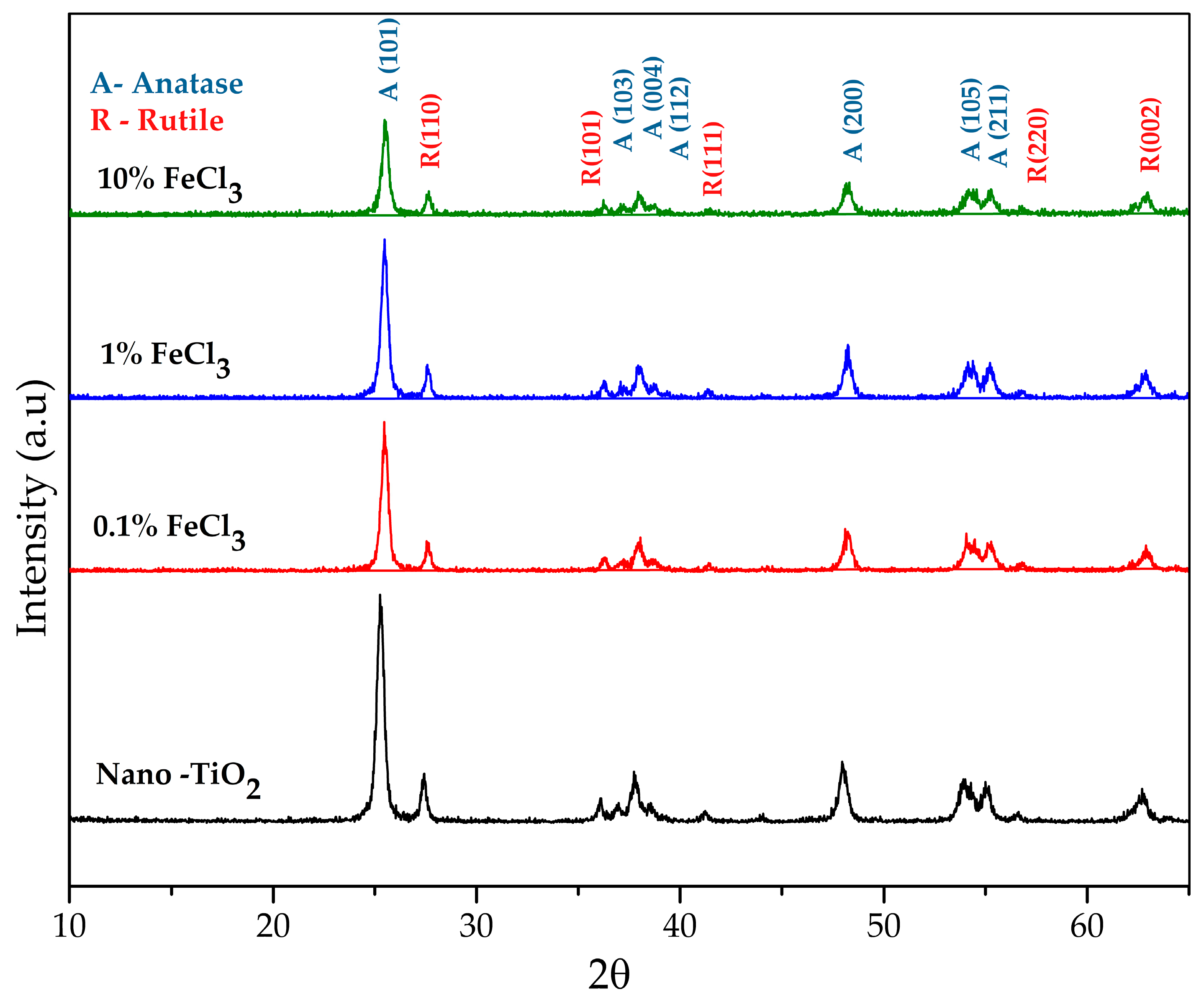
| Composition | Crystallite Size (nm) | |
|---|---|---|
| Anatase | Rutile | |
| Nano-TiO2 | 21.13 | 26.39 |
| 0.1% FeCl3 | 23.57 | 40.07 |
| 1% FeCl3 | 25.35 | 29.57 |
| 10% FeCl3 | 23.19 | 27.76 |
| Composition | Lattice Parameters (Å) | |||
|---|---|---|---|---|
| Anatase | Rutile | |||
| a | c | a | c | |
| Nano-TiO2 | 3.79 | 9.52 | 4.59 | 2.96 |
| 0.1% FeCl3 | 3.78 | 9.08 | 4.57 | 2.95 |
| 1% TiO2 | 3.77 | 9.19 | 4.57 | 2.95 |
| 10% FeCl3 | 3.76 | 9.30 | 4.56 | 2.95 |
| Composition | Unit Cell Volume (Å3) | XA | |
|---|---|---|---|
| Anatase | Rutile | ||
| Nano-TiO2 | 136.73 | 62.49 | 73.36 |
| 0.1% FeCl3 | 129.70 | 61.79 | 74.73 |
| 1% FeCl3 | 130.68 | 61.62 | 72.71 |
| 10% FeCl3 | 131.71 | 61.18 | 68.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margalho, É.M.; Lima, O., Jr.; Afonso, C.; Segundo, I.R.; Landi, S., Jr.; Freitas, E.; Costa, M.F.M.; Carneiro, J. Iron-Modified Nano-TiO2: Comprehensive Characterization for Enhanced Photocatalytic Properties. Photonics 2024, 11, 888. https://doi.org/10.3390/photonics11090888
Margalho ÉM, Lima O Jr., Afonso C, Segundo IR, Landi S Jr., Freitas E, Costa MFM, Carneiro J. Iron-Modified Nano-TiO2: Comprehensive Characterization for Enhanced Photocatalytic Properties. Photonics. 2024; 11(9):888. https://doi.org/10.3390/photonics11090888
Chicago/Turabian StyleMargalho, Élida M., Orlando Lima, Jr., Cátia Afonso, Iran Rocha Segundo, Salmon Landi, Jr., Elisabete Freitas, Manuel F. M. Costa, and Joaquim Carneiro. 2024. "Iron-Modified Nano-TiO2: Comprehensive Characterization for Enhanced Photocatalytic Properties" Photonics 11, no. 9: 888. https://doi.org/10.3390/photonics11090888
APA StyleMargalho, É. M., Lima, O., Jr., Afonso, C., Segundo, I. R., Landi, S., Jr., Freitas, E., Costa, M. F. M., & Carneiro, J. (2024). Iron-Modified Nano-TiO2: Comprehensive Characterization for Enhanced Photocatalytic Properties. Photonics, 11(9), 888. https://doi.org/10.3390/photonics11090888










