1. Introduction
Liquid targets occupy an intermediate position between gaseous and solid targets and are quite promising for use as laser-plasma sources (LPSs). Registration of the emission lines of a range of chemical elements that are difficult to obtain in the gas phase can become possible when using liquid targets. The use of liquids as LPS targets has its own advantages and disadvantages. Liquid targets have a greater density than gas targets, leading to more laser radiation being absorbed by the target. The practical convenience of using a liquid jet or liquid spray source lies in the possibility of separating the region of interaction with the laser beam from the nozzle exit. However, the formation of liquid targets is a rather complex technical task.
There are several types of liquid targets: constant flow jets [
1], cryogenic liquid jets [
2,
3], droplet targets [
4], and spray targets [
5,
6]. When using constant flow systems, it is necessary to use capillaries of a small cross-section to minimize the cost of consumables. However, this leads to a significant increase in the influence of surface forces on the separation of the jet from the nozzle during the exit process, and to instability of the position of the jet in space. Using pulsed systems allows for an increase in the nozzle cross-section while maintaining low liquid costs, thereby solving this problem. Spray targets are more often used to study substances that are difficult to reach in the gas phase and are solutions of solid chemical compounds in liquids. The spray target has an elongated shape; therefore, the laser focus can be located at a relatively large distance from the nozzle exit, and the average density of the substance at the focus will be higher compared to gaseous targets. In this investigation, a liquid spray target with a pulsed supply system of delivering the jet into a vacuum chamber was used.
In most cases designed for LPS, Nd:YAG lasers with pulse durations of 0.5–10 ns and pulse energies of about 1 J are used to excite targets [
7,
8]. This choice is due, for the most part, to the wide availability and comparative ease of working with such lasers. Although lasers with pulse durations of several picoseconds or some hundreds of femtoseconds would allow us to produce significantly greater power densities at their focus points, they are much less readily available in laboratories.
In this article, the results of investigations on the emission spectra in the spectral range of 10–20 nm for oxygen- and halogen-containing liquid targets are demonstrated. The main difference from previous studies is that we have determined the intensities of the registered emission lines in absolute units. The values of the corresponding conversion efficiencies are also given.
2. Research Stand
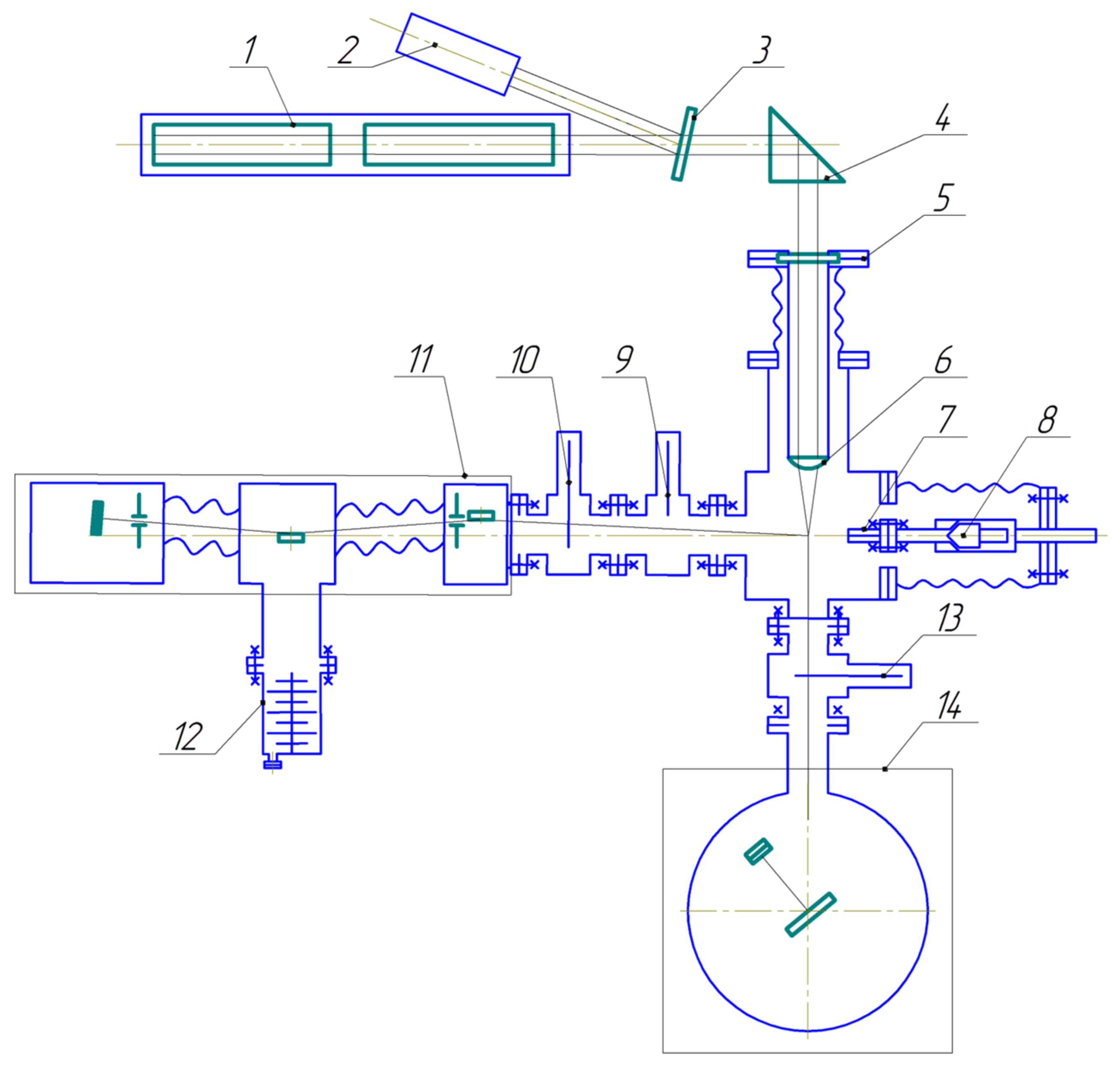
Experiments were carried out on the research stand, as shown in
Figure 1.
The research installation works as follows. The liquid used is forced out by gas under pressure through the quick-acting pulse valve 8, based on a Bosch 0 280 158 017 injector (Gerlingen, Germany), having one standard hole, 7, with a diameter of 225 μm. The liquid pressure at the input to the pulse valve is 4 bar, and the valve opening duration is 300 μs. The average diameter of liquid droplets in a spray is ~20 to 30 µm [
9]. A photograph of the liquid spray target is shown in
Figure 2.
The picture shows that the liquid jet has sharp boundaries where the liquid flows out of the valve nozzle. The jet spreads at a great distance from the nozzle.
The target was excited by focused radiation from an Nd:YAG laser with a pulse duration at half-maximum of 8.4 ns, a pulse energy of 0.8 J, and a frequency of 10 Hz. The laser radiation power density at the focal spot was ~2.6 × 10
12 W/cm
2. Referring to
Figure 1, the radiation from laser 1 goes to dividing plate 3, from where a small part of the beam goes to radiation power detector 2. The main part of the radiation passes through prism 4 and optical input 5, and goes to lens 6, which has a 45 mm focal length. The laser radiation is thereby focused on the liquid spray, causing its breakdown and the formation of a plasma. When taking into account all the optical elements on the path of the laser beam to the spark formation zone, the energy of laser radiation in the breakdown region is ~86% of that specified on the laser. The polychromatic soft X-rays and EUV radiation of the plasma pass through an electro-pneumatic vacuum shutter 9 and two free-hanging X-ray filters, 10, based on Mo/ZrSi
2, and enters the grating spectrometer–monochromator. A detailed description of the filters is given below. Here, the monochromatic soft X-rays and extreme ultraviolet (EUV) radiation are detected using a pulse detector. The detection system in this case is a photocathode coated with a CsI layer. The EUV radiation quanta striking the photocathode knocks photoelectrons out of it, which are then recorded by the VEU-7 secondary electron multiplier. Evacuation of the spectrometer–monochromator is carried out by the turbomolecular pump, 12. The spectral resolution was 0.04 nm in our experiments.
The calculated focal spot diameter is 66 µm. The size of the laser beam in the focal spot was determined by calculating the entire laser path, taking into account aberrations introduced by optical elements. After this, an experiment was carried out to burn thin films with laser radiation and then measure the resulting holes. The results of calculations and measurements coincided with an accuracy of about 10%.
The design and operating principles of the installation are described in more detail in [
10].
An absolute-calibrated Bragg spectrometer was used to measure the values of the emission radiation intensities in absolute units. A multilayer Mo/Be X-ray mirror was used as the monochromator. The two free-hanging Zr/ZrSi
2 film filters were used to reduce longwave radiation. One was placed on the entrance to the Bragg spectrometer and the other in front of its detector. A detailed description of the filters is given below. This combination of mirror and filters allowed us to specifically detect emission radiation of the plasma in the 10–20 nm spectral range, with an efficient reduction in the longer wavelength parts of the plasma radiation. A silicon photodiode with a sensitivity δ = 0.25 C/J was used as a soft X-ray radiation detector [
11]. The operating principles, design, and properties of the installation are described in more detail in [
12].
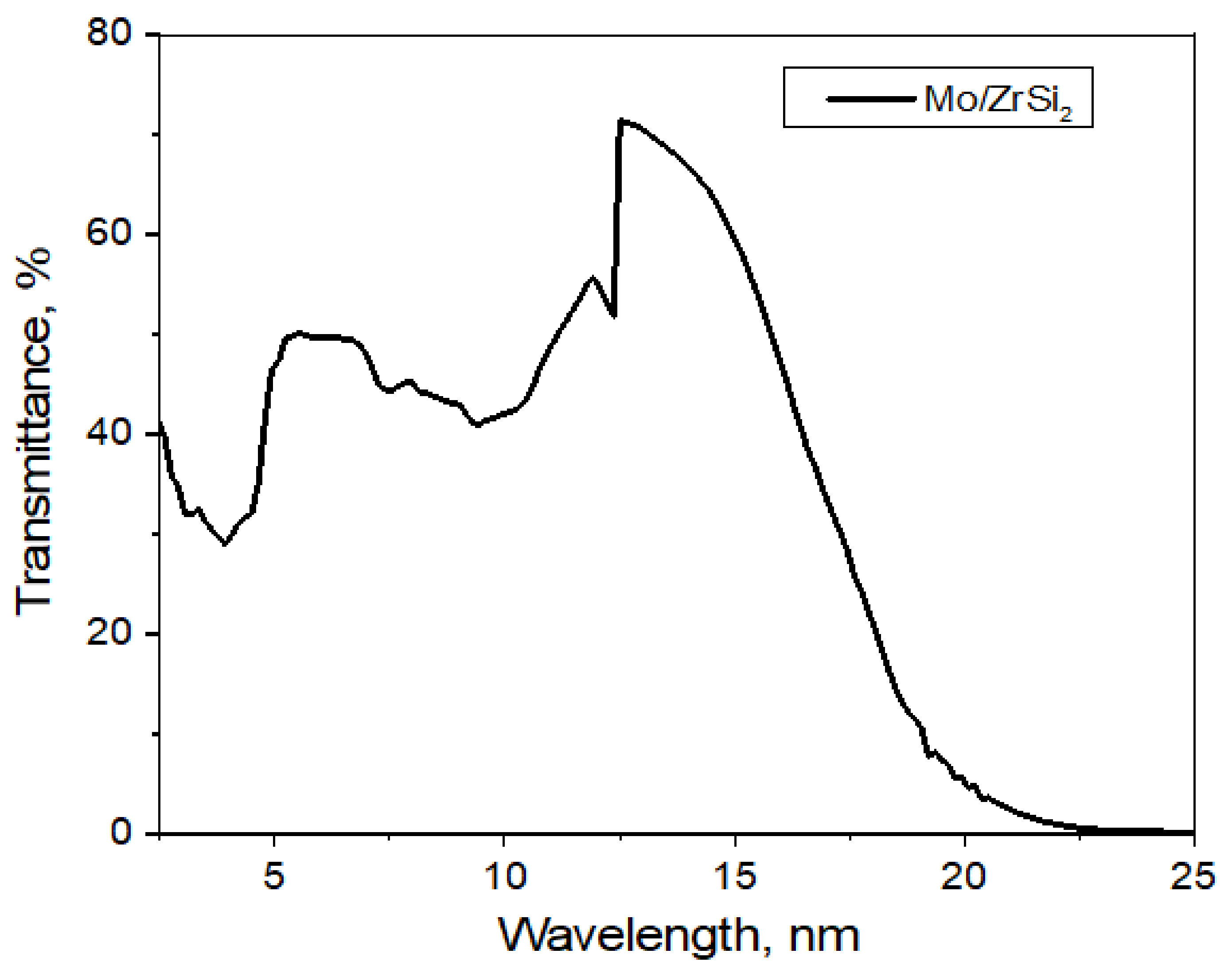
Film filters based on the Mo/ZrSi
2 structure, used in measurements on the grating spectrometer–monochromator, consist of the following layers: MoSi
2—3 nm, (Mo—2.5 nm; ZrSi
2—1.5 nm) × 11 periods, Mo—2.5 nm, MoSi
2—3 nm. This structure, its properties, and methods of preparation are described in detail in [
13]. The spectral dependence of the Mo/ZrSi
2 film transmittance is shown in
Figure 3.
This filter transmits radiation with a wavelength shorter than 20 nm quite well, which corresponds to the operating wavelength range of the spectrometer–monochromator 5–20 nm.
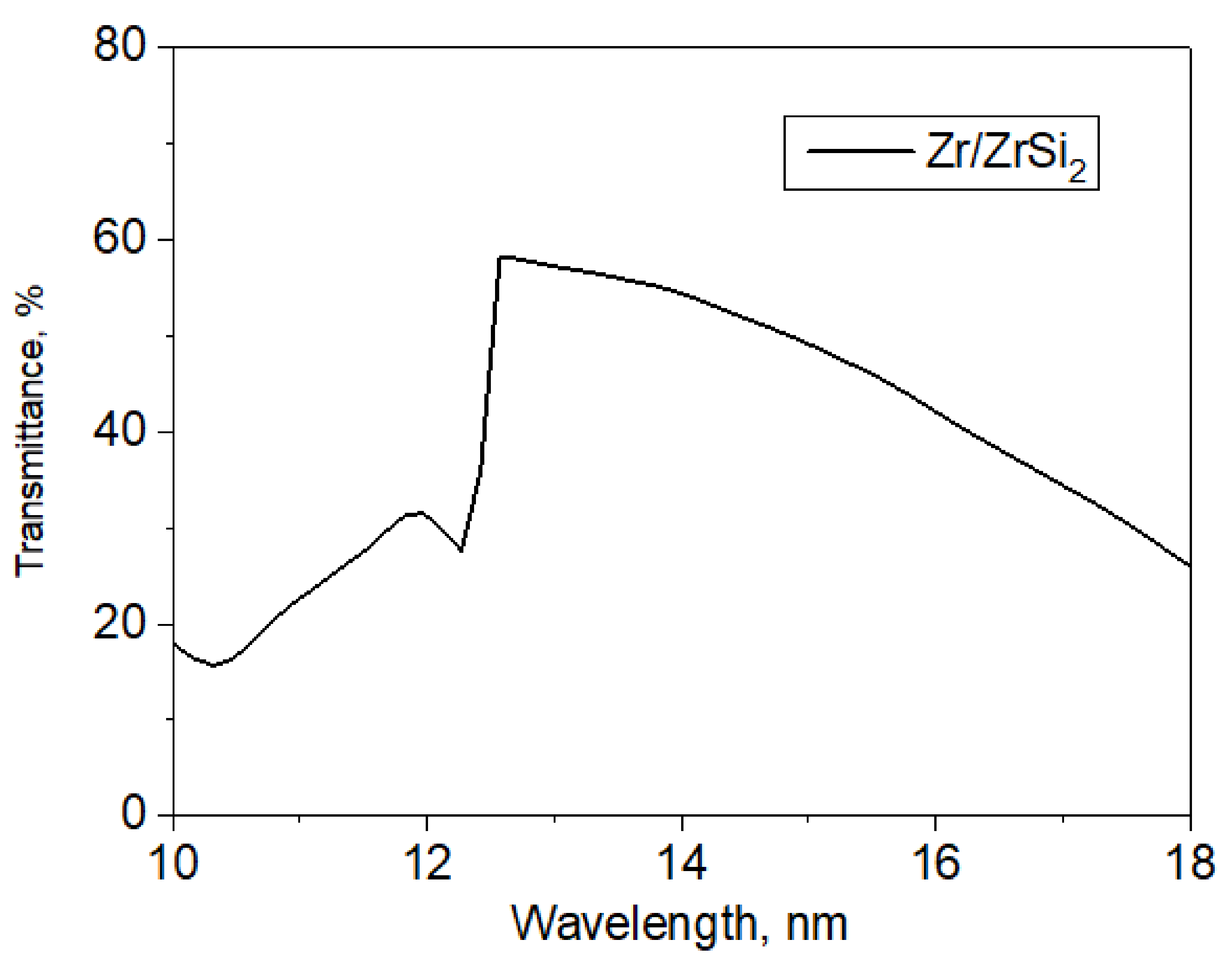
Film filters based on the Zr/ZrSi
2 structure, used in measurements on a mirror spectrometer, consist of the following layers: Zr/Si
2, (Zr—2.5 nm/ZrSi
2—2.5 nm) × 24 periods. The dependence of transmittance on wavelength for this type of filter is shown in
Figure 4.
This type of filter satisfactorily transmits radiation in the spectral range of 12.4–18 nm. For wavelengths shorter than 12.4 nm, the transmittance of this structure is noticeably reduced due to the absorption edge of the silicon included in the structure. Transmittances at certain wavelengths were taken into account when calculating emission intensities in absolute values.
3. Method for Calculating Intensities in Absolute Units
The method for determining the energy and number of photons emitted in a particular spectral band by a laser spark is described in [
12]. For the experiments directly carried out in this work, the energy and number of photons were calculated using the following formulas:
where E [J] is the energy emitted by the laser plasma in the band corresponding to the resolution of the Bragg spectrometer;
[nm] is the wavelength corresponding to the maximum signal in the experimental spectral band; α = 10
−11 [C/W] is the amplifier sensitivity; V [W] is the signal detected by the photodiode; γ = 5.45 × 10
−5 [Sr] is the solid angle over which the spark emission was observed by the detector; δ = 0.25 [C/J] is the photodiode sensitivity; R is the reflectivity of the Mo/Be multilayer X-ray mirror at wavelength
; T is the transmittance of the Zr/ZrSi
2 film filters at wavelength
; h is Planck’s constant [J/s]; and c [m/s] is the speed of light.
Thus, the EUV radiation intensities are calculated for one laser pulse in half a 2π sphere in the approximation of isotropic radiation and the absence of self-absorption of radiation in the target. These approximations are allowed in many works where conversion efficiencies were obtained [
14,
15], and therefore are convenient when making comparisons. It is also worth noting that in the case when only a separate, narrow emission line falls within the investigated spectral band, the intensity measured by the method, as described, will correspond solely to the intensity of this line. In all other cases, the intensity recorded will be for the whole spectral band, as determined by the resolution of the Bragg spectrometer. The resolution of the spectrometer is determined by the properties of the multilayer X-ray mirror used. The impact of the finite sizes of the input and diaphragm as well as the mechanical accuracy of the positioning of the mirror is insignificant.
4. Experimental Approach
4.1. Investigation with Alcohols
Investigation of alcohols as targets for LPS have previously been carried out [
4]. Their ease of pumping, convenient thermodynamic properties, and ready availability make alcohols good liquid targets. Soft X-rays and EUV radiation in the 10–20 nm spectral range from laser-created plasmas based on alcohols are formed by the emissions from oxygen ions.
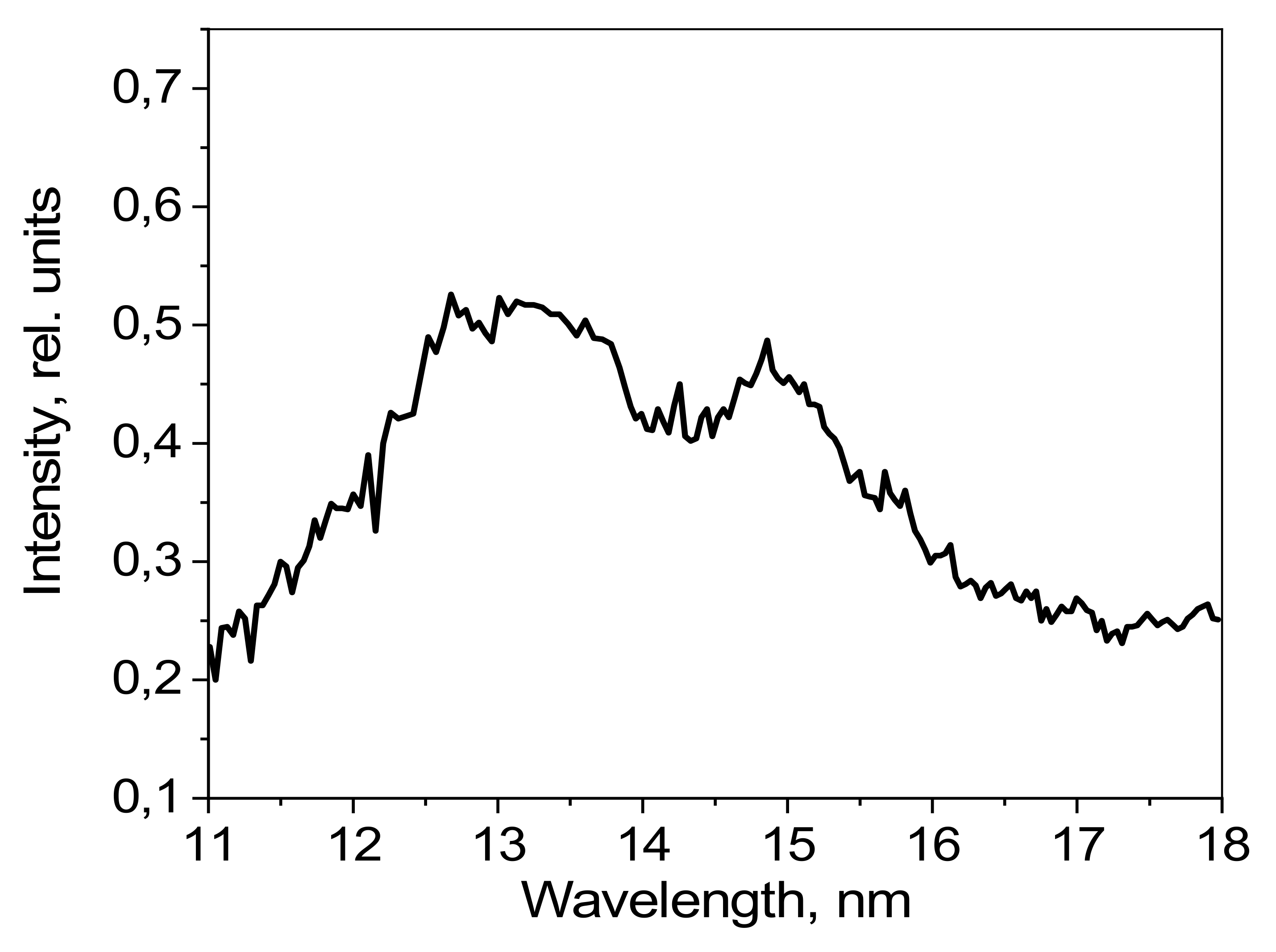
The emission spectrum of isopropyl alcohol in the 10–20 nm spectral range, as detected by a grating spectrometer, is shown in
Figure 5. The radiation intensity is given in relative units.
The measured spectra of methyl and ethyl alcohols show much resemblance to the spectrum of isopropyl alcohol (excepting small differences in the intensities of individual emission lines), and therefore they are not presented in this article.
Figure 5 shows several intense lines formed by the transitions of O-V and O-VI ions, observed in the 10–20 nm range. There are no lines visible for ionized carbon in this spectral range.
The interpretation of the observed lines was carried out in accordance with [
16].
Comparison was carried out of the intensities of the spectra observed with CO
2, and isopropyl and ethyl alcohols. The spectra of the two liquids were detected under similar excitation conditions. The CO
2 was passed through a supersonic conical nozzle with d
cr = 500 μm, under a pressure of 20 bar. The laser pulse energy was 0.8 J, and the pulse duration was τ = 8.4 ns. The results are shown in
Table 1. The intensities are given in relative units and normalized to the O-VI ion line at a wavelength of 12.98 nm.
When comparing the intensities of the O-V and O-VI lines in
Table 1, it is obvious that the laser spark reaches the highest temperature with ethyl alcohol as the target. A lower temperature was reached with isopropyl alcohol while the lowest occurred with the carbon dioxide gas jet target.
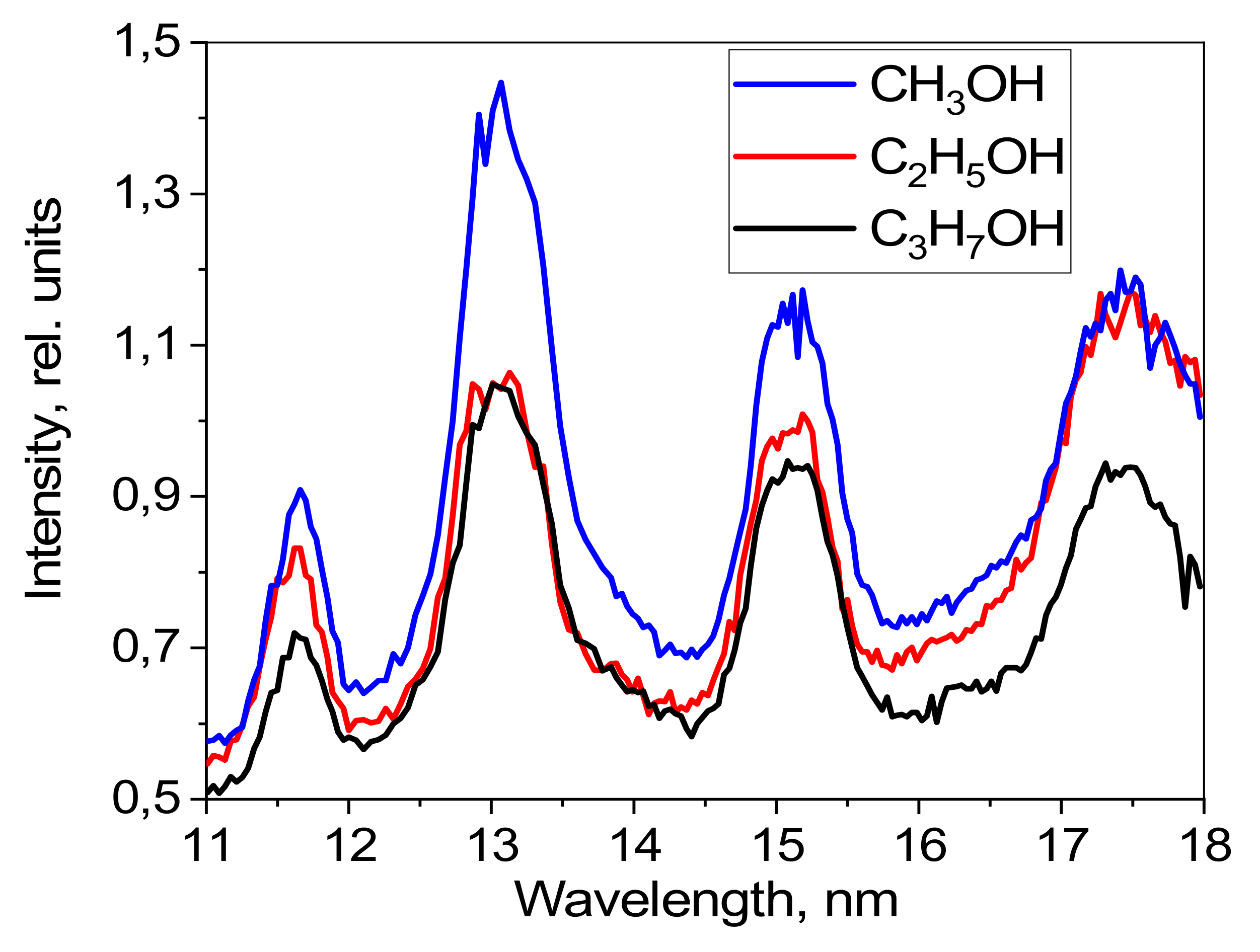
A separate investigation of the intensities in absolute units of radiation from targets based on the various alcohols was carried out using a mirror spectrometer. The typical alcohol emission spectra detected by this mirror spectrometer are shown in
Figure 6. The radiation intensity is given in relative units.
Figure 6 shows that the emission bands formed by the transitions of oxygen ions are wider compared to the bands seen in
Figure 5. This is because the resolution of the mirror spectrometer is worse than that of the grating spectrometer in this spectral range. The greatest radiation intensity was reached with excitation of the target based on methyl alcohol. Methyl alcohol is characterized by having a greater oxygen concentration compared to the other alcohols investigated, leading to its higher radiation intensity. A lower radiation intensity was observed with excitation of ethyl alcohol, and the lowest intensity was observed for excitation of isopropyl alcohol. These results therefore correlate with the oxygen concentrations within these alcohols.
The radiation intensities in absolute units of the alcohol emission lines and the conversion coefficients of laser radiation into EUV radiation at the corresponding wavelengths are given in
Table 2. The intensity values are for one laser pulse across the half 2π sphere in the approximation of isotropic radiation and in the absence of self-absorption of radiation by the target.
Table 2 shows the radiation intensities in the 11–18 nm spectral range when using liquid spray targets based on alcohols. The highest intensities for all studied alcohols are observed at a wavelength of 17.31 nm. The radiation intensities of these alcohol targets in the range of 10–20 nm correlate with the molecules’ oxygen concentrations as they decrease in the series: methyl–ethyl–isopropyl alcohol. The conversion coefficient under these conditions of excitation of various alcohol targets was ~0.005%, while the radiation intensity ranged from 1.0 × 10
12 to 4.6 × 10
12 ph/pulse at different wavelengths. Alcohols are quite useful targets for LPS, given that there is no risk of deposits forming on the optical elements. Thus, developing soft X-ray and EUV radiation laboratory sources based on alcohol targets is expedient.
4.2. Investigation of Dichloromethane CH2Cl2
Dichloromethane CH2Cl2 is a transparent, easily mobile, highly volatile, low-toxicity liquid with low reactivity in experimental conditions. The ease of pumping, acceptable thermodynamic properties, and ready availability make CH2Cl2 a good liquid target. The disadvantage of dichloromethane is its solvent ability, requiring the use of Fluoroplast-4 (PTFE) for all connections and seals.
The values of emission radiation in absolute units by LPS using targets based on dichloromethane, as far as we are aware, have not previously been investigated. The soft X-rays and EUV radiation for laser-created plasmas based on dichloromethane in the spectral range of 10–20 nm are the result of emissions from ionized chlorine.
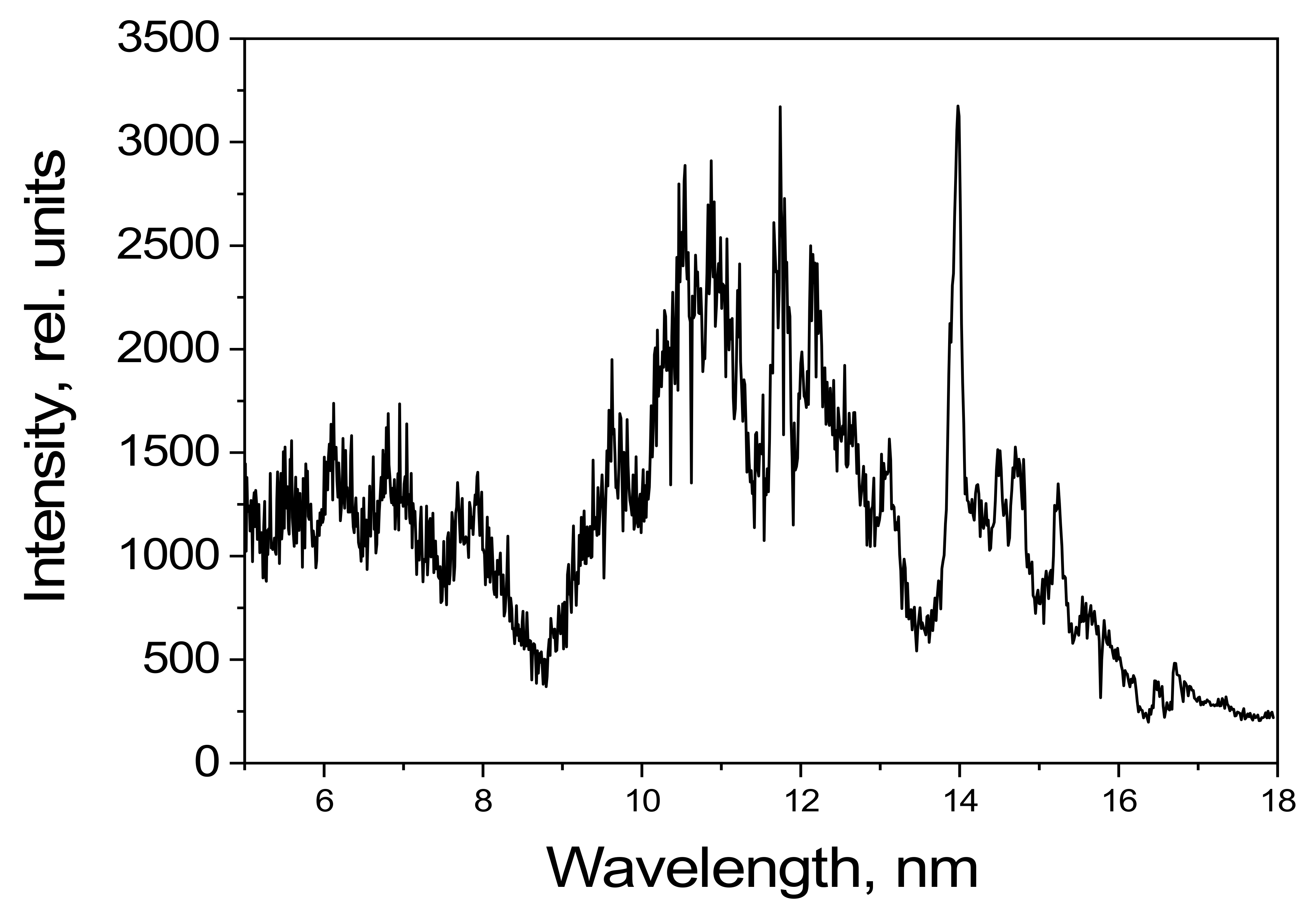
The emission spectrum of dichloromethane in the spectral range of 5–20 nm, as detected using the grating spectrometer, is shown in
Figure 7. The radiation intensity is given in relative units.
Several intense lines formed by transitions of Cl-VIII, Cl-VII, and Cl-IX ions can be observed in the 5–20 nm range. The emission lines of Cl-IX–Cl-XV ions, according to [
17,
18,
19,
20,
21], are few in number and of low intensity. There are no ionized carbon lines in the observed spectral range.
The interpretation of the observed lines was carried out in accordance with [
16,
17,
18,
19,
20,
21] and is given in [
22].
The typical dichloromethane emission spectrum, as detected by the mirror spectrometer, is shown in
Figure 8. The radiation intensity is given in relative units.
Figure 8 shows emission bands formed by the transition of chlorine ions that are wider than the bands in
Figure 7; this is due to the resolution of the mirror spectrometer being worse than that of the grating spectrometer in this spectral range.
The radiation intensities of some CH
2Cl
2 emission lines in absolute values and the conversion coefficients are given in
Table 3. The intensity values are for one laser pulse across the half 2π sphere in the approximation of isotropic radiation and with an absence of self-absorption of radiation by the spray target.
Table 3 shows that in terms of conversion efficiency at various wavelengths, amounting to ~0.005%, the dichloromethane target is comparable to the alcohol targets. Dichloromethane is therefore a useful target for LPS, given that there is also no risk of contamination of the optical elements. Thus, developing soft X-ray and EUV radiation laboratory sources based on dichloromethane targets is expedient.
4.3. Investigation of Methylene Bromide CH3Br
Methylene bromide CH3Br is a transparent, easily mobile, highly volatile liquid of relatively low toxicity, which is a very effective solvent with low reactivity in experimental conditions. The ease of pumping, acceptable thermodynamic properties, and ready availability make CH3Br a satisfactory liquid target. Methylene bromide’s disadvantage is its excellent solvent properties, requiring the use of Fluoroplast-4 connectors and seals. The CH3Br jet has a tendency to freeze while flowing into the vacuum chamber and therefore may give rise to deposits on the optical elements.
As far as we are aware, the emission radiation intensities generated by LPS using methylene bromide targets in absolute units have not previously been investigated. Soft X-rays and EUV radiation from laser-created plasmas based on CH3Br in the 10–18 nm spectral range are the result of emission radiation from ionized bromine.
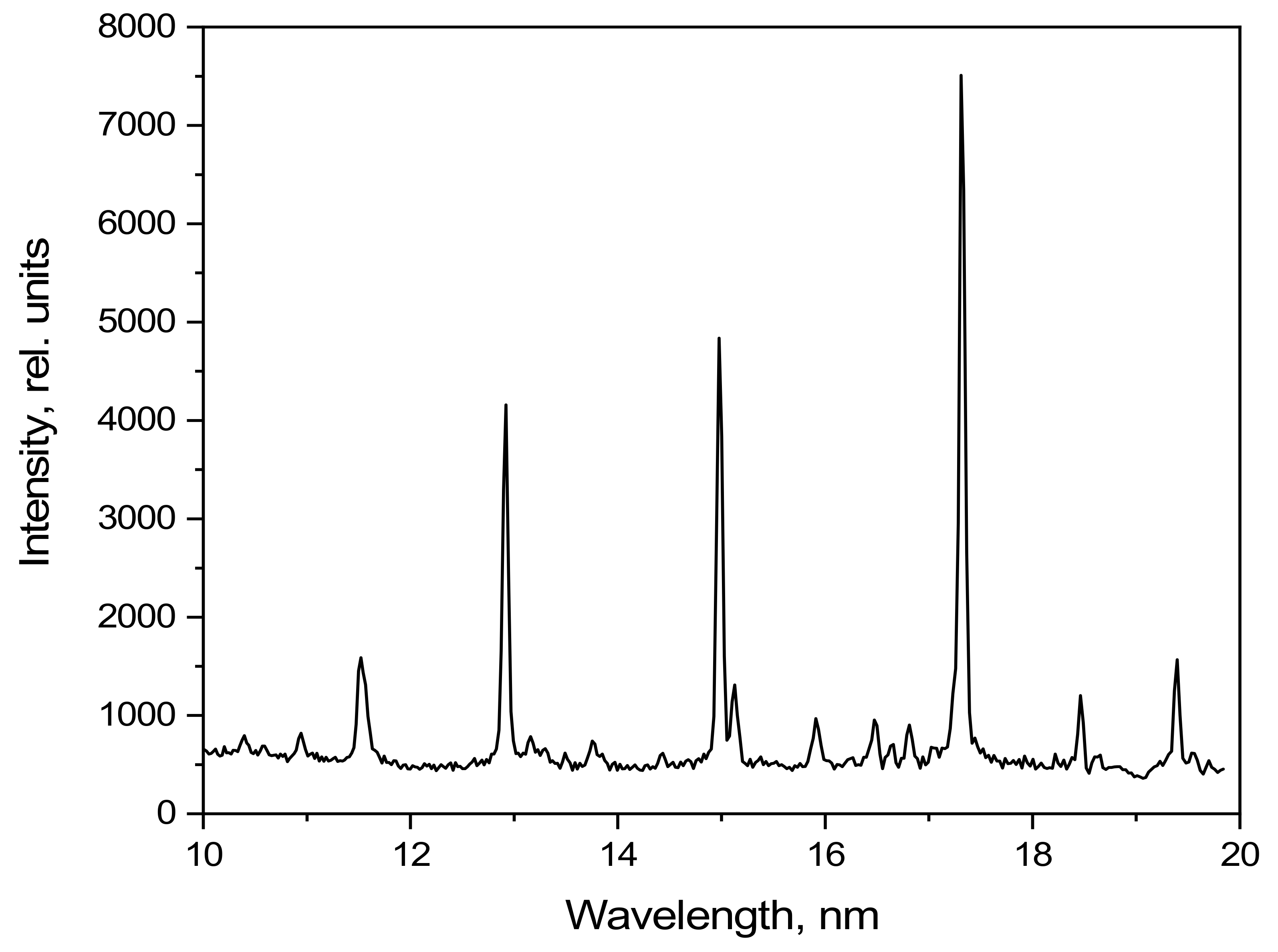
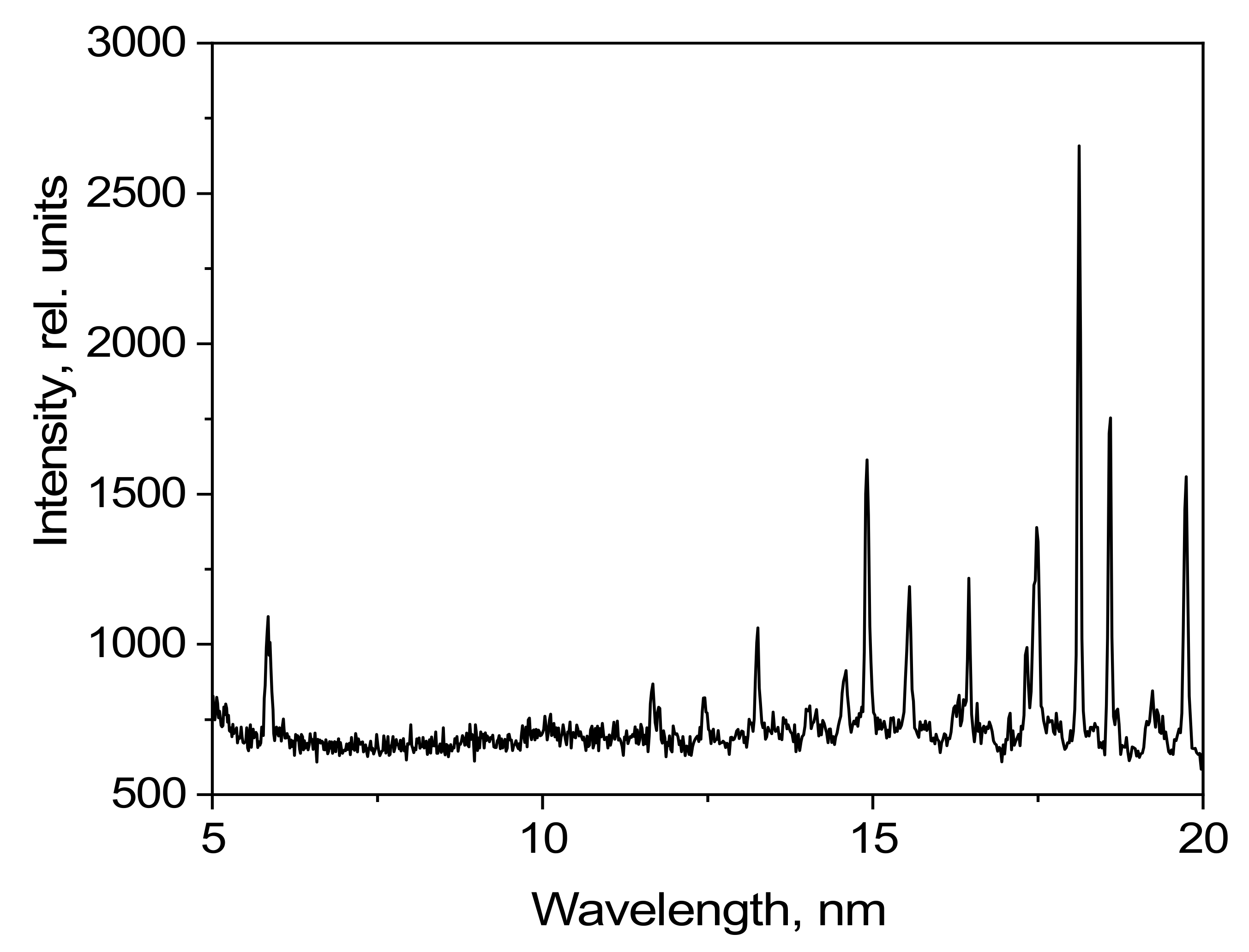
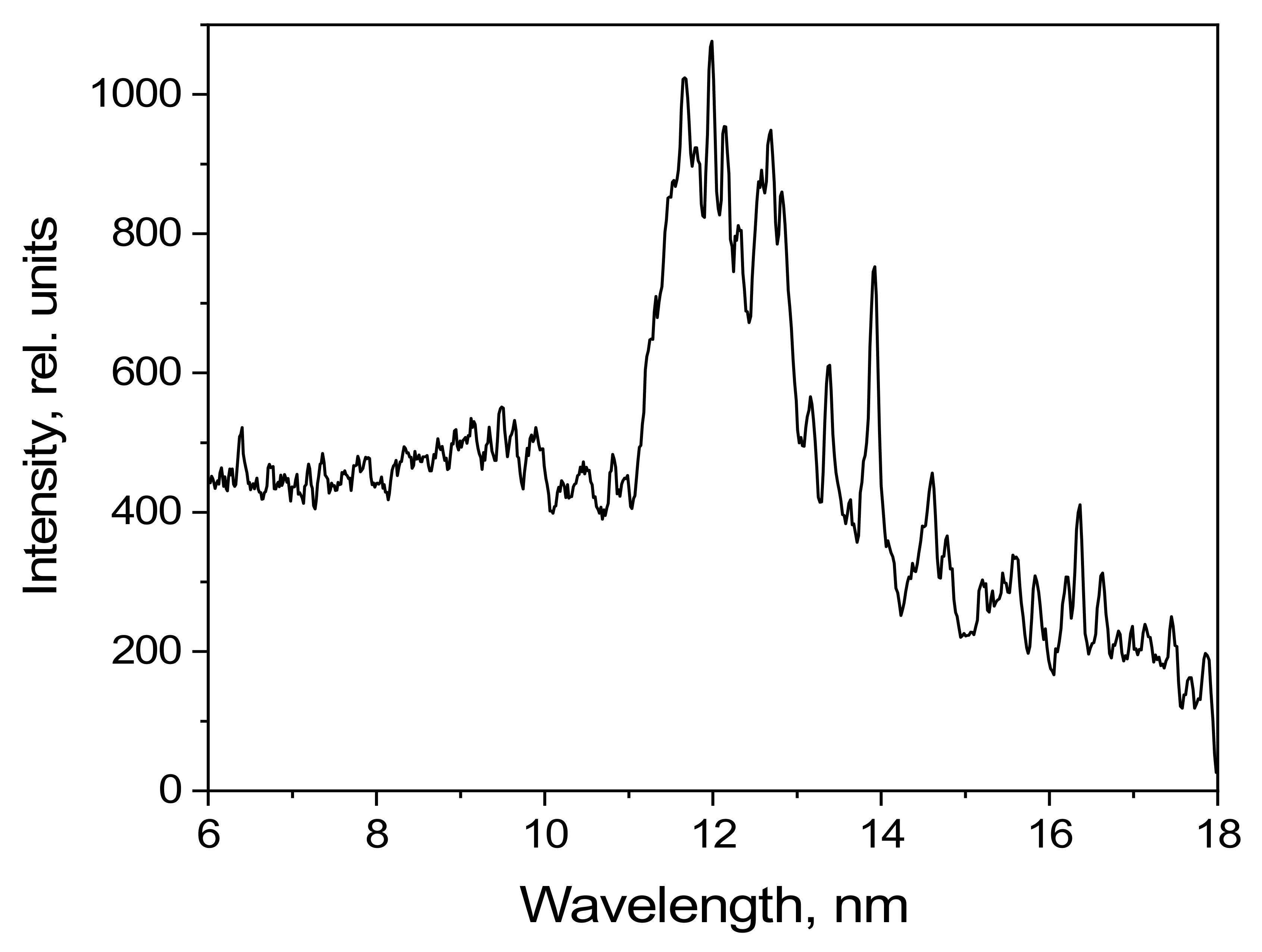
The emission spectrum of methylene bromide in the 5–18 nm spectral range as detected using the grating spectrometer is shown in
Figure 9. The radiation intensity is given in relative units.
Several of the high-intensity lines formed by transitions of Br-VII, Br-VIII, Br-IX, and Br-X ions can be observed in the 5–18 nm range. There are no ionized carbon lines in the observed spectral range.
The interpretation of the observed lines was carried out in accordance with [
16,
23,
24] and is given in [
22].
A comparison was undertaken of the spectra of the different bromine- and fluorine-containing targets. Additionally, spectra were recorded for the bromine-containing liquid C
2F
4Br
2 and the fluorine-containing gas CHF
3. The spectra of the liquids and of the CHF
3 were recorded under equivalent excitation conditions. The CHF
3 was made to flow through the supersonic conical nozzle of d
cr = 500 μm under a pressure of 25 bar. The laser pulse energy was 0.8 J, and the pulse duration was τ = 8.4 ns. The measured emission spectra are shown in
Figure 10. The intensities are given in relative units.
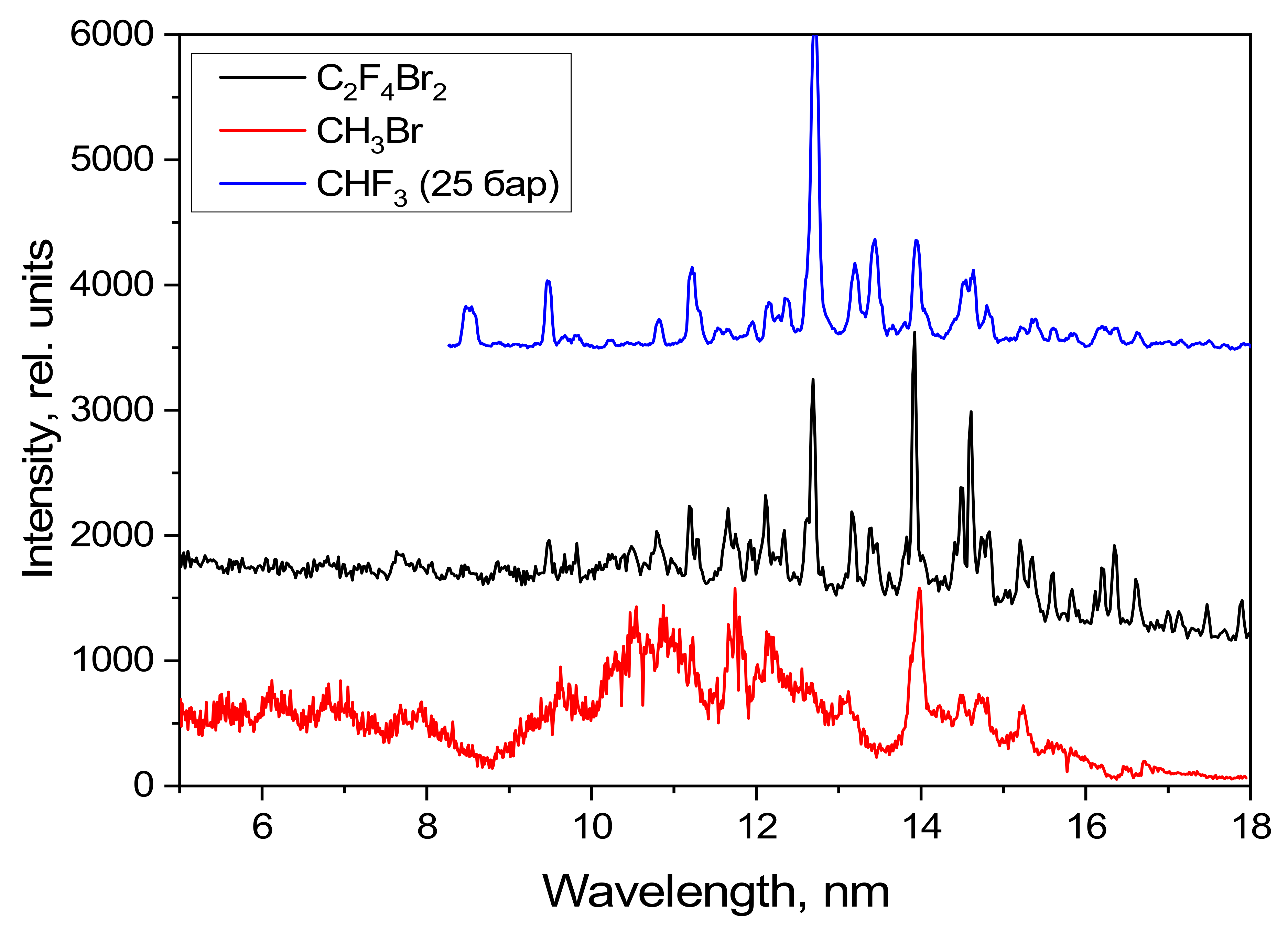
The emission spectrum of methylene bromide in the spectral range used is represented by ionized bromine lines, while the emission spectrum of C2F4Br2 in this spectral range is represented by lines from both bromine and fluorine ions. The emission spectrum of the CHF3 in the spectral range is represented by the ionized fluorine lines.
Figure 10 indicates that the C
2F
4Br
2 spectrum is essentially a superimposition of the CH
3Br and CHF
3 spectra, but that it also has its own features. The intensities of the bromine and fluorine lines, which correspond closely in wavelength, were increased for the excitation of C
2F
4Br
2. This phenomenon is most noticeable in the 13–18 nm range. However, the broad bromine emission bands and the fluorine lines in the 8–10 nm range disappear, indicating the lower plasma temperature of the target based on C
2F
4Br
2 compared to the separate CH
3Br and CHF
3 targets. It is also interesting that the broad bromine emission band in the 9–13 nm range disappears when using C
2F
4Br
2 instead of CH
3Br. This phenomenon indicates that it is pointless to use targets based on bromine if the compound also contains fluorine atoms.
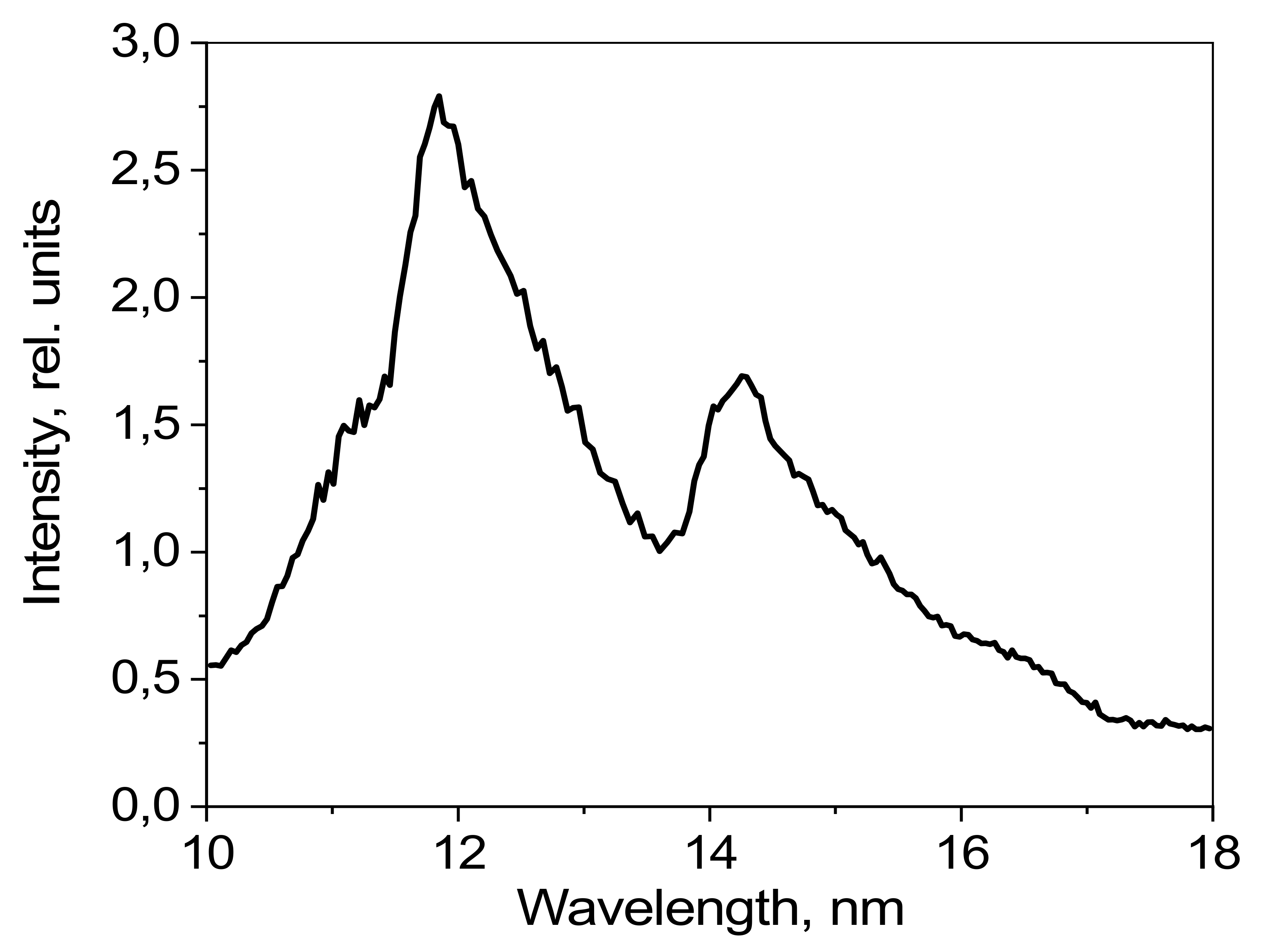
The emission spectrum of methylene bromide as detected using the mirror spectrometer is shown in
Figure 11. The radiation intensity is given in relative units.
Figure 11 shows that the emission bands formed by transitions of bromide ions are wider than those in
Figure 10, as the resolution of the mirror spectrometer is worse than that of the grating spectrometer in this spectral range.
The radiation intensities in absolute units of some of the CH
3Br emission lines and the conversion coefficients are given in
Table 4. The intensity values are for one laser pulse across the half 2π sphere in the approximation of isotropic radiation and in the absence of self-absorption of radiation by the spray target.
The table shows that the conversion coefficients upon excitation of a target based on methylene bromide are quite high and reach 0.18% at a wavelength of 11.75 nm. The emission spectra for bromine have been comparatively little studied in the 10–18 nm spectral range. Methylene bromide is an acceptable target for LPS.
4.4. Investigation of CF3CH2I
CF3CH2I is a yellowish, viscous, highly volatile, liquid of comparatively low toxicity, which is a very effective solvent, but with only low reactivity in experimental conditions. Its ease of pumping, satisfactory thermodynamic properties, and ready availability make CF3CH2I an adequate liquid target. CF3CH2I has a tendency to freeze as the jet flows into the vacuum chamber, and this can result in its deposition on the optical elements.
To the best of our knowledge, the values of emission radiation using LPS with targets based on CF3CH2I in absolute units have not previously been investigated. Soft X-rays and EUV radiation from a laser-created plasma based on CF3CH2I in the 10–18 nm spectral range are formed by the emission radiation from both ionized iodine and fluorine.
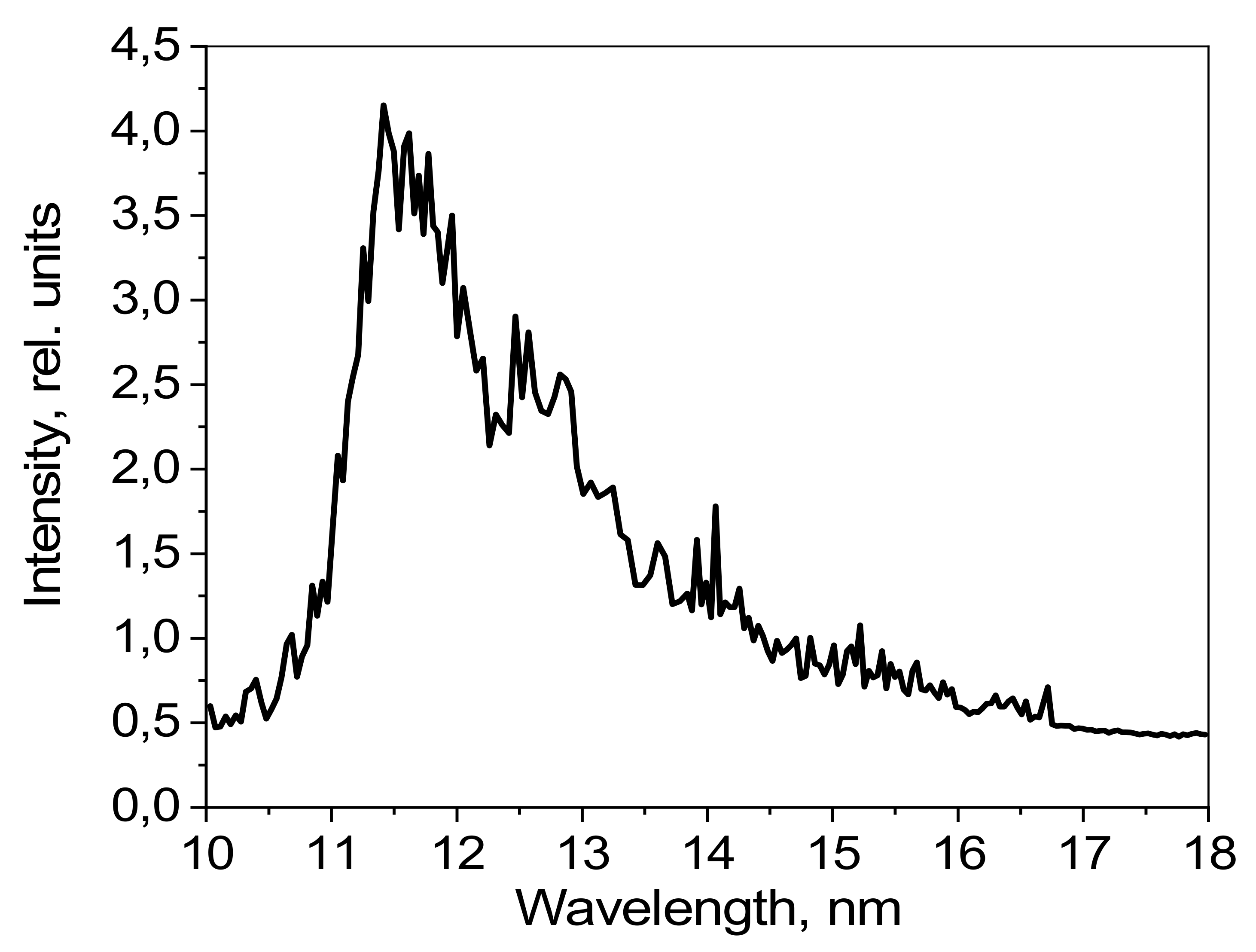
The emission spectrum of CF
3CH
2I in the 6–18 nm spectral range, as detected by the grating spectrometer–monochromator, is shown in
Figure 12. The radiation intensity is given in relative units.
Several high-intensity lines formed by the transitions of fluorine and iodine ions can be observed in the 6–18 nm range. In the 11–14 nm spectral range, the broad band with a maximum of around 11.8 nm corresponds to the emissions observed for iodine ions. Narrow peaks corresponding to fluorine ion emissions can be observed in the background of the band. There are no ionized carbon lines in the spectral range shown here.
No interpretation of the observed lines was carried out, because there is no information available about the emission spectra of iodine ions, although the thin lines were identified as corresponding to fluorine ions. The interpretation of the observed lines for fluorine ion emissions as part of the CHF
3 target is given in [
25].
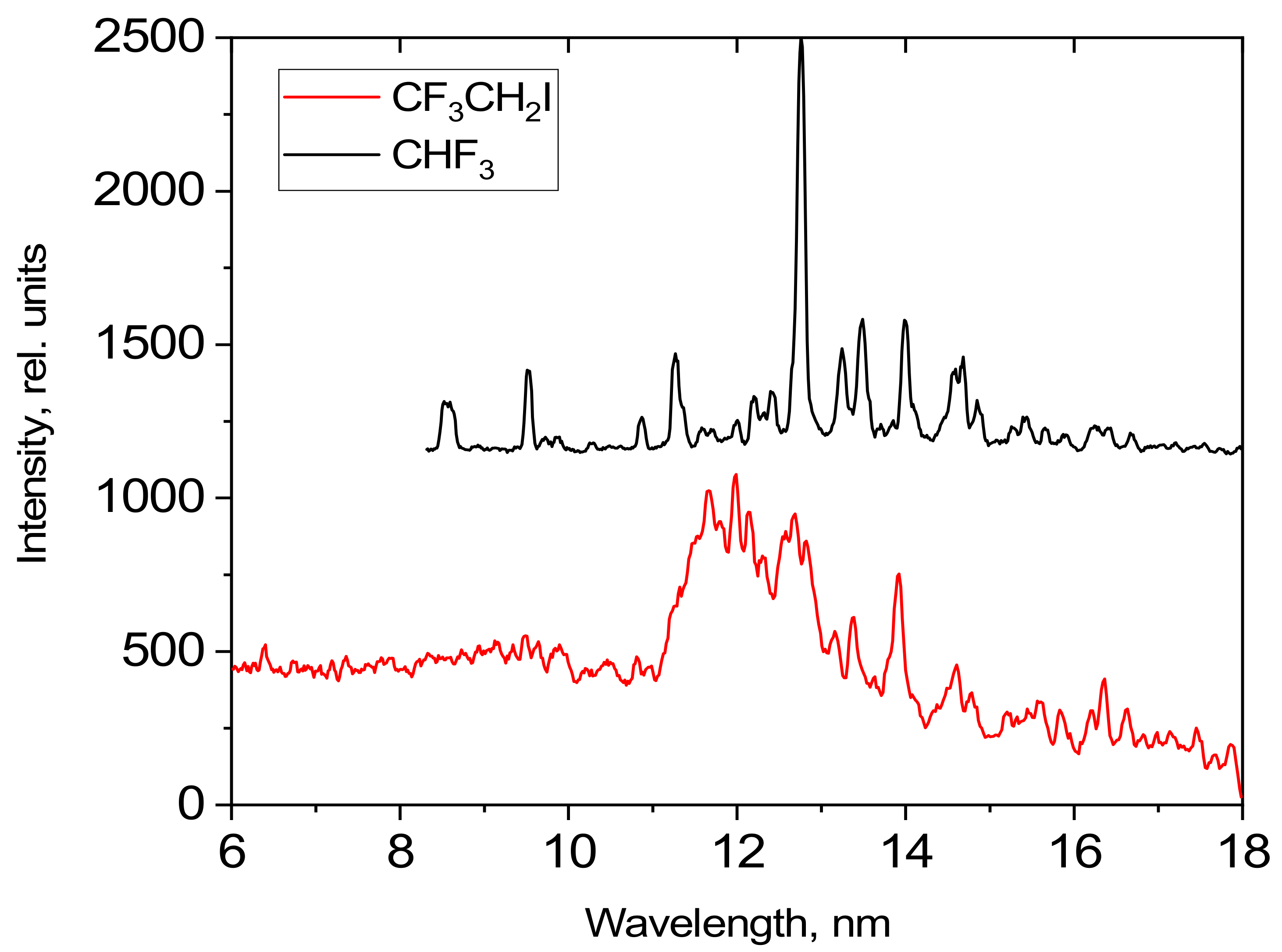
A comparison of the intensities of the spectra observed for CF
3CH
2I and CHF
3 was carried out. The gas was passed through the supersonic conical nozzle with a d
cr = 500 μm under a pressure of 25 bar. The laser pulse energy was 0.8 J. The detected emission spectra are shown in
Figure 13. The intensities are given in relative units.
A comparison with
Figure 12 indicates that the thin lines, in
Figure 13, correspond to the emissions seen in the CHF
3 gas target. The only feature contained in the CF
3CH
2I spectrum due to the presence of the iodine is the broad band in the 11–14 nm range, with a maximum of around 11.8 nm.
The typical emission spectrum of CF
3CH
2I as detected by the mirror spectrometer is shown in
Figure 14. The radiation intensity is given in relative units.
Figure 14 shows that the emission bands formed by the transitions of bromide ions are wide compared to the bands in
Figure 13, as the resolution of the mirror spectrometer is worse than that of the grating spectrometer in this spectral range.
The radiation intensities in absolute units of some of the CF
3CH
2I emission lines and the conversion coefficients are given in
Table 5. The intensity values are for one laser pulse across the half 2π sphere in the approximation of isotropic radiation and in the absence of self-absorption of radiation by the spray target.
The table shows that the conversion coefficient when exciting a target based on CF3CH2I is quite high. The iodine emission spectra in the 10–18 nm spectral range have not yet been studied, so this hampers the identification of the spectra of iodine-containing liquids. In addition, iodine is highly contaminant to the LPS setup, making it difficult to use iodine as a target for long periods of time. CF3CH2I therefore represents a poor target for use with LPS.
5. The Discussion of the Results
In the conducted studies of liquid spray targets for LPS based on methyl, ethyl, and isopropyl alcohols, and dichloromethane, methylene bromide, and CF3CH2I, the values of the radiation intensities in absolute units of the corresponding substances were obtained at individual wavelengths in the spectral range of 10–20 nm. Based on the experimental results, the conversion coefficients of laser radiation into short-wave radiation were calculated at certain wavelengths when using various liquid spray targets.
Alcohols as a target for liquid LPS have been studied previously [
4]. When alcohol targets are excited, EUV radiation is emitted by oxygen ions O-V and O-VI in the spectral range under study. Oxygen spectra were also obtained and studied using a CO
2 gas jet target [
12]. In [
12], the absolute value of the emission intensity of the oxygen ion O-VI at a wavelength of 12.98 nm was calculated to be 5.2 × 10
12 ph/pulse. The absolute value of the radiation intensity at a wavelength of 12.98 nm (see
Table 2) obtained in this work when using methyl alcohol as a target exceeds the value obtained with a gas jet target.
In [
26], a water jet target was studied when excited by an Nd-YLF laser with a duration of up to 3 ns and a focal spot of the order of 10 μm in diameter. Also, the article provides the obtained conversion coefficient at a wavelength of 13 nm in the whole 4π sphere, which amounted to 0.12%. Taking into account the excitation parameters and conversion coefficient, the absolute radiation intensity at a wavelength of 13 nm in [
26] was about 2.04 × 10
11 ph/pulse. The work also notes that the conversion coefficient strongly depends on both the energy of the exciting laser pulse and its duration. The conversion coefficient at a wavelength of 13 nm upon excitation of a target based on methyl alcohol, obtained in this work, is significantly lower, but the radiation intensity in absolute units turned out to be higher (see
Table 2).
Also in this work, values of the radiation intensity of oxygen-containing liquid jet targets at other wavelengths were obtained, which is a new experimental result.
When using dichloromethane as a target, EUV radiation in this spectral range is due to the emission of chlorine ions Cl-VIII, Cl-VII, and Cl-IX. Despite the large number of works with theoretical calculations of the spectra of chlorine, the emission spectra in the studied spectral range and the possibility of using chlorine and, in particular, dichloromethane as a chlorine-containing liquid as a target for LPS, have been studied relatively little in practice. In this work, the advantages of dichloromethane as a liquid target for laser excitation to generate EUV radiation were demonstrated, and the conversion coefficient at various wavelengths was ~0.005%, which creates the prerequisites for further research in this direction. Also, the given values of radiation intensities in absolute units are original experimental results.
When using methylene bromide as a target, EUV radiation in this spectral range is due to the emission of bromine ions Br-VII, Br-VIII, Br-IX, and Br-X. Despite the large number of works with calculations of bromine spectra, the emission spectra in the studied spectral range and the possibility of using bromine-containing liquids as a target for LPS have practically not been studied experimentally. In this work, the spectra of two bromine-containing liquids and their comparison were demonstrated, and the values of the emission intensities in absolute units of bromine in the composition of methylene bromide at wavelengths of 11.75 and 13.99 nm were obtained, which is an original and important experimental result. In this case, the conversion coefficient in the experiments performed reaches 0.0905% at a wavelength of 11.75 nm, which makes a liquid target based on methylene bromide quite promising for use in LPS.
Research on CF
3CH
2I is of particular importance since the spectra of iodine have been little studied and identified. Despite previous works with calculations of iodine spectra [
27,
28], when identifying the experimentally recorded spectrum of an iodine-containing liquid, it was not possible to decipher the observed lines. However, it is known that the broad emission band in the iodine spectrum in the wavelength range 11–14 nm corresponds to the UTA (Unresolved Transitions Array) band observed for xenon [
29]. For the first time, emission spectra of laser plasma containing highly charged iodine ions were recorded in the EUV spectral range, and measurements of the intensities of the recorded radiation in absolute values were carried out. The conversion coefficient at a wavelength of 11.44 nm was 0.348%, which makes a liquid target based on CF
3CH
2I quite promising for use in LPS.
Thus, this work obtained quite important and original scientific results. By using spray targets, higher absolute emission intensity values were obtained for some spectral bands in the wavelength range 10–20 nm. The emission spectra of laser plasma containing highly charged iodine ions were recorded for the first time.
6. Conclusions
In this study, investigations were carried out on liquid spray LPS targets based on methyl, ethyl, and isopropyl alcohols, and dichloromethane, methylene bromide, and CF3CH2I. The emission spectra of the plasmas based on the above substances in the 5–20 nm spectral range were detected under excitation by laser radiation having a pulse energy of 0.8 J and a pulse duration of 8.4 ns. The conversion efficiency in the experiments performed at various wavelengths was ~0.005% under excitation of alcohols and dichloromethane, up to ~0.09% for excitation of methylene bromide and 0.174% for excitation of CF3CH2I. Interpretation of the spectra was carried out and the absolute intensities of the emission lines detected in the 10–20 nm range were determined.
Methylene bromide and CF3CH2I are characterized by their tendency to freeze as the jet flows into the vacuum chamber, meaning that the use of these as targets can lead to heavy contamination of the optical surfaces. Nevertheless, the conversion coefficients and the intensities of laser-created plasma radiation in absolute units derived from these substances are the highest (~1013 to 1014 ph/pulse) when compared to the other liquids investigated (~1012 ph/pulse).
These research results will make it possible to choose appropriate liquid targets when designing different installations that are intended to generate LPS-created radiation in the soft X-ray and EUV ranges.
Author Contributions
Methodology, A.N.N. and A.A.P.; formal analysis, A.A.P.; investigation, A.N.N. and A.A.P.; data curation, A.N.N.; writing—preparation of the initial draft, A.N.N. and V.E.G.; writing—reviewing and editing, V.E.G.; supervision, N.I.C.; project administration, N.I.C.; acquisition of financing, N.I.C. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful for the support by the Center of Excellence «Center of Photonics» funded by The Ministry of Science and Higher Education of the Russian Federation, contract No. 075-15-2022-316.
Institutional Review Board Statement
Ethical review and approval not applicable for studies not involving humans or animals.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hansson, B.A.M.; Rymell, L.; Berglund, M.; Hertz, H.M. A Liquid-Xenon-Jet Laser-Plasma X-Ray and EUV Source. Microelectron. Eng. 2000, 53, 667–670. [Google Scholar] [CrossRef]
- Fogelqvist, E.; Kordel, M.; Selin, M.; Hertz, H.M. Stability of liquid-nitrogen-jet laser-plasma targets. J. Appl. Phys. 2015, 118, 174902. [Google Scholar] [CrossRef]
- Wieland, M.; Wilhein, T.; Faubel, M.; Ellert, C.; Schmidt, M.; Sublemontier, O. EUV and fast ion emission from cryogenic liquid jet target laser-generated plasma. Appl. Phys. B 2001, 72, 591–597. [Google Scholar] [CrossRef]
- Hertz, H.M.; Rymell, L.; Berglund, M.; Malmqvist, L. Debris-free soft X-ray generation using a liquid droplet laser-plasma target. Appl. Laser Plasma Radiat. II SPIE 1995, 2523, 88–93. [Google Scholar]
- Węgrzyński, Ł.; Bartnik, A.; Wachulak, P.; Fok, T.; Fiedorowicz, H. Laser-produced plasma soft X-ray source based on an aerosol target. Phys. Plasmas 2020, 27, 7. [Google Scholar] [CrossRef]
- Bartnik, A.; Skrzeczanowski, W.; Wachulak, P.; Fiedorowicz, H. EUV induced, low temperature plasmas, produced in an aerosol target. J. Instrum. 2020, 15, C02035. [Google Scholar] [CrossRef]
- Mann, K.R.; Kranzusch, S.; Eckert, G.; Peth, C.; Schaefer, B. Development of diagnostic tools for the EUV spectral range. Emerg. Lithogr. Technol. VI. SPIE 2002, 4688, 354–362. [Google Scholar]
- Hemminga, D.J.; Poirier, L.; Basko, M.M.; Hoekstra, R.; Ubachs, W.; Versolato, O.O.; Sheil, J. High-energy ions from Nd: YAG laser ablation of tin microdroplets: Comparison between experiment and a single-fluid hydrodynamic model. Plasma Sources Sci. Technol. 2021, 30, 105006. [Google Scholar] [CrossRef]
- Vitman, L.A.; Vitman, L.A.; Katsnelson, B.D.; Paleev, I.I. Spraying Liquid with Nozzles; Kutateladze, S.S., Ed.; State Energy Publishing House (USSR): Moscow, Russia, 1962. [Google Scholar]
- Nechay, A.N.; Perekalov, A.A.; Chkhalo, N.I.; Salashchenko, N.N.; Zabrodin, I.G.; Kaskov, I.A.; Pestov, A.Y. Modular installation for the formation and study of cluster beams of inert and molecular gases. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2019, 13, 83–92. [Google Scholar]
- Aruev, P.N.; Barysheva, M.M.; Ber, B.Y.; Zabrodskaya, N.V.; Zabrodskii, V.V.; Lopatin, A.Y.; Pestov, A.E.; Petrenko, M.V.; Polkovnikov, V.N.; Salashchenko, N.N.; et al. Silicon photodiode with selective Zr/Si coating for extreme ultraviolet spectral range. Quantum Electron. 2012, 42, 943–948. [Google Scholar] [CrossRef]
- Vodop’yanov, A.V.; Garakhin, S.A.; Zabrodin, I.G.; Zuev, S.Y.; Lopatin, A.Y.; Nechay, A.N.; Pestov, A.E.E.; Perekalov, A.A.; Pleshkov, R.S.; Polkovnikov, V.N.; et al. Measurements of the absolute intensities of the spectral lines of Kr, Ar and O ions in the wavelength range 10–18 nm under pulsed laser excitation. Quantum Electron. 2021, 51, 700–707. [Google Scholar] [CrossRef]
- Kranzusch, S.; Mann, K. Spectral characterization of EUV radiation emitted from a laser-irradiated gas puff target. Opt. Commun. 2001, 200, 223–230. [Google Scholar] [CrossRef]
- Kalmykov, S.G.; Butorin, P.S.; Sasin, M.E. Xe laser-plasma EUV radiation source with a wavelength near 11 nm—Optimization and conversion efficiency. J. Appl. Phys. 2019, 126, 10. [Google Scholar] [CrossRef]
- Abramov, I.S.; Gospodchikov, E.D.; Shalashov, A.G. Source of extreme ultraviolet radiation based on a discharge supported by a pulse of radiation from a terahertz free electron laser. Phys. Rev. Appl. 2018, 10, 034065. [Google Scholar] [CrossRef]
- Kelly, R.L.; Palumbo, L.P. Atomic and Ionic Emission Lines below 2000 Angstroms: Hydrogen through Krypton; Naval Research Laboratory: Washington, DC, USA, 1973; p. 7599. [Google Scholar]
- NIST. Atomic Spectra Database; NIST: Gaithersburg, MD, USA, 2009–2019. [CrossRef]
- Jupén, C.; Fremberg, J.; Fawcett, B. The spectrum and term system of Cl VII. Phys. Scr. 1984, 30, 260. [Google Scholar] [CrossRef]
- Jupén, C. The spectrum and term system of CI VIII. Phys. Scr. 1987, 36, 776. [Google Scholar] [CrossRef]
- Berry, H.G.; Desesquelles, J.; Cheng, K.T.; Schectman, R.M. Ne i-like resonance lines and Na i-like satellites in argon and chlorine. Phys. Rev. A 1978, 18, 546. [Google Scholar] [CrossRef]
- Kaufman, V.; Edl, B. Reference wavelengths from atomic spectra in the range 15 Å to 25000 Å. J. Phys. Chem. Ref. Data 1974, 3, 825–895. [Google Scholar] [CrossRef]
- Guseva, V.E.; Nechay, A.N.; Perekalov, A.A.; Salashchenko, N.N.; Chkhalo, N.I. Emission spectra of liquid jet targets of hexane C6H14, dichloromethane CH2Cl2, methylene bromide CH3Br in the range 4–20 nm under pulsed laser excitation. Opt. Spectrosc. 2022, 130, 7. [Google Scholar] [CrossRef]
- Joshi, Y.N.; Nencioni, A.; van Kleef, T.A.M. Eighth spectrum of bromine: Br viii. J. Opt. Soc. Am. B 1985, 2, 1650. [Google Scholar] [CrossRef]
- Joshi, Y.N.; Van Kleef, T.A.M. Ninth spectrum of bromine: Brix. Phys. Scr. 1981, 23, 249. [Google Scholar] [CrossRef]
- Guseva, V.E.; Nechay, A.N.; Perekalov, A.A.; Salashchenko, N.N.; Chkhalo, N.I. Emission Spectra of Molecular Gases CHF3, CCl2F2, SF6 in the Range 3–20 nm under Pulsed Laser Excitation Using Various Gas Jets as Targets. Opt. Spectrosc. 2022, 130, 391–397. [Google Scholar] [CrossRef]
- Vogt, U.; Stiel, H.; Will, I.; Nickles, P.V.; Sandner, W.; Wieland, M.; Wilhein, T. Influence of laser intensity and pulse duration on the extreme ultraviolet yield from a water jet target laser plasma. Appl. Phys. Lett. 2001, 79, 2336–2338. [Google Scholar] [CrossRef]
- Joshi, Y.N.; Van Kleef, T.A.M.; Mahajan, C.G. Eighth spectrum of iodine: I viii. J. Opt. Soc. Am. B 1987, 4, 1306–1308. [Google Scholar]
- Churilov, S.S.; Joshi, Y.N.; Gayazov, R.R. Revised and extended analyses of I ix and I x. J. Opt. Soc. Am. B 1998, 15, 1923–1931. [Google Scholar] [CrossRef]
- Blackburn, J.; Carroll, P.K.; Costello, J.; O’Sullivan, G. Spectra of Xe vii, viii, and ix in the extreme ultraviolet: 4d–mp, nf transitions. J. Opt. Soc. Am. 1983, 73, 1325–1329. [Google Scholar] [CrossRef]
Figure 1.
Diagram of research stand: 1—laser, 2—laser radiation power detector, 3—dividing plate, 4—prism, 5—optical input, 6—lens, 7—capillary, 8—quick-acting valve, 9—vacuum shutter, 10—free-hanging film filter, 11—grating spectrometer–monochromator, 12—turbomolecular pump, 13—free-hanging film filter, 14—spectrometer for studying absolute radiation intensities.
Figure 1.
Diagram of research stand: 1—laser, 2—laser radiation power detector, 3—dividing plate, 4—prism, 5—optical input, 6—lens, 7—capillary, 8—quick-acting valve, 9—vacuum shutter, 10—free-hanging film filter, 11—grating spectrometer–monochromator, 12—turbomolecular pump, 13—free-hanging film filter, 14—spectrometer for studying absolute radiation intensities.
Figure 2.
Photograph of liquid jet target when illuminated by a LED.
Figure 2.
Photograph of liquid jet target when illuminated by a LED.
Figure 3.
The spectral dependence of the Mo/ZrSi2 film transmittance.
Figure 3.
The spectral dependence of the Mo/ZrSi2 film transmittance.
Figure 4.
The spectral dependence of the Zr/ZrSi2 film transmittance.
Figure 4.
The spectral dependence of the Zr/ZrSi2 film transmittance.
Figure 5.
Emission spectrum of isopropyl alcohol under pulsed laser excitation.
Figure 5.
Emission spectrum of isopropyl alcohol under pulsed laser excitation.
Figure 6.
Emission spectra of alcohols detected using a mirror spectrometer.
Figure 6.
Emission spectra of alcohols detected using a mirror spectrometer.
Figure 7.
Emission spectrum of dichloromethane under pulsed laser excitation.
Figure 7.
Emission spectrum of dichloromethane under pulsed laser excitation.
Figure 8.
Dichloromethane emission spectrum detected using a mirror spectrometer.
Figure 8.
Dichloromethane emission spectrum detected using a mirror spectrometer.
Figure 9.
Emission spectrum of CH3Br under pulsed laser excitation.
Figure 9.
Emission spectrum of CH3Br under pulsed laser excitation.
Figure 10.
Emission spectra of the liquid targets CH3Br and C2F4Br2 and of the gas target, CHF3.
Figure 10.
Emission spectra of the liquid targets CH3Br and C2F4Br2 and of the gas target, CHF3.
Figure 11.
Emission spectrum of CH3Br detected using a mirror spectrometer.
Figure 11.
Emission spectrum of CH3Br detected using a mirror spectrometer.
Figure 12.
Emission spectrum of CF3CH2I under pulsed laser excitation.
Figure 12.
Emission spectrum of CF3CH2I under pulsed laser excitation.
Figure 13.
Emission spectra of a CF3CH2I liquid target and a CHF3 gas target.
Figure 13.
Emission spectra of a CF3CH2I liquid target and a CHF3 gas target.
Figure 14.
Emission spectrum of CF3CH2I as detected by a mirror spectrometer.
Figure 14.
Emission spectrum of CF3CH2I as detected by a mirror spectrometer.
Table 1.
Relative intensities of oxygen lines, observed from CO2, C3H7OH, and C2H5OH targets.
Table 1.
Relative intensities of oxygen lines, observed from CO2, C3H7OH, and C2H5OH targets.
| Wavelength, nm | Ion | Carbon Dioxide
CO2 | Ethyl Alcohol
C2H5OH | Isopropyl Alcohol
C3H7OH |
|---|
| 10.46 | O-VI | 0.035 | 0.068 | 0.053 |
| 11.00 | O-VI | 0.042 | 0.068 | 0.058 |
| 11.58 | O-VI | 0.260 | 0.273 | 0.289 |
| 12.98 | O-VI | 1 | 1 | 1 |
| 13.18 | O-V | 0.116 | 0.045 | 0.079 |
| 13.81 | O-V | 0.114 | 0.068 | 0.066 |
| 15.00 | O-VI | 0.323 | 1.160 | 1.160 |
| 15.15 | O-V | 0.142 | 0.195 | 0.211 |
| 15.89 | O-V | 0.071 | 0.114 | 0.126 |
| 16.46 | O-V | 0.052 | 0.114 | 0.105 |
| 16.80 | O-V | 0.028 | 0.114 | 0.079 |
| 17.31 | O-VI | 0.146 | 2.320 | 1.840 |
| 18.40 | O-VI | | 0.159 | 0.211 |
| 19.30 | O-V | | 0.270 | 0.316 |
Table 2.
Absolute values of radiation intensity and the conversion coefficients from different alcohol targets.
Table 2.
Absolute values of radiation intensity and the conversion coefficients from different alcohol targets.
| Wavelength, nm | Ion | Methyl | Ethyl | Isopropyl |
|---|
| CE, % | N, 1012 ph/pulse | CE, % | N, 1012 ph/pulse | CE, % | N, 1012 ph/pulse |
|---|
| 11.58 ± 0.55 | O-VI | 0.0042 | 1.7 ± 0.5 | 0.004 | 1.6 ± 0.5 | 0.0026 | 1.0 ± 0.3 |
| 12.98 ± 0.71 | O-VI | 0.0072 | 3.3 ± 1.0 | 0.0045 | 2.0 ± 0.6 | 0.0044 | 2.0 ± 0.6 |
| 15.01 ± 0.82 | O-VI/V | 0.0058 | 3.0 ± 0.9 | 0.0046 | 2.4 ± 0.7 | 0.0042 | 2.2 ± 0.6 |
| 17.31 ± 0.96 | O-VI | 0.0079 | 4.6 ± 1.4 | 0.005 | 3.1 ± 0.9 | 0.0052 | 3.1 ± 0.9 |
Table 3.
Absolute values of radiation intensity and the conversion coefficients for dichloromethane target.
Table 3.
Absolute values of radiation intensity and the conversion coefficients for dichloromethane target.
| Wavelength, nm | Ion | CE, % | N, 1012 ph/pulse |
|---|
| 13.31 ± 0.73 | Cl-VII | 0.0043 | 2.2 ± 0.6 |
| 14.94 ± 0.81 | Cl-VII | 0.0054 | 2.8 ± 0.8 |
Table 4.
Absolute values of radiation intensities and the conversion coefficients for methylene bromide target.
Table 4.
Absolute values of radiation intensities and the conversion coefficients for methylene bromide target.
| Wavelength, nm | Ion | CE, % | N, 1013 ph/pulse |
|---|
| 11.75 ± 0.57 | Br-IX | 0.0905 | 3.6 ± 1.1 |
| 13.99 ± 0.77 | Br-VIII | 0.0203 | 1.0 ± 0.3 |
Table 5.
Absolute values of radiation intensities for CF3CH2I.
Table 5.
Absolute values of radiation intensities for CF3CH2I.
| Wavelength, nm | Ion | CE, % | N, 1014 ph/pulse |
|---|
| 11.44 ± 0.51 | I-? | 0.174 | 0.7 ± 0.2 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).