Manganese-Doped Carbon Dots as a Promising Nanoprobe for Luminescent and Magnetic Resonance Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbon Dots Synthesis
2.3. Experimental Setup
3. Results and Discussion
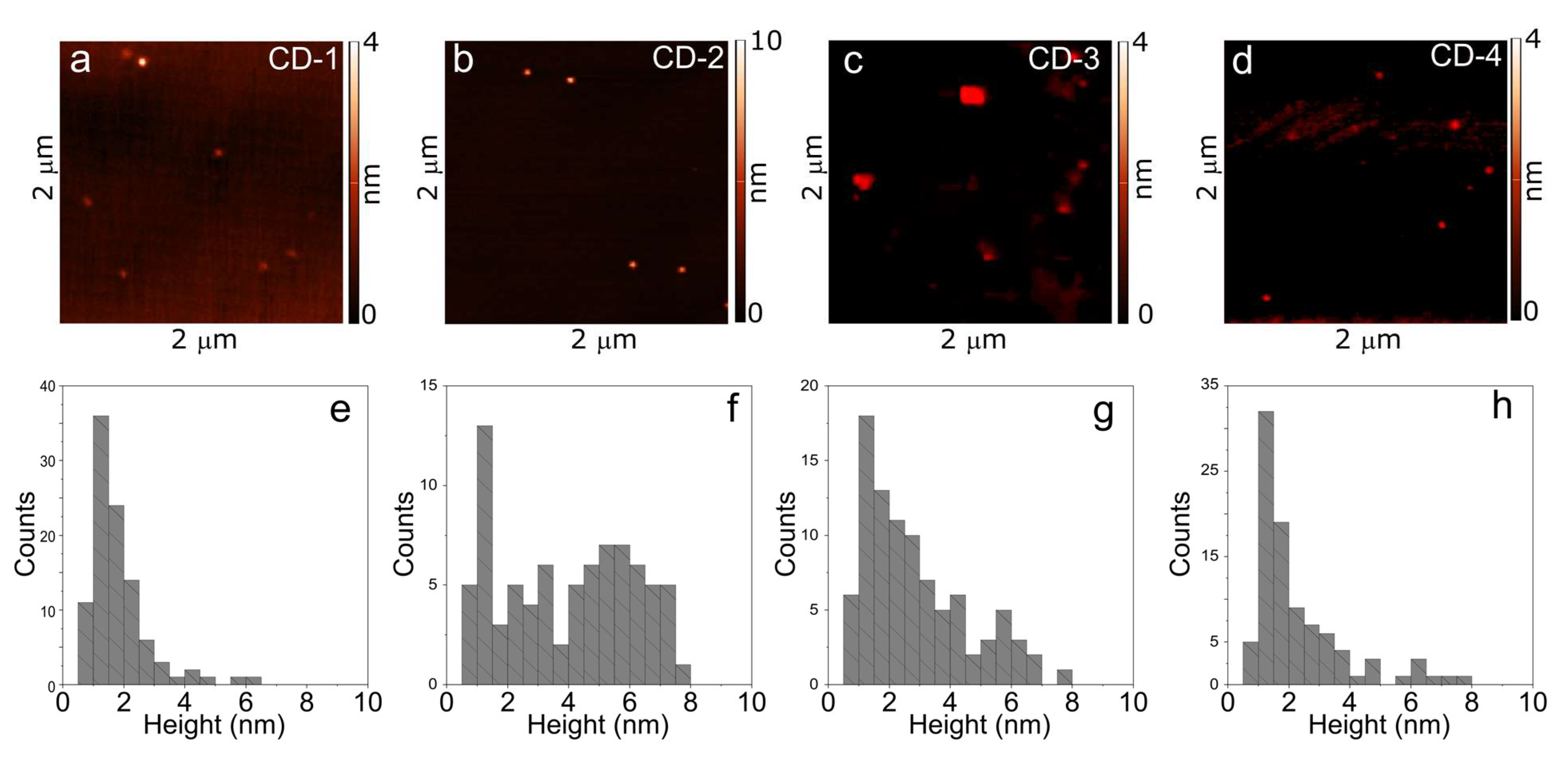
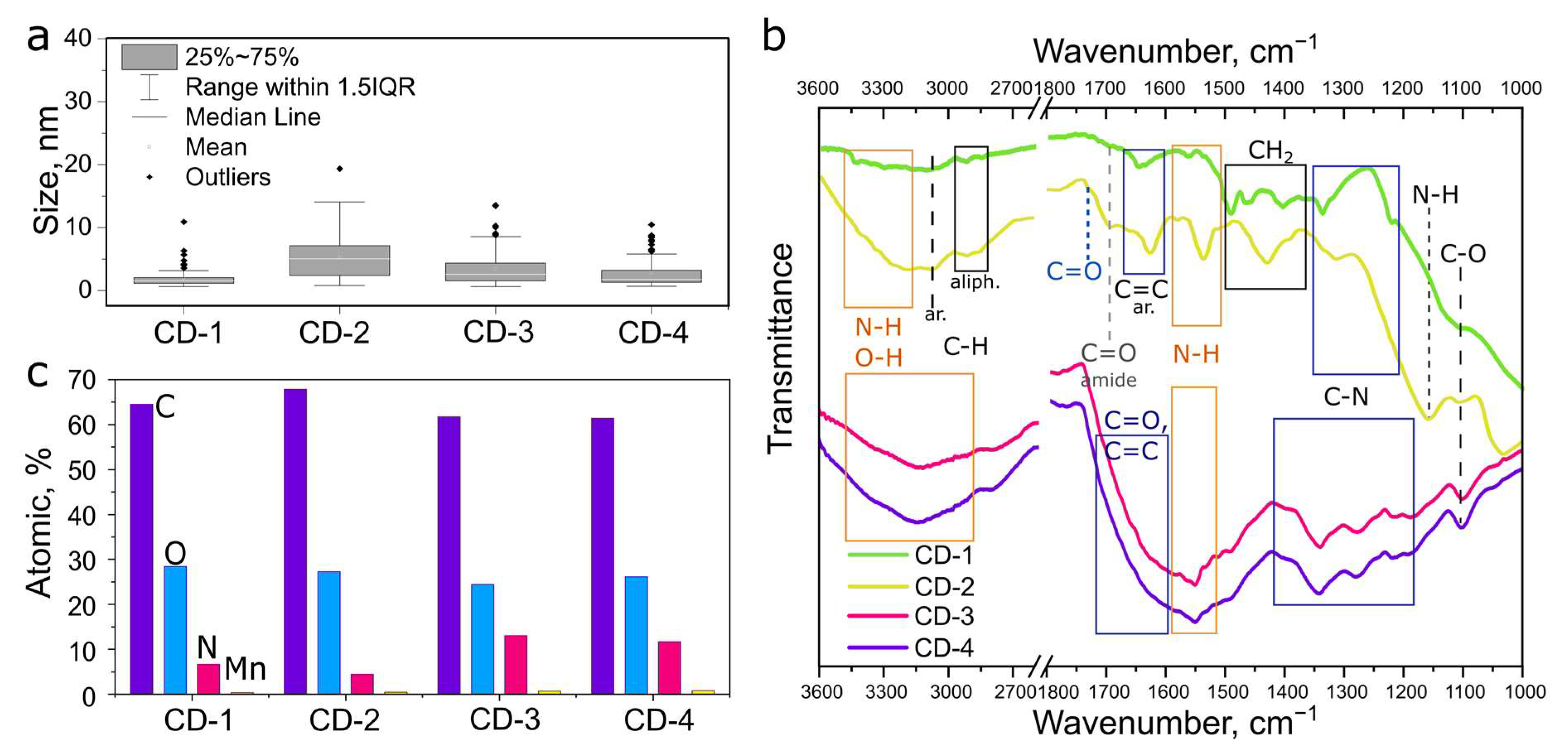
3.1. Morphology and Chemical Composition of CDs
3.2. Optical Properties and MR Performance
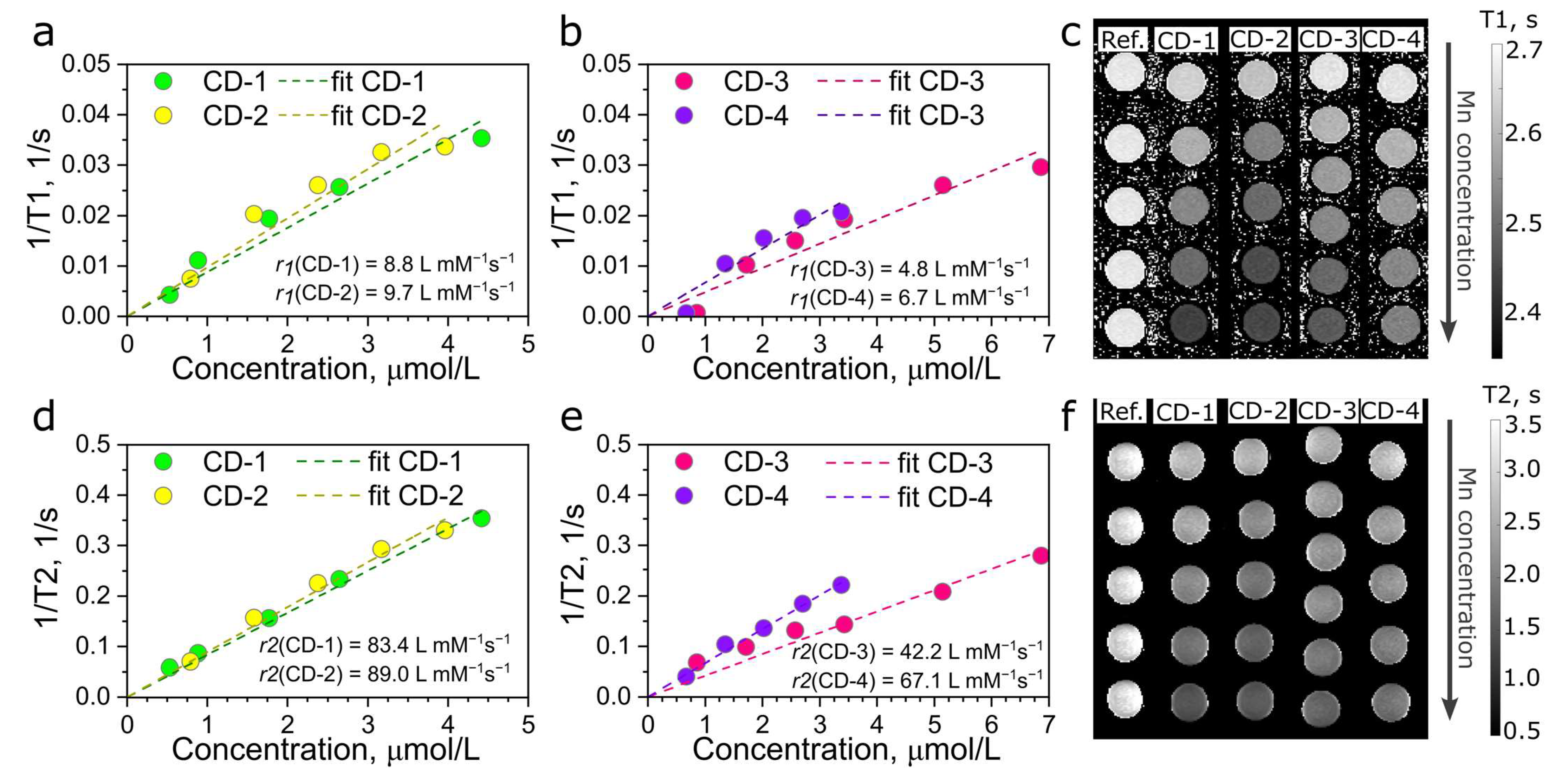
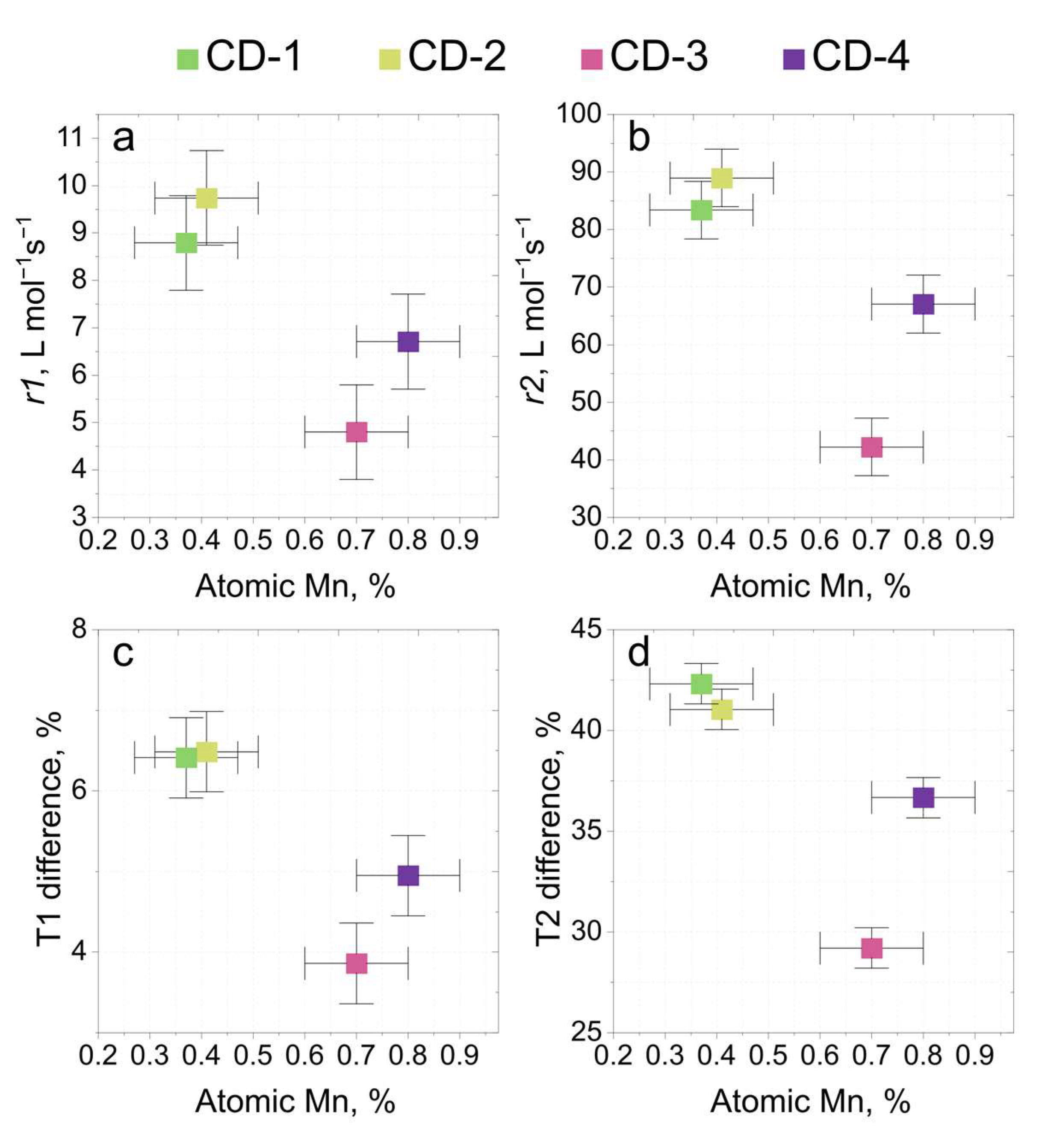
3.3. MR Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xu, P.; Zhang, X.; Long, W. The Synthetic Strategies, Photoluminescence Mechanisms and Promising Applications of Carbon Dots: Current State and Future Perspective. Carbon 2022, 186, 91–127. [Google Scholar] [CrossRef]
- Litvin, A.P.; Zhang, X.; Ushakova, E.V.; Rogach, A.L. Carbon Nanoparticles as Versatile Auxiliary Components of Perovskite-Based Optoelectronic Devices. Adv. Funct. Mater. 2021, 31, 2010768. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Ushakova, E.V.; Fedorov, A.V.; Rogach, A.L. Applications of Carbon Dots in Optoelectronics. Nanomaterials 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Falara, P.P.; Zourou, A.; Kordatos, K.V. Recent Advances in Carbon Dots/2-D Hybrid Materials. Carbon 2022, 195, 219–245. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Cai, C.; Lin, H. Conversion of Carbon Dots from Fluorescence to Ultralong Room-Temperature Phosphorescence by Heating for Security Applications. Adv. Mater. 2018, 30, 1800783. [Google Scholar] [CrossRef]
- Tian, Z.; Li, D.; Ushakova, E.V.; Maslov, V.G.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Rogach, A.L. Multilevel Data Encryption Using Thermal-Treatment Controlled Room Temperature Phosphorescence of Carbon Dot/Polyvinylalcohol Composites. Adv. Sci. 2018, 5, 1800795. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.; Tang, M.; Qiu, H. Tailored Fabrication of Carbon Dot Composites with Full-Color Ultralong Room-Temperature Phosphorescence for Multidimensional Encryption. Adv. Sci. 2022, 9, 2103833. [Google Scholar] [CrossRef]
- Su, W.; Wu, H.; Xu, H.; Zhang, Y.; Li, Y.; Li, X.; Fan, L. Carbon Dots: A Booming Material for Biomedical Applications. Mater. Chem. Front. 2020, 4, 821–836. [Google Scholar] [CrossRef]
- Khan, S.; Dunphy, A.; Anike, M.S.; Belperain, S.; Patel, K.; Chiu, N.H.L.; Jia, Z. Recent Advances in Carbon Nanodots: A Promising Nanomaterial for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 6786. [Google Scholar] [CrossRef]
- Li, S.; Wei, J.; Yao, Q.; Song, X.; Xie, J.; Yang, H. Emerging Ultrasmall Luminescent Nanoprobes for in Vivo Bioimaging. Chem. Soc. Rev. 2023, 52, 1672–1696. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, X.; Du, L.; Ye, M.; Lu, Y.; Xue, J.; Wu, J.; Shuai, X. Molecular Imaging Nanoprobes for Theranostic Applications. Adv. Drug Deliv. Rev. 2022, 186, 114320. [Google Scholar] [CrossRef]
- Gupta, B.D.; Pathak, A.; Shrivastav, A.M. Optical Biomedical Diagnostics Using Lab-on-Fiber Technology: A Review. Photonics 2022, 9, 86. [Google Scholar] [CrossRef]
- Yu, S.; Ding, L.; Lin, H.; Wu, W.; Huang, J. A Novel Optical Fiber Glucose Biosensor Based on Carbon Quantum Dots-Glucose Oxidase/Cellulose Acetate Complex Sensitive Film. Biosens. Bioelectron. 2019, 146, 111760. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zheng, C.; Yin, Z.; Wang, Z.; Wang, J.; Liu, J.; Luo, D.; Liu, Y.J. Hierarchically Ordered Silicon Metastructures from Improved Self-Assembly-Based Nanosphere Lithography. ACS Appl. Mater. Interfaces 2020, 12, 12345–12352. [Google Scholar] [CrossRef] [PubMed]
- Pisco, M.; Galeotti, F.; Grisci, G.; Quero, G.; Cusano, A. Self-Assembled Periodic Patterns on the Optical Fiber Tip by Microsphere Arrays. In Proceedings of the 24th International Conference on Optical Fibre Sensors, Curitiba, Brazil, 28 September–2 October 2015; Volume 9634, pp. 165–168. [Google Scholar] [CrossRef]
- Managò, S.; Quero, G.; Zito, G.; Tullii, G.; Galeotti, F.; Pisco, M.; De Luca, A.C.; Cusano, A. Tailoring Lab-on-Fiber SERS Optrodes towards Biological Targets of Different Sizes. Sens. Actuators B Chem. 2021, 339, 129321. [Google Scholar] [CrossRef]
- Huang, X.; Song, J.; Yung, B.C.; Huang, X.; Xiong, Y.; Chen, X. Ratiometric Optical Nanoprobes Enable Accurate Molecular Detection and Imaging. Chem. Soc. Rev. 2018, 47, 2873–2920. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, W.; Liang, X.; Wang, Y.R.; Xu, F.; Li, L.; Han, L.; Jiang, L.R. Construction and Application of Thrombin-Activated Fluorescence-SERS Dual-Mode Optical Nanoprobes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 293, 122513. [Google Scholar] [CrossRef]
- Ng, S.M. Carbon dots as optical nanoprobes for biosensors. In Nanobiosensors for Biomolecular Targeting, 1st ed.; Gopinath, S.C.B., Lakshmipriya, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–300. [Google Scholar] [CrossRef]
- Vedernikova, A.A.; Miruschenko, M.D.; Arefina, I.A.; Babaev, A.A.; Stepanidenko, E.A.; Cherevkov, S.A.; Spiridonov, I.G.; Danilov, D.V.; Koroleva, A.V.; Zhizhin, E.V.; et al. Dual-Purpose Sensing Nanoprobe Based on Carbon Dots from o-Phenylenediamine: PH and Solvent Polarity Measurement. Nanomaterials 2022, 12, 3314. [Google Scholar] [CrossRef]
- Ding, H.; Zhou, X.X.; Wei, J.S.; Li, X.B.; Qin, B.T.; Chen, X.B.; Xiong, H.M. Carbon Dots with Red/near-Infrared Emissions and Their Intrinsic Merits for Biomedical Applications. Carbon 2020, 167, 322–344. [Google Scholar] [CrossRef]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI Contrast Agents: Classification and Application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Hazardous Substances Data Bank (HSDB): 7547—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7547 (accessed on 6 April 2023).
- Kueny-Stotz, M.; Garofalo, A.; Felder-Flesch, D. Manganese-Enhanced MRI Contrast Agents: From Small Chelates to Nanosized Hybrids. Eur. J. Inorg. Chem. 2012, 2012, 1987–2005. [Google Scholar] [CrossRef]
- Nofiele, J.T.; Cheng, H.-L.M. Ultrashort Echo Time for Improved Positive-Contrast Manganese-Enhanced MRI of Cancer. PLoS ONE 2013, 8, e58617. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Hou, P.; Zhang, M.; Xu, K. One-Pot Preparation of Hydrophilic Manganese Oxide Nanoparticles as T1 Nano-Contrast Agent for Molecular Magnetic Resonance Imaging of Renal Carcinoma in Vitro and in Vivo. Biosens. Bioelectron. 2018, 102, 1–8. [Google Scholar] [CrossRef]
- Podyablonsky, A.S.; Belyanin, M.L.; Borodin, O.Y.; Belousov, M.V.; Brazovskiy, K.S.; Krivoshchekov, S.V.; Ussov, W.Y.; Shimanovskii, N.L. Paramagnetic Contrast Enhancement in MRI Imaging of Liver Using an Original Hepatotropic High-Affinity Agent GDOF-Mn-DTPA. Transl. Med. 2021, 8, 14–22. [Google Scholar] [CrossRef]
- Mnasri, W.; Parvizian, M.; Ammar-Merah, S. Design and Synthesis of Luminescent Lanthanide-Based Bimodal Nanoprobes for Dual Magnetic Resonance (MR) and Optical Imaging. Nanomaterials 2021, 11, 354. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; Zhao, S.; Xiao, J.F.; Lan, M.; Wang, B.; Zhang, K.; Song, X.; Zeng, L. Metal Ions-Doped Carbon Dots: Synthesis, Properties, and Applications. J. Chem. Eng. 2022, 430, 133101. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, Y.; Ding, D.; Lin, H.; Sun, W.; Wang, G.D.; Huang, W.; Zhang, W.; Lee, D.; Liu, G.; et al. Gadolinium-Encapsulated Graphene Carbon Nanotheranostics for Imaging-Guided Photodynamic Therapy. Adv. Mater. 2018, 30, 1802748. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yu, N.; Han, C.; Xie, T.; Dou, B.; Kong, Y.; Zuo, F.; Shi, M.; Xu, K. Preparation of Gadolinium Doped Carbon Dots for Enhanced MR Imaging and Cell Fluorescence Labeling. Biochem. Biophys. Res. Commun. 2019, 511, 207–213. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Zhang, D.; Gan, L.; Zhao, P.; Zhang, Y.; Zhang, Q.; Hua, M.; Jia, C. Gadolinium-Doped Carbon Quantum Dots Loaded Magnetite Nanoparticles as a Bimodal Nanoprobe for Both Fluorescence and Magnetic Resonance Imaging. Magn. Reason. Imaging 2020, 68, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cardo, L.; Martínez-Parra, L.; Cesco, M.; Echeverría-Beistegui, B.M.; Martínez-Moro, M.; Herrero-Álvarez, N.; Cabrerizo, M.; Carregal-Romero, S.; Ramos-Cabrer, P.; Ruiz-Cabello, J.; et al. Luminescent Carbon Nanodots Doped with Gadolinium (III): Purification Criteria, Chemical and Biological Characterization of a New Dual Fluorescence/MR Imaging Agent. Small 2023, 2206442. [Google Scholar] [CrossRef] [PubMed]
- Das, G.K.; Johnson, N.J.J.; Cramen, J.; Blasiak, B.; Latta, P.; Tomanek, B.; van Veggel, F.C.J.M. NaDyF 4 Nanoparticles as T 2 Contrast Agents for Ultrahigh Field Magnetic Resonance Imaging. J. Phys. Chem. Lett. 2012, 3, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Samanta, T.; Hazra, C.; Mahalingam, V. C-Dot Sensitized Eu 3+ Luminescence from Eu 3+ -Doped LaF 3 –C Dot Nanocomposites. NJC 2015, 39, 106–109. [Google Scholar] [CrossRef]
- Wu, F.; Yue, L.; Yang, L.; Wang, K.; Liu, G.; Luo, X.; Zhu, X. Ln(III) Chelates-Functionalized Carbon Quantum Dots: Synthesis, Optical Studies and Multimodal Bioimaging Applications. Colloids Surf. B Biointerfaces 2019, 175, 272–280. [Google Scholar] [CrossRef]
- Ji, Z.; Ai, P.; Shao, C.; Wang, T.; Yan, C.; Ye, L.; Gu, W. Manganese-Doped Carbon Dots for Magnetic Resonance/Optical Dual-Modal Imaging of Tiny Brain Glioma. ACS Biomater. Sci. Eng. 2018, 4, 2089–2094. [Google Scholar] [CrossRef]
- Jia, Q.; Ge, J.; Liu, W.; Zheng, X.; Chen, S.; Wen, Y.; Zhang, H.; Wang, P. A Magnetofluorescent Carbon Dot Assembly as an Acidic H2O2-Driven Oxygenerator to Regulate Tumor Hypoxia for Simultaneous Bimodal Imaging and Enhanced Photodynamic Therapy. Adv. Mater. 2018, 30, 1706090. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, T.; Sheng, B.; Wu, F.; Zhang, Q.; Wang, W.; Shen, J.; Zhou, N.; Sun, Y. Mn2+ Complex-Modified Polydopamine- and Dual Emissive Carbon Dots Based Nanoparticles for in Vitro and in Vivo Trimodality Fluorescent, Photothermal, and Magnetic Resonance Imaging. J. Chem. Eng. 2019, 373, 1054–1063. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, L.; Wu, D.; Zhang, H.; Lian, H.; Zhao, X.; Wu, A.; Zeng, L. Manganese-Doped Carbon Dots with Redshifted Orange Emission for Enhanced Fluorescence and Magnetic Resonance Imaging. ACS Appl. Bio. Mater. 2021, 4, 1969–1975. [Google Scholar] [CrossRef]
- Niu, D.; Luo, X.; Li, Y.; Liu, X.; Wang, X.; Shi, J. Manganese-Loaded Dual-Mesoporous Silica Spheres for Efficient T1- and T2-Weighted Dual Mode Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2013, 5, 9942–9948. [Google Scholar] [CrossRef]
- Torkashvand, N.; Sarlak, N. Fabrication of a Dual T1 and T2 Contrast Agent for Magnetic Resonance Imaging Using Cellulose Nanocrystals/Fe3O4 Nanocomposite. Eur. Polym. J. 2019, 118, 128–136. [Google Scholar] [CrossRef]
- Pan, C.; Lin, J.; Zheng, J.; Liu, C.; Yuan, B.; Akakuru, O.U.; Zubair Iqbal, M.; Fang, Q.; Hu, J.; Chen, J.; et al. An Intelligent T1–T2 Switchable MRI Contrast Agent for the Non-Invasive Identification of Vulnerable Atherosclerotic Plaques. Nanoscale 2021, 13, 6461–6474. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, S.; Hu, Y.; Deng, T.; Li, J. Microemulsion-Confined Biomineralization of PEGylated Ultrasmall Fe3O4 Nanocrystals for T2-T1 Switchable MRI of Tumors. Anal. Chem. 2021, 93, 14223–14230. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-K.; Tsai, W.-T. Effects of Temperature and Concentration on the Structure and Specific Capacitance of Manganese Oxide Deposited in Manganese Acetate Solution. J. Appl. Electrochem. 2004, 34, 953–961. [Google Scholar] [CrossRef]
- Outram, J.G.; Couperthwaite, S.J.; Millar, G.J. Investigation of Manganese Greensand Activation by Various Oxidants. J. Env. Chem. Eng. 2018, 6, 4130–4143. [Google Scholar] [CrossRef]
- Oku, M. X-ray Photoelectron Spectra of KMnO4 and K2MnO4 Fractured in Situ. J. Electron Spectros. Relat. Phenom. 1995, 74, 135–148. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Wang, B.; Wei, Z.; Sui, L.; Yu, J.; Zhang, B.; Wang, X.; Feng, S.; Song, H.; Yong, X.; Tian, Y.; et al. Electron–Phonon Coupling-Assisted Universal Red Luminescence of o-Phenylenediamine-Based Carbon Dots. Light. Sci. Appl. 2022, 11, 172. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, B.; Ushakova, E.V.; He, B.; Xing, G.; Tang, Z.; Rogach, A.L.; Qu, S.; Zhang, B.; Wang, B.; et al. Assignment of Core and Surface States in Multicolor-Emissive Carbon Dots. Small 2022, 2204158. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef] [PubMed]
- Tawagi, E.; Vollett, K.D.W.; Szulc, D.A.; Santerre, J.P.; Cheng, H.M. In Vivo MRI Tracking of Degradable Polyurethane Hydrogel Degradation In Situ Using a Manganese Porphyrin Contrast Agent. JMRI, 2023; in print. [Google Scholar] [CrossRef] [PubMed]
- Tiron, A.; Stan, C.S.; Luta, G.; Uritu, C.M.; Vacarean-Trandafir, I.-C.; Stanciu, G.D.; Coroaba, A.; Tiron, C.E. Manganese-Doped N-Hydroxyphthalimide-Derived Carbon Dots—Theranostics Applications in Experimental Breast Cancer Models. Pharmaceutics 2021, 13, 1982. [Google Scholar] [CrossRef] [PubMed]
- Pintaske, J.; Martirosian, P.; Graf, H.; Erb, G.; Lodemann, K.P.; Claussen, C.D.; Schick, F. Relaxivity of Gadopentetate Dimeglumine (Magnevist), Gadobutrol (Gadovist), and Gadobenate Dimeglumine (MultiHance) in Human Blood Plasma at 0.2, 1.5, and 3 Tesla. Investig. Radiol. 2006, 41, 213–221. [Google Scholar] [CrossRef]
- Anbu, S.; Hoffmann, S.H.L.; Carniato, F.; Kenning, L.; Price, T.W.; Prior, T.J.; Botta, M.; Martins, A.F.; Stasiuk, G.J. A Single-Pot Template Reaction towards a Manganese-Based T1 Contrast Agent. Angew. Chem. Int. Ed. 2021, 60, 10831–10839. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Chen, X.; Xu, Y.; Li, H. Mn(II)-Coordinated Fluorescent Carbon Dots: Preparation and Discrimination of Organic Solvents. Opt. Mater. 2018, 78, 118–125. [Google Scholar] [CrossRef]
- Lee, B.H.; Hasan, M.T.; Lichthardt, D.; Gonzalez-Rodriguez, R.; Naumov, A.V. Manganese-Nitrogen and Gadolinium-Nitrogen Co-Doped Graphene Quantum Dots as Bimodal Magnetic Resonance and Fluorescence Imaging Nanoprobes. Nanotechnology 2021, 32, 095103. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Gedda, G.; Girma, W.M.; Yen, C.L.; Ling, Y.C.; Chang, J.Y. Magnetofluorescent Carbon Dots Derived from Crab Shell for Targeted Dual-Modality Bioimaging and Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 13887–13899. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanidenko, E.A.; Vedernikova, A.A.; Badrieva, Z.F.; Brui, E.A.; Ondar, S.O.; Miruschenko, M.D.; Volina, O.V.; Koroleva, A.V.; Zhizhin, E.V.; Ushakova, E.V. Manganese-Doped Carbon Dots as a Promising Nanoprobe for Luminescent and Magnetic Resonance Imaging. Photonics 2023, 10, 757. https://doi.org/10.3390/photonics10070757
Stepanidenko EA, Vedernikova AA, Badrieva ZF, Brui EA, Ondar SO, Miruschenko MD, Volina OV, Koroleva AV, Zhizhin EV, Ushakova EV. Manganese-Doped Carbon Dots as a Promising Nanoprobe for Luminescent and Magnetic Resonance Imaging. Photonics. 2023; 10(7):757. https://doi.org/10.3390/photonics10070757
Chicago/Turabian StyleStepanidenko, Evgeniia A., Anna A. Vedernikova, Zilya F. Badrieva, Ekaterina A. Brui, Saikho O. Ondar, Mikhail D. Miruschenko, Olga V. Volina, Aleksandra V. Koroleva, Evgeniy V. Zhizhin, and Elena V. Ushakova. 2023. "Manganese-Doped Carbon Dots as a Promising Nanoprobe for Luminescent and Magnetic Resonance Imaging" Photonics 10, no. 7: 757. https://doi.org/10.3390/photonics10070757
APA StyleStepanidenko, E. A., Vedernikova, A. A., Badrieva, Z. F., Brui, E. A., Ondar, S. O., Miruschenko, M. D., Volina, O. V., Koroleva, A. V., Zhizhin, E. V., & Ushakova, E. V. (2023). Manganese-Doped Carbon Dots as a Promising Nanoprobe for Luminescent and Magnetic Resonance Imaging. Photonics, 10(7), 757. https://doi.org/10.3390/photonics10070757





