1. Introduction
The application of touchscreens continues to grow worldwide, replacing conventional keyboards [
1,
2,
3]. They are used in private notebooks, smartphones and tablets, in public computers such as ATMs or ticket machines, and also increasingly in medical devices. Potentially pathogenic microorganisms, can always be transmitted by touching these screens, which is especially concerning regarding nosocomial infections in the medical sector [
4]. Bacterial strains, including the so-called ESKAPE pathogens (
Enterococcus faecium,
Staphylococcus aureus,
Klebsiella pneumoniae,
Acinetobacter baumannii,
Pseudomonas aeruginosa, and
Enterobacter spp.), were found on touchscreens in all studies conducted to date, both outside and inside the healthcare sector. Staphylococci, which include the methicillin resistant
Staphylococcus aureus, were consistently found [
5]. To prevent the transmission of pathogens via touchscreens, there would have to be a regular disinfection process between users. An automatic self-disinfection would reduce the effort enormously and guarantee the execution of the procedure. This process needs to be fast enough to not disrupt the user experience.
Most microorganisms are sensitive to UVC radiation, which describes non-ionizing electromagnetic radiation with a wavelength between 100 nm and 280 nm [
6]. As illustrated in
Figure 1, this radiation is absorbed by DNA and RNA. Microorganisms can suffer death through UVC irradiation. For more complex, multicellular organisms such as humans, UVC irradiation can lead to inflammations of the upper skin layers and increased risk of skin cancer [
6,
7,
8,
9].
UVA (315–400 nm) and visible light (380–780 nm) generally require much higher irradiation doses and thus longer exposure durations [
11,
12]. In a previous publication, a staphylococcal reduction of 90% was reached after 15 h through blue light emitted by the device’s own screen [
13]. With additional UVA irradiation, this was reduced to 1 h, which still is too long for the application of frequently used devices [
14]. A much faster disinfection was reached using 236 nm Far-UVC LEDs. A 90% reduction in
Staphylococcus carnosus was achieved in only 10 s on a small quartz plate of 5 × 5 cm
2, which acted as touchscreen model without real touchscreen functionality [
15]. Unfortunately, as Far-UVC LEDs are currently still very expensive, inefficient, and short-lived, they are not yet applicable on a large scale.
Consequently, in this study, conventional UVC LEDs shall be used for the desired touchscreen disinfection because its emitters are cheaper and more durable than Far-UVC and enable a more efficient disinfection than UVA LEDs. To irradiate the front glass of the touchscreen, UVC LEDs are placed around its edge and radiate into the pane laterally. Due to internal total reflection, the front glass acts as a light guide, thus theoretically, no radiation exits through the pane’s surface (aside from the evanescent waves). Contamination of the front glass’ surface leads to a restriction of internal total reflection on its surface and thus UVC irradiation of the contaminant, leading to disinfection. In addition to this, the contaminant is irradiated through the evanescent field at the quartz glass/air border. Because of its high UVC transparency, quartz glass is used as material for the front glass.
Previously, a functional touchscreen computer system with the above-described front glass disinfection was developed. This system utilized a near-infrared touchscreen frame in front of the screen’s front glass [
16]. In contrast, this study aims to advance this system by implementing a projective capacitive (=PCAP) touch sensor into the self-disinfecting touchscreen system. This sensor technology is, along with the resistive touchscreen technology, most widely used [
2,
17]. To ensure total reflection inside the quartz front glass, an air gap between it and the PCAP panel has to be ensured. Additionally, a safety shutdown function shall protect the user of the touchscreen from the UVC hazards mentioned above. Our hypothesis is that it is possible to achieve a fast bacterial reduction with internal UVC LEDs within less than a minute and without endangering the user.
2. Materials and Methods
2.1. System Design and Construction
As a host device for the self-disinfecting touchscreen, the cryotherapy device “Cryo7” by Zimmer MedizinSysteme GmbH (Neu-Ulm, Germany) was selected. The touchscreen disinfection system is designed as follows:
In front of the display and the touch panel, a 4 mm thick quartz glass pane with an area of 236 × 169 mm2 (rounding excluded) was mounted additionally as a “second front glass”. The quartz glass “FN-08” was sourced from GVB GmbH (Herzogenrath, Germany) and laser cut into shape to ensure highly transparent edges.
A total of 36 UVC-emitting LEDs “C3535DUVC-QB-Q5-D” from Luckylight Electronics Co., Ltd. (Shenzhen, China), specified for a wavelength of 275 nm, were mounted onto long, narrow printed circuit boards (PCBs), which themselves were mounted around three edges of the quartz front glass (left, right, and bottom), as is schematically presented in
Figure 2. The size of the LED housings was 3.5 × 3.5 × 1.4 mm
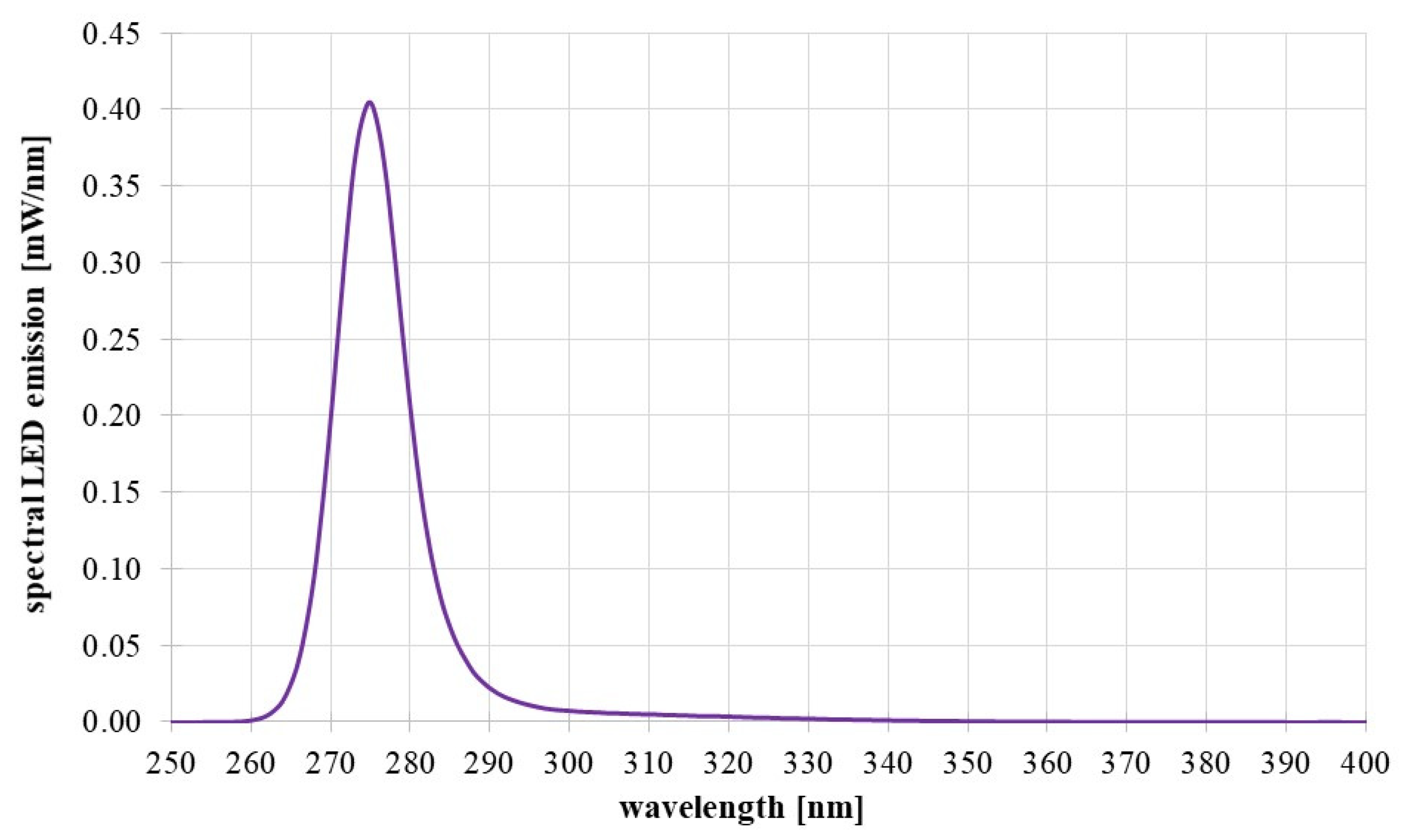
3 each. The internal LED chips were one order of magnitude smaller. The LED emission spectrum, measured with a calibrated spectrometer type CAS140D of Instrument Systems (Munich, Germany) is given in
Figure 3.
One LED PCB carried 12 UVC LEDs. Additional LEDs in the visible spectrum were placed on the PCBs to visualize an irradiation in progress. For this application, the color violet was chosen to complement the design of the Cryo7’s user interface.
All LEDs radiated into the front glass via an air gap. An optical adhesive or mechanical bonding was not applied. A distance of 0.35 mm from the UVC LED’s casing to the glass edge led to the irradiation of the glass edge with an angular range of approximately 160°. The UVC LEDs datasheet states its emission angle as 120°. Therefore, it is assumed that most of the emitted radiation is coupled into the front glass.
The installed UVC LEDs each emitted a radiant power of ~4 mW when driven with a LED current of 30 mA. This led to a total emitted radiant power of 36 × 4 mW = 144 mW. It is assumed that all emitted radiation is coupled into the front glass. The selected LEDs also had a built-in UVA emitter, which was not used in the following experiments. To define the LED current, temperature measurements of the LED housings were performed. To dissipate their generated heat, the LED-PCBs were embedded into an aluminum frame around the quartz front glass using thermal conductive glue.
The aluminum frame also held the quartz front glass in place and ensured an air gap of 0.125 mm between the front quartz glass and the capacitive touch panel.
To fixate the quartz glass firmly in place, surface contact was unavoidable. To minimize radiation loss, contact surfaces between the quartz glass and the aluminum frame were designed to be as small as possible and covered with polytetrafluoroethylene (PTFE), which is a highly UVC-reflective polymer [
18]. The aluminum frame was anodized black and thus absorbed any radiation, which did not couple into the quartz glass.
For the touch panel, a PCAP sensor from Apex Material Corporation Technology’s (Keelung City, Taiwan) PenMount 25xx series was used. This sensor was authorized for medical use. It was laminated onto a 1.1 mm thin and chemically strengthened carrier glass. The PCAP sensor was simulated and parameterized together with the quartz glass prior to assembly using the manufacturer-provided software “PCI Utility V2.0.1”.
2.2. System Control and Safety Concept
The UVC and violet LEDs were driven by a microcontroller circuit, which communicated with the host device Cryo7 of the touchscreen system to integrate the disinfection process seamlessly into the user experience. For this, the microcontroller was operated in combination with an LED driver and an RS232 transceiver.
To protect the touchscreen user from a potentially harmful dose of UVC radiation, a two-stage safety shutdown feature, visualized in
Figure 4, was implemented. Behind the front glass, the infrared proximity sensor “VCNL4030X01-GS08” from Vishay Intertechnology Inc. (Malvern, PA, USA) was integrated into the system to recognize movements towards the touchscreen during irradiation of the front glass. In case the first safety stage was bypassed, the second mechanism came into effect, which worked in communication with the Cryo7. A touch control input during irradiation was registered by the host device, which sent an error message to the disinfection systems microcontroller interrupting the irradiation.
2.3. Investigation of the Homogeneity of the Front Glass Irradiation
To evaluate the homogeneity of the irradiation of the front glasses surface, a picture of the prototype during irradiation was taken in a dark room. The radiation, left through the quartz glass’ surface, could be captured by a sensitive camera, because UVC LEDs emitted light, although very weak, in the visible spectrum. Because only the UVC homogeneity was of interest, the visible violet LEDs were disabled for this photograph. The camera model “D5600” from Nikon Corporation (Tokio, Japan) was used with settings adjusted to aperture: f/16, shutter speed: 1 s and sensitivity ISO 6400. To enhance the visibility even more, the image parameters “brightness” and “exposure” were turned up in the Windows Photo Editor.
Using the software “ImageJ V1.8” [
20], an analysis of the gray values, thus its relative brightness, was performed. The values were averaged horizontally and vertically.
2.4. Contamination of the Samples
To carry out the following microbial measurements for verifying the system’s disinfection capability, the touchscreen had to be contaminated first. Thus, a method for homogeneous spraying of bacterial suspension onto the touchscreen was needed.
As test organism, the non-pathogenic
Staphylococcus carnosus (
S. carnosus, DSM 20501), was chosen, because regarding its sensitivity to UVC irradiation, it can be used as a surrogate for the pathogenic
Staphylococcus aureus, a well-known ESKAPE pathogen [
21]. The suspension was produced by propagating
S. carnosus at 37 °C in a trypticase soy yeast extract medium (“M92”) [
22] until an optical density of 0.5 at 600 nm was reached. After that, the bacteria were washed in phosphate-buffered saline.
Before spraying the suspension, the surfaces were disinfected using 70% ethanol. To distribute this suspension on the quartz glass, a microcontroller-driven air pressure system with the ultrasonic nebulizer “SK508” from CY Spray (Dongguan City, China) was used. The nebulizer system was operated at 2 bar for 1.2 s. This led to an applied suspension volume of 1.75 mL. Based on previous work [
23], through this method of contamination, a bacterial concentration of 10
6 CFU/cm
2 on the quartz surface was produced. After spraying, the surfaces were left to dry before sample taking.
2.5. Measurement and Verification of the Bacterial Reduction
Following, the above-described prototype was tested regarding bacteria through irradiation of its front glass.
After contamination of the touchscreen prototype, the number of bacteria surviving on its surface was determined at 150, 500, and 750-millisecond irradiation intervals. As an unirradiated control, an additional glass pane was contaminated as well. This pane was not irradiated and only probed at the beginning and the end of each test cycle. To ensure reliable results, at the start and after each irradiation interval, three samples were taken.
To determine the number of bacteria on the surfaces, they were segmented in 4 × 4 cm
2 squares, as presented in
Figure 5a. For taking samples, the collection and transport System “eSwab” from Copan Italia s.p.a. (Brescia, Italy) was used. For each sample taken, 5 µL of its buffer solution was transferred onto the sample square, distributed, and taken up again. These sample bacterial suspensions were spread on agar plates at various dilution levels and incubated at 37 °C for 24 h. The visible emerged colonies in the agar plates then were counted. The sample squares were chosen in a random order.
The whole test run was performed three times independently from each other. For each exposure time, the mean values and the error bars of the observed bacterial reduction were then calculated.
2.6. Measurement of the Radiant Power in Front of the Quartz Glass
Because of material impurities of the quartz pane, its not ideally even surfaces, and unavoidable contaminations, a small part of the coupled radiation escaped towards the user and the touch pane.
The former was measured, as depicted in
Figure 5b, to evaluate the user hazard. Measurements of the irradiance at distances of 1 mm and 11 mm above the contaminated front glass were taken. For this, the Optometer X1 from Gigahertz Optik GmbH (Türkenfeld, Germany) was used. Here too, the measurement was conducted three times, and the results were averaged.
Directive 2006/25/EG of the European Parliament [
24] prescribes a maximum UVC exposure dose of
Heff,dose limit = 3
per 8 h workday. With the measured data, the maximum duration of staying in front of the front glass during irradiation at specific distances could be calculated using Formula (1).
4. Discussion
4.1. Homogenity of the Front Glass Irradiation
A standard deviation of 7% and 8% over the whole touchscreen area is acceptable, therefore, the irradiation’s homogeneity on the glasses surface, is sufficient. The drop in irradiance towards the center of the front glass is likely due to radiation loss through absorption inside the quartz glass and unavoidable contamination. Nevertheless, the determined values are not significant.
To achieve this homogeneity, quartz glass was used as front glass due to its high transparency deep into the UVC wavelengths. However, its material properties are not ideal for application as a touchscreen front. Quartz glass is significantly more brittle than what is typically used for device front glasses and on top of that is very expensive [
25].
4.2. Bacterial Reduction on the Touchscreen
With
S. carnosus, a very fast disinfection of the front glass was observed. Previous research revealed, that
S. carnosus is a suitable surrogate for the ESKAPE pathogen
S. aureus regarding UVC inactivation [
22]. However, the reductions in further microorganisms are currently being analyzed.
The contamination of the front glass through spraying with bacterial suspension and letting it dry represents real-world contamination only to a limited extent. Bacteria, left on the touchscreen by the touch of a finger, often are inside a greasy film, which itself can absorb UVC radiation.
The here applied bacterial starting concentrations of 10
6/cm
2 were also not realistic but allowed us to assess the potential of this disinfection approach. Previously published concentrations on touchscreens were usually in the range of about 10/cm
2 up to 600/cm
2 for outliers [
5]. Therefore, a bacterial reduction of 3.5 log levels would result in no surviving microorganism.
4.3. Radiant Power in Front of the Quartz Glass and User Safety
Due to the potential health risks to the user from UVC radiation, the maximum daily exposure times were calculated for distances of 1 and 11 mm from the touch screen. With the above-described two-staged safety feature of the system in place, daily UVC exposure durations per user of 1.3 min or longer at these small distances are estimated as very unlikely. During irradiation, a user’s hand cannot come within a few millimeters of the front glass without triggering the safety irradiation shutdown. Thus, the observed hazard only remains during specific fault cases. In the event of a malfunctioning proximity sensor, a high number of 1 s disinfection cycles can still be tolerated in close proximity to the front glass.
To reduce the potential exposure further, through proximity measurement and communication of the self-disinfecting touchscreen system with its host device, disinfection can be timed specifically to avoid the presence of a user during UVC irradiation.
The infrared proximity sensor is well-suited for this application but could be replaced by an ultra-sensitive PCAP sensor in the future. There are already systems in the market that feature a gesture control in front of the touchscreen.
So far, the safety discussion focused on hands and skin exposure limits and the eye safety was ignored. However, the above-mentioned 275 nm daily exposure limit of 3 mJ/cm
2 is not only true for skin, but also for eyes [
24]. As the distance between eyes and the touchscreen should be much larger than the distance between hands and the touchscreen, the eye irradiation is much lower than the skin irradiation. Therefore, the skin safety for the user includes the eye safety.
5. Conclusions
This study describes the development and testing of a capacitive touchscreen that can disinfect itself very fast by short UVC irradiation of its front glass. A bacterial reduction of 3.5 log levels (99.97%) in under 1 s was reached, without observing a feared sensitivity decrease of the touchscreen. Instead, the opposite was the case. The touchscreen can even be controlled with multiple layers of rubber gloves.
A two-stage safety shutdown function for user protection was implemented in combination with a visible light irradiation to visualize a disinfection in progress and thus warn the user. We therefore confirmed our hypothesis that it is possible to achieve a fast bacterial reduction without endangering humans.
Further development, such as using less harmful Far-UVC emitters, implementing the above-mentioned capacitive proximity detection, utilizing thinner quartz front glass, and replacing the air gap between quartz glass and touch panel with a comparable separating medium will be investigated. Additionally, further measurements with different test microorganisms are already being conducted.