Abstract
Traumatic brain injury (TBI) is a common cause of neurologic morbidity for which few effective therapies exist, especially during the chronic stage. A potential therapy for chronic TBI is transcranial photobiomodulation (tPBM). tPBM is a noninvasive neuromodulation technique that uses light to stimulate the cortex and increase blood flow and metabolism while also enhancing cognition and improving affect. There has been much work focusing on the efficacy of tPBM in acute TBI in small animals, but much less work has focused on chronic TBI. Patients with chronic TBI manifest microvascular injury, which may serve as a modifiable treatment target for tPBM. There is a need to study and improve tPBM, as the currently implemented protocols targeting microvascular injury have been relatively unsuccessful. This review includes 16 studies, which concluded that after tPBM application, there were improvements in neuropsychological outcomes in addition to increases in cerebral blood flow. However, these conclusions are confounded by differing tPBM parameters, small sample sizes, and heterogenous TBI populations. While these results are encouraging, there is a need to further understand the therapeutic potential of tPBM in chronic TBI.
1. Introduction
1.1. Background and Purpose of Review
Traumatic brain injury (TBI) is a common public health problem that requires long-term management [1]. TBI is considered a chronic, evolving disease process [2] and is the cause of long-term disability in at least 2% of the United States’ population [3]. There are very limited therapeutic options to improve functional recovery in TBI, especially in the chronic stage of TBI. Transcranial photobiomodulation (tPBM) shows substantial promise as a potential therapeutic intervention after TBI. tPBM is a noninvasive brain stimulation method that uses light to stimulate, heal, regenerate, and protect injured or degenerating brain tissue [4]. tPBM is a promising tool to boost neurometabolic activity and has potential beneficial neurophysiological and functional effects for TBI patients [4]. However, the optimal dose in terms of strength, duration, and frequency of illumination, as well as the neural mechanisms underlying beneficial cognitive effects, are not well understood.
The growing literature has demonstrated both cognitive and brain activity changes in response to tPBM administration in healthy and neuropsychiatric populations [5,6]. A recent review focused on tPBM in acute TBI in small animal studies [7], but there are not currently any reviews explicitly focusing on the therapeutic potential of tPBM in chronic TBI in humans. Chronic TBI is not studied in small animal models due to the limitations of a shortened lifespan after TBI in these small animal models. Previous reviews have neither disentangled chronic TBI from acute TBI nor acknowledged the heterogeneity of TBI or the differences in injury severity. Further, chronic TBI patients manifest microvascular injury, which can vary across injury severity [8] and may result in varying treatment responses to tPBM application. Patients in the chronic stage after TBI suffer from microvascular deficits including hypoperfusion [9], altered cerebrovascular reactivity [10], and reduced blood–brain barrier integrity [11], which may serve as modifiable treatment targets for tPBM. This review aims to evaluate the current literature focusing on tPBM in the chronic TBI population, examining both the neural and cognitive effects as well as the varying laser or light-emitting diode (LED) parameters used across studies. In addition, this review acknowledges the differences in TBI severity and proposes microvascular injury as a target for tPBM.
1.2. Physiological Mechanisms of Transcranial Photobiomodulation
tPBM penetrates the skull and stimulates brain tissue through a nonthermal mechanism [12]. tPBM activates light- and heat-sensitive ion channels and is also absorbed by cytochrome c oxidase (CCO), the terminal enzyme in the mitochondrial electron transport chain, leading to increased energy and brain metabolism. The absorption of near-infrared (NIR) light has been shown in numerous tissue culture and animal studies to upregulate CCO activity [13,14,15,16,17], increase production of nitric oxide (NO) [13,18], generate reactive oxygen species [19,20], activate mitochondrial deoxyribonucleic acid (DNA) replication [21], increase early response genes [22], and increase the rate of cortical adenosine triphosphate (ATP) production [15,17], which then leads to a number of other beneficial secondary neuronal and tissue-specific processes. This mechanism has been illustrated in the figures by Hamblin (i.e., Figure 1 and Figure 2) [4]. A study including human participants [23] has reported a temporal increase in CCO, oxygenated hemoglobin, and total hemoglobin during and after tPBM in a dose-dependent manner over time. The most commonly reported neurophysiological consequence of tPBM is an increase in cerebral blood flow (CBF) in pre-clinical and clinical models [4], presumably as a consequence of the increased release of NO. tPBM’s effect on CBF in healthy and clinical populations [4,6] makes it a promising intervention for individuals with deficits in CBF, such as chronic TBI.
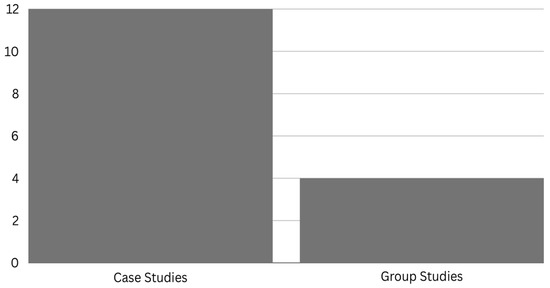
Figure 1.
Number of case and group studies in chronic TBI.
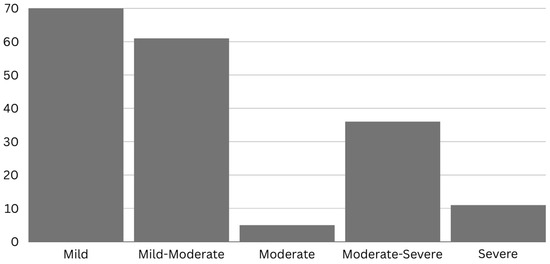
Figure 2.
Number of individuals with chronic TBI included in previous studies across severities.
1.3. Microvascular Injury as a Potential Target for Transcranial Photobiomodulation in Chronic Traumatic Brain Injury
Microvascular injury is one of the many neuropathological consequences of TBI [24]. Impaired CBF is a biomarker of microvascular disruption (i.e., traumatic microvascular injury [25]) and has clinical importance in TBI because of its relationship with metabolic alterations [26], cognitive and functional outcomes [27,28,29,30,31], and the onset of neurodegenerative diseases [32,33,34,35,36,37,38]. In a recent study, a widespread reduction was demonstrated among moderate–severe TBI patients in the early chronic phase of injury, which predicted the ensuing cognitive recovery trajectory [30]. CBF has also become recognized as a prognostic biomarker of neurodegeneration, as reductions in CBF often precede and predict structural atrophy and cognitive decline [32,39]. Impaired CBF has been demonstrated in both the acute and chronic phases of injury [9,26,27,29,30,31,40,41,42]. Due to the relationship between CBF and neural health and behavior, targeting impaired CBF may help enhance microvascular function and will likely have cognitive and behavioral implications.
Microvascular injury persists into the chronic phase of TBI [9,25,27,30] and currently there are few interventions available to halt the progression of microvascular injury. The existing literature [9,27,30] strongly implicates microvascular injury, including CBF alterations, in TBI as an important contributing factor to clinical outcomes and cognitive consequences of TBI, which can precede further neurodegeneration [34]. Interestingly, tPBM may have a greater effect on damaged tissues than healthy tissues, justifying an approach that selectively targets impaired cortical tissue [43]. As explained above, light stimulation releases NO from CCO, resulting in direct vasodilatory effects and increased mitochondrial ATP production by reversing the NO-dependent inhibition of CCO. Damaged cells are likely to have higher concentrations of NO than healthy cells [43]; thus, damaged cells undergo a greater release from the effects of NO upon stimulation. There has even been evidence that laser acupuncture applied to the feet of healthy participants is able to activate cortical regions using two variations of laser stimulation [44]. A recent study in patients with chronic TBI found that tPBM increased CBF in hypoperfused areas of the brain [45]. Another case study in TBI patients also found increased perfusion after tPBM in regions that were underperfused [46]. And so, there is a need to explore novel methods of treating microvascular injury in chronic TBI, where there are limited interventions available.
2. Methods
The relevant articles were searched in PubMed using the search terms “photobiomodulation” or “low-level laser therapy (LLLT)” and “traumatic brain injury”, for all articles published in English between 1950 and 2023. Additional studies were identified through manual searches of references in detected articles or in previous reviews using Google Scholar and Scopus. Studies were excluded if they were not written in English, reported other medical conditions, such as Alzheimer’s disease, or the population studied was identified as acute traumatic brain injury. In total, 16 studies were included in this review, consisting of 12 case reports/series and 4 clinical studies.
3. Previous Literature
3.1. Overview of Previous Literature
Most of the previous works exploring the effects of tPBM involving the chronic TBI population are case studies or single case reports (Figure 1) and focus primarily on mild TBI (Figure 2). The chronicity studied the most in the previous literature is around 1-year post-injury (Figure 3), and there is much variability between studies. To our knowledge, no studies have been conducted on chronic TBI using tPBM between 111 months [47] and 372 months post-injury [46] (or about 10 to 30 years). This literature documents behavioral (e.g., sleep, mood), cognitive, and some brain activity and blood flow changes after tPBM administration across a range of tPBM parameters (detailed in each of the tables below). However, much of the literature only includes behavioral or cognitive outcome measures post-administration. Few of these studies are blinded or randomized controlled trials. Building on the promising results detailed below, future research should include more properly controlled, blinded, randomized controlled trials with optimized implementation of neuroimaging metrics to better understand the mechanisms of the effect of tPBM chronic TBI. Specific details for tPBM administration and a summary of participants included in each study included below can be found in the corresponding tables.
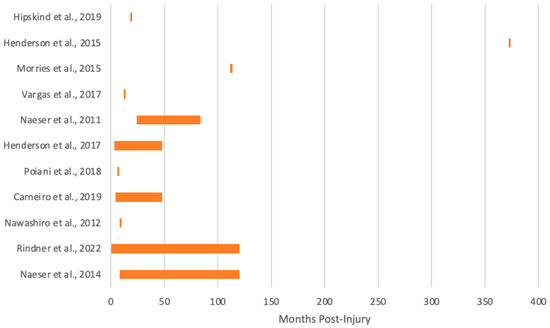
Figure 3.
Months post-injury of chronic TBI cohorts in the previous literature [45,46,47,48,49,50,51,52,53,54,55].
3.2. Transcranial Photobiomodulation in Chronic Mild TBI
The literature on tPBM for chronic mild TBI has a strong focus on cognitive and neuropsychiatric outcomes post-treatment, with one of the most widely reported effects of tPBM being enhanced cognition. Naeser et al. [55] reported data on 11 chronic mild TBI subjects with non-penetrating injuries who showed a significant increase in executive functioning and decrease in post-traumatic stress disorder (PTSD)-related symptoms following 18 treatment sessions in a pilot, open-protocol study (Table 1). tPBM was administered 10 months to 8 years following mild TBI occurrence. All participants displayed persistent cognitive dysfunction, and four experienced multiple concussions. LED cluster heads were applied for 10 min to 11 scalp placements (13 Joules (J)/cm2). Treatments were administered 3 days a week with a minimum of 48 h between sessions over the course of 6 weeks. Neuropsychological testing was performed at four points throughout the study: pre-LED treatment, 1 week, 1 month, and 2 months following the 18th treatment administration. The results showed a significant linear trend in the effect of treatment for inhibition (Stroop test for executive function); inhibition switching (Stroop test Trial 4); and verbal learning and memory (California Verbal Learning Test, Long Delay Free Recall). All four participants, originally reporting symptoms of PTSD according to established Monson criteria, reported a clinically meaningful decrease in symptoms post-LED treatment. This demonstrated promising results in improving cognition and PTSD symptoms in individuals with chronic mild TBI incurred at varying time points post-injury [55]. An abstract from Liebel et al. [56], reported improvement in many metrics among a cohort of 49 athletes who had a history of concussive and/or sub-concussive hits in response to tPBM with 810 nm LEDs (Table 2). After undergoing active tPBM for 8 weeks, the athletes showed improvements, including a reduction in psychiatric symptoms and improved sleep quality, reaction time, and grip strength. The effect of tPBM on TBI-induced motor deficits remains an area that is underexplored among the body of literature [57].

Table 1.
Transcranial photobiomodulation studies in chronic mild TBI.
Table 1.
Transcranial photobiomodulation studies in chronic mild TBI.
| Mild TBI | |||
|---|---|---|---|
| Title | Naeser et al., 2014 [55] | Naeser et al., 2011 [49] | Naeser et al., 2023 [58] |
| Chronicity | 10 months–8 years. | 2 and 7 years. | 35 to 55 years prior, multiple head traumas took place during their football careers. |
| Mechanism of Injury | Mixed: motor vehicle accidents, concussions, and blast injuries. | Participant 1: Motor vehicle accidentParticipant 2: Multiple concussions with and without loss of consciousness. | Football/contact sports injury. |
| Sample Size | 11 | 2 | 4 |
| Age (years) | 26–62 | 52 and 59 | 55–74 |
| Sex | 6 males | 2 females | 4 males |
| tPBM Delivery | LED | LED | LED (tPBM and iPBM) |
| Duration | 20 min | 5:10 min gradually increased to 12:54 min; 7 min increased to 10 min. | Protocol A: 40 min, 633, and 870 nm tPBM. Protocol B: 20 min, 810 nm, tPBM, and iPBM; 25 min, 633 nm, and iPBM. Protocol C: 10 min tPBM on midline with 5 LED cluster heads, 12 min tPBM, and 5 LED cluster heads, each side of the head. |
| Target Region(s) | Midline from front-to-back hairline and bilaterally over frontal, parietal, and temporal areas. | Bilaterally and over midline sagittal areas. | Protocol A: Whole head. Protocol B: Default mode network and olfactory bulbs. Protocol C: Whole head. |
| tPBM Mode | Continuous | Continuous | Protocol A: Continuous. Protocol B: Pulsed; 40 Hz. Protocol C: Continuous. |
| Wavelength (nm) | 633 and 870 | 633 and 870 | Protocol A: 2 sets of 6 LED cluster heads composed of 9, 633 nm diodes and 52, 870 nm diodes in each LED cluster head. Protocol B: 4, 810 nm single tPBM diodes with 1, 810 nm iPBM, and all pulsed at 40 Hz; 1 iPBM single diode, 633 nm, and continuous. Protocol C: Each LED cluster head: 34, 660 nm, and 35, 850 nm diodes; 5 LED cluster heads on midline, and 5 LED cluster heads on each side. |
| Cortical Irradiance (mW/cm2) | 22.2 | 22.2 and 25.8 | Protocol A: 22.2 mW/cm2 per LED cluster head. Protocol B: 810 nm, default mode network tPBM, 75 mW/cm2, Mesial Prefrontal Cortex; 100 mW/cm2, Precuneus; L and R Angular gyrus; iPBM, 25 mW/cm2. and a separate iPBM, 633 nm 8 mW/cm2. Protocol C: 41 mW/cm2, midline; 35 mW/cm2, sides. |
| Number of Sessions | 18 | Participant 1: Approximately 31 in clinic (following this, participant self-treated at home daily for 5 years). Participant 2: 28. | 18 |
| Outcome | Improved executive function; decreased PTSD symptoms. | Participant 1: Improved self-awareness, and improved inhibition of angry outbursts. Participant 2: Medical disability discontinued; returned to full time work; improved executive function, memory, and inhibition; improved social behavior; and reduced PTSD symptoms. | Improved neuropsychological measures, including executive function, attention, and verbal learning/memory; decreased PTSD, depression, pain, and improved sleep; increased functional connectivity in salience network; increased n- acetyl-aspartate, a measure of mitochondrial oxygenation, in Anterior Cingulate Cortex. |
| Side Effects | None. | None. | None. |
In the Vargas et al. [48] study with 11 cases who had subjective memory complaints, the etiology of three cases was mild TBI occurring about one year prior (Table 2). In all three of these cases, cognitive improvement was noted in response to five sessions of tPBM. Laser stimulation was administered once a week for 5 weeks by applying coherent 1064 nm infrared laser light using a well-collimated laser diode. The laser was administered continuously to a uniform area of 13.6 cm2 at a power output of 3.4 Watts (W) and a cumulative fluence (energy density) per site of 60 J/cm2. The target of laser administration was the right prefrontal cortex, through the right forehead, on the standard 10-20 EEG placement system. Stimulation was alternated every minute between medial and lateral sites for a total of 4 min per site (816 J). Cognitive performance, particularly sustained attention and visual working memory, was assessed using the Psychomotor Vigilance Task (PVT) and the Delayed Match-to-Sample Memory Task (DMS). Testing occurred at six time points: baseline evaluation, pre-treatment, and following each of the five weekly treatment sessions. Improvement was demonstrated by all participants across all cognitive measures. This study reported improvement in PVT lapses but not PVT reaction time, and borderline DMS correct responses were demonstrated acutely following the single treatment administration at week 1. Improvement expanded to include PVT reaction time, PVT lapses, and DMS correct responses following long-term tPBM (after treatment administrations at weeks 2–5). Cognitive improvement was associated with decreases in blood oxygen level-dependent (BOLD) MRI signal in the targeted areas of the right prefrontal cortex. The change in task-induced BOLD signal demonstrates the ability of tPBM to alter cerebrovascular physiological responses in vivo and is an intermediate-term effect during the two-back task versus the zero back task. The group previously showed that increased oxygen consumption and oxygenated hemoglobin in the prefrontal cortex were seen after tPBM, which helps to explain the decrease in BOLD signal. The results from this study expanded on previous research by demonstrating improvements in cognition following single-dose administrations and illustrate the potential for repeated, weekly treatments to induce clinically relevant improvements in cognitive performance and associated neural changes indicating potential improvement in cognition.
Case reports of chronic mild TBI have also reported cognitive changes using at-home tPBM devices. Chao et al. [59] published a case study on two subjects with Gulf War Illness, one of which also had sustained a mild TBI in the past (Table 2). The individual with mild TBI showed a decrease in sleep difficulties and mood-cognitive symptoms in addition to decreased pain and fatigue—differences prominent enough for the subject to be reclassified from severe chronic multisymptomatic illness to mild–moderate chronic multisymptomatic illness. The 810 nm LED treatment was applied transcranially and intranasally (intranasal tPBM or iPBM) every other day for 12 weeks in the patient’s home, demonstrating the utility of tPBM as a successful therapeutic that requires minimal clinician involvement. Naeser et al. [49] reported two cases of chronic mild TBI patients wherein nightly at-home transcranial application of 870 nm LEDs bilaterally and to midline sagittal areas yielded significant cognitive improvement (Table 1). One subject noted improved self-awareness and inhibition of angry thoughts. The other was able to discontinue medical disability and return to work due to improved executive function, memory, inhibition, and social behavior, along with reduced PTSD symptoms. One of these subjects noted regression when treatment was stopped for more than two weeks.
In another case series, Naeser and colleagues [58] observed significant improvements in cognition and behavior/mood in four ex-football players who met the National Institutes of Health criteria for Possible Chronic Traumatic Encephalopathy (CTE) and elucidated potential neurobiological mechanisms leading to clinical improvements (Table 1). The players initially received in-clinic, transcranial LED all over the head, where LED cluster heads with wavelengths of red, plus NIR (850 or 870 nm), depending on the device type), were applied midline, front to back, as well as left and the right sides, three times per week for six weeks. A total of 26 J/cm2 was administered at each LED cluster head placement on the scalp, at each treatment session. Resting-state functional connectivity MRI scans (rs-fcMRI) and magnetic resonance spectroscopy (MRS) scans were obtained, as well as an array of neuropsychological assessment batteries and behavior/mood questionnaires pre- and post-tPBM at 1 week, 1 month, and 2 months post the final, 18th treatment. There were significant improvements in cognition and PTSD, depression, pain, and sleep at 1 week and/or 1 month post-tPBM series. The rs-fcMRI scans showed a significant correlation between increased salience network functional connectivity and improved executive function, attention, PTSD, pain, and sleep, as well as between central executive network functional connectivity, verbal learning and memory, and depression. The MRS studies showed an increase in the metabolite, n-acetyl-aspartate (NAA), a marker correlated with oxygenation in the mitochondria of neural cells, in the anterior cingulate gyrus at 1 week and 6 weeks post-tPBM. Taken together, the results from Naeser et al. [58] demonstrated the potential mechanisms by which tPBM works to exert beneficial effects on the brain. This study is one of the few that has explored brain-behavior relationships in chronic TBI. Future research may benefit from incorporating neuroimaging measures to further our understanding of the neural effects of tPBM on chronic mild TBI, which may underlie the cognitive and behavioral changes seen post-treatment.

Table 2.
Transcranial photobiomodulation studies in chronic mild TBI continued.
Table 2.
Transcranial photobiomodulation studies in chronic mild TBI continued.
| Mild TBI | |||
|---|---|---|---|
| Title | Liebel et al., 2022 [56] | Vargas et al., 2017 [48] | Chao et al. 2020 [60] |
| Chronicity | Not reported; former athletes. | 12 months or less. | 6 concussions: most recent was 1 month pre-treatment. |
| Mechanism of Injury | Multiple concussions or sub-concussive events. | - | Multiple concussions. |
| Sample Size | 49 | 12 (3 with TBI) | 1 |
| Age (years) | - | 49–90 | 23 |
| Sex | - | 5 males, 7 females. 2 males with TBI; 1 female with TBI. | Male. |
| tPBM Delivery | LED (tPBM and iPBM) | Laser | LED (tPBM and iPBM) |
| Duration | - | 8 min | 20 min |
| Target Region(s) | - | Right forehead | Default mode network |
| tPBM Mode | Pulsed (40 Hz) | Continuous | Pulsed (10 and 40 Hz) |
| Wavelength (nm) | 810 | 1064 | 810 |
| Cortical Irradiance (mW/cm2) | - | 250 | 100 |
| Number of Sessions | Not reported; 8-week duration. | 5 | 38 |
| Outcome | Reduced depression, PTSD, and and adjustment symptoms; improved sleep quality, reaction times, and nondominant hand grip strength. | Improved cognitive scores. | Improved neuropsychological scores and functional connectivity and increased brain volume. |
| Side Effects | None. | None. | Mild headaches noted with the 40 Hz frequency. |
3.3. Transcranial Photobiomodulation in Chronic Mild–Moderate TBI
The commonly reported effects of tPBM in the chronic mild–moderate TBI population are changes in psychiatric symptoms, affect, and sleep (please refer to Table 3). Henderson et al. [50] explored symptoms of depression in a population of 39 chronic mild–moderate TBI subjects after tPBM treatment and observed that 32 of the 39 subjects showed Quick Inventory of Depressive Symptoms (QIDS) scores indicative of a remission from depressive symptoms. Prior to beginning tPBM, all subjects met QIDS criteria for mild to severe depression. A total of 69% of participants had undergone previous antidepressant trials, with all continuing to report depressive symptoms. Each subject received multi-Watt tPBM using NIR lasers (810/980 nm at 8–15 W) applied to the forehead and temporal regions bilaterally for 9–12 min to each area. The application to each area lasted 9–12 min, with total individual session times of 30 min. The number of treatments varied, with improvement occurring between 8 and 34 treatments. QIDS was administered before and after treatment courses. A total of 92% of subjects experienced a significant decrease in depressive symptomology, and 82% of patients indicated remission. Overall, the mean QIDS scores decreased from baseline to endpoint. Follow-up interviews conducted at 2, 6, and 12 months, and for five participants, 55 months, support the assertion that the therapeutic effects of tPBM may be lasting well beyond the course of treatment. It is notable that while 77% of subjects endorsed suicidal ideation at baseline, only two subjects (non-responders) endorsed such ideation at follow-up. The present study highlights the results across a range of tPBM durations, but with consistent data indicating the efficacy of varied courses of tPBM in improving depression symptoms.

Table 3.
Transcranial photobiomodulation studies in chronic mild–moderate TBI.
In a retrospective case series report, Morries and colleagues [47] detailed the effects of tPBM on mood, cognitive, and physical symptoms of 10 patients with chronic mild–moderate TBI who were sequentially treated in-clinic. The participants had mild–moderate chronic TBI with an average time since injury of 9.3 years and were treated with at least 10 sessions of tPBM with wavelengths of 810 nm or 980 nm resulting in delivered fluences ranging from 55 J/cm2 to 81 J/cm2 over the duration of 10 min each session. The patients’ clinical symptoms were monitored using the QIDS-Self Report (QIDS-SR), Beck Depression Inventory (BDI), and patient and spousal diaries of symptoms and subjective progression. They reported significant improvements in clinical TBI symptoms (e.g., insomnia, nightmares, headaches, suicidal ideation, and anxiety) in all 10 patients. Additionally, marked improvements in depressive symptoms, as demonstrated by significant decreases in BDI scores and in QIDS-SR scores, were observed. The patients also reported improvements in cognition and life quality, as reflected by both increased desire to and success in returning to work.
Hipskind and colleagues [45] demonstrated the influence of pulsed red/NIR tPBM with LEDs on cognitive abilities in 12 veterans who had incurred a TBI a minimum of 18 months prior to participation in the study. All participants were Caucasian males, between 21 and 55 years of age. The subjects were treated with an FDA-cleared tPBM with NIR device designed to address the heterogeneity of TBI across subjects by targeting the entire cranium. The device contained two separate neoprene pads embedded with alternating rows of 180 red (629 nm) and 222 (850 nm) LEDs generating 3.3 W pulsed power output, providing 519 cm2 of coverage. 20-min tPBM sessions were administered three times per week for six consecutive weeks, totaling 18 sessions. Red/NIR light was pulsed at 3 different frequencies (73, 587, and 1175 Hz) for 6.7 min, each at 34% duty cycle. The results revealed increases demonstrated across six neuropsychological scales following tPBM. Significant increases were seen within three of five California Verbal Learning Test Second Edition subtests and three of the seven Wechsler Adult Intelligence Scale Fourth Edition subtests. Additionally, eight participants exhibited increased regional CBF as demonstrated by quantitative SPECT imaging analysis in hypoperfused areas of their brains. These findings suggest not only that pulsed transcranial red/NIR light therapy can improve cognitive deficits associated with TBI but also emphasize neural mechanisms that may be central to the etiology of such deficits, including alterations in regional CBF and related cerebral metabolic function.
3.4. Transcranial Photobiomodulation in Chronic Moderate TBI
A case report by Chao et al. [60] provides information on neural changes that may underlie overt improvements in TBI clinical symptoms (Table 4). The authors reported on changes in structural and functional brain characteristics measured by both structural (T1) and functional (echo planar imaging and arterial spin labeled) MRI, as well as changes in neuropsychological performance. The individual included in this case report was a professional hockey player with multiple concussions. The participant underwent 8 weeks of tPBM treatments administered every other day using at-home, commercially available devices that deliver 810 nm of light for 20 min to the DMN, resulting in 240 J of energy delivered per session. Structural and functional MRI scans were conducted before and after tPBM had concluded. The authors noted increased brain volumes, improved FC, and increased cerebral perfusion following tPBM, along with improvements in the cognitive domains of verbal learning and memory, executive functioning, attention, and processing speed [60].

Table 4.
Transcranial photobiomodulation studies in chronic moderate TBI.
Bogdanova et al. [61] described effects on persistent cognitive symptoms associated with two cases of moderate TBI, including improved sleep, verbal memory, episodic function, and decreased symptoms of PTSD and depression, in response to eighteen sessions of transcranial LED treatment (Table 4). The individuals received 18 sessions of transcranial LED therapy three times per week for 6 weeks using an FDA-cleared, non-significant risk device. Standardized neuropsychological, neuropsychiatric, and sleep measures were administered to both participants at three time points: prior to tPBM, mid-tPBM, and one week following completion of tPBM. Both individuals included in this case report exhibited improved sleep according to their actigraphy total sleep following the completion of treatment. One subject demonstrated additional improvement in executive function, verbal memory, and sleep efficiency, while the other subject demonstrated additional improvement on measures of PTSD severity and depression. This case study demonstrates the potential for noninvasive transcranial red/NIR LED tPBM to elicit improvement in both cognitive and neuropsychiatric symptoms characteristic of TBI.
Henderson and colleagues [46] reported a comparable result in their case study, as they found changes in blood flow via SPECT imaging along with decreased symptoms of anxiety, headache, and insomnia and improvements in measures of cognition and quality of life in response to 20 sessions of tPBM over the course of three months with both 810 nm and 980 nm lasers (Table 4). The individual showed increased CBF 2 months after the last of the 20 sessions of tPBM within the left and right frontal cortices, left and right temporal cortices, within the foci of injury (to a lesser extent), regions adjacent to the foci of injury, and areas of distant regions of cortex that had previously been hypoperfused. These results emphasize the ability of tPBM to target both cognitive and physiological manifestations of TBI. It is notable that the quantifiable neuropsychological improvements observed in this case study were accompanied by the patient’s subjective report of improved quality of life. This demonstration of high-power NIR’s impact on pragmatic patient outcomes calls for further exploration of tPBM as an intervention for TBI in a larger-scale, controlled study design.
3.5. Transcranial Photobiomodulation in Chronic Moderate–Severe TBI
While there were no results available at the time that the current review was written, Poiani et al. [51] published the protocol of their randomized controlled trial, which examines the effects of tPBM with LEDs on inhibitory attentional control in 36 patients with moderate-severe TBI (Table 5). Participants who meet eligibility criteria will be randomized to either the active or the sham arm of the treatment phase, during which the active arm consists of light with a 632 nm wavelength and a total expected dose per session of 3.74 J/cm2 per 30-min session. Both the active and the sham arm of the treatment phases consist of eighteen tPBM sessions administered three times per week over the course of 6 weeks. Patients will complete psychiatric inventories (e.g., BDI) to collect data on symptoms of anxiety and depression, as well as neuropsychological assessments to collect data on the cognitive domains of attention, executive functioning, memory, fluency, and visuospatial construction, both immediately before randomization and after the treatment phase concludes. The authors expect tPBM to improve the cognitive and depressive symptoms of patients with chronic moderate-severe TBI.

Table 5.
Transcranial photobiomodulation studies in chronic moderate–severe TBI.
3.6. Transcranial Photobiomodulation in Chronic Severe TBI
The study published by Carneiro et al. [52] provides further evidence for tPBM-induced changes in hemodynamics within a cohort of 10 patients with chronic severe TBI (Table 6). All 10 patients received tPBM at 630 nm wavelength irradiated over a total surface area of 400 cm2, with a total fluency of 3.74 J/cm2, delivered during a single course of 30-min session. The patients received 3 treatment sessions per week for 6 weeks, resulting in 18 sessions received in total. Using transcranial doppler ultrasound, this study demonstrated increased CBF and cerebral oxygenation. In addition, the authors observed trends of improvement in visuospatial memory, information processing speed, and verbal learning and memory measured through neuropsychological assessments. The combination of neuroimaging effects and symptomatic improvement in these studies demonstrates the utility of tPBM as a potential therapeutic intervention for chronic moderate–severe TBI, but more research is needed to understand the clinical utility of tPBM in chronic TBI as well as to optimize tPBM parameters and protocols.

Table 6.
Transcranial photobiomodulation studies in chronic severe TBI.
Increases in CBF were also seen in a case report of a patient with severe TBI in a vegetative state. Nawashiro et al. [53] observed a 20% increase in regional CBF of the target region, accompanied by an improvement in neurological function evidenced by movement of the subject’s left hand and arm that was previously absent (Table 6). This case study involved 146 sessions of 850 nm LED treatments on the left and right forehead areas of the patient (30 min per session). Sessions were performed twice daily for 73 days. They additionally reported a CBF increase 30 min after the 146th tPBM treatment in the vegetative patient. These results, with consideration of the severity of TBI and the vegetative state of the subject, provide a novel demonstration of the promise for increases in CBF associated with tPBM treatment to have a meaningful therapeutic impact in severe instances of TBI and impaired consciousness.
3.7. Transcranial Photobiomodulation in Chronic TBI across Mixed Severities
Rindner et al. [54] studied the therapeutic effects of tPBM with laser stimulation in 11 patients diagnosed with chronic TBI at different severities, ranging from one to multiple concussions and motor vehicle accidents with and without loss of consciousness, and observed neuropsychological testing performance, self-reported global change rating, and mood (Table 7). All 11 participants underwent five to eight 20-min sessions of a continuous-wave 1064 nm tPBM delivered to the bilateral frontal cortex (Brodmann area 10). Cortical mapping was accomplished using structural MRIs. The authors implemented the global rating of change as the primary outcome measure post-tPBM, as well as mood questionnaires and neuropsychological testing batteries as the secondary outcome measures. They reported that seven of nine subjects who had been diagnosed with TBI of mixed severity experienced clinically meaningful changes in wellbeing in response to eight sessions of tPBM, which were accompanied by improvements in memory and reductions in anxiety and depression symptoms. Notably, significant improvement was found among a wide range of injury chronicity in this study, with a chronicity range from two months to six years among the subjects who showed improvement, providing further evidence of tPBM’s potential efficacy in treating patients with a wide range of TBI chronicity.

Table 7.
Transcranial photobiomodulation studies in chronic TBI with mixed severities.
4. Discussion
4.1. Summary
The literature regarding tPBM and patients with TBI suffers from a small sample size, an open-label and non-randomized design, a lack of control groups, highly heterogeneous time post-injury, varying subgroups of TBI, including a range of injury severities, and a paucity of neuroimaging metrics (relying mainly on cognitive measures). A majority of studies have included individuals with mild chronic TBI (Figure 2), with inconsistency on how injury severity was defined and little-to-no information on etiology, presence of brain lesions, or occurrence of diffuse axonal injury. Out of the 16 studies reviewed, only 4 were not case studies (Figure 1); however, all had very promising results. There is a need in the field for more clinical trials and randomized controlled studies to address the potential clinical utility of tPBM for chronic TBI.
Some studies, however, have claimed to account for the individual differences of patients with TBI in tPBM studies by using whole-brain LED caps [45]. Two studies have acknowledged baseline hypoperfusion in one TBI patient with chronic moderate TBI [46] and 12 military veterans with chronic TBI [45]. The one chronic moderate TBI patient’s SPECT images showed increases in CBF two months after the last tPBM treatment within the foci of injury (i.e., the right temporal and right lateral frontal cortices), regions adjacent to those foci, and distal cortical areas that were previously hypoperfused at baseline. A total of 8 of 12 chronic military veterans’ SPECT images showed increases in regional CBF post-tPBM in areas that displayed baseline hypoperfusion. The very small pool of tPBM studies, including neuroimaging metrics, underscores the need for empirical studies to directly examine the clinically relevant acute effects and the underlying neural mechanisms of tPBM in TBI. There is a need for studies to explore the mechanisms of tPBM in chronic TBI using animal models, although there are limitations to utilizing chronic models of TBI in mice. There is also a need to understand the mechanisms of tPBM in chronic TBI populations using a clinical trial with a well-characterized cohort. While the studies described in this review are promising, there is a need for future studies to continue to explore these questions using well-controlled, randomized controlled clinical trials.
4.2. A Potential Target for Chronic TBI Using Transcranial Photobiomodulation
The efficacy of tPBM in acute TBI has been extensively studied only in research with small animals, not humans (e.g., [62,63]). There has been much work focusing on the efficacy of tPBM in acute TBI in animal models; however, thus far, only one study has been conducted in humans (acute, moderate TBI) [64]. Conversely, there have been six tPBM studies, including chronic TBI in humans. These studies are open-protocol but have reported significant improvements in these mild–moderate TBI participants [45,47,48,53,54,56]. In some of the cases, tPBM was initiated even 20 or 30 years after the first TBI. Since most of the previous tPBM studies with chronic, mild–moderate TBI cases were open-protocol, there is a need to determine the necessary sample sizes and tPBM protocols for future studies. In addition, there is a need for sham-controlled studies with large numbers of chronic (and acute) mild–moderate TBI in humans.
The efficacy of tPBM in acute TBI has been extensively studied only in research with small animals, not humans (e.g., [62,63]). There has been much work focusing on the efficacy of tPBM in acute TBI in animal models; however, thus far, only one study has been conducted in humans (acute, moderate TBI) [64]. Conversely, there have been six tPBM studies, including chronic TBI in humans. These studies are open-protocol but have reported significant improvements in these mild–moderate TBI participants [45,47,48,53,54,56]. In some of the cases, tPBM was initiated even 20 or 30 years after the first TBI. Since most of the previous tPBM studies with chronic, mild–moderate TBI cases were open-protocol, there is a need to determine the necessary sample sizes and tPBM protocols for future studies. In addition, there is a need for sham-controlled studies with large numbers of chronic (and acute) mild–moderate TBI in humans.
Previous studies have demonstrated changes in CBF post-tPBM treatments in TBI after a varying number of sessions. The beneficial effects from Naeser et al. [58] wore off after 2 months without continued tPBM. Cases who then continued with tPBM treatments at home, treating only the cortical node areas of the default mode network with NIR, 810 nm LEDs pulsed at 40 Hz, again showed significant improvements, similar to what had been observed at 1 week and 1 month after treating the whole head, in the initial in-clinic treatment series. The fall-off in cognition and behavior/mood after 2 months without continued LED treatments in the ex-football players was a pattern previously observed with mild to moderate–severe dementia cases, likely Alzheimer’s Disease (AD) treated with LEDs [65]. Both patient populations, where there was initial improvement after 18 tPBM treatments but later decline, likely had a progressive neurodegenerative disease but different etiologies. Conversely, chronic, mild TBI cases, however, who had suffered TBI from motor vehicle accidents or single sports injuries and were treated with tPBM in a separate study, retained their significant improvements even 2 months after the final, 18th in-office LED treatment [55]; these chronic TBI cases did not have a progressive neurodegenerative disease. Another study showed that an individual with chronic moderate TBI also displayed increased CBF 2 months after the last of 20 tPBM sessions [46]. Cases with a progressive neurodegenerative disease, such as possible CTE or AD, are likely to require on-going, long-term tPBM. While shorter-term increases in CBF have been associated with both behavior and cognition, the longer-term influences of tPBM on CBF are unknown but an important area for future tPBM research.
4.3. A Potential Target for Chronic TBI Using Transcranial Photobiomodulation
A microvascular injury can serve as a potential target for tPBM. Due to the lack of information on etiology, the presence of brain lesions, or the occurrence of diffuse axonal injury, it is unknown whether the targeted regions are damaged or not. The current literature that has included target engagement as an aim of their study has mainly targeted networks, such as the default mode network and salience network. Future studies should include target engagement strategies, including identifying impaired tissue (e.g., hypoperfused cortical areas) and then targeting areas with microvascular injury. It is also important to denote the areas with focal lesions, which are likely to not respond to tPBM administration. However, increasing regional CBF within the penumbra surrounding the edges of a focal area of infarction, where there is compromised brain tissue, may be seen after tPBM; this has been demonstrated in patients with chronic stroke using a specific protocol (protocol D) [66]. In order to target regions with microvascular injury that may respond to tPBM, future research should use patient-specific methods of quantifying the degree of impairment in local tissue defined against the normative distribution of healthy control subjects [9,30]. Another option for choosing specific target engagement regions could be identifying regions with impaired neuronal activity within a functional network while still taking into account regions with diffuse and focal lesions, which previous studies have not achieved.
4.4. Conclusions
Overall, the literature has suggested that tPBM has the potential to be beneficial for individuals with chronic TBI, both behaviorally and physiologically. In addition to decreases in CBF, there are other impairments, such as blood–brain barrier disruption and the glymphatic system [67], associated with behavioral deterioration that may also benefit from tPBM treatment [68,69]. More randomized controlled trials are needed to understand the mechanisms of tPBM in chronic TBI and how increasing blood flow and brain activity are related to behavioral changes post-tPBM by including neuroimaging measures. It is known that cognitive enhancement post-tPBM is related to prefrontal cerebrovascular oxygenation in healthy adults [70]. However, it is unknown whether tPBM can benefit damaged tissue in chronic TBI, which would also benefit from more research on individualized target engagement. Future research should try to replicate these findings in individuals with impairments in prefrontal cerebrovasculature, such as chronic TBI.
Author Contributions
Conceptualization, N.L.G. and J.J.K.; writing—original draft preparation, N.L.G., N.L.R., X.S. and A.L.P.; writing—review and editing, P.C., D.V.I., J.J.K., N.L.G. and R.D.-A.; visualization, N.L.G.; supervision, J.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Results from search strategies above can be shared upon request.
Conflicts of Interest
Dr. Cassano consulted for Janssen Research and Development and Niraxx Light Therapeutics Inc. Dr. Cassano was funded by PhotoThera Inc, LiteCure LLC and Cerebral Sciences Inc to conduct studies on transcranial photobiomodulation. Dr. Cassano is a co-founder, shareholder, and board director of Niraxx Inc. Dr. Cassano has filed several patents related to the use of near-infrared light in psychiatry. In the last 10 years, Dr. Iosifescu has served as a consultant for Alkermes, Allergan, Angelini, Autobahn, Axsome, Biogen, Boehringer Ingelheim, the Centers for Psychiatric Excellence, Clexio, Delix, Jazz, Lundbeck, Neumora, Otsuka, Precision Neuroscience, Relmada, Sage Therapeutics, and Sunovion. He has received grant support (paid to his institutions) from Alkermes, AstraZeneca, BrainsWay, LiteCure, NeoSync, Otsuka, Roche, and Shire. The remaining authors declare no conflict of interest.
References
- Willemse-van Son, A.H.P.; Ribbers, G.M.; Verhagen, A.P.; Stam, H.J. Prognostic factors of long-term functioning and productivity after traumatic brain injury: A systematic review of prospective cohort studies. Clin. Rehabil. 2007, 21, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.J.; McCormick, W.C.; Kagan, S.H. Traumatic Brain Injury in Older Adults: Epidemiology, Outcomes, and Future Implications. J. Am. Geriatr. Soc. 2006, 54, 1590–1595. [Google Scholar] [CrossRef]
- Bigler, E.D. Traumatic brain injury, neuroimaging, and neurodegeneration. Front. Hum. Neurosci. 2013, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Ding, Z.; Chan, A.S. Can transcranial photobiomodulation improve cognitive function? A systematic review of human studies. Ageing Res. Rev. 2023, 83, 101786. [Google Scholar] [CrossRef]
- Dole, M.; Auboiroux, V.; Langar, L.; Mitrofanis, J. A systematic review of the effects of transcranial photobiomodulation on brain activity in humans. Rev. Neurosci. 2023, 34, 671–693. [Google Scholar] [CrossRef]
- Stevens, A.R.; Hadis, M.; Milward, M.; Ahmed, Z.; Belli, A.; Palin, W.; Davies, D.J. Photobiomodulation in Acute Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Neurotrauma 2022, 40, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Sandsmark, D.K.; Bashir, A.; Wellington, C.L.; Diaz-Arrastia, R. Cerebral Microvascular Injury: A Potentially Treatable Endophenotype of Traumatic Brain Injury-Induced Neurodegeneration. Neuron 2019, 103, 367–379. [Google Scholar] [CrossRef]
- Gaggi, N.L.; Ware, J.B.; Dolui, S.; Brennan, D.; Torrellas, J.; Wang, Z.; Whyte, J.; Diaz-Arrastia, R.; Kim, J.J. Temporal dynamics of cerebral blood flow during the first year after moderate-severe traumatic brain injury: A longitudinal perfusion MRI study. NeuroImage Clin. 2023, 37, 103344. [Google Scholar] [CrossRef]
- Amyot, F.; Kenney, K.; Moore, C.; Haber, M.; Turtzo, L.C.; Shenouda, C.; Silverman, E.; Gong, Y.; Qu, B.X.; Harburg, L.; et al. Imaging of cerebrovascular function in chronic traumatic brain injury. J. Neurotrauma 2018, 35, 1116–1123. [Google Scholar] [CrossRef]
- Hay, J.R.; Johnson, V.E.; Young, A.M.H.; Smith, D.H.; Stewart, W. Blood-brain barrier disruption Is an early event that may persist for many years after traumatic brain injury in humans. J. Neuropathol. Exp. Neurol. 2015, 74, 1147–1157. [Google Scholar] [PubMed]
- Dmochowski, G.M.; Shereen, A.D.; Berisha, D.; Dmochowski, J.P. Near-Infrared Light Increases Functional Connectivity with a Non-thermal Mechanism. Cereb. Cortex Commun. 2020, 1, tgaa004. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Chen, A.C.-H.; Carroll, J.D.; Hamblin, M.R. Biphasic Dose Response in Low Level Light Therapy. Dose-Response 2009, 7, 358–383. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Mechanisms of Low-Power Laser Light Action on Cellular Level; Karu, T.I., Lubart, R., Eds.; SPIE: Amsterdam, The Netherlands, 2000; pp. 1–17. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Kolyakov, S.F.; Afanasyeva, N.I. Absorption measurements of a cell monolayer relevant to phototherapy: Reduction of cytochrome c oxidase under near IR radiation. J. Photochem. Photobiol. B 2005, 81, 98–106. [Google Scholar] [CrossRef]
- Wong-Riley, M.T.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation Directly Benefits Primary Neurons Functionally Inactivated by Toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.P.; Lo, S.C.L.; Siu, F.K.W.; So, K.-F. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg. Med. 2002, 31, 283–288. [Google Scholar] [CrossRef]
- Fujimaki, Y.; Shimoyama, T.; Liu, Q.; Umeda, T.; Nakaji, S.; Sugawara, K. Low-Level Laser Irradiation Attenuates Production of Reactive Oxygen Species by Human Neutrophils. J. Clin. Laser Med. Surg. 2003, 21, 165–170. [Google Scholar] [CrossRef]
- Liang, H.L.; Whelan, H.T.; Eells, J.T.; Wong-Riley, M.T.T. Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience 2008, 153, 963–974. [Google Scholar] [CrossRef]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Regulation of miRNA Expression by Low-Level Laser Therapy (LLLT) and Photodynamic Therapy (PDT). Int. J. Mol. Sci. 2013, 14, 13542–13558. [Google Scholar] [CrossRef]
- Greco, M.; Vacca, R.A.; Moro, L.; Perlino, E.; Petragallo, V.A.; Marra, E.; Passarella, S. Helium-Neon laser irradiation of hepatocytes can trigger increase of the mitochondrial membrane potential and can stimulate c-fos expression in a Ca2+-dependent manner. Lasers Surg. Med. 2001, 29, 433–441. [Google Scholar] [CrossRef]
- Wang, X.; Tian, F.; Reddy, D.D.; Nalawade, S.S.; Barrett, D.W.; Gonzalez-Lima, F.; Liu, H. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study. J. Cereb. Blood Flow Metab. 2017, 37, 3789–3802. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.M.; Marklund, N.; Lebold, D.; Thompson, H.J.; Pitkanen, A.; Maxwell, W.L.; Longhi, L.; Laurer, H.; Maegele, M.; Neugebauer, E.; et al. Experimental models of traumatic brain injury: Do we really need to build a better mousetrap? Neuroscience 2005, 136, 971–989. [Google Scholar] [CrossRef] [PubMed]
- Kenney, K.; Haber, M.; Amyot, F.; Davis, C.; Pronger, A.; Moore, C.; Diaz-Arrastia, R. Cerebral Microvascular Injury in Traumatic Brain Injury. J. Neurol. Neuromed. 2016, 6, 40–46. [Google Scholar]
- Soustiel, J.F.; Glenn, T.C.; Shik, V.; Boscardin, J.; Mahamid, E.; Zaaroor, M. Monitoring of cerebral blood flow and metabolism in traumatic brain injury. J. Neurotrauma 2005, 22, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Whyte, J.; Patel, S.; Europa, E.; Slattery, J.; Coslett, H.B.; Detre, J.A. A Perfusion fMRI Study of the Neural Correlates of Sustained-Attention and Working-Memory Deficits in Chronic Traumatic Brain Injury. Neurorehabil. Neural Repair 2012, 26, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Obrist, W.D.; Langfitt, T.W.; Jaggi, J.L.; Cruz, J.; Gennarelli, T.A. Cerebral blood flow and metabolism in comatose patients with acute head injury: Relationship to intracranial hypertension. J. Neurosurg. 1984, 61, 241–253. [Google Scholar] [CrossRef]
- Ding, K.; Tarumi, T.; Tomoto, T.; Mccolloster, M.; Le, T.; Dieppa, M.; Diaz-Arrastia, R.; Bell, K.; Madden, C.; Cullum, C.M.; et al. Impaired cerebral blood flow regulation in chronic traumatic brain injury. Brain Res. 2020, 1743, 146924. [Google Scholar] [CrossRef]
- Ware, J.B.; Dolui, S.; Duda, J.; Gaggi, N.L.; Choi, R.; Detre, J.; Whyte, J.; Diaz-Arrastia, R.; Kim, J.J. Relationship of Cerebral Blood Flow to Cognitive Function and Recovery in Early Chronic Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2180–2187. [Google Scholar] [CrossRef]
- Hlatky, R.; Contant, C.F.; Diaz-Marchan, P.; Valadka, A.B.; Robertson, C.S. Significance of a reduced cerebral blood flow during the first 12 hours after traumatic brain injury. Neurocrit. Care 2004, 1, 69–83. [Google Scholar] [CrossRef]
- Benedictus, M.R.; Leeuwis, A.E.; Binnewijzend, M.A.; Kuijer, J.P.; Scheltens, P.; Barkhof, F.; van der Flier, W.M.; Prins, N.D. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. Eur. Radiol. 2017, 27, 1169–1175. [Google Scholar] [CrossRef]
- Chao, L.L.; Buckley, S.T.; Kornak, J.; Schuff, N.; Madison, C.; Yaffe, K.; Miller, B.L.; Kramer, J.H.; Weiner, M.W. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis. Assoc. Disord. 2010, 24, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cejudo, J.; Wisniewski, T.; Marmar, C.; Zetterberg, H.; Blennow, K.; de Leon, M.J.; Fossati, S. Traumatic Brain Injury and Alzheimer’s Disease: The Cerebrovascular Link. EBioMedicine 2018, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Shively, S.; Scher, A.I.; Perl, D.P.; Diaz-Arrastia, R. Dementia resulting from traumatic brain injury: What is the pathology? Arch. Neurol. 2012, 69, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Wilson, L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Xekardaki, A.; Rodriguez, C.; Montandon, M.L.; Toma, S.; Tombeur, E.; Herrmann, F.R.; Zekry, D.; Lovblad, K.O.; Barkhof, F.; Giannakopoulos, P.; et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology 2015, 274, 490–499. [Google Scholar] [CrossRef]
- Hwang, J.; Castelli, D.M.; Gonzalez-Lima, F. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med. Sci. 2016, 31, 1151–1160. [Google Scholar] [CrossRef]
- Marion, D.W.; Darby, J.; Yonas, H. Acute regional cerebral blood flow changes caused by severe head injuries. J. Neurosurg. 1991, 74, 407–414. [Google Scholar] [CrossRef]
- Stamatakis, E.A.; Tyler, L.K. Identifying lesions on structural brain images—Validation of the method and application to neuropsychological patients. Brain Lang. 2005, 94, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Jullienne, A.; Obenaus, A.; Ichkova, A.; Savona-Baron, C.; Pearce, W.J.; Badaut, J. Chronic cerebrovascular dysfunction after traumatic brain injury: Cerebrovascular Dysfunction After TBI. J. Neurosci. Res. 2016, 94, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-W.; Wu, J.-H.; Hsieh, C.-H.; Wang, Q.-F.; Chen, J.-H. Different Brain Network Activations Induced by Modulation and Nonmodulation Laser Acupuncture. Evid. Based Complement. Alternat. Med. 2011, 2011, 951258. [Google Scholar] [CrossRef]
- Hipskind, S.G.; Grover, F.L.; Fort, T.R.; Helffenstein, D.; Burke, T.J.; Quint, S.A.; Bussiere, G.; Stone, M.; Hurtado, T. Pulsed Transcranial Red/Near-Infrared Light Therapy Using Light-Emitting Diodes Improves Cerebral Blood Flow and Cognitive Function in Veterans with Chronic Traumatic Brain Injury: A Case Series. Photomed. Laser Surg. 2018, pho.2018.4489. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.A.; Morries, L.D. SPECT Perfusion Imaging Demonstrates Improvement of Traumatic Brain Injury with Transcranial Near-infrared Laser Phototherapy. Adv. Mind Body Med. 2015, 29, 27–33. [Google Scholar] [PubMed]
- Morries, L.; Cassano, P.; Henderson, T.A. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 2015, 11, 2159. [Google Scholar] [CrossRef]
- Vargas, E.; Barrett, D.W.; Saucedo, C.L.; Huang, L.D.; Abraham, J.A.; Tanaka, H.; Haley, A.P.; Gonzalez-Lima, F. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med. Sci. 2017, 32, 1153–1162. [Google Scholar] [CrossRef]
- Naeser, M.A.; Saltmarche, A.; Krengel, M.H.; Hamblin, M.R.; Knight, J.A. Improved Cognitive Function after Transcranial, Light-Emitting Diode Treatments in Chronic, Traumatic Brain Injury: Two Case Reports. Photomed. Laser Surg. 2011, 29, 351–358. [Google Scholar] [CrossRef]
- Henderson, T.A.; Morries, L.D. Multi-Watt Near-Infrared Phototherapy for the Treatment of Comorbid Depression: An Open-Label Single-Arm Study. Front. Psychiatry 2017, 8, 187. [Google Scholar] [CrossRef]
- Poiani, G.D.C.R.; Zaninotto, A.L.; Carneiro, A.M.C.; Zangaro, R.A.; Salgado, A.S.I.; Parreira, R.B.; de Andrade, A.; Teixeira, M.J.; Paiva, W.S. Photobiomodulation using low-level laser therapy (LLLT) for patients with chronic traumatic brain injury: A randomized controlled trial study protocol. Trials 2018, 19, 17. [Google Scholar] [CrossRef]
- Carneiro, A.M.C.; Poiani, G.C.; Zaninnoto, A.L.; Lazo Osorio, R.; Oliveira, M.L.; Paiva, W.S.; Zângaro, R.A. Transcranial Photobiomodulation Therapy in the Cognitive Rehabilitation of Patients with Cranioencephalic Trauma. Photobiomodulation Photomed. Laser Surg. 2019, 37, 657–666. [Google Scholar] [CrossRef]
- Nawashiro, H.; Wada, K.; Nakai, K.; Sato, S. Focal Increase in Cerebral Blood Flow after Treatment with Near-Infrared Light to the Forehead in a Patient in a Persistent Vegetative State. Photomed. Laser Surg. 2012, 30, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Rindner, E.S.; Haroon, J.M.; Jordan, K.G.; Mahdavi, K.D.; Surya, J.R.; Zielinski, M.A.; Habelhah, B.; Venkatraman, V.; Becerra, S.A.; Chan, L.; et al. Transcranial Infrared Laser Stimulation for the Treatment of Traumatic Brain Injury: A Case Series. J. Lasers Med. Sci. 2022, 13, e65. [Google Scholar] [CrossRef]
- Naeser, M.A.; Zafonte, R.; Krengel, M.H.; Martin, P.I.; Frazier, J.; Hamblin, M.R.; Knight, J.A.; Meehan, W.P., 3rd; Baker, E.H. Significant Improvements in Cognitive Performance Post-Transcranial, Red/Near-Infrared Light-Emitting Diode Treatments in Chronic, Mild Traumatic Brain Injury: Open-Protocol Study. J. Neurotrauma 2014, 31, 1008–1017. [Google Scholar] [CrossRef]
- Liebel, S.W.; Johnson, P.K.; Lindsey, H.M.; Russell, H.A.; Hovenden, E.S.; Velez, C.; Carr, L.S.; Wilde, E.A.; Tate, D.F. A-25 Transcranial Photobiomodulation Treatment Effects in Former Athletes with Repetitive Head Hits. Arch. Clin. Neuropsychol. 2022, 37, 1066. [Google Scholar] [CrossRef]
- Chao, L.L. Effects of Home Photobiomodulation Treatments on Cognitive and Behavioral Function, Cerebral Perfusion, and Resting-State Functional Connectivity in Patients with Dementia: A Pilot Trial. Photobiomodul. Photomed. Laser Surg. 2019, 37, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Naeser, M.A.; Martin, P.I.; Ho, M.D.; Krengel, M.H.; Bogdanova, Y.; Knight, J.A.; Hamblin, M.R.; Fedoruk, A.E.; Poole, L.G.; Cheng, C.; et al. Transcranial Photobiomodulation Treatment: Significant Improvements in Four Ex-Football Players with Possible Chronic Traumatic Encephalopathy. J. Alzheimers Dis. Rep. 2023, 7, 77–105. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.L. Improvements in Gulf War Illness Symptoms after Near-Infrared Transcranial and Intranasal Photobiomodulation: Two Case Reports. Mil. Med. 2019, 184, e568–e574. [Google Scholar] [CrossRef]
- Chao, L.L.; Barlow, C.; Karimpoor, M.; Lim, L. Changes in Brain Function and Structure After Self-Administered Home Photobiomodulation Treatment in a Concussion Case. Front. Neurol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Bogdanova, Y.; Martin, P.; Ho, M.; Krengel, M.; Ho, V.; Yee, M.; Knight, J.; Hamblin, M.; Naeser, M. LED Therapy Improves Sleep and Cognition in Chronic Moderate TBI: Pilot Case Studies. Arch. Phys. Med. Rehabil. 2014, 95, e77. [Google Scholar] [CrossRef][Green Version]
- Oron, A.; Oron, U.; Streeter, J.; de Taboada, L.; Alexandrovich, A.; Trembovler, V.; Shohami, E. Low-Level Laser Therapy Applied Transcranially to Mice following Traumatic Brain Injury Significantly Reduces Long-term Neurological Deficits. J. Neurotrauma 2007, 24, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Vatansever, F.; Huang, L.; Hamblin, M.R. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J. Biomed. Opt. 2014, 19, 108003. [Google Scholar] [CrossRef] [PubMed]
- Figueiro Longo, M.G.; Tan, C.O.; Chan, S.T.; Welt, J.; Avesta, A.; Ratai, E.; Mercaldo, N.D.; Yendiki, A.; Namati, J.; Chico-Calero, I.; et al. Effect of Transcranial Low-Level Light Therapy vs Sham Therapy Among Patients with Moderate Traumatic Brain Injury: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2017337. [Google Scholar] [CrossRef]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Photomed. Laser Surgery 2017, 35, 432–441. [Google Scholar] [CrossRef]
- Naeser, M.A.; Ho, M.D.; Martin, P.I.; Hamblin, M.R.; Koo, B.-B. Increased Functional Connectivity within Intrinsic Neural Networks in Chronic Stroke Following Treatment with Red/Near-Infrared Transcranial Photobiomodulation: Case Series with Improved Naming in Aphasia. Photobiomodul. Photomed. Laser Surg. 2020, 38, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.; Zhou, L.; Ozsahin, I.; Wang, X.H.; Garetti, J.; Zetterberg, H.; Blennow, K.; Jamison, K.; de Leon, M.J.; Li, Y.; et al. Glymphatic clearance estimated using diffusion tensor imaging along perivascular spaces is reduced after traumatic brain injury and correlates with plasma neurofilament light, a biomarker of injury severity. Brain Commun. 2023, 5, fcad134. [Google Scholar] [CrossRef]
- Formolo, D.A.; Yu, J.; Lin, K.; Tsang, H.W.H.; Ou, H.; Kranz, G.S.; Yau, S.Y. Leveraging the glymphatic and meningeal lymphatic systems as therapeutic strategies in Alzheimer’s disease: An updated overview of nonpharmacological therapies. Mol. Neurodegener. 2023, 18, 26. [Google Scholar] [CrossRef]
- Salehpour, F.; Khademi, M.; Bragin, D.E.; DiDuro, J.O. Photobiomodulation Therapy and the Glymphatic System: Promising Applications for Augmenting the Brain Lymphatic Drainage System. Int. J. Mol. Sci. 2022, 23, 2975. [Google Scholar] [CrossRef]
- Holmes, E.; Barrett, D.W.; Saucedo, C.L.; O’Connor, P.; Liu, H.; Gonzalez-Lima, F. Cognitive Enhancement by Transcranial Photobiomodulation Is Associated with Cerebrovascular Oxygenation of the Prefrontal Cortex. Front. Neurosci. 2019, 13, 1129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).