Polycationic Photosensitizers as Effective Anticancer Agents That Destroy Cancer Stem Cells, Cancer Vascularization and Induce Protective Desmoplastic Reaction around Lung Cancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Photosensitizers
2.2. Tumor Model
2.3. Photodynamic Studies
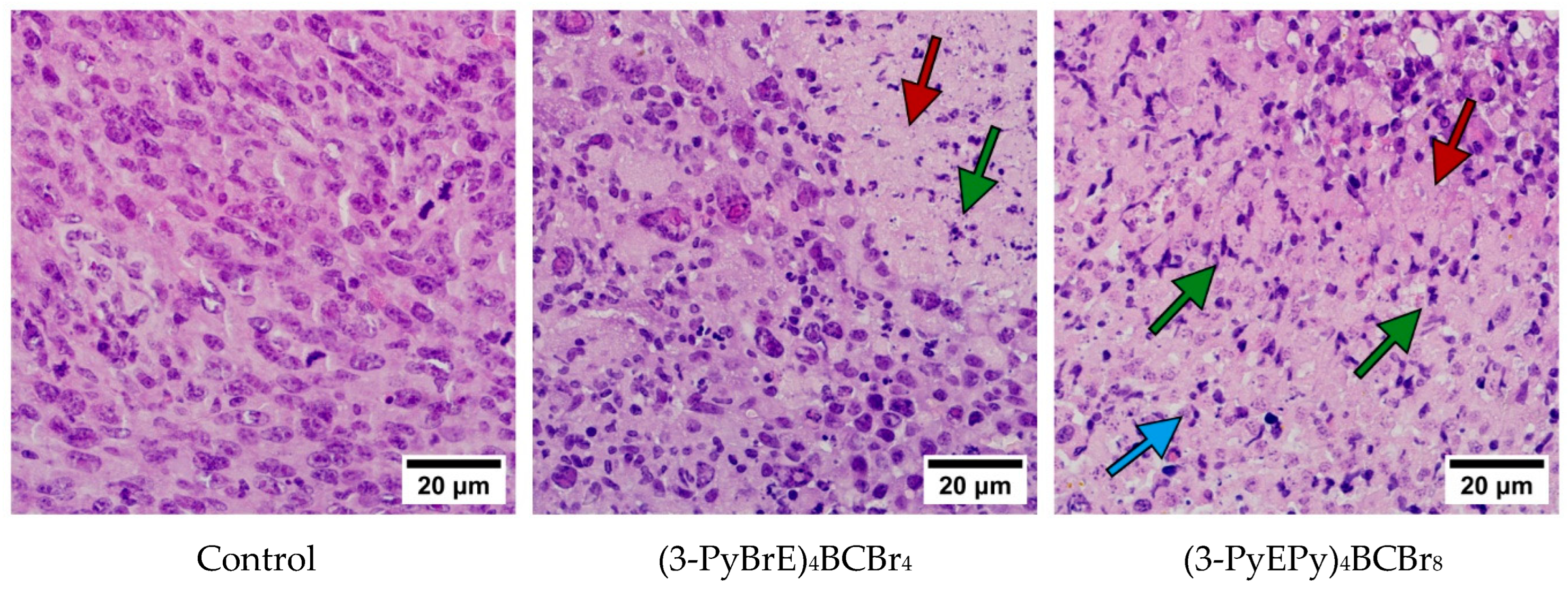
2.4. Morphological Analysis
2.5. Immunohistochemistry (IHC)
2.6. Statistical Analysis of Morphological Data
3. Results
3.1. Macro-Criteria of Antitumor Efficacy of Photosensitizers
3.2. Evaluation of the Antitumor Efficacy of PSs Based on the Results of Morphological and Immunohistochemical Studies of Tumor Tissues
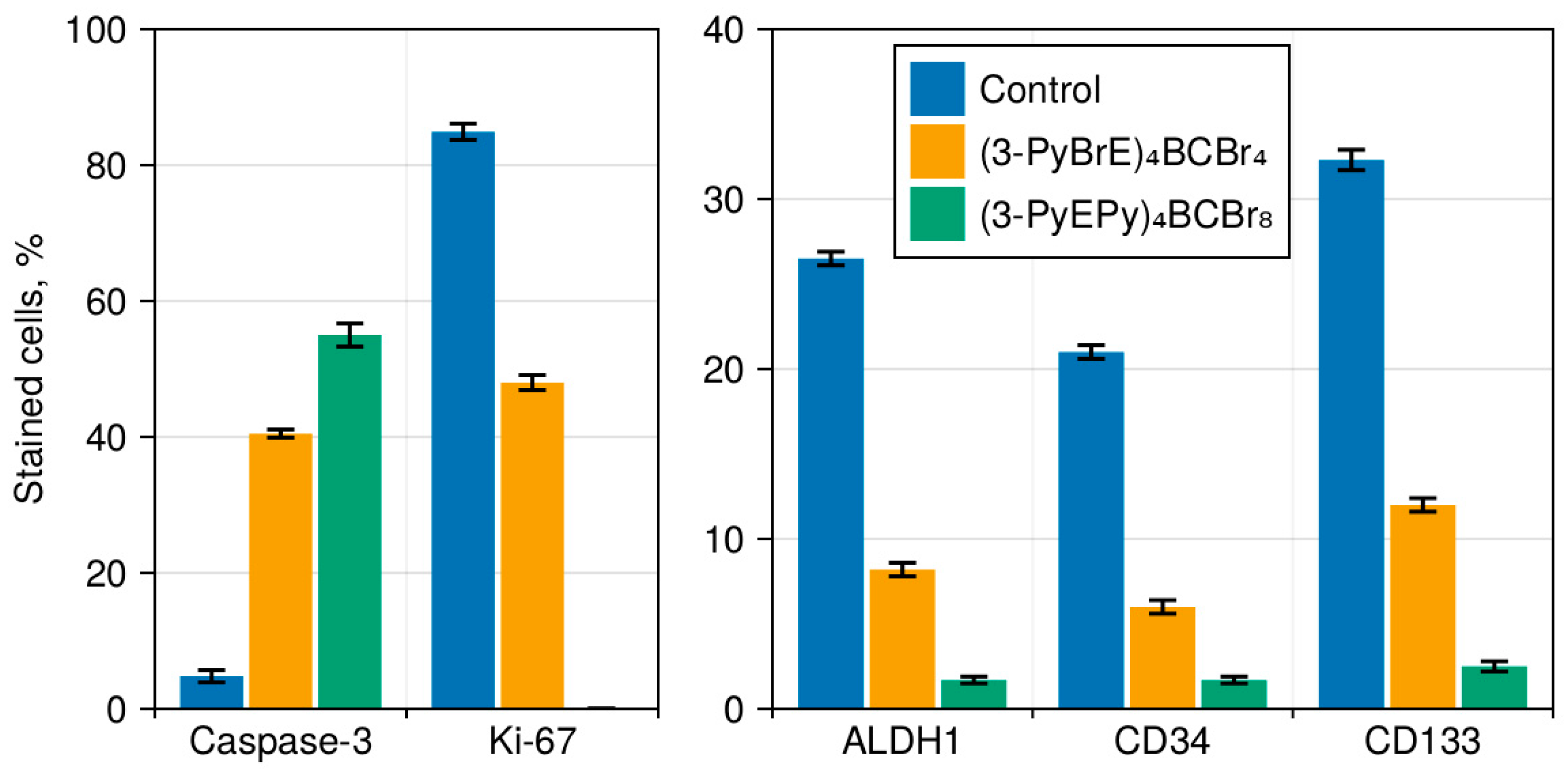
3.3. The Immunohistochemical Examination of the Pathomorphosis of LLC
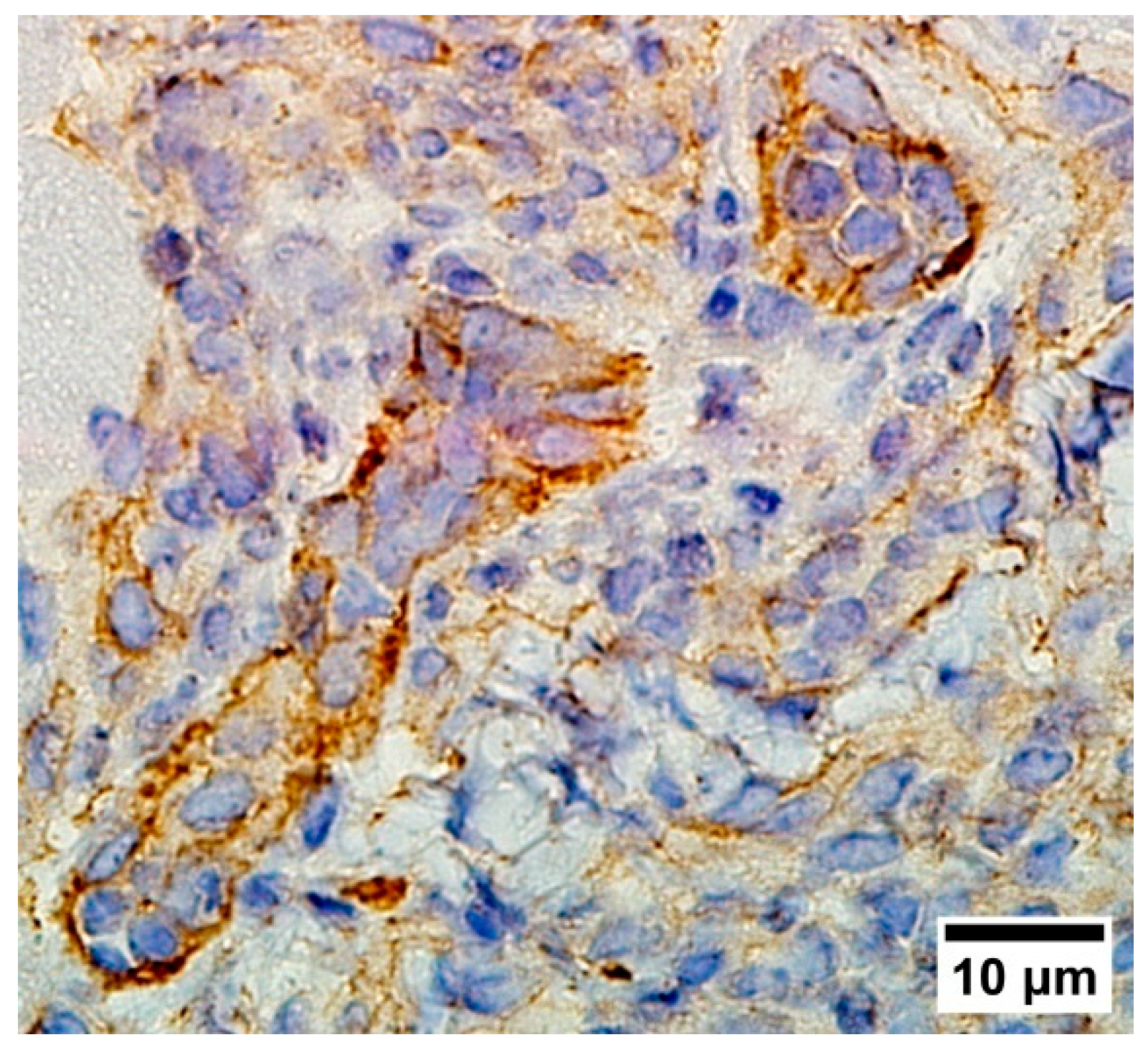
3.4. Cancer Stem Cells in LLC
3.5. Vascularisation in Tumor Tissue
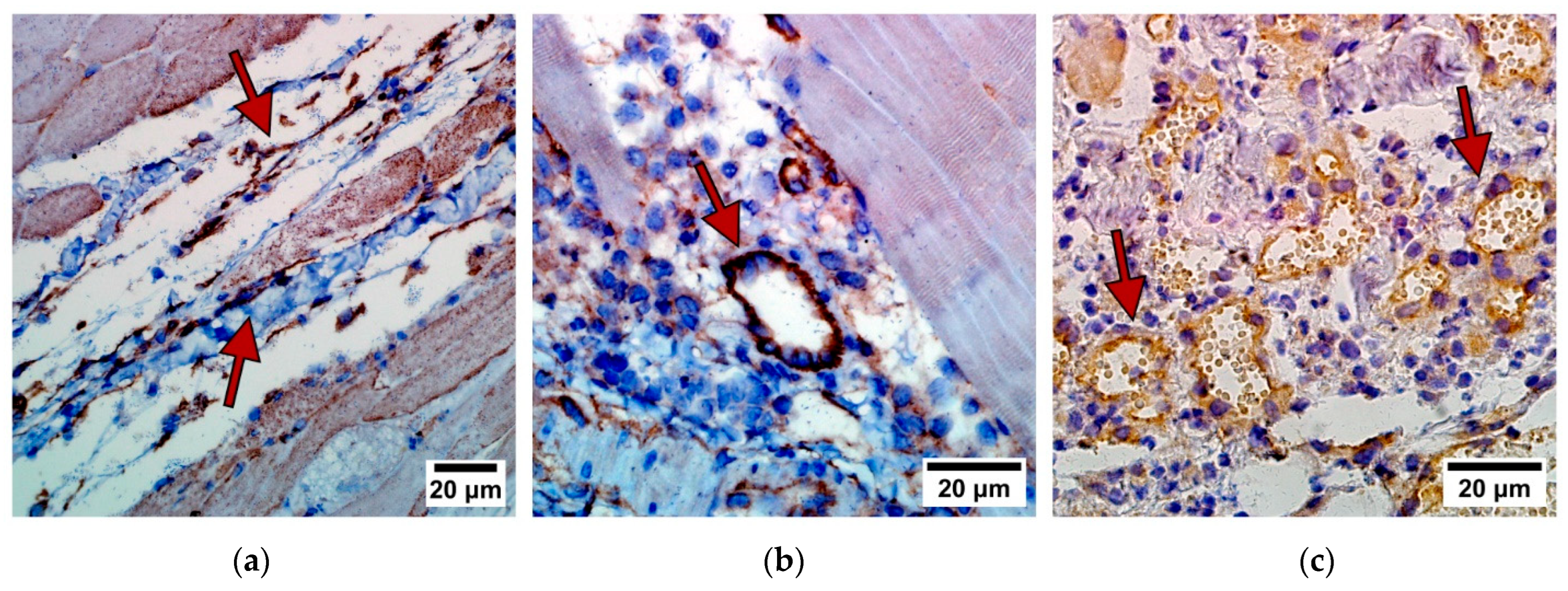
3.6. Desmoplastic Reaction in the Border of Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Averyanov, A. (Ed.) Difficult to Diagnose Rare Diffuse Lung Disease; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-815375-8. [Google Scholar]
- Goldblum, J.R.; Lamps, L.W.; McKenney, J.K.; Myers, J.L.; Ackerman, L.V.; Rosai, J. (Eds.) Rosai and Ackerman’s Surgical Pathology—2 Volume Set, 11th ed.; Elsevier: Philadelphia, PA, USA, 2018; ISBN 978-0-323-26339-9. [Google Scholar]
- Kumar, V.; Abbas, A.K.; Aster, J.C.; Turner, J.R.; Perkins, J.A.; Robbins, S.L.; Cotran, R.S. (Eds.) Robbins & Cotran Pathologic Basis of Disease, 10th ed.; Elsevier: Philadelphia, PA, USA, 2021; ISBN 978-0-323-53113-9. [Google Scholar]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24. [Google Scholar] [CrossRef] [PubMed]
- Prabavathy, D.; Swarnalatha, Y.; Ramadoss, N. Lung Cancer Stem Cells—Origin, Characteristics and Therapy. Stem Cell Investig. 2018, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Caon, I.; Bartolini, B.; Parnigoni, A.; Caravà, E.; Moretto, P.; Viola, M.; Karousou, E.; Vigetti, D.; Passi, A. Revisiting the Hallmarks of Cancer: The Role of Hyaluronan. Semin. Cancer Biol. 2020, 62, 9–19. [Google Scholar] [CrossRef] [PubMed]
- De Aberasturi, A.L.; Redrado, M.; Villalba, M.; Larzabal, L.; Pajares, M.J.; Garcia, J.; Evans, S.R.; Garcia-Ros, D.; Bodegas, M.E.; Lopez, L.; et al. TMPRSS4 Induces Cancer Stem Cell-like Properties in Lung Cancer Cells and Correlates with ALDH Expression in NSCLC Patients. Cancer Lett. 2016, 370, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, I.S.; Wei, S.-J.; Kang, M.H. Role of OCT4 in Cancer Stem-like Cells and Chemotherapy Resistance. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2020, 1866, 165432. [Google Scholar] [CrossRef]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.-W.; Buchanan, P. Lung Cancer: Biology and Treatment Options. Biochim. Biophys. Acta BBA—Rev. Cancer 2015, 1856, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Gopalan, V.; Smith, R.A.; Lam, A.K.-Y. Translational Potential of Cancer Stem Cells: A Review of the Detection of Cancer Stem Cells and Their Roles in Cancer Recurrence and Cancer Treatment. Exp. Cell Res. 2015, 335, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Wang, K.-P.; Mo, J.-G.; Xiong, L.; Wen, Y. Photodynamic Therapy Regulates Fate of Cancer Stem Cells through Reactive Oxygen Species. World J. Stem Cells 2020, 12, 562–584. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Jackson, J.D.; Zheng, X.; Pandey, S.K.; Pandey, R.K. Substrate Affinity of Photosensitizers Derived from Chlorophyll-a: The ABCG2 Transporter Affects the Phototoxic Response of Side Population Stem Cell-like Cancer Cells to Photodynamic Therapy. Mol. Pharm. 2010, 7, 1789–1804. [Google Scholar] [CrossRef][Green Version]
- Crous, A.; Dhilip Kumar, S.S.; Abrahamse, H. Effect of Dose Responses of Hydrophilic Aluminium (III) Phthalocyanine Chloride Tetrasulphonate Based Photosensitizer on Lung Cancer Cells. J. Photochem. Photobiol. B Biol. 2019, 194, 96–106. [Google Scholar] [CrossRef]
- Chizenga, E.P.; Chandran, R.; Abrahamse, H. Photodynamic Therapy of Cervical Cancer by Eradication of Cervical Cancer Cells and Cervical Cancer Stem Cells. Oncotarget 2019, 10, 4380–4396. [Google Scholar] [CrossRef]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The Role of Photodynamic Therapy on Multidrug Resistant Breast Cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Tumangelova-Yuzeir, K.; Minkin, K.; Angelov, I.; Ivanova-Todorova, E.; Kurteva, E.; Vasilev, G.; Arabadjiev, J.; Karazapryanov, P.; Gabrovski, K.; Zaharieva, L.; et al. Alteration of Mesenchymal Stem Cells Isolated from Glioblastoma Multiforme under the Influence of Photodynamic Treatment. Curr. Issues Mol. Biol. 2023, 45, 2580–2596. [Google Scholar] [CrossRef]
- Kogan, E.A.; Meerovich, G.A.; Karshieva, S.S.; Makarova, E.A.; Romanishkin, I.D.; Akhlyustina, E.V.; Meerovich, I.G.; Zharkov, N.V.; Demura, T.A.; Chen, Z.-L.; et al. On the Mechanisms of Photodynamic Action of Photosensitizers Based on Polycationic Derivatives of Synthetic Bacteriochlorin against Human Lung Cancer Cells A549 (in Vitro Study). Photodiagnosis Photodyn. Ther. 2022, 39, 102955. [Google Scholar] [CrossRef] [PubMed]
- Koudinova, N.V.; Pinthus, J.H.; Brandis, A.; Brenner, O.; Bendel, P.; Ramon, J.; Eshhar, Z.; Scherz, A.; Salomon, Y. Photodynamic Therapy with Pd-bacteriopheophorbide (TOOKAD): Successful in Vivo Treatment of Human Prostatic Small Cell Carcinoma Xenografts. Int. J. Cancer 2003, 104, 782–789. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The Role of Porphyrin Chemistry in Tumor Imaging and Photodynamic Therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Yakubovskaya, R.I.; Plotnikova, E.A.; Plyutinskaya, A.D.; Morozova, N.B.; Chissov, V.I.; Makarova, E.A.; Dudkin, S.V.; Lukyanets, E.A.; Vorozhtsov, G.N. Photophysical Properties and in Vitro and in Vivo Photoinduced Antitumor Activity of Cationic Salts of Meso-Tetrakis(N-Alkyl-3-Pyridyl)Bacteriochlorins. J. Photochem. Photobiol. B Biol. 2014, 130, 109–114. [Google Scholar] [CrossRef]
- Kogan, E.A.; Meerovich, G.A.; Karshieva, S.S.; Makarova, E.A.; Romanishkin, I.D.; Akhlyustina, E.V.; Meerovich, I.G.; Zharkov, N.V.; Koudan, E.V.; Demura, T.A.; et al. Photodynamic Therapy of Lung Cancer with Photosensitizers Based on Polycationic Derivatives of Synthetic Bacteriochlorin (Experimental Study). Photodiagnosis Photodyn. Ther. 2023, 42, 103647. [Google Scholar] [CrossRef]
- Makarova, E.A.; Lukyanets, E.A.; Tiganova, I.G.; Romanova, Y.M.; Meerovich, G.A.; Loschenov, V.B.; Alekseeva, N.V.; Akhlyustina, E.V. Photosensitizers for Photodynamic Inactivation of Bacteria, Including in Biofilms. RU Patent #2670201, 13 June 2018. Available online: https://patents.google.com/patent/RU2670201C1/en (accessed on 8 April 2024).
- Yakubovskaya, R.I.; Kazachkina, N.I.; Karmakova, T.A.; Morozova, N.B.; Pankratov, A.A.; Plyutinskaya, A.D.; Feofanov, A.V.; Chissov, V.I.; Tikhomirova, A.V. Methodological Recommendations for the Study of Photoinduced Antitumor Properties of Pharmaceuticals. In Guidelines for Conducting Preclinical Studies of Pharmaceuticals; Grif&K: Tula, Russia, 2012; Volume 1, pp. 655–669. [Google Scholar]
- Kumar, G.L.; Rudbeck, L. (Eds.) Immunohistochemical Staining Methods, 5th ed.; DAKO Corporation: Glostrup, Denmark, 2009. [Google Scholar]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Zheng, H.; Pomyen, Y.; Hernandez, M.O.; Li, C.; Livak, F.; Tang, W.; Dang, H.; Greten, T.F.; Davis, J.L.; Zhao, Y.; et al. Single-cell Analysis Reveals Cancer Stem Cell Heterogeneity in Hepatocellular Carcinoma. Hepatology 2018, 68, 127–140. [Google Scholar] [CrossRef]
- Orekhov, P.S.; Kholina, E.G.; Bozdaganyan, M.E.; Nesterenko, A.M.; Kovalenko, I.B.; Strakhovskaya, M.G. Molecular Mechanism of Uptake of Cationic Photoantimicrobial Phthalocyanine across Bacterial Membranes Revealed by Molecular Dynamics Simulations. J. Phys. Chem. B 2018, 122, 3711–3722. [Google Scholar] [CrossRef]
- Li, F.; Xu, J.; Liu, S. Cancer Stem Cells and Neovascularization. Cells 2021, 10, 1070. [Google Scholar] [CrossRef]





| Group | TGImax, % | ALS, Days | LSI, % | Surviving Mice, % |
|---|---|---|---|---|
| Control | — | 28.3 ± 6 | — | 0 |
| (3-PyBrE)4BCBr4 | 80 | 56.0 ± 18.7 | 97 | 16.7 |
| (3-PyEPy)4BCBr8 | 85 | 67.2 ± 20.8 | 137 | 50 |
| Group | Necrosis, % | Surviving Cancer Tissue, % | Mitosis, % |
|---|---|---|---|
| Control | 25.0 | 75.0 | 13.4 ± 1.3 |
| (3-PyBrE)4BCBr4 | 69.0 | 31.0 | 2.4 ± 0.2 |
| (3-PyEPy)4BCBr8 | 97.5 | 2.5 | 0.3 ± 0.2 |
| Group | Cancer Invasiveness, Points | Desmoplastic Reaction, Points |
|---|---|---|
| Control | 6.0 | 2.0 |
| (3-PyBrE)4BCBr4 | 2.0 | 6.0 |
| (3-PyEPy)4BCBr8 | 0.0 | 8.0 |
| Group | Interval after Irradiation, Days | ||
|---|---|---|---|
| 3 | 7 | 14 | |
| Control | 17 | 20 | 18 |
| (3-PyBrE)4BCBr4 | 7 | 5 | 3 |
| (3-PyEPy)4BCBr8 | 3 | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogan, E.; Meerovich, G.; Karshieva, S.; Makarova, E.; Romanishkin, I.; Akhlyustina, E.; Meerovich, I.; Zharkov, N.; Kharnas, S.; Levkin, V.; et al. Polycationic Photosensitizers as Effective Anticancer Agents That Destroy Cancer Stem Cells, Cancer Vascularization and Induce Protective Desmoplastic Reaction around Lung Cancers. Photonics 2024, 11, 485. https://doi.org/10.3390/photonics11060485
Kogan E, Meerovich G, Karshieva S, Makarova E, Romanishkin I, Akhlyustina E, Meerovich I, Zharkov N, Kharnas S, Levkin V, et al. Polycationic Photosensitizers as Effective Anticancer Agents That Destroy Cancer Stem Cells, Cancer Vascularization and Induce Protective Desmoplastic Reaction around Lung Cancers. Photonics. 2024; 11(6):485. https://doi.org/10.3390/photonics11060485
Chicago/Turabian StyleKogan, Evgeniya, Gennady Meerovich, Saida Karshieva, Elena Makarova, Igor Romanishkin, Ekaterina Akhlyustina, Irina Meerovich, Nikolay Zharkov, Sergey Kharnas, Vladimir Levkin, and et al. 2024. "Polycationic Photosensitizers as Effective Anticancer Agents That Destroy Cancer Stem Cells, Cancer Vascularization and Induce Protective Desmoplastic Reaction around Lung Cancers" Photonics 11, no. 6: 485. https://doi.org/10.3390/photonics11060485
APA StyleKogan, E., Meerovich, G., Karshieva, S., Makarova, E., Romanishkin, I., Akhlyustina, E., Meerovich, I., Zharkov, N., Kharnas, S., Levkin, V., Demura, S., Chen, Z., Loschenov, V., & Reshetov, I. (2024). Polycationic Photosensitizers as Effective Anticancer Agents That Destroy Cancer Stem Cells, Cancer Vascularization and Induce Protective Desmoplastic Reaction around Lung Cancers. Photonics, 11(6), 485. https://doi.org/10.3390/photonics11060485






