Caries Preventive Action of Nd:YAG and Fluoride in Three Different pH Conditions: FTIR Spectroscopy and SEM Evaluation
Abstract
:1. Introduction
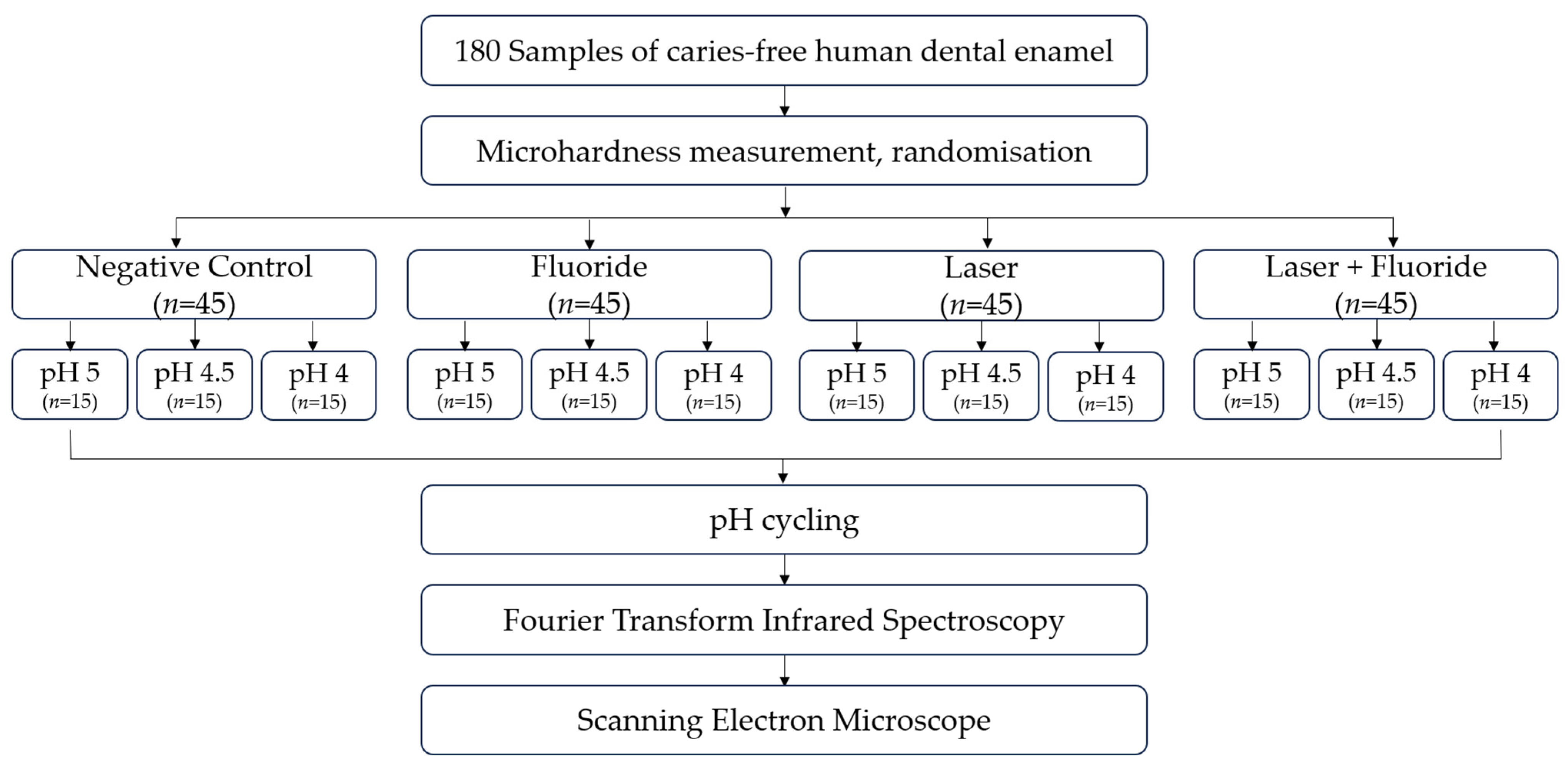
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Preparation
2.3. Experimental Groups
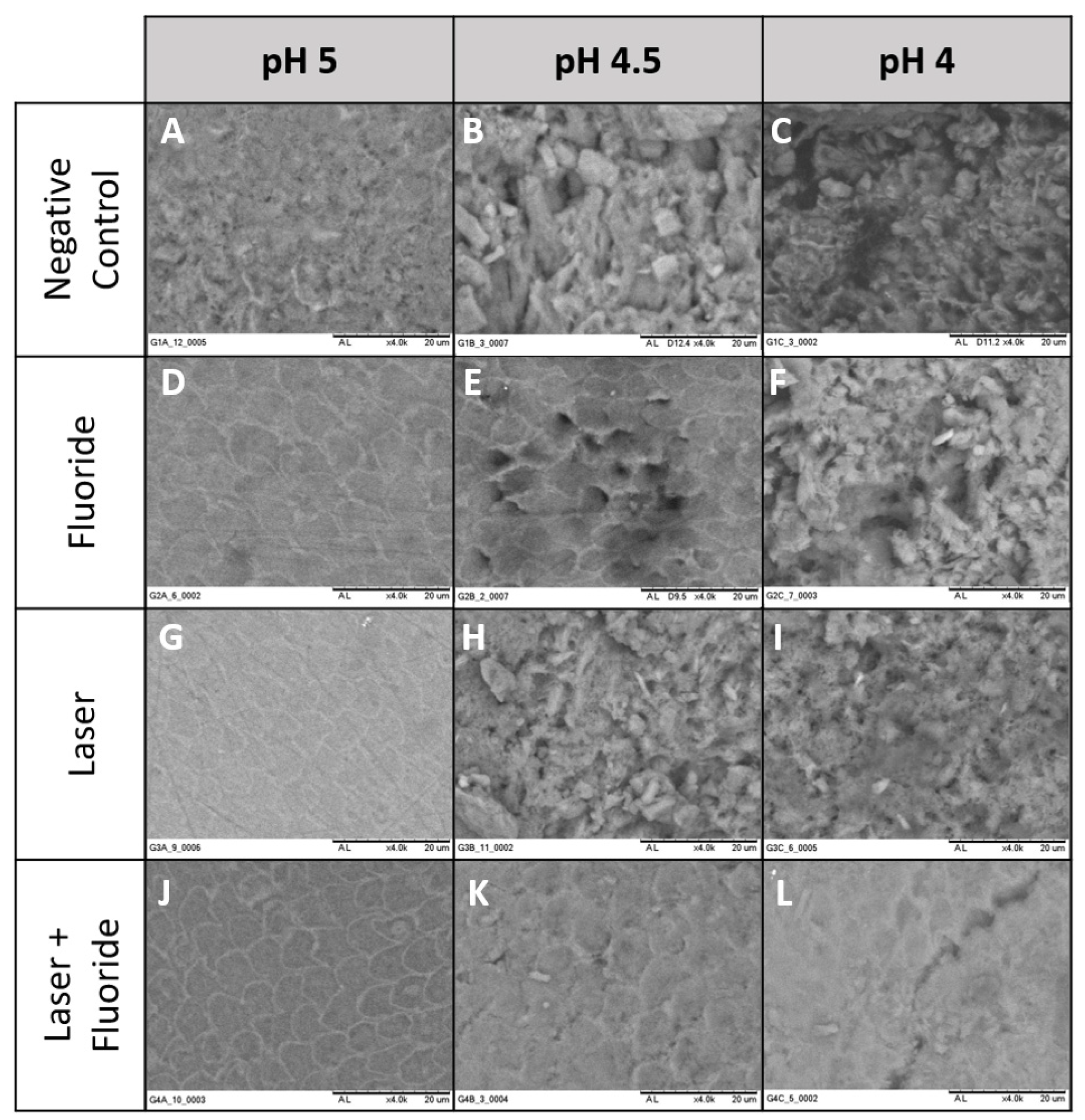
- Negative Control: Non-treated
- Fluoride: Topical application of fluoride (FFA 12.300 µF−/g)
- Laser: Irradiation with Nd:YAG (84.9 J/cm2)
- Laser + Flouride: Irradiation with Nd:YAG (84.9 J/cm2) and topical application of fluoride (FFA 12.300 µF−/g)
- pH 5: The enamel surface tends to demineralize in the form of hydroxyapatite below a critical pH, but not in the form of fluorapatite, which is more acid resistant.
- pH 4.5: The enamel surface tends to demineralize in the form of hydroxyapatite and in the form of fluorapatite below a critical pH.
- pH 4: This pH was used to investigate the acid resistance of hydroxyapatite and fluorapatite forms in the presence of laser irradiation.
2.4. Treatment of Samples
2.5. pH Cycling
2.6. Fourier Transform Infrared Spectroscopy
2.7. Scanning Electron Microscope
2.8. Statistical Tests
3. Results
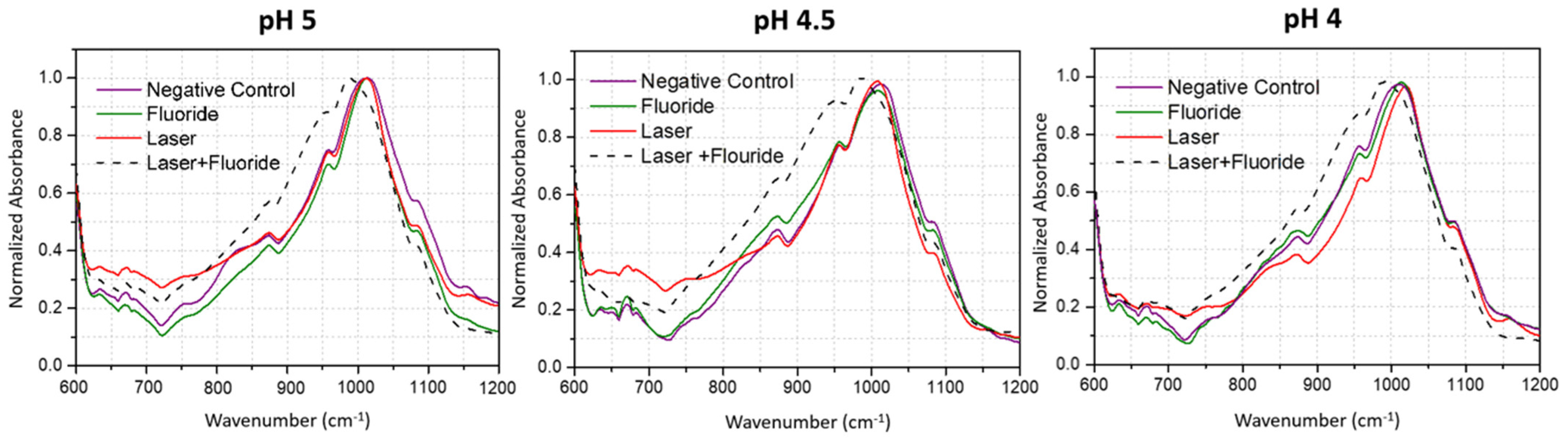
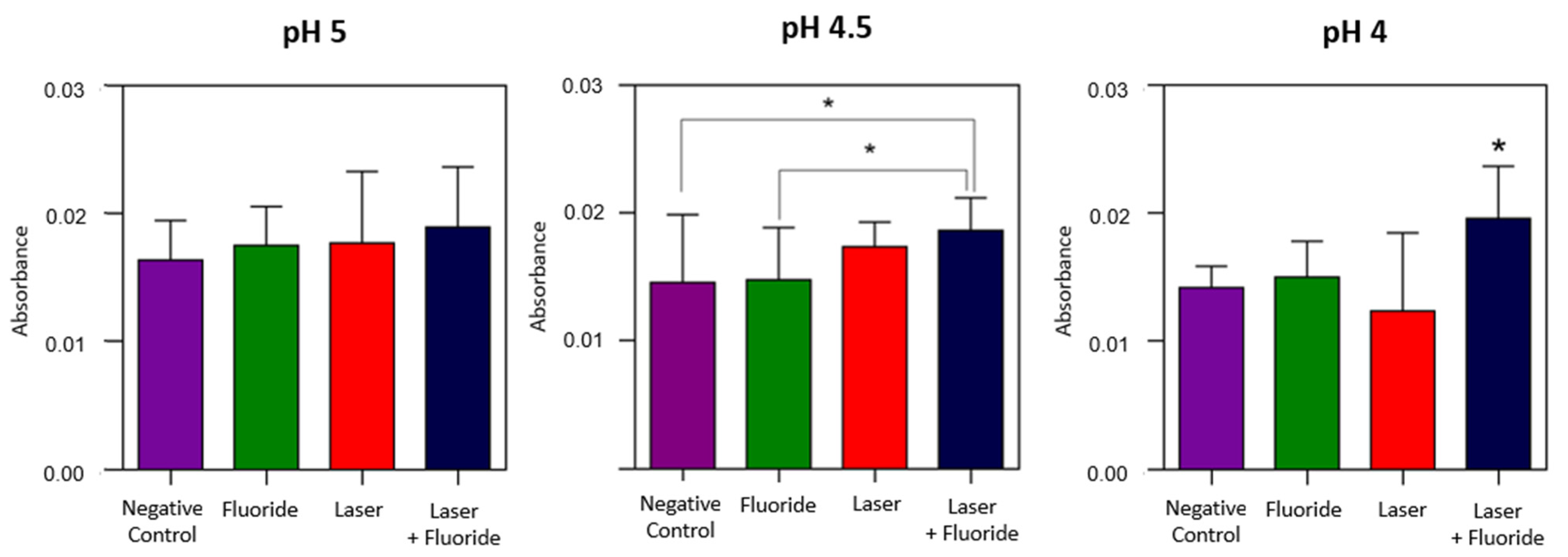
3.1. Fourier Transform Infrared Spectroscopy
Phosphate and Carbonate Analysis
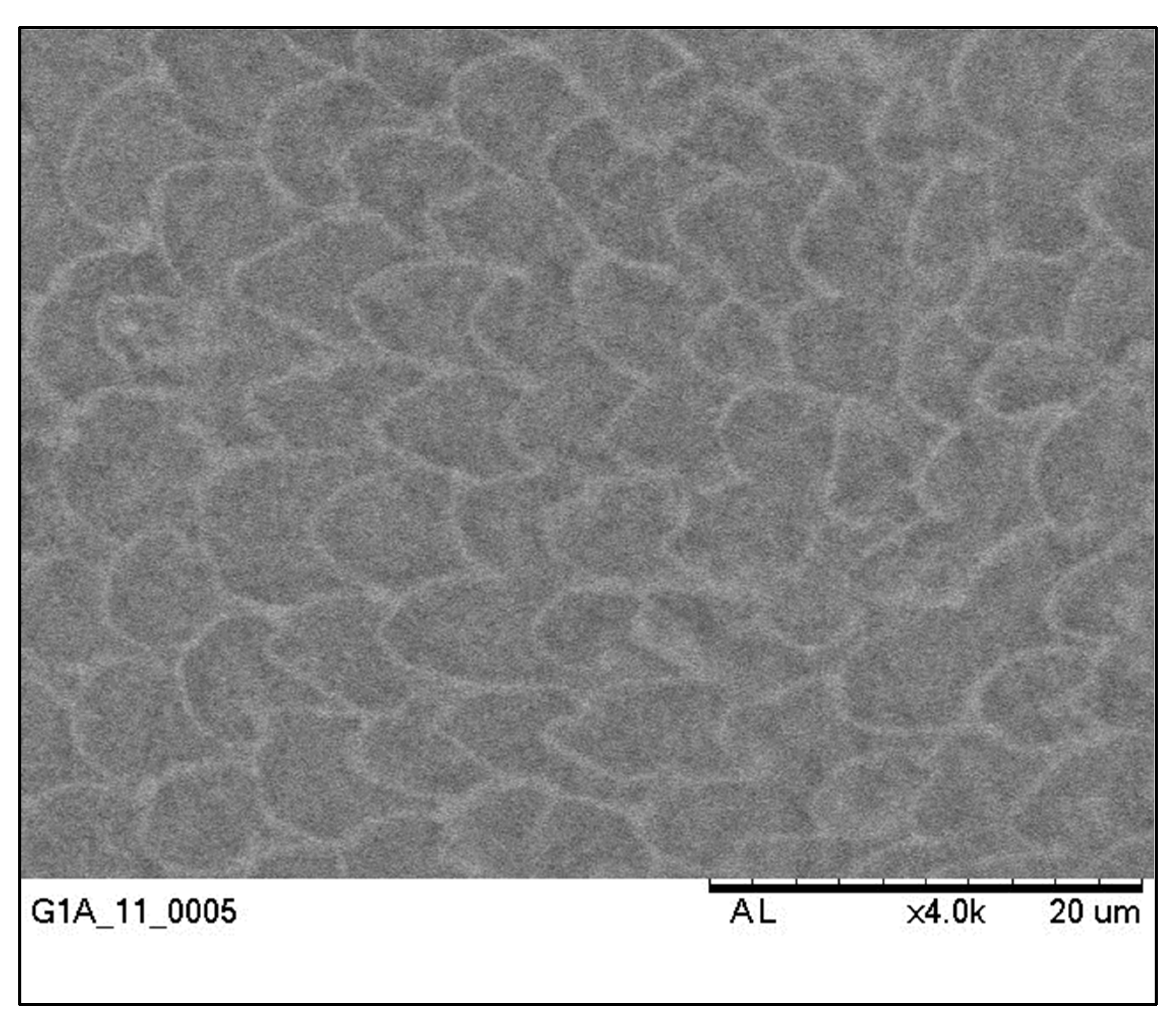
3.2. Scanning Electron Microscope
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, A.P.M.; Da Silva, E.G.; Gonçalves, S.H.F.; Huhtala, M.F.R.L.; Martinho, F.C.; Gonçalves, S.E.D.P.; Torres, C.R.G. Relationship between patient’s education level and knowledge on oral health preventive measures. Int. Dent. Med. J. Adv. Res. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- World Health Organization. Oral Health. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 23 May 2023).
- Fejerskov, O.; Nyvad, B.; Kidd, E. Dental Caries: The Disease and Its Clinical Management, 3rd ed.; Wiley Blackwell: Oxford, UK, 2015. [Google Scholar]
- Buzalaf, M.A.R.; Pessan, J.P.; Honorio, H.M.; Ten Cate, J.M. Mechanisms of action of fluoride for caries control. Monogr. Oral. Sci. 2011, 22, 97–114. Available online: https://www.karger.com/Article/Abstract/325151 (accessed on 1 June 2023).
- Tenuta, L.M.A.; Cury, J.A. Fluoride: Its role in dentistry. Braz. Oral Res. 2010, 24, 9–17. [Google Scholar] [CrossRef]
- Robinson, C. Fluoride and the caries lesion: Interactions and mechanism of action. Eur. Arch. Paediatr. Dent. 2009, 10, 136–140. [Google Scholar] [CrossRef]
- Neev, J.; White, J.M.; Goodis, H.E. Thermal characteristics during Nd:YAG and CO2 laser application to enamel and dentin. Laser Surg. Adv. Charact. Ther. Syst. IV 1994, 2128, 424–430. [Google Scholar] [CrossRef]
- Ana, P.A.; Bachmann, L.; Zezell, D.M. Lasers effects on enamel for caries prevention. Laser Phys. 2006, 16, 865–875. [Google Scholar] [CrossRef]
- Raucci-Neto, W.; de Castro-Raucci, L.M.S.; Lepri, C.P.; Faraoni-Romano, J.J.; da Silva, J.M.G.; Palma-Dibb, R.G. Nd:YAG laser in occlusal caries prevention of primary teeth: A randomized clinical trial. Lasers Med. Sci. 2013, 30, 761–768. [Google Scholar] [CrossRef]
- Zezell, D.M.; Boari, H.G.D.; Ana, P.A.; Eduardo, C.d.P.; Powell, G.L. Nd:YAG laser in caries prevention: A clinical trial. Lasers Surg. Med. 2009, 41, 31–35. [Google Scholar] [CrossRef]
- Zezell, D.M.; Silva, M.R.; Castro, P.A.A.; Silva, T.M.; Goncalves, S.E.P. Nd:YAG laser on dental enamel in the reduction of artificial caries demineralization. In Optics InfoBase Conference Papers; Optica Publishing Group: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Dias-Moraes, M.C.; Castro, P.A.A.; Pereira, D.L.; Ana, P.A.; Freitas, A.Z.; Zezell, D.M. Assessment of the preventive effects of Nd:YAG laser associated with fluoride on enamel caries using optical coherence tomography and FTIR spectroscopy. PLoS ONE 2021, 16, e0254217. [Google Scholar] [CrossRef]
- Agnol, M.A.D.; Battiston, C.; Tenuta, L.M.A.; Cury, J.A. Fluoride Formed on Enamel by Fluoride Varnish or Gel Application: A Randomized Controlled Clinical Trial. Caries Res. 2021, 56, 73–80. [Google Scholar] [CrossRef]
- Rabelo, J.S.; Ana, P.A.; Benetti, C.; Valério, M.E.G.; Zezell, D.M. Changes in dental enamel oven heated or irradiated with Er,Cr:YSGG laser. Analysis by FTIR. Laser Phys. 2010, 20, 871–875. [Google Scholar] [CrossRef]
- Corrêa-Afonso, A.M.; Bachmann, L.; de Almeida, C.G.; Corona, S.A.M.; Borsatto, M.C. FTIR and SEM analysis of CO2 laser irradiated human enamel. Arch. Oral Biol. 2012, 57, 1153–1158. [Google Scholar] [CrossRef]
- Lopes, C.D.C.A.; Limirio, P.H.J.O.; Novais, V.R.; Dechichi, P. Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- White, D.; Featherstone, J. A Longitudinal Microhardness Analysis of Fluoride Dentifrice Effects on Lesion Progression in vitro. Caries Res. 1987, 21, 502–512. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Tenuta, L.M.A.; Filho, A.P.R.; Cury, J.A. Protocols to Study Dental Caries In Vitro: Microbial Caries Models. Methods Mol. Biol. 2019, 1922, 357–368. [Google Scholar] [CrossRef]
- Boari, H.G.D.; Ana, P.A.; Eduardo, C.P.; Powell, G.L.; Zezell, D.M. Absorption and thermal study of dental enamel when irradiated with Nd:YAG laser with the aim of caries prevention. Laser Phys. 2009, 19, 1463–1469. [Google Scholar] [CrossRef]
- Seino, P.Y.; Freitas, P.M.; Marques, M.M.; de Souza Almeida, F.C.; Botta, S.B.; Moreira, M.S.N.A. Influence of CO2 (10.6 μm) and Nd:YAG laser irradiation on the prevention of enamel caries around orthodontic brackets. Lasers Med. Sci. 2015, 30, 611–616. [Google Scholar] [CrossRef]
- Featherstone, J.; O’Reilly, M.; Shariati, M.; Brugler, S. Enhancement of remineralization in vitro and in vivo. In Leach SA. Factors Affecting de- and Remineralization of the Teeth; IRL Press: Oxford, UK, 1986; pp. 23–24. [Google Scholar]
- Argenta, R.M.O.; Tabchoury, C.P.M.; Cury, J.A. A modified pH-cycling model to evaluate fluoride effect on enamel demineralization. Pesqui. Odontol. Bras. 2003, 17, 241–246. [Google Scholar] [CrossRef]
- Raghis, T.R.; Mahmoud, G.; Hamadah, O. Effectiveness of laser irradiation in preventing enamel demineralization during orthodontic treatment: A systematic review. Dent. Med. Probl. 2018, 55, 321–332. [Google Scholar] [CrossRef]
- Pereira, D.; Freitas, A.; Bachmann, L.; Benetti, C.; Zezell, D.; Ana, P. Variation on Molecular Structure, Crystallinity, and Optical Properties of Dentin Due to Nd:YAG Laser and Fluoride Aimed at Tooth Erosion Prevention. Int. J. Mol. Sci. 2018, 19, 433. [Google Scholar] [CrossRef]
- Chen, C.-C.; Huang, S.-T. The Effects of Lasers and Fluoride on the Acid Resistance of Decalcified Human Enamel. Photomed. Laser Surg. 2009, 27, 447–452. [Google Scholar] [CrossRef]
- Corrêa-Afonso, A.M.; Bachmann, L.; de Almeida, C.G.; Dibb, R.G.P.; Borsatto, M.C. Loss of structural water and carbonate of Nd:YAG laser-irradiated human enamel. Lasers Med. Sci. 2014, 30, 1183–1187. [Google Scholar] [CrossRef]
- Castellan, C.S.; Luiz, A.C.; Bezinelli, L.M.; Lopes, R.M.; Mendes, F.M.; Eduardo, C.D.P.; Freitas, P.M.D. In Vitro Evaluation of Enamel Demineralization after Er:YAG and Nd:YAG Laser Irradiation on Primary Teeth. Photomed. Laser Surg. 2007, 25, 85–90. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kimura, Y.; Yamada, Y.; Kawanaka, T.; Matsumoto, K.; de Oliveira, R.M.; de Souza, V.M.; Esteves, C.M.; Lima-Arsati, Y.B.d.O.; Cassoni, A.; et al. Effect of Pulsed Nd:YAG Laser Irradiation on Acid Demineralization of Enamel and Dentin. J. Clin. Laser Med. Surg. 2001, 19, 105–108. [Google Scholar] [CrossRef]
- Harris, D.M.; Fried, D. Pulsed Nd:YAG laser selective ablation of surface enamel caries: I. Photoacoustic response and FTIR spectroscopy. In Lasers in Dentistry VI; SPIE: San Jose, CA, USA, 2000; Volume 3910, pp. 164–171. [Google Scholar] [CrossRef]
- Huang, G.F.; Lan, W.H.; Guo, M.K.; Chiang, C.-P. Synergistic effect of Nd:YAG laser combined with fluoride varnish on inhibition of caries formation in dental pits and fissures in vitro. J. Formos. Med. Assoc. 2001, 100, 181–186. Available online: http://www.fma.org.tw/jfma/PDF/2001-100/issue_3/Article_6.pdf (accessed on 1 June 2023).
- Harazaki, M.; Hayakawa, K.; Fukui, T.; Isshiki, Y.; Powell, L.G. The Nd-YAG Laser is Useful in Prevention of Dental Caries During Orthodontic Treatment. Bull. Tokyo Dent. Coll. 2001, 42, 79–86. [Google Scholar] [CrossRef]
- Yamada, M.K.; Uo, M.; Ohkawa, S.; Akasaka, T.; Watari, F. Three-dimensional topographic scanning electron microscope and Raman spectroscopic analyses of the irradiation effect on teeth by Nd:YAG, Er: YAG, and CO2 lasers. J. Biomed. Mater. Res. 2004, 71B, 7–15. [Google Scholar] [CrossRef]
- Deandrade, L.E.H.; Lizarelli, R.F.Z.; Pelino, J.E.P.; Bagnato, V.S.; Oliveira, O.B., Jr. Enamel caries resistance accidentally irradiated by the Nd:YAG laser. Laser Phys. Lett. 2007, 4, 457–463. [Google Scholar] [CrossRef]

| Phosphate | |||
| Group | pH 5 | pH 4.5 | pH 4 |
| Negative Control | 0.0136 ± 0.0031 | 0.0146 ± 0.0052 a | 0.0142 ± 0.0017 a |
| Fluoride | 0.0175 ± 0.0030 | 0.0148 ± 0.0041 a | 0.0150 ± 0.0028 a |
| Laser | 0.0177 ± 0.0056 | 0.0173 ± 0.0019 ab | 0.0124 ± 0.0061 a |
| Laser + Fluoride | 0.0189 ± 0.0047 | 0.0186 ± 0.0025 b | 0.0196 ± 0.0041 b |
| Carbonate | |||
| Group | pH 5 | pH 4.5 | pH 4 |
| Negative Control | 0.0023 ± 0.0003 | 0.0034 ± 0.0008 | 0.0041 ± 0.0004 |
| Fluoride | 0.0025 ± 0.0003 | 0.0032 ± 0.0009 | 0.0042 ± 0.0009 |
| Laser | 0.0024 ± 0.0008 | 0.0024 ± 0.0006 | 0.0042 ± 0.0009 |
| Laser + Fluoride | 0.0018 ± 0.0005 | 0.0030 ± 0.0005 | 0.0036 ± 0.0008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caramel-Juvino, A.; Zanini, N.A.; Avelino, S.G.; Fontes-Oliveira, Y.R.; Germano, G.C.M.; Eduardo, C.d.P.; Zezell, D.M. Caries Preventive Action of Nd:YAG and Fluoride in Three Different pH Conditions: FTIR Spectroscopy and SEM Evaluation. Photonics 2023, 10, 985. https://doi.org/10.3390/photonics10090985
Caramel-Juvino A, Zanini NA, Avelino SG, Fontes-Oliveira YR, Germano GCM, Eduardo CdP, Zezell DM. Caries Preventive Action of Nd:YAG and Fluoride in Three Different pH Conditions: FTIR Spectroscopy and SEM Evaluation. Photonics. 2023; 10(9):985. https://doi.org/10.3390/photonics10090985
Chicago/Turabian StyleCaramel-Juvino, Amanda, Nathalia A. Zanini, Sabrina Gardiano Avelino, Yasmin Reis Fontes-Oliveira, Gleice Conceição Mendonça Germano, Carlos de Paula Eduardo, and Denise Maria Zezell. 2023. "Caries Preventive Action of Nd:YAG and Fluoride in Three Different pH Conditions: FTIR Spectroscopy and SEM Evaluation" Photonics 10, no. 9: 985. https://doi.org/10.3390/photonics10090985
APA StyleCaramel-Juvino, A., Zanini, N. A., Avelino, S. G., Fontes-Oliveira, Y. R., Germano, G. C. M., Eduardo, C. d. P., & Zezell, D. M. (2023). Caries Preventive Action of Nd:YAG and Fluoride in Three Different pH Conditions: FTIR Spectroscopy and SEM Evaluation. Photonics, 10(9), 985. https://doi.org/10.3390/photonics10090985






