New Yellow Azo Pyridone Derivatives with Enhanced Thermal Stability for Color Filters in Image Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis
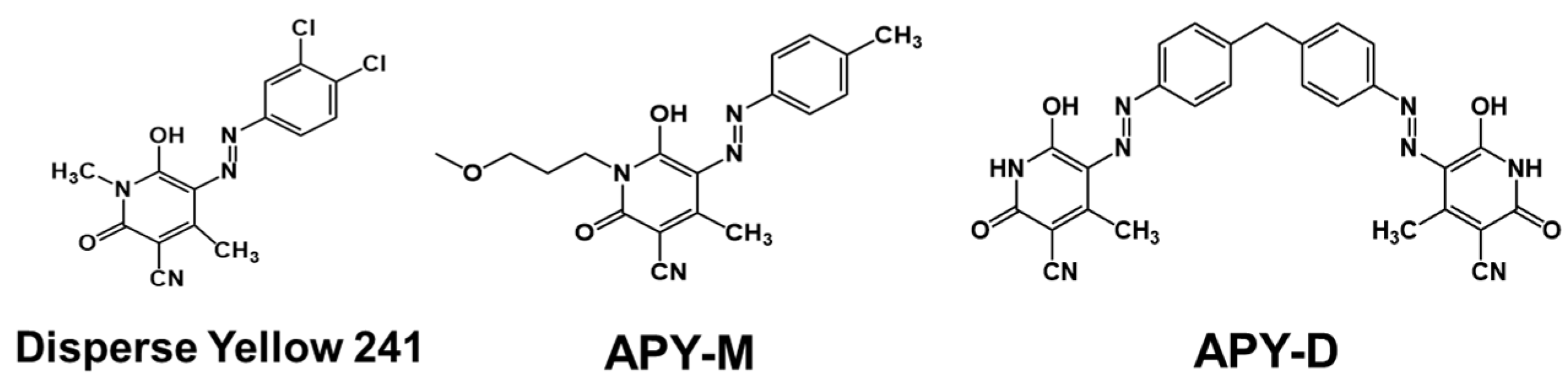
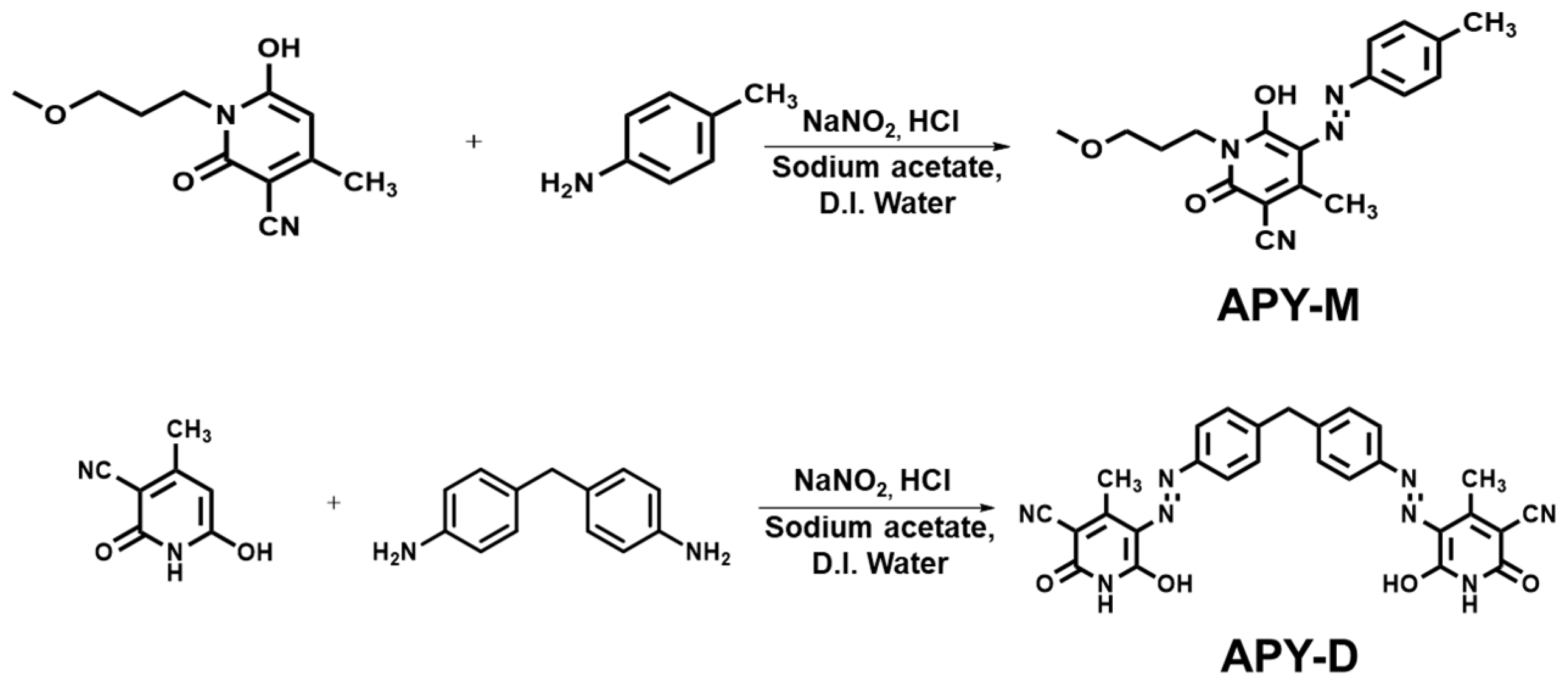
2.2.1. Synthesis of (E)-6-hydroxy-1-(3-methoxypropyl)-4-methyl-2-oxo-5-(p-tolyldiazenyl)-1,2-dihydropyridine-3-carbonitrile, APY-M
2.2.2. Synthesis of 5,5′-((1E,1′E)-(methylenebis(4,1-phenylene))bis(diazene-2,1-diyl))bis(6-hydroxy-4-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile), APY-D
2.3. Fabrication of Film and Color Filters
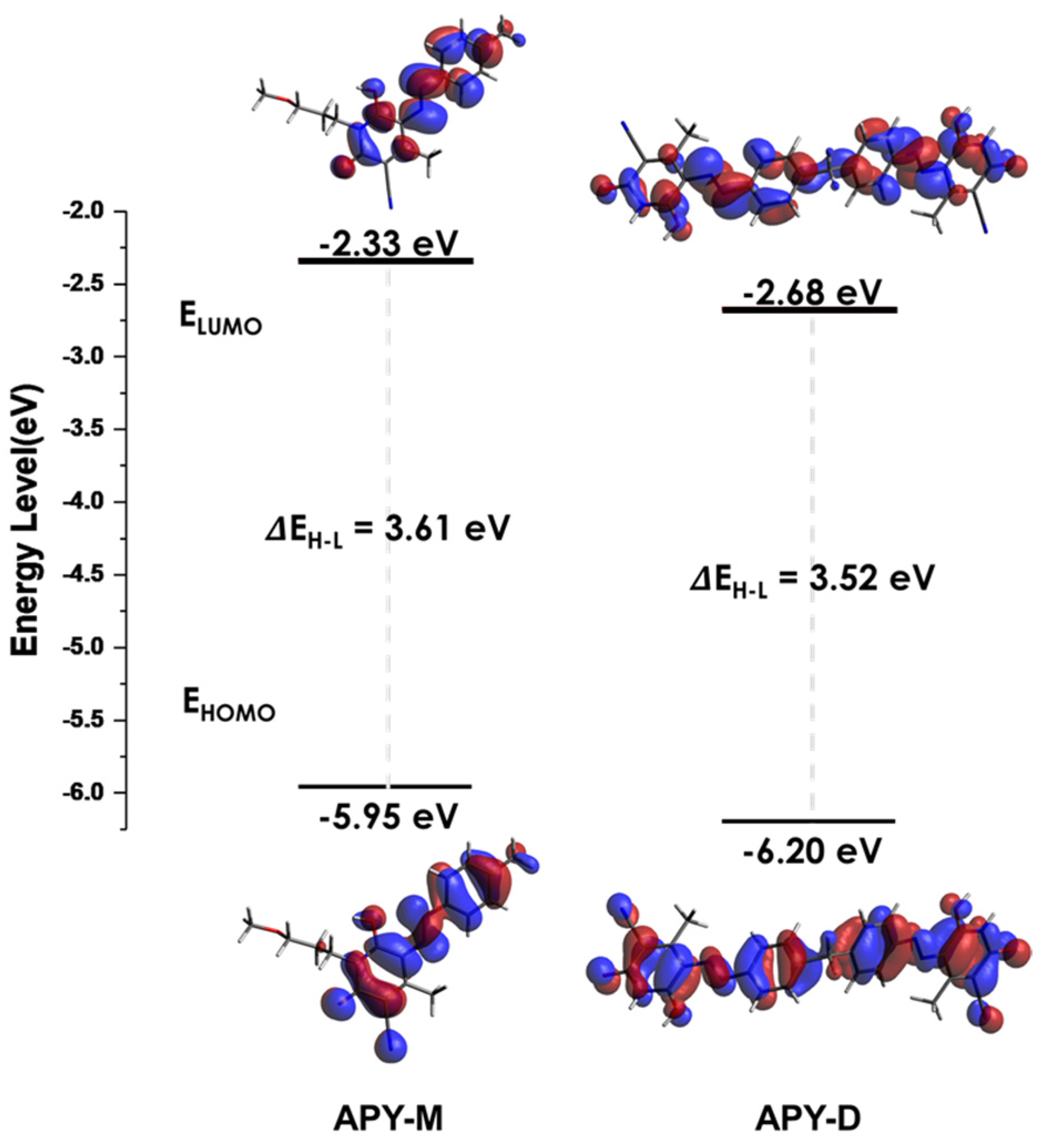
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, S.; Zhang, W.; Wang, Z.; Yassar, A.; Chen, J.; Zeng, M.; Yi, Z. Preparation of Dye Semiconductors via Coupling Polymerization Catalyzed by Two Catalysts and Application to Transistor. Molecules 2023, 29, 71. [Google Scholar] [CrossRef]
- Taguchi, H.; Enokido, M. Technology of color filter materials for image sensor. Red 2017, 10502, 3216. [Google Scholar]
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and Pigments; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ashida, T. Development of color resists containing novel dyes for liquid crystal displays. Sumitomo Kagaku 2013, 1, 521–523. [Google Scholar]
- Fontaine, R. In The state-of-the-art of mainstream CMOS image sensors. In Proceedings of the International Image Sensors Workshop, Vaals, The Netherlands, 8–11 June 2015; International Image Sensors Society: Vaals, The Netherlands, 2015; pp. 6–12. [Google Scholar]
- Chen, Q.; Hu, X.; Wen, L.; Yu, Y.; Cumming, D.R. Nanophotonic Image Sensors. Small 2016, 12, 4922–4935. [Google Scholar] [CrossRef]
- Kim, C.; Hong, J.; Jang, J.; Lee, G.-Y.; Kim, Y.; Jeong, Y.; Lee, B. Freeform metasurface color router for deep submicron pixel image sensors. Sci. Adv. 2024, 10, eadn9000. [Google Scholar] [CrossRef]
- Li, S.; Gao, C.; Xue, J.; Xin, H.; Li, H.; Zhang, J. Red phenanthrenequinone dyes with high thermal and photo-stability for LCD color filters. Dye. Pigment. 2024, 224, 112023. [Google Scholar] [CrossRef]
- Kelley, A.T.; Alessi, P.J.; Fornalik, J.E.; Minter, J.R.; Bessey, P.G.; Garno, J.C.; Royster, T.L., Jr. Investigation and application of nanoparticle dispersions of pigment yellow 185 using organic solvents. Acs Appl. Mater. Interfaces 2010, 2, 61–68. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, B.-J.; An, S.-O.; Lee, J.-H.; Choi, J.-H. The synthesis of red dyes based on diketo-pyrrolo-pyrrole chromophore to improve heat stability and solubility for colour filter fabrication. Dye. Pigment. 2020, 174, 108053. [Google Scholar] [CrossRef]
- Do Kim, Y.; Kim, J.P.; Kwon, O.S.; Cho, I.H. The synthesis and application of thermally stable dyes for ink-jet printed LCD color filters. Dye. Pigment. 2009, 81, 45–52. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, H.J.; Lee, H.K.; Hwang, T.G.; Namgoong, J.W.; Lee, J.M.; Kim, S.; Kim, J.P. A study of the diimmonium dyes employing bis (fluorosulfonyl) imide anions for NIR absorbing film of CMOS image sensor. Dye. Pigment. 2021, 190, 109288. [Google Scholar] [CrossRef]
- Namgoong, J.W.; Kim, H.M.; Kim, S.H.; Yuk, S.B.; Choi, J.; Kim, J.P. Synthesis and characterization of metal phthalocyanine bearing carboxylic acid anchoring groups for nanoparticle dispersion and their application to color filters. Dye. Pigment. 2021, 184, 108737. [Google Scholar] [CrossRef]
- Jang, S.H.; Lee, G.; Lee, S.Y.; Kim, S.H.; Lee, W.; Jung, J.W.; Kim, J.P.; Choi, J. Synthesis and characterisation of triphenylmethine dyes for colour conversion layer of the virtual and augmented reality display. Dye. Pigment. 2022, 204, 110419. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, H.M.; Kim, S.; Kim, W.; Kim, M.S.; Yoon, J.H.; Choi, W.J.; Jeong, D.H.; Kim, J.P. Development of thermally-stable NIR absorbing films based on heptamethine cyanine dyes with bistriflimide anion. Prog. Org. Coat. 2023, 178, 107473. [Google Scholar] [CrossRef]
- Sokolova, N.; Kovzhina, L.; Dmitrieva, N.; Karsakova, T. Lightfastness of polymeric color filters with metal-containing dyes and heterocyclic azo dyes as absorbing agents. Russ. J. Appl. Chem. 2003, 76, 114–116. [Google Scholar] [CrossRef]
- Oliveira, E.; Bértolo, E.; Núñez, C.; Pilla, V.; Santos, H.M.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Djafari, J.; Capelo, J.L.; Lodeiro, C. Green and red fluorescent dyes for translational applications in imaging and sensing analytes: A dual-color flag. ChemistryOpen 2018, 7, 9–52. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, T.G.; Kim, S.H.; Namgoong, J.W.; Kim, J.E.; Sakong, C.; Choi, J.; Lee, W.; Kim, J.P. Synthesis of high-soluble and non-fluorescent perylene derivatives and their effect on the contrast ratio of LCD color filters. Dye. Pigment. 2017, 136, 836–845. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, C.K.; Jeong, S.H.; Jaung, J.Y. Synthesis and characterization of novel blue azo-dye derivatives containing acrylate group for dye-based color filters. J. Soc. Inf. Disp. 2010, 18, 994–1009. [Google Scholar] [CrossRef]
- Porobić, S.J.; Božić, B.Đ.; Dramićanin, M.D.; Vitnik, V.; Vitnik, Ž.; Marinović-Cincović, M.; Mijin, D.Ž. Absorption and fluorescence spectral properties of azo dyes based on 3-amido-6-hydroxy-4-methyl-2-pyridone: Solvent and substituent effects. Dye. Pigment. 2020, 175, 108139. [Google Scholar] [CrossRef]
- Mijin, D.Ž.; Ušćumlić, G.S.; Valentić, N.V. History, Synthesis and Properties of Azo Pyridone Dyes. In Textiles: History, Properties and Performance and Applications; Nova Publishing: Hauppauge, NY, USA, 2014; pp. 157–186. [Google Scholar]
- Matijević, B.; Mrđan, G.; Lađarević, J.; Valentić, N.; Mijin, D.; Apostolov, S.; Vaštag, Đ. Synthesis and solvatochromism of some hydroxy substituted phenyl azo pyridone dyes. Zaštita Mater. 2023, 64, 444–451. [Google Scholar] [CrossRef]
- Lee, J. Development of Color Photoresist Material and Color Filter Technology for Image Sensor; The Ministry of Trade, Industry & Energy (MOTIE, Korea): Seoul, Republic of Korea, 2022.
- Park, S.; Kim, J.H.; Park, S.; Mahendra, G.; Lee, J.; Park, J. New Yellow Aromatic Imine Derivatives Based on Organic Semiconductor Compounds for Image Sensor Color Filters. Appl. Chem. Eng. 2023, 34, 590–595. [Google Scholar]
- Ren, S.; Wang, S.; Chen, J.; Yi, Z. Design of Novel Functional Conductive Structures and Preparation of High-Hole-Mobility Polymer Transistors by Green Synthesis Using Acceptor-Donor-Acceptor Strategies. Polymers 2024, 16, 396. [Google Scholar] [CrossRef]
- Ren, S.; Habibi, A.; Ni, P.; Zhang, Y.; Yassar, A. Tuning the Photophysical Properties of Acceptor-Donor-Acceptor Di-2-(2-oxindolin-3-ylidene) Malononitrile Materials via Extended pi-Conjugation: A Joint Experimental and Theoretical Study. Materials 2023, 16, 6410. [Google Scholar] [CrossRef]
- Kim, W.S.; Yoon, H.I.; Lee, J.M.; Hwang, T.G.; Kim, H.M.; Lee, H.K.; Kim, S.; Choi, W.J.; Kim, J.P. Substituents effects on properties of perylene dyes for spectrum conversion film. Dye. Pigment. 2023, 209, 110845. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, C.Y.; Yao, W.; Song, C.J.; Jaung, J.Y. Synthesis of yellow pyridonylazo colorants and their application in dye–pigment hybrid colour filters for liquid crystal display. Color. Technol. 2016, 133, 158–164. [Google Scholar] [CrossRef]
- Park, S.; Godi, M.; Khairnar, N.; Dae, S.; Kwon, H.; Park, S.; Lee, H.; Lee, K.; Park, J. Enhanced Optical and Thermal Properties of a Novel Yellow Quinophthalone Derivative for Color Filter Colorants in Image Sensors. Phys. Status Solidi (A) 2024, 2300888. [Google Scholar] [CrossRef]
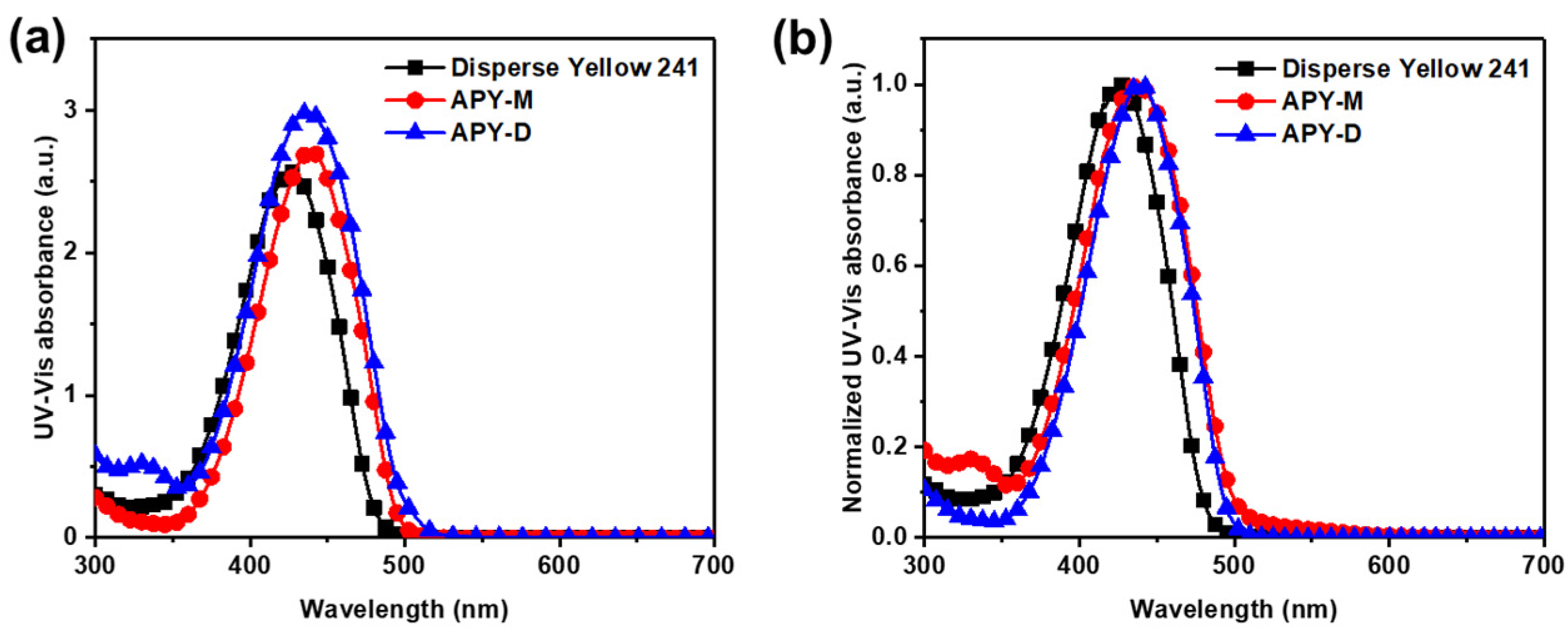


| λmax [nm] | FWHM a [nm] | εmax b [L/mol cm] | |
|---|---|---|---|
| Disperse Yellow 241 | 425 | 72 | 2.6 × 105 |
| APY-M | 437 | 80 | 2.7 × 105 |
| APY-D | 435 | 73 | 3.0 × 105 |
| Transmittance [%] | ||
|---|---|---|
| 435 nm | 530 nm | |
| Disperse Yellow 241 | 0.34 | 99.8 |
| APY-M | 0.21 | 99.9 |
| APY-D | 0.10 | 97.1 |
| Disperse Yellow 241 | APY-M | APY-D | |
|---|---|---|---|
| Solubility [wt%] | 1.2 | 1.3 | 1.0 |
| Td a [°C] | |
|---|---|
| Disperse yellow 241 | 216 |
| APY-M | 248 |
| APY-D | 266 |
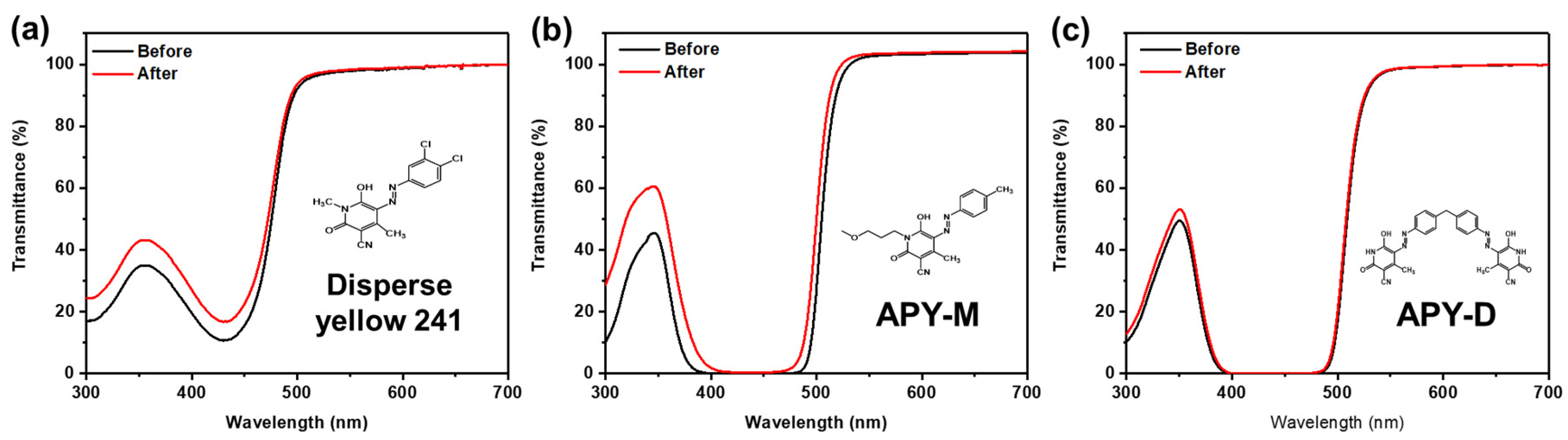
| Transmittance [%] | |||
|---|---|---|---|
| 435 nm | 530 nm | ||
| Disperse Yellow 241 | Before | 11.0 | 97.2 |
| After | 17.1 | 97.7 | |
| APY-M | Before | 0.1 | 99.9 |
| After | 0.3 | 100 | |
| APY-D | Before | 0.04 | 92.3 |
| After | 0.07 | 93.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Park, S.; Oh, S.; Kwon, H.; Lee, H.; Lee, K.; Yoon, C.; Park, J. New Yellow Azo Pyridone Derivatives with Enhanced Thermal Stability for Color Filters in Image Sensors. Photonics 2024, 11, 989. https://doi.org/10.3390/photonics11100989
Park S, Park S, Oh S, Kwon H, Lee H, Lee K, Yoon C, Park J. New Yellow Azo Pyridone Derivatives with Enhanced Thermal Stability for Color Filters in Image Sensors. Photonics. 2024; 11(10):989. https://doi.org/10.3390/photonics11100989
Chicago/Turabian StylePark, Sunwoo, Sangwook Park, Saeyoung Oh, Hyukmin Kwon, Hayoon Lee, Kiho Lee, Chun Yoon, and Jongwook Park. 2024. "New Yellow Azo Pyridone Derivatives with Enhanced Thermal Stability for Color Filters in Image Sensors" Photonics 11, no. 10: 989. https://doi.org/10.3390/photonics11100989
APA StylePark, S., Park, S., Oh, S., Kwon, H., Lee, H., Lee, K., Yoon, C., & Park, J. (2024). New Yellow Azo Pyridone Derivatives with Enhanced Thermal Stability for Color Filters in Image Sensors. Photonics, 11(10), 989. https://doi.org/10.3390/photonics11100989






