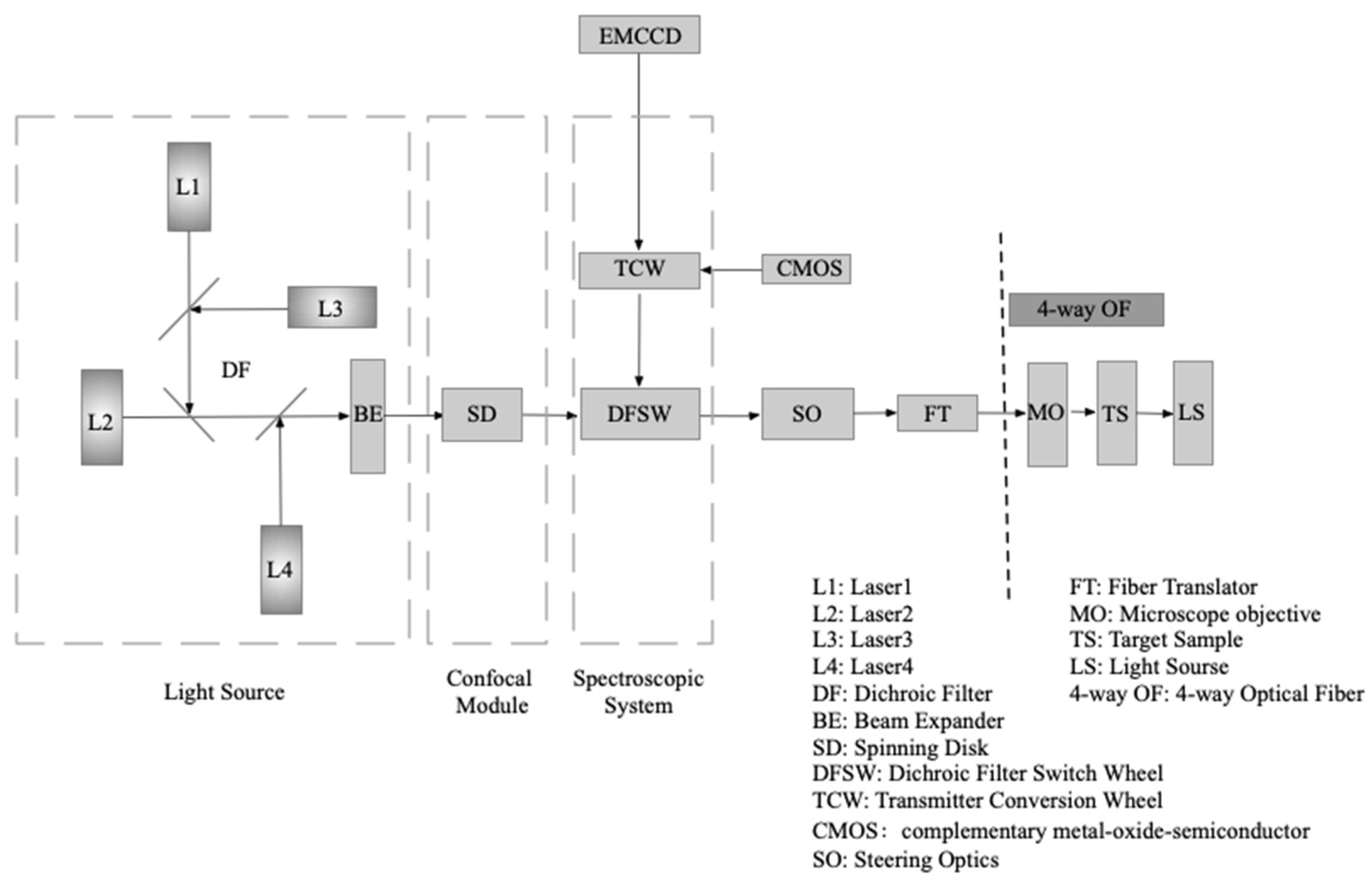
The main function of confocal microscopy imaging is completed by the scanning module, and its main structure is a spinning disk and drive motor customized for the system parameters, as shown in
Figure 1. A spinning disk with a pinhole sequence as the scanning unit is also called a Nipkow spinning disk, named after the scientist Paul Nipkow, who first proposed it. In 1967, Edgar and Petráň modified the Nipkow spinning disk and designed a spinning disk with a sequence of pinholes arranged in an Archimedes spiral. In this arrangement, in the light path, the spinning disk not only serves as the illumination pinhole of the microscope, but also as the imaging pinhole conjugate to the sample with respect to the objective lens [
7]. Whether the pinhole of the spinning disk is replaced by a strip [
8] or the scanning structure of a single spinning disk is replaced by a coupled double disk [
9], the confocal microscope can only be adjusted in the spatial structure to improve the signal-to-noise ratio and spatial resolution of the image. The basic principle still follows the confocal principle proposed by Nipkow, that is, the detection and illumination planes are in a conjugate relationship, and both are in focus mode. The principle of confocal imaging can be expressed as
Here,
is the point source function;
and
are the illumination and detection PSF (point spread function),
is the aperture function of the pinhole; and
is the final PSF of the system. The pinhole is used as a detection and filtering element at the same time, and the out-of-focus signal outside the scanning plane can be effectively filtered out. It also has a three-dimensional imaging function as a slice element. When the aperture of the pinhole is infinitely small, its aperture function can be simplified into an impact function. At this point, (1) can be expressed as
At this time, the PSF of the confocal system can be increased to 1.4 times that of the widefield in both the axial and lateral directions [
10]. However, in practical applications, the smaller the aperture of the pinhole is, the weaker the signal that can be detected and the worse the signal-to-noise ratio will be, which affects the improvement in the resolution. Experiments have shown that the best balance between the signal-to-noise ratio and resolution can be achieved at 0.6–0.8 AU (Airy unit) [
11]. It can be seen from Formula (2) that the main factors affecting the imaging performance of the point-scanning system are the parameters of the illumination system and the detection system. Spinning-disk confocal microscopy (SDCM) improves on both of these based on the point-scanning system. Firstly, the quantum efficiency (QE) of an enhanced charge-coupled device (CCD) and a new complementary metal–oxide–semiconductor (CMOS) camera can reach 65 to 90%, which is much higher than that in photomultiplier tubes (20%) or avalanche photodiodes (40%). This high-sensitivity detection device can reduce the exposure time required for an experiment, achieve a higher frame rate at the same signal-to-noise ratio, and make it possible to collect images of living cells. Secondly, through the pinhole disk, thousands of beams can simultaneously irradiate and scan the sample. These beams rotate with the spinning disk to scan the same point on the sample multiple times in one cycle of the entire camera exposure, thus improving the mechanical movement form of single-point scanning, which is efficient and fast, and the illumination intensity can be reduced by more than one thousand times. However, it should be noted that in the design process, although the number of pinholes in the spinning disk usually ranges from 2000 to 20,000, the transmittance of the disk surface is still below 5% for fluorescence imaging, and the image blurring caused by expanding the diameter of the pinhole needs to be considered. Furthermore, as the excitation and emission light pass through the pinholes at the same time, the positioning of the pinholes requires high precision. In addition, the crosstalk effect between multiple pinholes is the most important factor affecting the axial resolution of the signal and blurring the background. Therefore, a balance must be struck between the pinhole spacing and the intensity of crosstalk in the background. The parameters of the scanning system directly determine the quality of the image, as will be discussed in detail below.
3.1. Pinhole Spacing
The pinholes of the spinning disk are arranged at equal intervals on its outer periphery in the form of an Archimedes spiral. The number of pinholes determines the amount of light passing through, and the spacing of the pinholes affects the quality of imaging. The denser the pinholes, the higher the transmittance of the disk surface, and the larger the pinhole spacing, the better the axial resolution. T represents the transmission efficiency of the pinhole, then:
Here,
D represents the pinhole diameter, and
S represents the pinhole spacing. It can be seen that the transmittance of the spinning disk is not high. Taking the normal
in biology as an example, when
is 1/5, the transmittance is only 4%. Continuing to reduce the pinholes’ spacing has an impact on the axial resolution, allowing more emitted light to return through adjacent pinholes, especially when observing thick samples. However, if the diameter of the pinhole is increased, the axial resolution of the system will decrease. In the system, the lateral resolution is described by the Abbe equation, which is defined as a function of the wavelength of the emitted light and the numerical aperture of the objective lens:
In biological fluorescence confocal microscopy, if the diameter of the pinhole is smaller than the diameter of an Airy disk, the crosstalk between the holes causes the overall performance of the confocal microscope to rapidly drop, and the axial resolution approaches that of a widefield microscope. When the pinhole diameter exceeds two Airy disk diameters, the detector can capture a tiny image that is only a few pixels in size from the light emitted by each pinhole. Within this range, the pinhole spacing should be expanded as much as possible to reduce the occurrence of crosstalk between the holes.
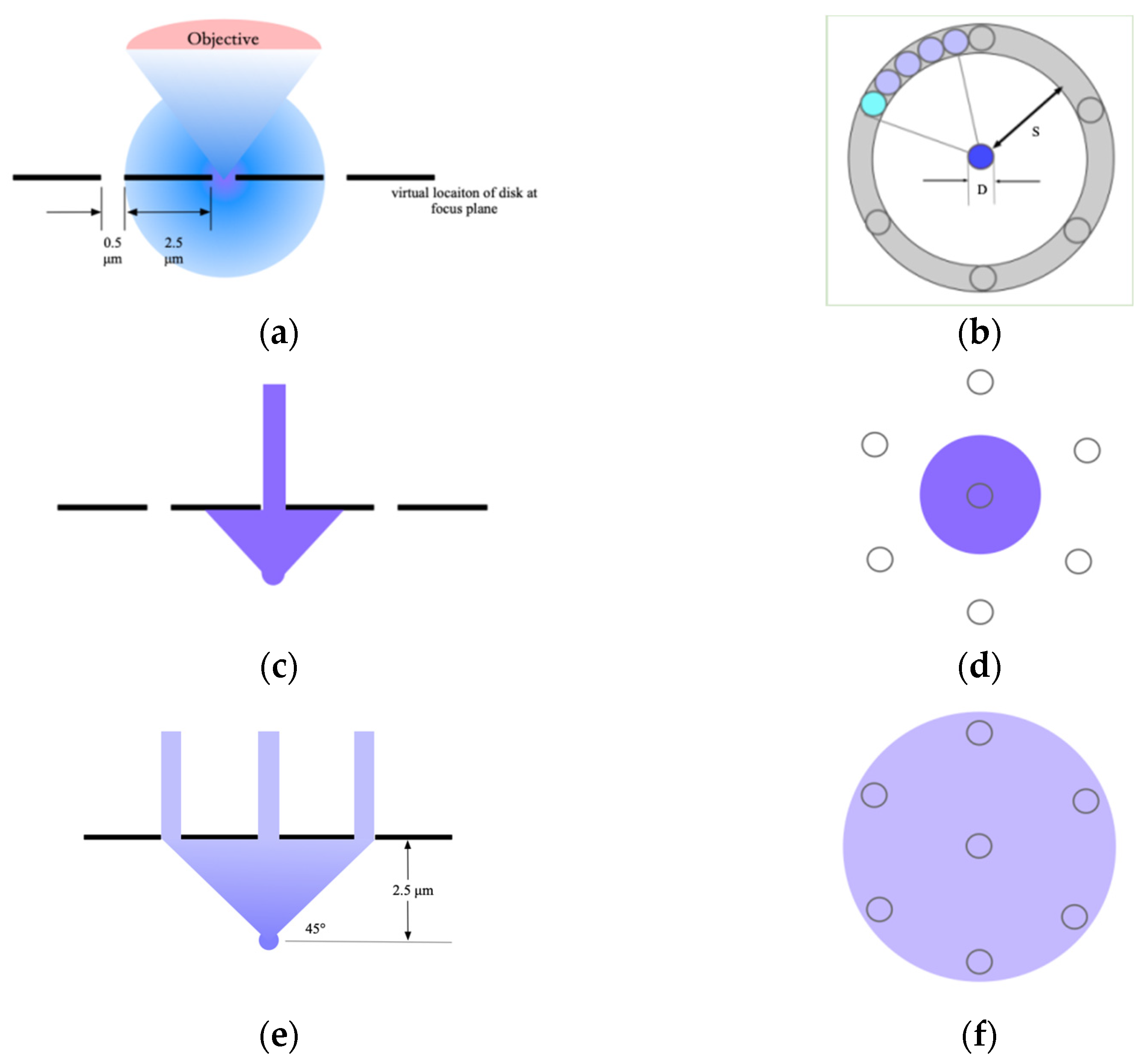
We discuss the occurrence of crosstalk near the spinning disk with a model in which the numerical aperture (
NA) is 1.0 and the diameter,
, is 1 Airy. As shown in
Figure 2a, the fluorescent spot is located in the focal plane. At this time, the central main maximum value of the Airy disk emitted by the point light source can almost completely pass through the pinhole and be collected by the detector. The light distribution on the disk observed in the optical path is shown in
Figure 2b. At this time,
is about 1/5, and the total transmission efficiency is about 4%, which is in line with the transmission efficiency of most disk scanning structures used in biological applications. When the stage moves downwards, the position of the point light source drops below the focal plane, and the amount of light passing through the pinhole in the center of the spinning disk decreases, as shown in
Figure 2c. At this time, most of the light beams that can be accepted by the objective lens and leave the point light source at any angle are located on the back of the spinning disk, as shown in
Figure 2d.
When the point light source is 2.5 mm away from the focal plane,
S is the same as the amount of defocus. At this time, a part of the light from the point light source is able to pass through a pinhole in the first circular ring around the pinhole. The strength of the light beam reaching the detector from the wrong pinhole is six times that of the light beam reaching the detector from the correct pinhole. The photons that were originally only distributed in the spot with a diameter of 0.5 mm are now scattered on a spot with a diameter that is five times that of the original and an area that is 25 times that of the original. The attenuation of the emission end and the excitation end is the same. At this time, the number of photons per unit area on the disk is 1/625 of that at the focal point. It can be seen from
Figure 2b that only about
D/S of the light incident on the torus with diameter
S and width
D actually passes through the torus. As the point source continues to move away from the spinning disk, the number of photons reaching the detector continues to decrease until some beams begin to pass through pinholes in the second ring around the central pinhole, which are at a distance of about 4–5 mm. When the value of
NA is high, this happens with a low amount of defocus, and vice versa.
However, in actual operation, it is impossible to infinitely expand the pinhole spacing in order to pursue an excessively high axial optical resolution. As the pinhole spacing increases, the light transmittance of the pinhole disk gradually decreases, which not only reduces the efficiency of the light source, but also causes an excessive amount of light reflected from the disk to enter the detector and form background noise. At the same time, a smaller amount of transmitted light affects the fluorescence excitation and reduces the fluorescence intensity. These two disadvantages ultimately affect the detector ratio, thereby reducing the image resolution. What we ultimately care about is the clarity of the image presented to us rather than just the optical resolution, so it makes no sense to sacrifice the detector’s signal-to-noise ratio too much in order to improve optical resolution. Therefore, the values of the pinhole spacing and pinhole diameter depend on the detector performance, sample characteristics, and excitation light source.
On the basis of the axial PSF of a single-pinhole scanning fluorescence confocal microscope, the PSF of a multiple-pinhole scanning fluorescence confocal microscope was analyzed. Considering the crosstalk of the focal plane and the crosstalk of stray out-of-focus light, the axial intensity point spread function of the multi-pinhole scanning fluorescence confocal microscope is expressed as:
where
is the defocus correction parameter and
is the transmittance of the spinning disk.
In order to compare the influence of the crosstalk caused by different pinhole spacings on the axial intensity point spread function of the system, two commonly used pinhole disks were selected for analysis, and their parameters are shown in
Table 1.
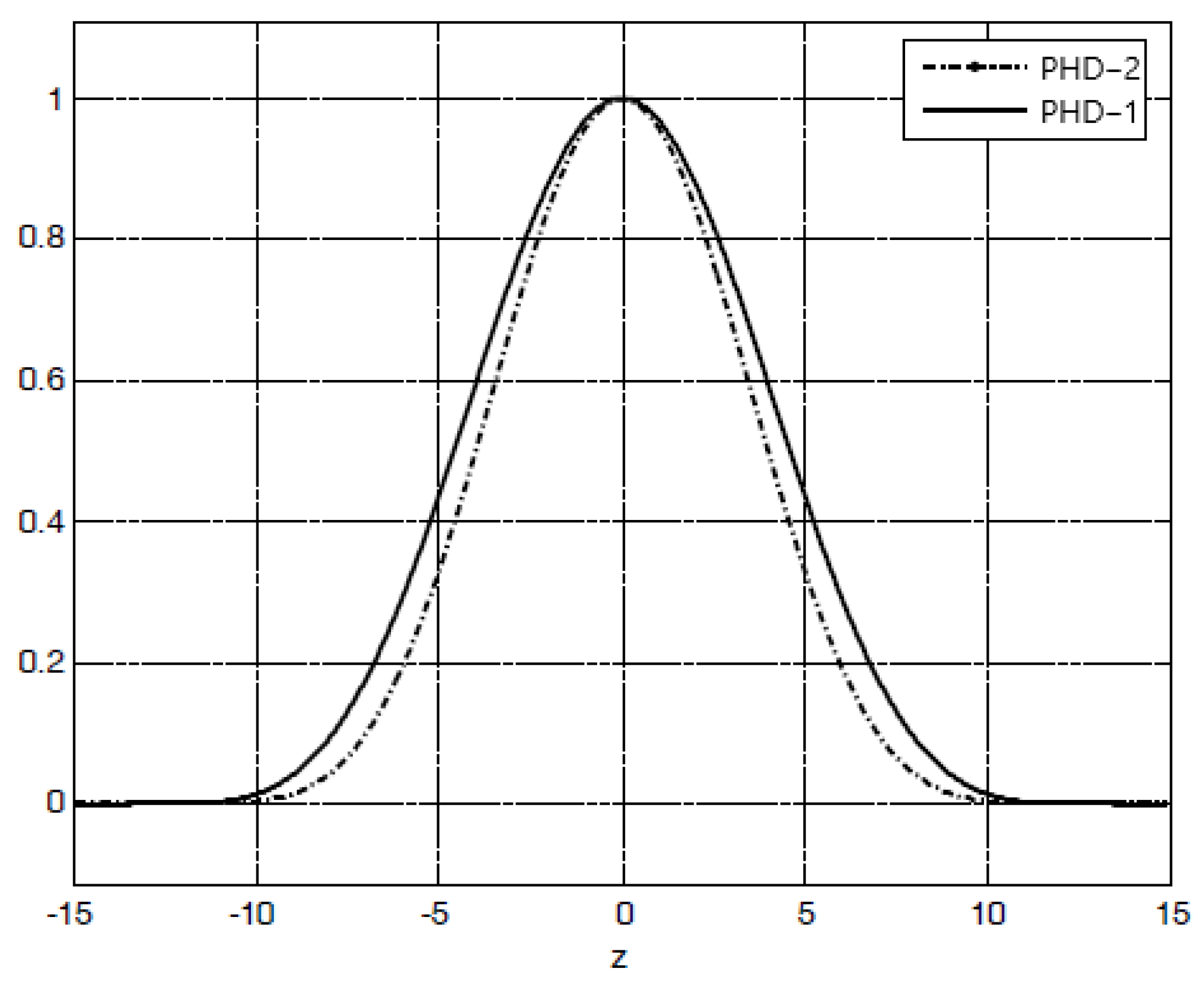
We put the above parameters into Formulas (5) and (6) and drew their respective intensity point diffusion curves, as shown in
Figure 3. It can be seen from the figure that the intensity point spread function of pinhole disk 2 (PHD-2) was narrower than that of pinhole disk 1 (PHD-1). The normalized full widths at half maximum of the obtained intensity point spread curves of the two were 8.13 and 9.21, respectively. Compared to PHD-1, when using PHD-2, the system resolution was increased by about 11%.
In the system, the objective lens with the highest magnification is given priority. Considering the imaging situation with a 40× objective lens, the NA is 0.6, and the defocus distance is 5.2 μm. That is to say, no crosstalk will occur when the system observes a sample of 10.4 μm, which is close to the thickness value of the observed system sample. When the pinhole size is 50 μm (as shown below), the pinhole spacing is 250 μm, which meets the design requirements.
3.2. Pinhole Size
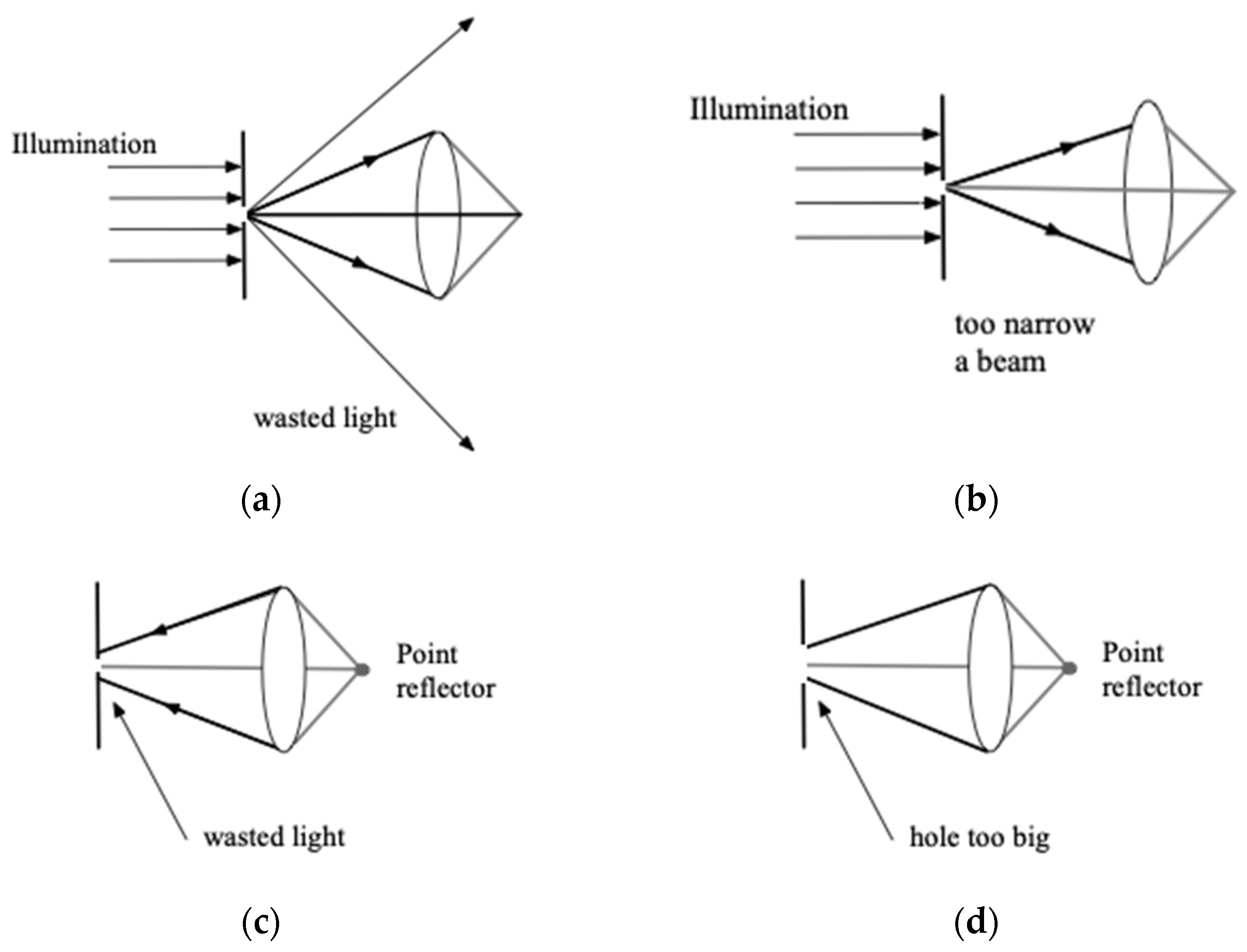
Next, we analyze the light transmission process with different pinhole sizes, as shown in
Figure 4.
When the pinhole is too small (
Figure 4a,c), the transmittance of the pinhole is reduced, generating a diffraction effect at the pinhole. Its size exceeds the entrance pupil of the objective lens, causing part of the light to be wasted. When the diffracted light passes through, the pinholes that are too small simultaneously block the diffracted light, reducing the signal-to-noise ratio of the system. When the pinhole is too large (
Figure 4b,d), the incident light is collimated and may not be completely diffracted, and the light spot cannot cover the entire entrance pupil. The amount of diffracted light increases, but the resolution of the
z-axis becomes relatively worse [
12]. Only when the diameter of the pinhole is the optimal value can the excitation light evenly cover the entire entrance pupil, and the pinhole can receive the emitted light to the maximum extent. At this time, the system can achieve its relatively ideal resolution.
In confocal microscopy imaging, in the optical path, each pinhole on the spinning disk has the same diameter and is fixed as an excitation and detection pinhole at the same time. The excitation light irradiating the sample is no longer a beam of light transmitted by a single diffraction point but is simultaneously illuminated by thousands of excitation pinholes. This makes the pinhole size even more important. Usually, the optimal size of the pinhole is determined using the Fraunhofer formula, which is the FWHM of the Airy disk size [
13,
14,
15]:
Here,
represents the diameter of the pinhole,
M represents the magnification of the objective lens,
NA represents the numerical aperture of the objective lens, and
is the wavelength of the reflected light. In the formula, the pinhole diameter is defined as the full width at half maximum of the Airy disk, and this allows about 75% of the light at the central main maximum to pass through. In biology, considering the low light transmission efficiency of biological samples, the pinhole diameter is defined as the distance between the first-order zero points of the Airy disk, which is approximately twice that of Formula (7):
It can be seen that in a confocal microscope with the same fixed-pinhole diameter, different
M/NA ratios are required depending on the different excitation light sources to achieve the optimal confocal effect. However, in practical applications, the pinhole diameter of the pinhole disk in a system is often the optimal pinhole diameter corresponding to the high-magnification objective lens, so the system may sacrifice its resolution performance when using a low-magnification objective lens. This is a problem that is common in confocal microscopy based on fixed-diameter pinhole disks, but it can usually be ignored when observing live targets. For reference, in commercial single-disk confocal microscopes, high-magnification and high-
NA objectives are usually given priority to meet design requirements, and the maximum fixed-pinhole size is about 50–70 μm. The above formula was used to calculate the optimal pinhole diameter corresponding to each microscopic objective lens in the system when imaging, as shown in
Table 2.
According to the calculation results in the table, the pinhole disk diameter that was finally determined for the system was 50 μm.
3.3. Quantum Efficiency
The original detector used in spinning-disk confocal microscopy (SDCM) was an enhanced camera. It is still currently used as a detector for spinning-disk synchronized-timing video signals in Yokogawa’s core CSU series, with a frame rate of 50 or 60 Hz. The frame rate of 50 Hz is the field rate for the European interlaced video standard CCIR, while 60 Hz is the rate for the American and Japanese RS170 standards. Enhanced cameras have a limited dynamic range and are sensitive and vulnerable to damage in indoor ambient light, making them unsuitable for general use. The timescale and dynamic range of the phenomena of life in living cells place higher requirements on detectors, and the low light level used in SDCM limits the frame rates of detectors. However, Andor Technology launched the electron-multiplying CCD (EMCCD), which was not only able to overcome read noise through on-chip electron multiplication gain, but also provided the highest QE (>90%) at an acquisition speed of hundreds of frames per second [
16,
17,
18]. Since then, the deeply cooled EMCCD has become the optimal choice for SDCM detectors, making SDCM the best tool for live-cell imaging.
In recent years, Coates released a scientific complementary metal–oxide–semiconductor (scientific CMOS or sCMOS) for microscopic imaging [
19]. Unlike the CCD and EMCCD, the sCMOS mainly utilizes advances in high-density planar semiconductor processing technology to integrate complex circuits onto the same chip as the photoelectric sensor array. A high-density embedded readout circuit, amplifiers, and analog-to-digital converters enable each row of the sensor to be read out via an independent channel while operating in parallel. Therefore, even moderate pixel readout speeds can be used to achieve a low readout noise and yield very high frame rates (hundreds of fps on full chips and thousands of fps on cropped target regions) when executed in parallel. This parallel planar processing feature enables the sCMOS to be used to create powerful sensor arrays. Currently, the peak QE of the sCMOS with the highest quantum efficiency can reach 95%.
In order to fulfill the imaging requirements of the composite laser confocal microscopy module and match the electronic design of the entire system at the same time, the system adopts the CCD97 and Mt9p031v303 on-chip CMOS active-pixel digital image sensors from the British company e2v. CCD97 is a thin back-illuminated frame transfer EMCCD with 512 × 512 effective pixels and a pixel size of 16 × 16 μm. It has both normal-mode and multiplication-mode imaging methods, with two corresponding readout amplifiers. When cooling at −40 °C in doubling mode, its detection capability can reach 30 photons/pixel/s (the integration time is 0.1 s), thus meeting the needs of low-light detection, and its quantum efficiency can reach over 90%, to meet the need for a high frame rate. The effective number of pixels of Mt9p031v303 is 2592 × 1944, the pixel size is 2.2 × 2.2 μm, the full resolution can reach 14 fps, and the low pixel resolution can reach 53 fps. The two work together in different modes to meet the requirements of high-resolution and high-speed confocal imaging in different scenarios.