Photoactive Properties of Transport Sol-Gel Layers Based on Strontium Titanate for Perovskite Solar Cells
Abstract
1. Introduction
2. Experimental
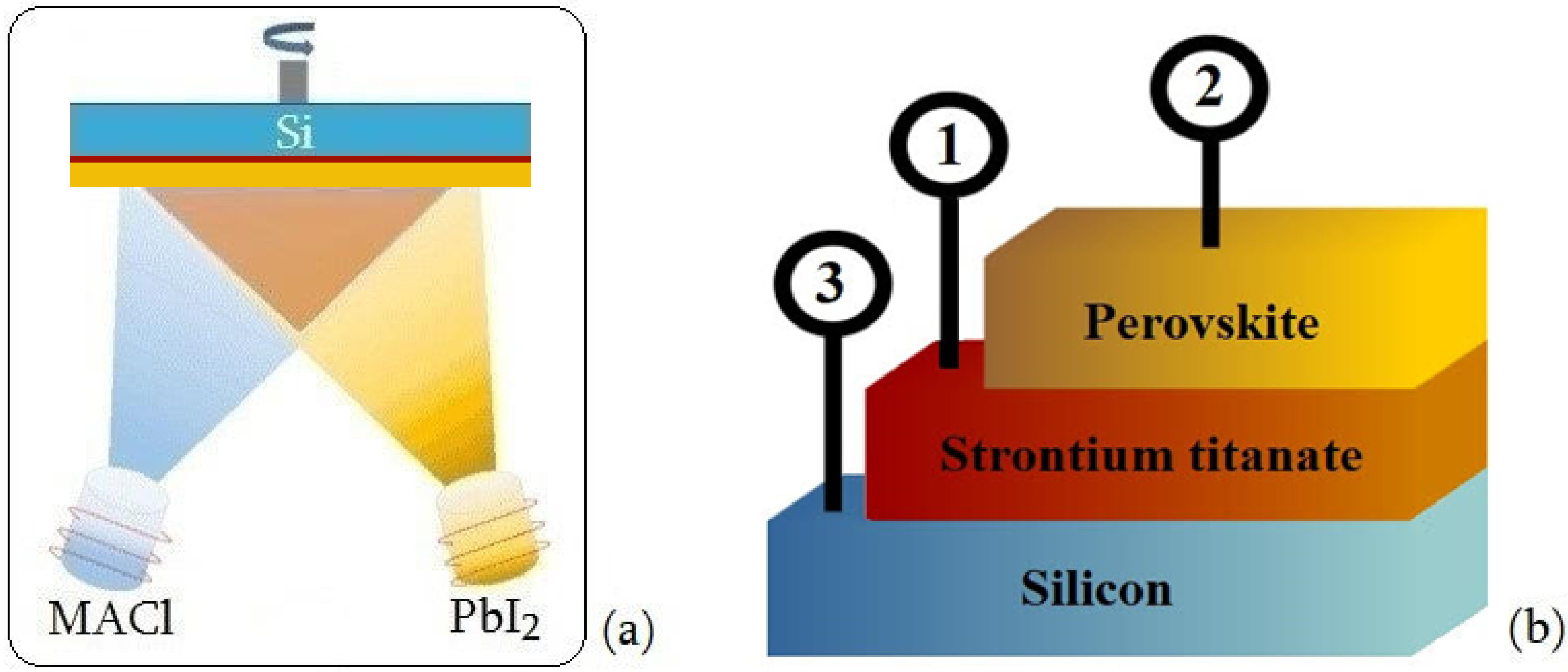
2.1. Sol–Gel Synthesis of SrTiO3:xNb Films
2.2. Preparation of Perovskite Layers
2.3. Measurements
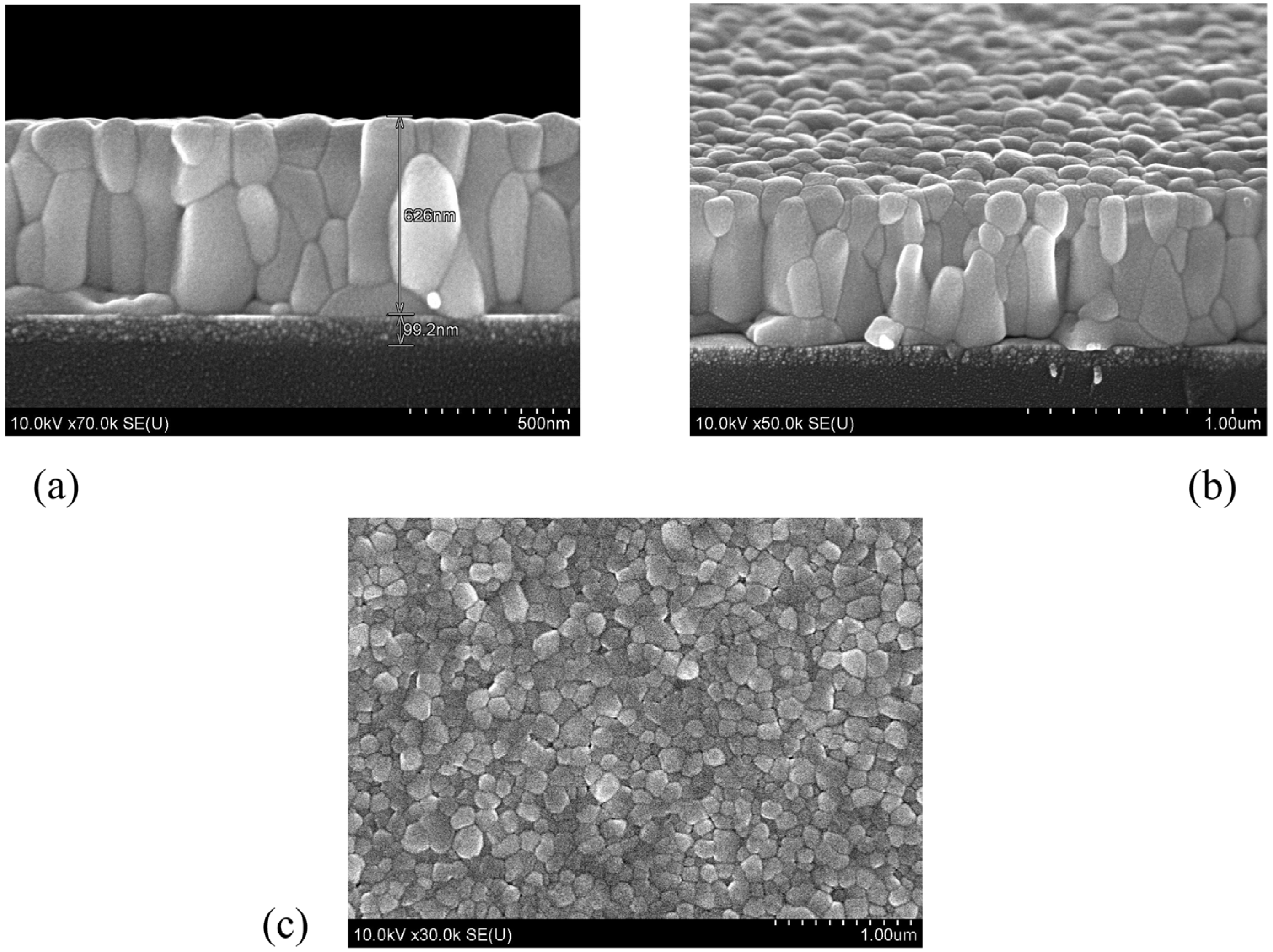
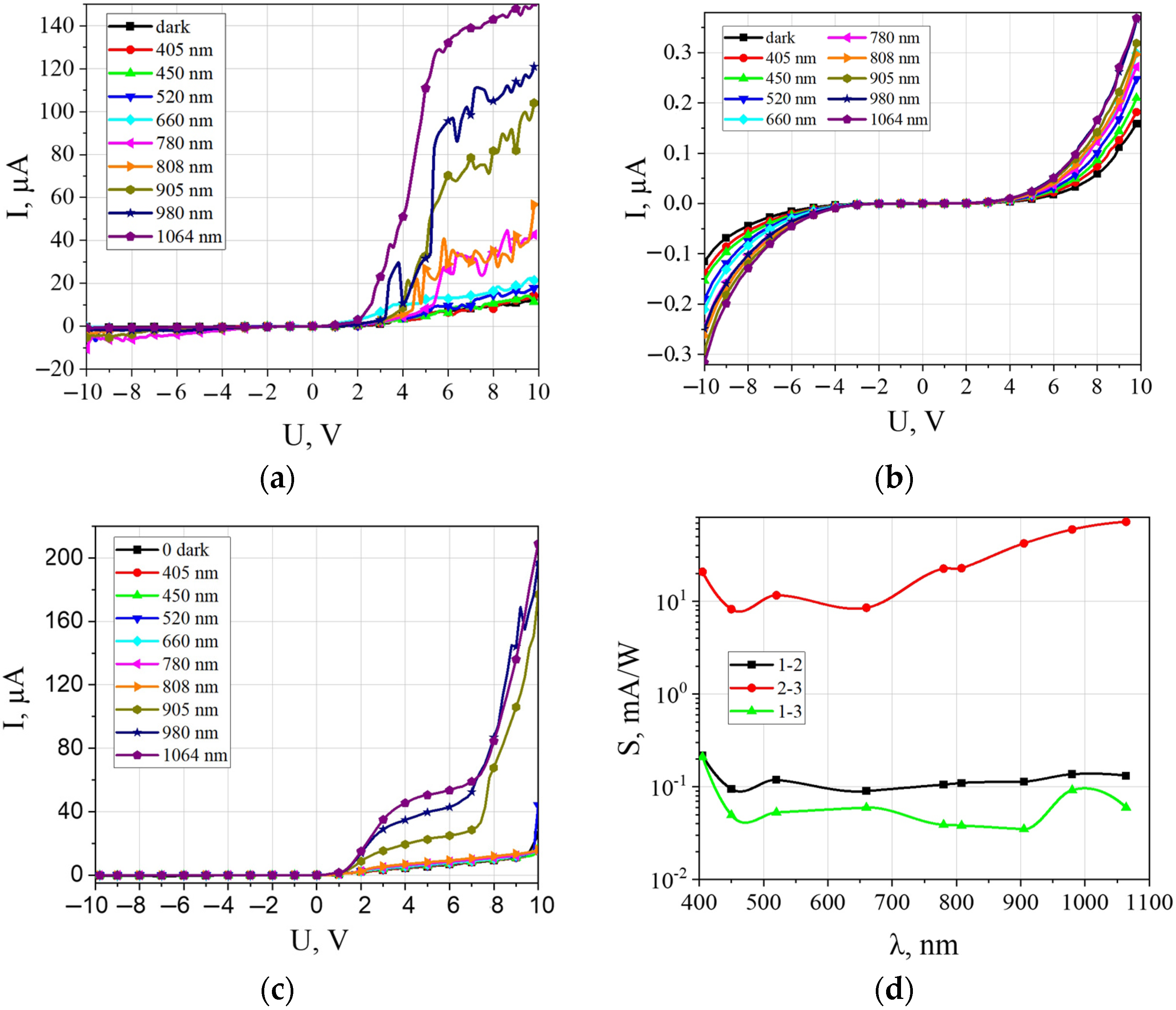
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaseashta, A.; Ayvazyan, G.; Khudaverdyan, S.; Matevosyan, L. Structural and optical properties of vacuum-evaporated mixed-halide perovskite layers on nanotextured black silicon. Phys. Status Solidi RRL 2023, 17, 2200482. [Google Scholar] [CrossRef]
- Ayvazyan, G.Y.; Kovalenko, D.L.; Lebedev, M.S.; Matevosyan, L.A.; Semchenko, A.V. Investigation of the structural and optical properties of silicon-perovskite structures with a black silicon layer. J. Contemp. Phys. 2022, 57, 274. [Google Scholar] [CrossRef]
- Kumar, V.; Pandey, A.; Kumar, L.; Singh, M.; Kumar, A.; Kishor, S.; Jain, K.; Singh, K.S.; Singh, B.P. Experimental and theoretical investigations on fullerene (C60) induced compact CH3NH3PbI3 perovskite thin films. Phys. Scr. 2022, 97, 075809. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Qaid, S.M.H.; Hezam, M.; Bedja, I.; Ghaithan, H.M.; Aldwayyan, A.S. Effect of deposition method on the structural and optical properties of CH3NH3PbI3 perovskite thin films. Opt. Mater. 2020, 103, 109836. [Google Scholar] [CrossRef]
- Ayvazyan, G.; Vaseashta, A.; Gasparyan, F.; Khudaverdyan, S. Effect of thermal annealing on the structural and optical properties of black silicon. J. Mater. Sci. Mater. Electron. 2022, 33, 17001–17010. [Google Scholar] [CrossRef]
- Cojocaru, L.; Wienands, K.; Kim, T.W.; Uchida, S.; Bett, A.J.; Rafizadeh, S.; Goldschmidt, J.C.; Glunz, S.W. Detailed investigation of evaporated perovskite absorbers with high crystal quality on different substrates. ACS Appl. Mater. Interfaces 2018, 10, 26293–26302. [Google Scholar] [CrossRef] [PubMed]
- Guesnay, Q.; Sahli, F.; Ballif, C.; Jeangros, Q. Vapor deposition of metal halide perovskite thin films: Process control strategies to shape layer properties. APL Mater. 2021, 9, 100703. [Google Scholar] [CrossRef]
- Semchenko, A.V.; Sidsky, V.V.; Bdikin, I.; Gaishun, V.E.; Kopyl, S.; Kovalenko, D.L.; Pakhomov, O.; Khakhomov, S.A.; Kholkin, A.L. Nanoscale Piezoelectric Properties and Phase Separation in Pure and La-Doped BiFeO3 Films Prepared by Sol–Gel Method. Materials 2021, 14, 1694. [Google Scholar] [CrossRef] [PubMed]
- Khakhomov, S.A.; Semchenko, A.V.; Sidsky, V.V.; Vaskevich, V.V.; Maevsky, A.A.; Tyulenkova, O.I.; Gaishun, V.E.; Kovalenko, D.L.; Pakhomov, O.V.; Es’kov, A.V.; et al. Influence of the composition and conditions of the sol-gel process on the properties of barium-strontium titanate ferroelectric thin films. Probl. Phys. Math. Tech. 2021, 4, 45–50. [Google Scholar] [CrossRef]
- Malyutina-Bronskaya, V.V.; Semchenko, A.V.; Sidsky, V.V.; Fedorov, V.E. Properties of ZnO: Er3+ films obtained by the sol–gel method. Semiconductors 2017, 51, 392–395. [Google Scholar] [CrossRef]
- Rogachev, A.; Luca, D.; Gaishun, V.; Semchenko, A.; Sidsky, V.; Tyulenkova, O.; Kovalenko, D. Sol-Gel Synthesis of Functional Nanostructured Materials for Electronic Devices. Adv. Mater. Res. 2015, 1117, 164–167. [Google Scholar] [CrossRef]
- Khakhomov, S.A.; Sidsky, V.V.; Semchenko, A.V.; Gaishun, V.E.; Kovalenko, D.L. Synthesis of RE-Doped YAG Scintillators by Sol-Gel Method. In Proceedings of the Education Inter-Academia 2019—The 18th International Conference on Global Research, Budapest and Balatonfüred, Hungary, 4–7 September 2019; pp. 1–5. [Google Scholar]
- Lee, W.; Yoo, S.; Yoon, K.J.; Yeu, I.W.; Chang, H.J.; Choi, J.-H.; Hoffmann-Eifert, S.; Waser, R.; Hwang, C.S. Resistance switching behavior of atomic layer deposited SrTiO3 film through possible formation of Sr2Ti6O13 or Sr1Ti11O20 phases. Sci. Rep. 2016, 6, 20550. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Nakayama, A.; Niimi, H.; Kageyama, K.; Takagi, H. Resistance switching and retention behaviors in polycrystalline La-doped SrTiO3 ceramics chip devices. J. Appl. Phys. 2008, 104, 053712. [Google Scholar] [CrossRef]
- Sohrabi Anaraki, H.; Gaponenko, N.V.; Litvinov, V.G.; Ermachikhin, A.V.; Kolos, V.V.; Pyatlitski, A.N.; Ivanov, V.A. Low-resistance and high-resistance states in strontium titanate films formed by the sol–gel method. Phys. Solid State 2015, 57, 2030–2033. [Google Scholar] [CrossRef]
- Katsu, H.; Tanaka, H.; Kawai, T. Anomalous Photoconductivity in SrTiO3. Jpn. J. Appl. Phys. 2000, 39, 2657. [Google Scholar] [CrossRef]
- Tarun, M.C.; Selim, F.A.; McCluskey, M.D. Persistent photoconductivity in strontium titanate. Phys. Rev. Lett. 2013, 111, 187403. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, M.; Gaponenko, N.; Litvinov, V.; Ermachikhin, A.; Chubenko, E.; Borisenko, V.; Mukhin, N.; Radyush, Y.; Tumarkin, A.; Gagarin, A. Structural Dependent Eu3+ Luminescence, Photoelectric and Hysteresis Effects in Porous Strontium Titanate. Materials 2020, 13, 5767. [Google Scholar] [CrossRef]
- Son, J.; Moetakef, P.; Jalan, B.; Bierwagen, O.; Wright, N.J.; Engel-Herbert, R.; Stemmer, S. Epitaxial SrTiO3 films with electron mobilities exceeding 30,000 cm2 V−1 s−1. Nat. Mater. 2010, 9, 482–484. [Google Scholar] [CrossRef]
- Bera, A.; Wu, K.; Sheikh, A.; Alarousu, E.; Mohammed, O.F.; Wu, T. Perovskite oxide SrTiO3 as an efficient electron transporter for hybrid perovskite solar cells. J. Phys. Chem. C 2014, 118, 28494–28501. [Google Scholar] [CrossRef]
- Kalabukhov, A.; Gunnarsson, R.; Börjesson, J.; Olsson, E.; Claeson, T.; Winkler, D. Effect of oxygen vacancies in the SrTiO3 substrate on the electrical properties of the LaAlO3/SrTiO3 interface. arXiv 2006. [Google Scholar] [CrossRef]
- Ohtomo, A.; Hwang, H.Y. Growth mode control of the free carrier density in Sr TiO3−δ films. J. Appl. Phys. 2007, 102, 083704. [Google Scholar] [CrossRef]
- Ayvazyan, G.; Hakhoyan, L.; Dashtoyan, H.; Matevosyan, L. Preparation and Investigation of Vacuum-Deposited CH3NH3PbI3−xClx Perovskite Films on Black Silicon. J. Contemp. Phys. 2023, 58, 85–91. [Google Scholar] [CrossRef]
- Ngqoloda, S.; Arendse, C.J.; Guha, S.; Muller, T.F.; Klue, S.C.; Magubane, S.S.; Oliphant, C.J. Mixed-halide perovskites solar cells through PbICl and PbCl2 precursor films by sequential chemical vapor deposition. Sol. Energy 2021, 215, 179–188. [Google Scholar] [CrossRef]
- Tufte, O.N.; Chapman, P.W. Electron mobility in semiconducting strontium titanate. Phys. Rev. 1967, 155, 796. [Google Scholar] [CrossRef]
- Rusevich, L.L.; Tyunina, M.; Kotomin, E.A.; Nepomniashchaia, N.; Dejneka, A. The electronic properties of SrTiO3−δ with oxygen vacancies or substitutions. Sci. Rep. 2021, 11, 23341. [Google Scholar] [CrossRef]
- Spasović, S.; Paunović, N.; Popović, D.; Dojčilović, J. Infrared and dielectrical prorerties of SrTiO3:Nd. Mater. Sci. Forum 2006, 518, 471–476. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semchenko, A.V.; Ayvazyan, G.Y.; Malyutina-Bronskaya, V.V.; Khakhomov, S.A.; Kovalenko, D.L.; Boiko, A.A.; Sidski, V.V.; Nestsiaronak, A.V.; Mayevsky, A.A.; Danilchenko, K.D.; et al. Photoactive Properties of Transport Sol-Gel Layers Based on Strontium Titanate for Perovskite Solar Cells. Photonics 2023, 10, 845. https://doi.org/10.3390/photonics10070845
Semchenko AV, Ayvazyan GY, Malyutina-Bronskaya VV, Khakhomov SA, Kovalenko DL, Boiko AA, Sidski VV, Nestsiaronak AV, Mayevsky AA, Danilchenko KD, et al. Photoactive Properties of Transport Sol-Gel Layers Based on Strontium Titanate for Perovskite Solar Cells. Photonics. 2023; 10(7):845. https://doi.org/10.3390/photonics10070845
Chicago/Turabian StyleSemchenko, Alina V., Gagik Y. Ayvazyan, Viktoriya V. Malyutina-Bronskaya, Sergei A. Khakhomov, Dmitry L. Kovalenko, Andrei A. Boiko, Vitali V. Sidski, Anton V. Nestsiaronak, Alexander A. Mayevsky, Konstantin D. Danilchenko, and et al. 2023. "Photoactive Properties of Transport Sol-Gel Layers Based on Strontium Titanate for Perovskite Solar Cells" Photonics 10, no. 7: 845. https://doi.org/10.3390/photonics10070845
APA StyleSemchenko, A. V., Ayvazyan, G. Y., Malyutina-Bronskaya, V. V., Khakhomov, S. A., Kovalenko, D. L., Boiko, A. A., Sidski, V. V., Nestsiaronak, A. V., Mayevsky, A. A., Danilchenko, K. D., Zhigulin, D. V., Pilipenko, V. A., Subasri, R., & Gaponenko, N. V. (2023). Photoactive Properties of Transport Sol-Gel Layers Based on Strontium Titanate for Perovskite Solar Cells. Photonics, 10(7), 845. https://doi.org/10.3390/photonics10070845








