Light Disturbance Analysis and Applications
Abstract
1. Introduction
2. Concepts and Definitions
2.1. Disability Glare
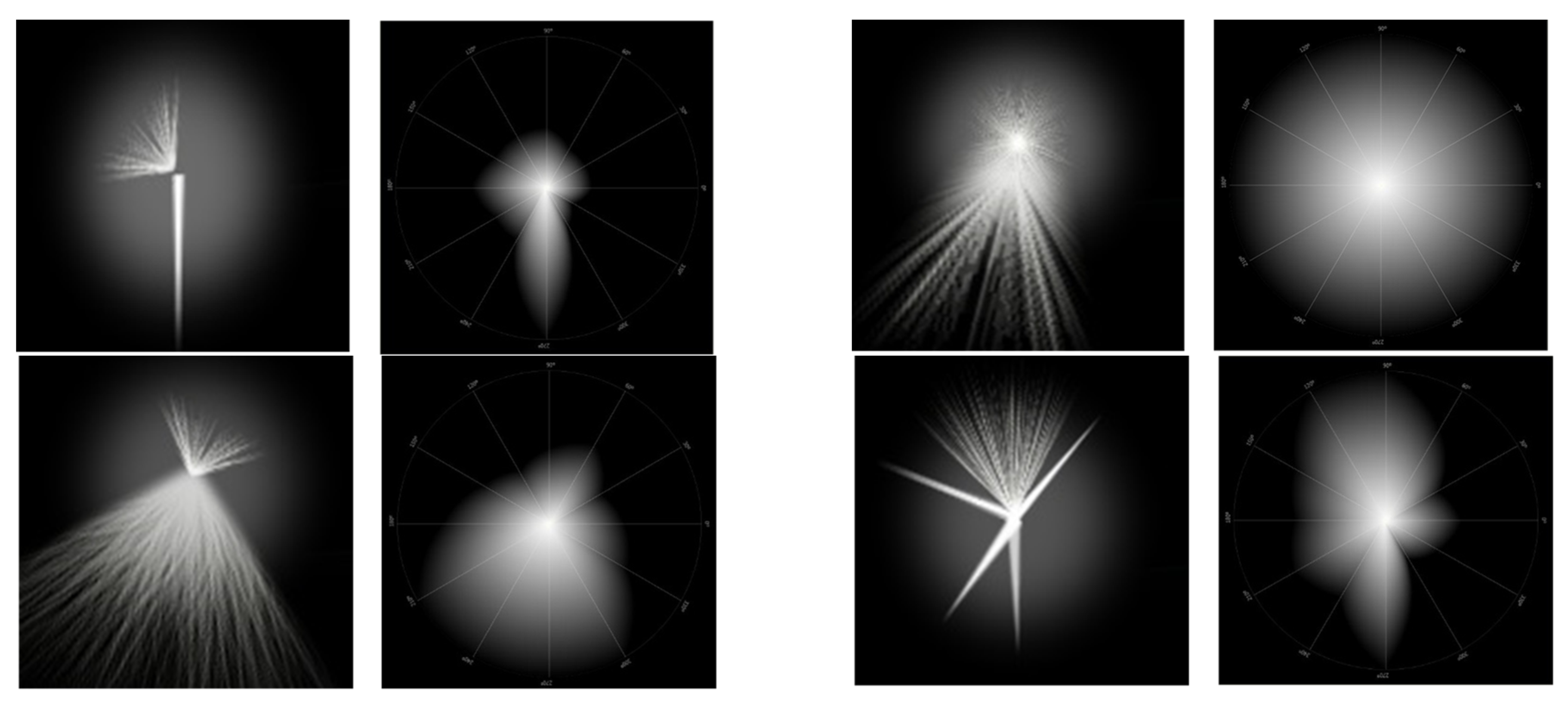
2.2. Starburst
2.3. Halo

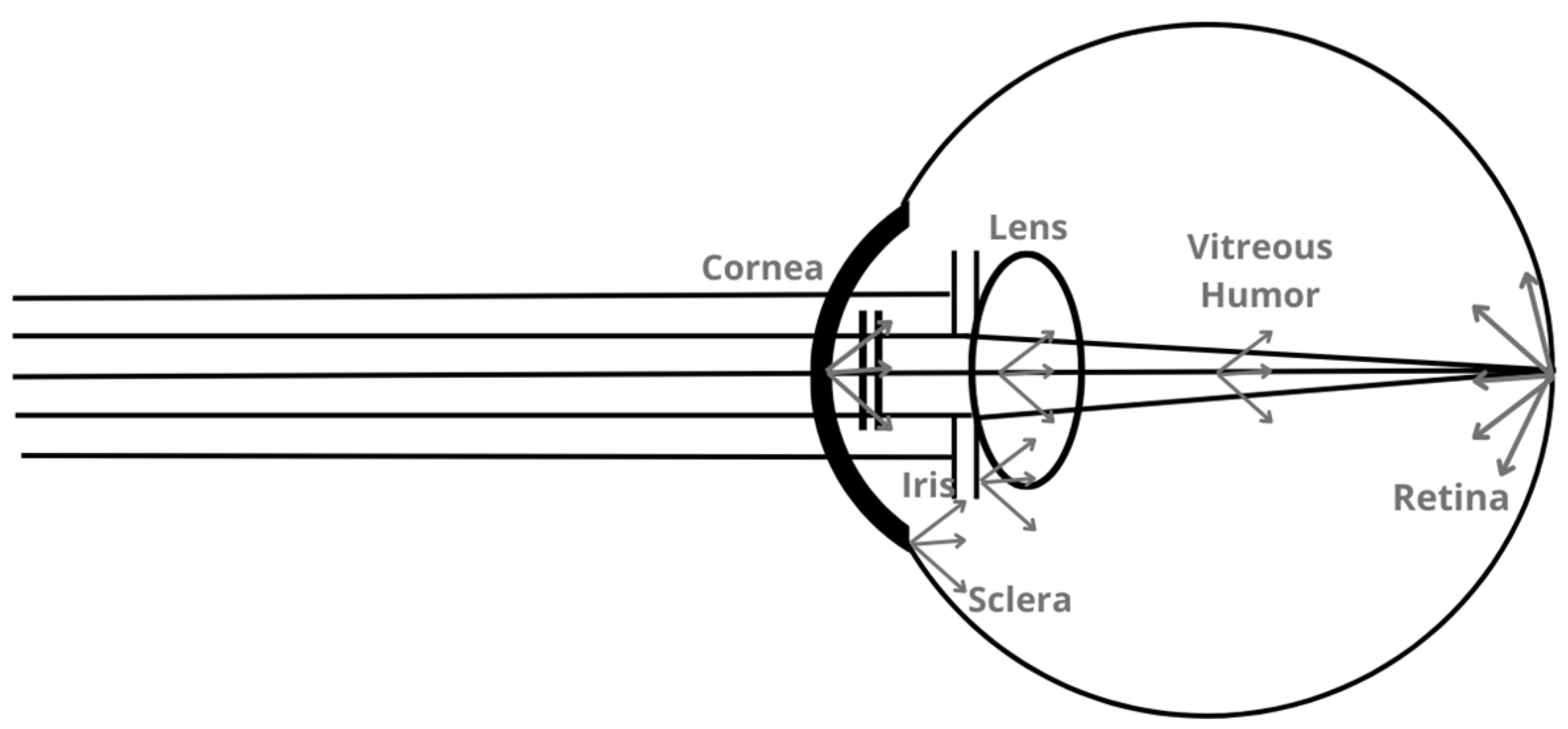
2.4. Ocular Scattering
3. Methods to Measure Night Vision Disturbances
3.1. Night Vision Recording Chart
3.2. Simulators
3.3. Direct Compensation Method
Conventional Straylight Meter (CSLM) and Computer-Implemented Straylight Meter (NSLM)
3.4. Compensation Comparison Method
3.5. Night Vision Test
3.6. Starlight System
3.7. Gutiérrez Halometer
3.8. Vision Monitor (Metrovision)
3.9. Aston Halometer
3.10. Rostock Glare Perimeter
3.11. Halometer: Halo v1.0
3.12. Light Disturbance Analyzer
- Distortion Area (DA): This is the result of the sum of the areas of all the sectors formed between each pair of semi-meridians under analysis and is measured in mm2.
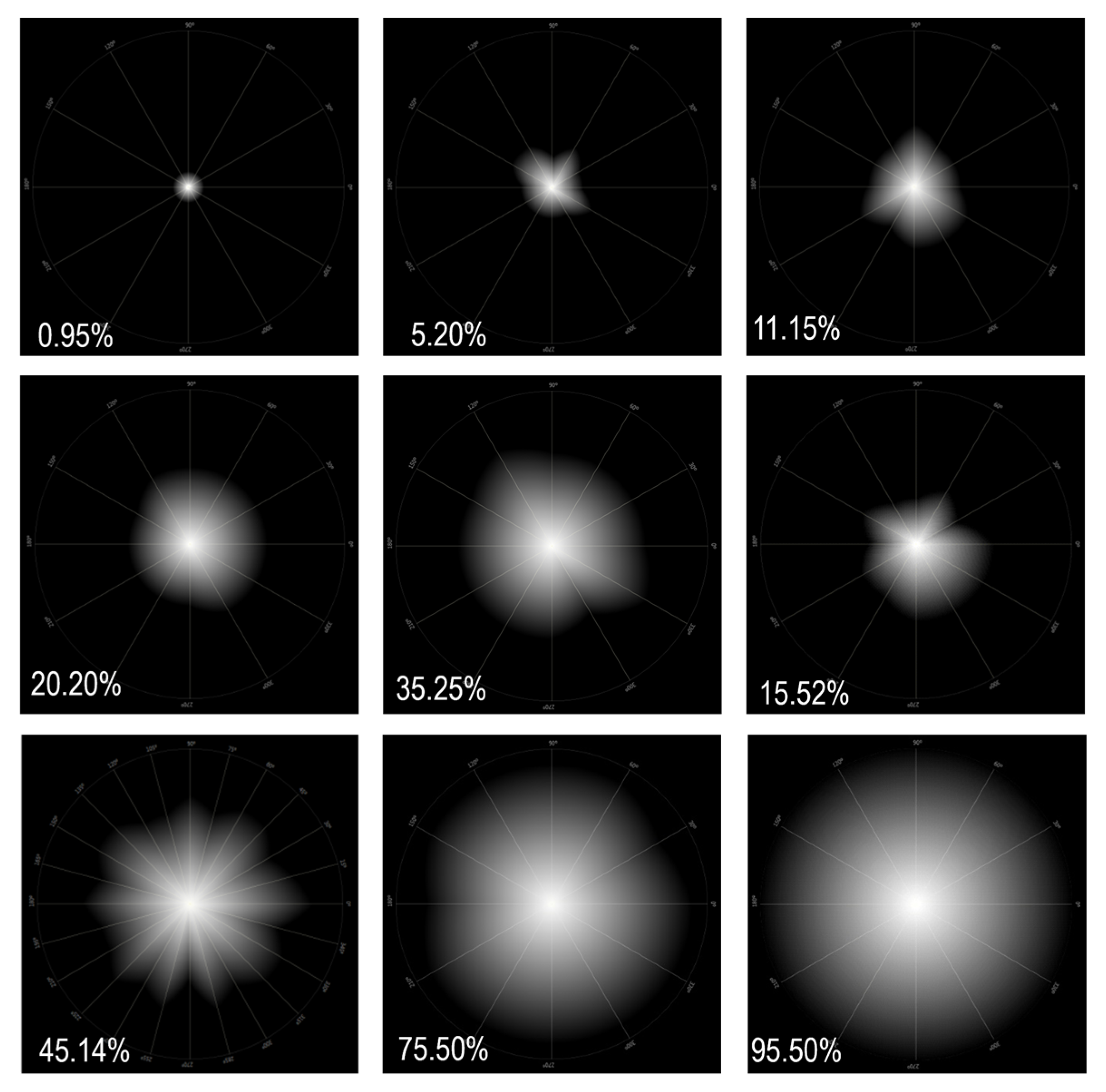
- Light Distortion Index (LDI): This is the main parameter and is calculated from the ratio between the area not seen by the subject and the total area explored and is expressed as a percentage. It is indicative of the area that is not visible due to the impairment of light distortion phenomena. Higher LDI values are understood as a lower ability to discriminate small stimuli surrounding the central light source and, therefore, the greater the light disturbance induced by the central light source; Figure 4.
- Best Fit Circle Radius (BFCRad): This corresponds to the radius of the circle that best fits the distortion area, whose value is equal to the average length of the disturbance along each semi-meridian under study, presented in mm.
- Coordinates of the Best Fit Circle (XCoord e YCoord): These are the cartesian coordinates of the center of the screen in degrees.
- Best Fit Circle Center Orientation (BFCOrient): This is the angle of the BFC center from the origin of the coordinates, which corresponds to the center of the screen in degrees.
- BFC Irregularity (BFCIrreg): This is the sum of the deviations between the actual distortion area and the outer perimeter of the BFC along all semi-meridians. It is the sum of the positive and negative values depending on whether the distortion limit is inside or outside the perimeter of the BFC and is measured in mm.
- BFC Irreg Standard Deviation (BFCIrregSD): This is the standard deviation of the BFC Irreg. It determines the degree of asymmetry of the distortion area limited from a perfectly circular shape and is measured in mm. Higher values correspond to more irregular distortion [56].
4. Advantages and Applications of LDA in Clinical Practice
4.1. Ablative Refractive Surgery and Intraocular Lenses
4.2. Applications on Contact Lens
4.2.1. Scleral Lenses
4.2.2. Orthokeratology
4.2.3. Contact Lenses for Presbyopia and Myopia Control
4.2.4. Changes in Tear Film
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan-Paul, N.I.; Li, J.; Miller, J.S.; Florakis, G.J. Night Vision Disturbances after Corneal Refractive Surgery. Surv. Ophthalmol. 2002, 47, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Artal, P. Optics of the Eye and Its Impact in Vision: A Tutorial. Adv. Opt. Photonics 2014, 6, 340. [Google Scholar] [CrossRef]
- Marcos, S. Image Quality of the Human Eye, Vol.43, No. 2. In International Ophthalmology Clinics; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 43–62. [Google Scholar]
- Piñero, D.P.; Ortiz, D.; Alio, J.L. Ocular Scattering. Optom. Vis. Sci. 2010, 87, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Mainster, M.A.; Turner, P.L. Retinal Injuries from Light: Mechanisms, Hazards, and Prevention. Retina 2006, 2, 1857–1870. [Google Scholar]
- Mainster, M.A.; Turner, P.L. Glare’s Causes, Consequences, and Clinical Challenges after a Century of Ophthalmic Study. Am. J. Ophthalmol. 2012, 153, 587–593. [Google Scholar] [CrossRef]
- Thibos, L.N. Principles of Hartmann-Shack Aberrometry. J. Refract. Surg. 2000, 16, S563–S565. [Google Scholar] [CrossRef]
- Hart, R.W.; Farrell, R.A. Light Scattering in the Cornea. J. Opt. Soc. Am. 1969, 59, 766–774. [Google Scholar] [CrossRef]
- Van den Berg, T.J.T.P.; Spekreijse, H. Light Scattering Model for Donor Lenses as a Function of Depth. Vis. Res. 1999, 39, 1437–1445. [Google Scholar] [CrossRef]
- Mohammadi, S.F.; Khorrami-Nejad, M.; Hamidirad, M. Posterior Corneal Astigmatism: A Review Article. Clin. Optom. 2019, 11, 85–96. [Google Scholar] [CrossRef]
- Nishida, T. The Cornea: Stasis and Dynamics. Nihon. Ganka Gakkai Zasshi 2008, 112, 179–212; discussion 213. [Google Scholar]
- Spadea, L.; Maraone, G.; Verboschi, F.; Vingolo, E.M.; Tognetto, D. Effect of Corneal Light Scatter on Vision: A Review of the Literature. Int. J. Ophthalmol. 2016, 9, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, N.; Barbato, A.; Giannotti, R.; Komaiha, C.; Lenarduzzi, F. Age-Related Changes in the Kinetics of Human Lenses: Prevention of the Cataract. Int. J. Ophthalmol. 2016, 9, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Siew, E.L.; Opalecky, D.; Bettelheim, F.A. Light Scattering of Normal Human Lens. II. Age Dependence of the Light Scattering Parameters. Exp. Eye Res. 1981, 33, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, T.J.T.P.; Van Rijn, L.J.; Kaper-Bongers, R.; Vonhoff, D.J.; Völker-Dieben, H.J.; Grabner, G.; Nischler, C.; Emesz, M.; Wilhelm, H.; Gamer, D.; et al. Disability Glare in the Aging Eye. Assessment and Impact on Driving. J. Optom. 2009, 2, 112–118. [Google Scholar] [CrossRef]
- Artal, P.; Benito, A.; Pérez, G.M.; Alcón, E.; de Casas, Á.; Pujol, J.; Marín, J.M. An Objective Scatter Index Based on Double-Pass Retinal Images of a Point Source to Classify Cataracts. PLoS ONE 2011, 6, e16823. [Google Scholar] [CrossRef]
- Jinabhai, A.; O’Donnell, C.; Radhakrishnan, H.; Nourrit, V. Forward Light Scatter and Contrast Sensitivity in Keratoconic Patients. Contact Lens Anterior Eye 2012, 35, 22–27. [Google Scholar] [CrossRef]
- Fernández, J.; Rodríguez-Vallejo, M.; Martínez, J.; Burguera, N.; Piñero, D.P. Long-Term Efficacy, Visual Performance and Patient Reported Outcomes with a Trifocal Intraocular Lens: A Six-Year Follow-Up. J. Clin. Med. 2021, 10, 2009. [Google Scholar] [CrossRef]
- Lim, D.H.; Lyu, I.J.; Choi, S.H.; Chung, E.S.; Chung, T.Y. Risk Factors Associated with Night Vision Disturbances after Phakic Intraocular Lens Implantation. Am. J. Ophthalmol. 2014, 157, 135–141.e1. [Google Scholar] [CrossRef]
- Nieto-Bona, A.; Lorente-Velázquez, A.; Collar, C.V.; Nieto-Bona, P.; Mesa, A.G. Intraocular Straylight and Corneal Morphology Six Months after LASIK. Curr. Eye Res. 2010, 35, 212–219. [Google Scholar] [CrossRef]
- Silva-Leite, S.; Amorim-de-Sousa, A.; Queirós, A.; González-Méijome, J.M.; Fernandes, P. Peripheral Refraction and Visual Function of Novel Perifocal Ophthalmic Lens for the Control of Myopia Progression. J. Clin. Med. 2023, 12, 1435. [Google Scholar] [CrossRef]
- Fernandes, P.; Amorim-de-Sousa, A.; Queirós, A.; Escandón-Garcia, S.; McAlinden, C.; González-Méijome, J.M. Light Disturbance with Multifocal Contact Lens and Monovision for Presbyopia. Contact Lens Anterior Eye 2018, 41, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Anera, R.G.; Villa, C.; Jiménez, J.R.; Gutierrez, R. Effect of LASIK and Contact Lens Corneal Refractive Therapy on Higher Order Aberrations and Contrast Sensitivity Function. J. Refract. Surg. 2009, 25, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Minasyan, H.; Richer, S.P. Cataract Halos: A Driving Hazard in Aging Populations. Implication of the Halometer DG Test for Assessment of Intraocular Light Scatter. Appl. Ergon. 2009, 40, 545–553. [Google Scholar] [CrossRef]
- Cerviño, A.; Villa-Collar, C.; Gonzalez-Meijome, J.M.; Ferrer-Blasco, T.; García-Lázaro, S. Retinal Straylight and Light Distortion Phenomena in Normal and Post-LASIK Eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Santolaria Sanz, E.; Cerviño, A.; Queirós, A.; Villa-Collar, C.; Lopes-Ferreira, D.; González-Méijome, J.M. Short-Term Changes in Light Distortion in Orthokeratology Subjects. BioMed Res. Int. 2015, 2015, 278425. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Gutiérrez, R.; Jiménez, J.R.; González-Méijome, J.M. Night Vision Disturbances after Successful LASIK Surgery. Br. J. Ophthalmol. 2007, 91, 1031–1037. [Google Scholar] [CrossRef]
- Amorim-de-Sousa, A.; Macedo-De-Araújo, R.; Fernandes, P.; Queirós, A.; González-Méijome, J.M. Impact of Defocus and High-Order Aberrations on Light Disturbance Measurements. J. Ophthalmol. 2019, 2019, 2874036. [Google Scholar] [CrossRef]
- Meikies, D.; van der Mooren, M.; Terwee, T.; Guthoff, R.F.; Stachs, O. Rostock Glare Perimeter: A Distinctive Method for Quantification of Glare. Optom. Vis. Sci. 2013, 90, 1143–1148. [Google Scholar] [CrossRef]
- Buckhurst, P.J.; Naroo, S.A.; Davies, L.N.; Shah, S.; Buckhurst, H.; Kingsnorth, A.; Drew, T.; Wolffsohn, J.S. Tablet App Halometer for the Assessment of Dysphotopsia. J. Cataract Refract. Surg. 2015, 41, 2424–2429. [Google Scholar] [CrossRef]
- Mangione, C.M.; Lee, P.P.; Gutierrez, P.R.; Spritzer, K.; Berry, S.; Hays, R.D. Development of the 25-Item National Eye Institute Visual Function Questionnaire. Arch. Ophthalmol. 2001, 119, 1050–1058. [Google Scholar] [CrossRef]
- McAlinden, C.; Pesudovs, K.; Moore, J.E. The Development of an Instrument to Measure Quality of Vision: The Quality of Vision (QoV) Questionnaire. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5537–5545. [Google Scholar] [CrossRef] [PubMed]
- Klyce, S.D. Night Vision Disturbances after Refractive Surgery: Haloes Are Not Just for Angels. Br. J. Ophthalmol. 2007, 91, 992–993. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brito, P.; Salgado-Borges, J.; Neves, H.; Gonzalez-Meijome, J.; Monteiro, M. Light-Distortion Analysis as a Possible Indicator of Visual Quality after Refractive Lens Exchange with Diffractive Multifocal Intraocular Lenses. J. Cataract Refract. Surg. 2015, 41, 613–622. [Google Scholar] [CrossRef] [PubMed]
- García-Marqués, J.V.; Macedo-De-Araújo, R.J.; McAlinden, C.; Faria-Ribeiro, M.; Cerviño, A.; González-Méijome, J.M. Short-Term Tear Film Stability, Optical Quality and Visual Performance in Two Dual-Focus Contact Lenses for Myopia Control with Different Optical Designs. Ophthalmic Physiol. Opt. 2022, 42, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.; Jiménez, J.R.; Villa, C.; Valverde, J.A.; Anera, R.G. Simple Device for Quantifying the Influence of Halos after Lasik Surgery. J. Biomed. Opt. 2003, 8, 663. [Google Scholar] [CrossRef]
- Vos, J.J. Disability Glare-a State of Art Report. CIE J. 1984, 3, 39–53. [Google Scholar]
- Vos, J.J. Report on Disability Glare. CIE Collect. 1999, 135, 1–9. [Google Scholar]
- Regan, D. The Charles F. Prentice Award Lecture 1990: Specific Tests and Specific Blindnesses: Keys, Locks, and Parallel Processing. Optom. Vis. Sci. 1991, 68, 489–512. [Google Scholar] [CrossRef]
- O’Brart, D.P.; Lohmann, C.P.; Fitzke, F.W.; Klonos, G.; Corbett, M.C.; Kerr-Muir, M.G.; Marshall, J. Disturbances in Night Vision after Excimer Laser Photorefractive Keratectomy. Eye 1994, 8, 46–51. [Google Scholar] [CrossRef][Green Version]
- O’Brart, D.P.; Lohmann, C.P.; Fitzke, F.W.; Smith, S.E.; Kerr-Muir, M.G.; Marshall, J. Night Vision after Excimer Laser Photorefractive Keratectomy: Haze and Halos. Eur. J. Ophthalmol. 1994, 4, 43–51. [Google Scholar] [CrossRef]
- Jewelewicz, D.A.; Evans, R.; Chen, R.; Trokel, S.; Florakis, G.J. Evaluation of Night Vision Disturbances in Contact Lens Wearers. CLAO J. 1998, 24, 107–110. [Google Scholar] [PubMed]
- Oliver, K.M.; Hemenger, R.P.; Corbett, M.C.; O’Brart, D.P.; Verma, S.; Marshall, J.; Tomlinson, A. Corneal Optical Aberrations Induced by Photorefractive Keratectomy. J. Refract. Surg. 1997, 13, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Rajan, M.S.; O’Brart, D.; Jaycock, P.; Marshall, J. Effects of Ablation Diameter on Long-Term Refractive Stability and Corneal Transparency after Photorefractive Keratectomy. Ophthalmology 2006, 113, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.H.; Huggins, L.K. Understanding the Role of IOL Optics in Postoperative Vision Complaints. Rev. Optom. 2018, 48–51. [Google Scholar]
- Van den Berg, T.J.T.P.; IJspeert, J.K.; de Waard, P.W. Dependence of Intraocular Straylight on Pigmentation and Light Transmission through the Ocular Wall. Vis. Res. 1991, 31, 1361–1367. [Google Scholar] [CrossRef]
- van den Berg, T.J.T.P. Analysis of Intraocular Straylight, Especially in Relation to Age. Optom. Vis. Sci. 1995, 72, 52–59. [Google Scholar] [CrossRef]
- Ginis, H.S.; Perez, G.M.; Bueno, J.M.; Pennos, A.; Artal, P. Wavelength Dependence of the Ocular Straylight. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3702–3708. [Google Scholar] [CrossRef]
- Kohnen, T.; Suryakumar, R. Measures of Visual Disturbance in Patients Receiving Extended Depth-of-Focus or Trifocal Intraocular Lenses. J. Cataract Refract. Surg. 2021, 47, 245–255. [Google Scholar] [CrossRef]
- El Naggar, F.; Alnassar, A.; Tarib, I.; Breyer, D.R.H.; Gerl, M.; Kretz, F.T. Enhancing Intermediate Vision in Different Working Distances with a Novel Enhanced Depth of Focus Intraocular Lens (EDOF). EC Ophthalmol. 2018, 9, 94–99. [Google Scholar]
- Van den Berg, T.J.T.P.; Ijspeert, J.K. Clinical Assessment of Intraocular Stray Light. Appl. Opt. 1992, 31, 3694. [Google Scholar] [CrossRef]
- Kojima, T.; Hasegawa, A.; Hara, S.; Horai, R.; Yoshida, Y.; Nakamura, T.; Dogru, M.; Ichikawa, K. Quantitative Evaluation of Night Vision and Correlation of Refractive and Topographical Parameters with Glare after Orthokeratology. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Puell, M.C.; Pérez-Carrasco, M.J.; Barrio, A.; Antona, B.; Palomo-Alvarez, C. Normal Values for the Size of a Halo Produced by a Glare Source. J. Refract. Surg. 2013, 29, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.J.; Ortiz, C.; Pozo, A.M.; Anera, R.G.; Soler, M. A Visual Test Based on a Freeware Software for Quantifying and Displaying Night-Vision Disturbances: Study in Subjects after Alcohol Consumption. Theor. Biol. Med. Model. 2014, 11, S1. [Google Scholar] [CrossRef] [PubMed]
- Alba-Bueno, F.; Garzón, N.; Vega, F.; Poyales, F.; Millán, M.S. Patient-Perceived and Laboratory-Measured Halos Associated with Diffractive Bifocal and Trifocal Intraocular Lenses. Curr. Eye Res. 2018, 43, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Neves, H.; Macedo-de-Araújo, R.; Rico-del-Viejo, L.; da-Silva, A.C.; Queirós, A.; González-Méijome, J.M. Validation of a Method to Measure Light Distortion Surrounding a Source of Glare. J. Biomed. Opt. 2015, 20, 075002. [Google Scholar] [CrossRef] [PubMed]
- Florakis, G.J.; Jewelewicz, D.A.; Michelsen, H.E.; Trokel, S.L. Evaluation of Night Vision Disturbances. J. Refract. Corneal Surg. 1994, 10, 333–338. [Google Scholar] [CrossRef]
- Wachler, B.S.B.; Durrie, D.S.; Assil, K.K.; Krueger, R.R. Improvement of Visual Function with Glare Testing after Photorefractive Keratectomy and Radial Keratotomy. Am. J. Ophthalmol. 1999, 128, 582–587. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; González-Méijome, J.M.; Vida, R.S.; Gupta, R. Changes in Light Disturbance Analyzer Evaluation in SMILE for High Myopia and Astigmatism. J. Refract. Surg. 2022, 38, 725–732. [Google Scholar] [CrossRef]
- Ukai, Y.; Okemoto, H.; Seki, Y.; Nakatsugawa, Y.; Kawasaki, A.; Shibata, T.; Mito, T.; Kubo, E.; Sasaki, H. Quantitative Assessment of Photic Phenomena in the Presbyopia-Correcting Intraocular Lens. PLoS ONE 2021, 16, e0260406. [Google Scholar] [CrossRef]
- van den Berg, T.J.T.P. Importance of Pathological Intraocular Light Scatter for Visual Disability. Doc. Ophthalmol. 1986, 61, 327–333. [Google Scholar] [CrossRef]
- van den Berg, T.J.T.P. Measurement of the Straylight Function of the Eye in Cataract and Other Optical Media Disturbances by Means of a Direct Compensation Method. Investig. Ophthalmol. Vis. Sci. 1987, 28, 397. [Google Scholar]
- Van Rijn, L.J.; Nischler, C.; Gamer, D.; Franssen, L.; De Wit, G.; Kaper, R.; Vonhoff, D.; Grabner, G.; Wilhelm, H.; Völker-Dieben, H.J.; et al. Measurement of Stray Light and Glare: Comparison of Nyktotest, Mesotest, Stray Light Meter, and Computer Implemented Stray Light Meter. Br. J. Ophthalmol. 2005, 89, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Franssen, L.; Coppens, J.E.; Van den Berg, T.J.T.P. Compensation Comparison Method for Assessment of Retinal Straylight. Investig. Ophthalmol. Vis. Sci. 2006, 47, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Pieh, S.; Lackner, B.; Hanselmayer, G.; Zöhrer, R.; Sticker, M.; Weghaupt, H.; Fercher, A.; Skorpik, C. Halo Size under Distance and near Conditions in Refractive Multifocal Intraocular Lenses. Br. J. Ophthalmol. 2001, 85, 816–821. [Google Scholar] [CrossRef]
- Alarcón, A.; Anera, R.G.; Villa, C.; Jiménez del Barco, L.; Gutierrez, R. Visual Quality after Monovision Correction by Laser in Situ Keratomileusis in Presbyopic Patients. J. Cataract Refract. Surg. 2011, 37, 1629–1635. [Google Scholar] [CrossRef]
- Anera, R.G.; Castro, J.J.; Jiménez, J.R.; Villa, C.; Alarcón, A. Optical Quality and Visual Discrimination Capacity after Myopic LASIK with a Standard and Aspheric Ablation Profile. J. Refract. Surg. 2011, 27, 597–601. [Google Scholar] [CrossRef]
- Castro, J.J.; Jiménez, J.R.; Ortiz, C.; Alarcón, A.; Anera, R.G. New Testing Software for Quantifying Discrimination Capacity in Subjects with Ocular Pathologies. J. Biomed. Opt. 2011, 16, 015001. [Google Scholar] [CrossRef]
- Linhares, J.M.M.; Neves, H.; Lopes-Ferreira, D.; Faria-Ribeiro, M.; Peixoto-De-Matos, S.C.; Gonzalez-Meijome, J.M. Radiometric Characterization of a Novel LED Array System for Visual Assessment. J. Mod. Opt. 2013, 60, 1136–1144. [Google Scholar] [CrossRef]
- Monsálvez-Romín, D.; González-Méijome, J.M.; Esteve-Taboada, J.J.; García-Lázaro, S.; Cerviño, A. Light Distortion of Soft Multifocal Contact Lenses with Different Pupil Size and Shape. Contact Lens Anterior Eye 2020, 43, 130–136. [Google Scholar] [CrossRef]
- Escandón-García, S.; Ribeiro, F.J.; McAlinden, C.; Queirós, A.; González-Méijome, J.M. Through-Focus Vision Performance and Light Disturbances of 3 New Intraocular Lenses for Presbyopia Correction. J. Ophthalmol. 2018, 2018, 6165493. [Google Scholar] [CrossRef]
- Salgado-Borges, J.; Dias, L.; Costa, J. Light Distortion and Ocular Scattering with Glistening and Aberration-Free Pseudophakic IOL: A Pilot Study. J. Emmetropia 2015, 6, 127–132. [Google Scholar]
- Macedo-de-Araújo, R.; Ferreira-Neves, H.; Rico-del-Viejo, L.; Peixoto-de-Matos, S.C.; González-Méijome, J.M. Light Distortion and Spherical Aberration for the Accommodating and Nonaccommodating Eye. J. Biomed. Opt. 2016, 21, 075003. [Google Scholar] [CrossRef]
- Santolaria-Sanz, E.; Cerviño, A.; González-Méijome, J.M. Corneal Aberrations, Contrast Sensitivity, and Light Distortion in Orthokeratology Patients: 1-Year Results. J. Ophthalmol. 2016, 2016, 8453462. [Google Scholar] [CrossRef]
- Martino, F.; Pereira-da-Mota, A.F.; Amorim-de-Sousa, A.; Castro-Torres, J.J.; González-Méijome, J.M. Pupil Size Effect on Binocular Summation for Visual Acuity and Light Disturbance. Int. Ophthalmol. 2023, 43, 2183–2195. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Vargas, V.; Plaza-Puche, A.B.; Alió, J.L. Long-Term Results of a Diffractive Trifocal Intraocular Lens: Visual, Aberrometric and Patient Satisfaction Results. Eur. J. Ophthalmol. 2020, 30, 201–208. [Google Scholar] [CrossRef]
- Escandón-García, S.; Ribeiro, F.; McAlinden, C.; González Méijome, J.M. Light Distortion as an Indicator of Adaptation to Multifocality after Refractive Lens Exchange (RLE). Biomed. J. Sci. Tech. Res. 2021, 37, 29583–29591. [Google Scholar] [CrossRef]
- Alió, J.L.; Plaza-Puche, A.B.; Alió del Barrio, J.L.; Amat-Peral, P.; Ortuño, V.; Yébana, P.; Al-Shymali, O.; Vega-Estrada, A. Clinical Outcomes with a Diffractive Trifocal Intraocular Lens. Eur. J. Ophthalmol. 2018, 28, 419–424. [Google Scholar] [CrossRef]
- Escandón-García, S.; Ribeiro, F.; McAlinden, C.; González-Méijome, J.M. Attenuation of Light Disturbances and Subjective Complains after 6 Months with New Generation Multifocal IOLs. Biomed. J. Sci. Tech. Res. 2021, 37, 29097–29106. [Google Scholar] [CrossRef]
- Macedo-de-Araújo, R.J.; Faria-Ribeiro, M.; McAllinden, C.; Van der Worp, E.; González-Méijome, J.M. Optical Quality and Visual Performance for One Year in a Sample of Scleral Lens Wearers. Optom. Vis. Sci. 2020, 97, 775–789. [Google Scholar] [CrossRef]
- Llorente, L.; Barbero, S.; Cano, D.; Dorronsoro, C.; Marcos, S. Myopic versus Hyperopic Eyes: Axial Length, Corneal Shape and Optical Aberrations. J. Vis. 2004, 4, 288–298. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, K.; Jin, Y.; Niu, Y.; Zuo, T. Changes of Higher Order Aberration with Various Pupil Sizes in the Myopic Eye. J. Refract. Surg. 2003, 19, S270–S274. [Google Scholar] [CrossRef]
- Pereira-da-Mota, A.F.; Costa, J.; Amorim-de-sousa, A.; González-Méijome, J.M.; Queirós, A. The Impact of Overnight Orthokeratology on Accommodative Response in Myopic Subjects. J. Clin. Med. 2020, 9, 3687. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pomeda, A.; Fernandes, P.; Amorim-de-Sousa, A.; González-Méijome, J.M.; Prieto-Garrido, F.L.; Pérez-Sánchez, B.; Villa-Collar, C. Light Disturbance Analysis in the Controlled Randomized Clinical Trial MiSight® Assessment Study Spain (MASS). Contact Lens Anterior Eye 2019, 42, 200–205. [Google Scholar] [CrossRef] [PubMed]
- García-Marqués, J.V.; Macedo-De-Araújo, R.J.; Cerviño, A.; García-Lázaro, S.; McAlinden, C.; González-Méijome, J.M. Comparison of Short-Term Light Disturbance, Optical and Visual Performance Outcomes between a Myopia Control Contact Lens and a Single-Vision Contact Lens. Ophthalmic Physiol. Opt. 2020, 40, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Amorim-de-Sousa, A.; Faria-Ribeiro, M.; Pauné, J.; González-Méijome, J.M.; Queirós, A. Visual Performance and High-Order Aberrations with Different Contact Lens Prototypes with Potential for Myopia Control. Curr. Eye Res. 2020, 45, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Valle, D.; Arriola-Villalobos, P.; García-Vidal, S.E.; Sánchez-Pulgarín, M.; Borrego Sanz, L.; Gegúndez-Fernández, J.A.; Benitez-Del-Castillo, J.M. Effect of Lubricating Eyedrops on Ocular Light Scattering as a Measure of Vision Quality in Patients with Dry Eye. J. Cataract Refract. Surg. 2012, 38, 1192–1197. [Google Scholar] [CrossRef]
- Talens-Estarelles, C.; Mechó-García, M.; McAlinden, C.; Cerviño, A.; García-Lázaro, S.; González-Méijome, J.M. Changes in Visual Function and Optical and Tear Film Quality in Computer Users. Ophthalmic Physiol. Opt. 2023, 43, 885–897. [Google Scholar] [CrossRef]
- Vargas, V.; Ferreira, R.; Del Barrio, J.L.A.; Alió, J.L. Visual Outcomes, Patient Satisfaction, and Light Distortion Analysis after Blended Implantation of Rotationally Asymmetric Multifocal Intraocular Lenses. J. Refract. Surg. 2020, 36, 796–803. [Google Scholar] [CrossRef]
- Lajara-Blesa, J.; Rodríguez-Izquierdo, M.Á.; Vallés-San-Leandro, L.; Jutley, G.; de los Remedios Ortega-García, M.; Zapata-Díaz, J.F. Standard Clinical Outcomes, Light Distortion, Stereopsis, and a Quality-of-Life Assessment of a New Binocular System of Complementary IOLs. J. Refract. Surg. 2023, 39, 654–661. [Google Scholar] [CrossRef]
- Fernández, J.; Burguera, N.; Rocha-de-Lossada, C.; Rachwani-Anil, R.; Rodríguez-Vallejo, M. Influence of a Multifocal Intraocular Lens Centration and Eye Angles on Light Distortion and Ocular Scatter Index. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 2291–2299. [Google Scholar] [CrossRef]

| Test | Parameter Measured | Brief Description |
|---|---|---|
| Night Vision Recording Chart (NVRC) [1] | Size of halos and presence of starburst or other image degradations | Patients are asked to draw or describe their visual disturbances when looking at a light source, providing a subjective representation of their NVD. |
| Simulators [49,50] | Perception of photic phenomena (halos, glare, and starburst) | Software that simulates night driving or other scenarios where NVD might be pronounced, allowing patients to adjust settings to match their perception of disturbances, thereby quantifying the severity and nature of their NVD. |
| Van den Berg Straylight Meter [51] | Retinal straylight (disability glare) | Measurement of the light scatter in the eye, contributing to reduced contrast sensitivity and increased glare. |
| Night Vision Test [52] | Size of the glare | Evaluates the size of glare perceived by the patient, offering a quantitative measure of this specific night vision disturbance. |
| Starlight System [27] | Quantitative assessment of halos | Offers a quantitative measure of halo size around light sources, useful for understanding the extent of this common night vision disturbance. |
| Gutiérrez Halometer [36] | Effects of halos | Specifically designed to assess the impact of halos on vision, providing a disturbance index based on the patient’s perception under low-light conditions. |
| Vision Monitor (Metrovision) [53] | Size of halos | Measures the size of halos induced by glare sources, using circular white light sources to generate glare and assess its effect on vision. |
| Aston Halometer [30] | Extent of halos | Utilize a central LED and mobile tablet to quantify and analyze the extent of dysphotopsias, including halos, in various directions of vision. |
| Rostock Glare Perimeter [29] | Quantify the effects of glare | Measures the subject’s ability to distinguish the marker’s brightness from the light source. |
| Halometer: Halo v1.0 [54,55] | Size and intensity of the halos and glare | During the procedure, the subject identifies peripheral stimuli that appear randomly around a central point of high luminosity, displayed on a dark background of a monitor. |
| Light Disturbance Analyzer [34,56] | Determines the size, shape, and regularity of light distortion | Quantifies the distortion caused by light, providing metrics on the size, shape, and regularity of phenomena like halos and starbursts, and is based on a predefined algorithm assessing the distribution of light in the visual field. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-de-Carvalho, R.S.; Macedo-de-Araújo, R.J.; González-Méijome, J.M. Light Disturbance Analysis and Applications. Photonics 2024, 11, 905. https://doi.org/10.3390/photonics11100905
Alves-de-Carvalho RS, Macedo-de-Araújo RJ, González-Méijome JM. Light Disturbance Analysis and Applications. Photonics. 2024; 11(10):905. https://doi.org/10.3390/photonics11100905
Chicago/Turabian StyleAlves-de-Carvalho, Rafaela S., Rute J. Macedo-de-Araújo, and José M. González-Méijome. 2024. "Light Disturbance Analysis and Applications" Photonics 11, no. 10: 905. https://doi.org/10.3390/photonics11100905
APA StyleAlves-de-Carvalho, R. S., Macedo-de-Araújo, R. J., & González-Méijome, J. M. (2024). Light Disturbance Analysis and Applications. Photonics, 11(10), 905. https://doi.org/10.3390/photonics11100905





