Abstract
The quality of synthesized materials is affected by various factors such as the prehistory of substances used and the synthesis technology. Most methods for synthesizing luminescent ceramics based on metal oxides rely on high-temperature heating to facilitate the necessary exchange of elements between precursor particles. However, a promising alternative method involves the direct application of a powerful high-energy radiation flux, which stimulates different processes. The formation of ceramics through this method occurs in a highly ionized medium, which may produce different results from those achieved through thermal exposure. This paper reports the findings of a study that explores the relationship between the morphology and luminescent properties of YAG:Ce ceramics and the characteristics of Y2O3 and Al2O3 oxides used in the synthesis, such as dispersity and activator concentration. The results indicate that the morphology of the synthesized ceramic samples is significantly affected by the dispersity of the powder mixture used.
1. Introduction
Phosphors based on yttrium aluminum garnet doped with cerium—YAG:Ce (Y3A5O12:Ce)—are highly promising for use in light-emitting diodes (LEDs) [1,2]. LEDs incorporating YAG:Ce phosphors have demonstrated luminous efficiency (LE) of up to 150 lm/W, with a theoretical maximum of 250–300 lm/W [3]. The improved conversion efficiency of UV radiation from the LED chip to light by the phosphor could lead to a significant increase in luminous efficiency. Therefore, there is considerable research underway to discover new and effective phosphors, with a focus on metal oxides.
Typically, phosphors are complex, multiphase materials containing activators and modifiers. The synthesis of refractory oxide materials is time-consuming and labor-intensive, and various substances are used to facilitate the synthesis process. These substances must subsequently be removed from the formed phosphor, adding further complexity to the process. Technological challenges make it difficult to optimize promising phosphors and synthesis processes, and thus research constantly focuses on improving existing technologies and developing new ones [4,5,6,7,8,9,10]. Novel methods, such as combustion [11,12] and spark plasma sintering (SPS) [13,14], have been developed to accelerate synthesis rates. However, these methods have the disadvantage of utilizing additional substances during synthesis, leading to uncontrolled amounts of remaining elements that can affect functional properties.
Recent studies have shown that YAG:Ce ceramics can be synthesized from refractory metal oxides using a powerful electron beam without the need for additional substances to facilitate the synthesis [15,16]. These studies have demonstrated the possibility of synthesizing ceramics in times shorter than 1 s, making it a highly attractive method for synthesizing YAG:Ce phosphors. Moreover, it has been found that the fusion processes induced by radiation are fundamentally different from thermally stimulated ones. The properties of the initial substances, including their phase state, purity, and morphology, strongly influence the synthesis results, making the optimization of radiation-assisted synthesis a complex and multifactorial task that requires numerous experimental studies.
This work is focused on investigating the dependence of the results of radiation-assisted synthesis on the prehistory of precursors. It is essential to understand the underlying mechanisms of radiation-assisted synthesis to optimize the process and improve the quality of synthesized YAG:Ce phosphors. The findings of this study could lead to the development of more effective synthesis methods and help to overcome the technological challenges associated with synthesizing promising phosphors based on metal oxides.
2. Materials and Methods
2.1. Materials
The existing research on the radiation-assisted synthesis of YAG:Ce ceramics has demonstrated that the resultant morphology and luminescent properties are contingent upon the prehistory of the chosen raw materials. To elucidate the underlying patterns of this dependency, a novel method is proposed.
Initially, the raw materials were selected based on the superior, albeit diverse, properties observed in prior experiments. These materials included Y2O3 grades ITO/I and ITO/V, Al2O3 grades F800–F1200, and nanopowders. A concise description of the employed materials is provided in Table 1.

Table 1.
Information about the used oxide materials.
A homogeneous mixture was prepared for the synthesis, consisting of Al2O3 (43%) and Y2O3 (57%). During the preparation process, Ce2O3 was introduced to the mixture in the form of Ce2O3 powder, constituting 0.1–1% of the total mass of the primary composition, to obtain activated ceramics. No additional substances were incorporated into the mixture.
To account for the potential influence of irradiation regimes, a series of experiments were conducted under identical conditions for the synthesis of the ceramics. Throughout the synthesis in a crucible, a range of samples was generated, numbering from one to 10–15. The table delineates the sample series numbers by the classification system adopted by the authors.
Before synthesis, the morphology of the used Y2O3 and Al2O3 powders was studied using the µVizo (LOMO) optical miMicroVisionImages of samples were taken with an optical microscope at magnifications of 10×, 40×, and 100×. The research results are shown in Figure 1.
The experimental data obtained on the granulometric composition of yttrium oxide (Y2O3) and aluminum oxide (Al2O3) particles are found to be in good agreement with the information provided in the respective material passports. A significant portion of Y2O3 particles falls within the size range of 2–4 µm, which is consistent with the passport data. Although larger particles may possess greater mass, this observation does not contradict the presented information. The particle sizes of Al2O3 grades F600–F1200 also concur with the passport data. These particles exhibit fragment-like shapes with often pronounced facets, indicating a crystalline structure. Conversely, nanooxide powders display significantly different morphologies, resembling accumulations of nanoparticles or droplets. These particles do not exhibit microcrystalline characteristics, with droplet sizes ranging from a few units up to 10 microns.
2.2. Synthesis
For the synthesis process, a prepared mixture was poured into a massive copper crucible to facilitate heat dissipation from the emerging ceramics. The crucible dimensions were as follows: a base area of 120 × 50 mm2 and a height of 40 mm. The synthesis was carried out by directly applying an electron beam from the ELV-6 accelerator of the INP SB RAS, named after Budker, with an energy of 1.4 MeV and a power density of 18–25 kW/cm2 on the mixture near the target surface. The Gaussian beam was scanned transversely relative to the crucible at a frequency of 50 Hz and an amplitude of 50 mm. The crucible was moved relative to the scanning beam at a speed of 10 mm/s, with a total electron flux exposure time of 10 s on the charge within the crucible.
A total of 19 ceramic mixture variants, derived from Y2O3 and Al2O3 powders with different prehistories (as indicated in Table 1), were synthesized. Each variant produced several complex-shaped ceramic samples within the crucible. Series 276–280, 284–287, 290–291, and 299–300 were synthesized under identical conditions to assess potential differences caused by unaccounted factors. The remaining series were synthesized from Y2O3 and Al2O3 powders with differing prehistories or activator concentrations. All synthesis experiments maintained consistent radiation exposure conditions: an electron energy of 1.4 MeV and an electron beam power density of 25 kW/cm2 at the target surface. Figure 2 illustrates examples of the obtained sample series in crucibles and randomly selected samples from each series.
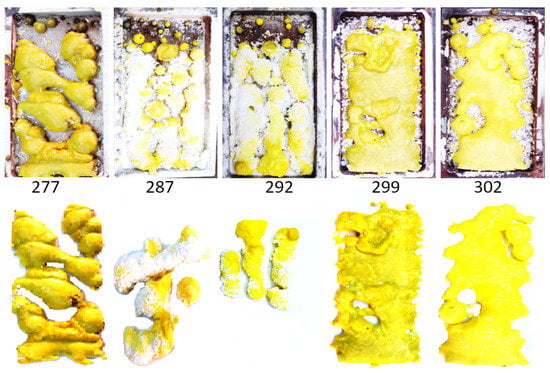
Figure 2.
Images of some series of samples in crucibles (upper row) and examples of samples of these series (lower row) synthesized from Y2O3 and Al2O3 powders of a different prehistory.
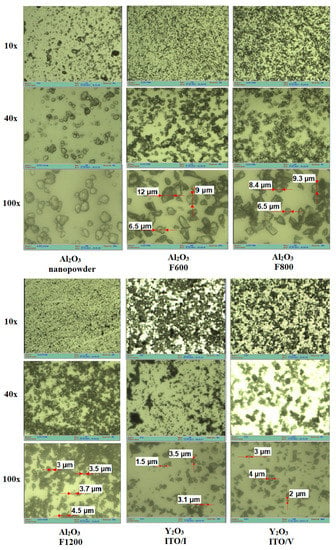
Figure 1.
Micrographs of the Y2O3 and Al2O3 powders used for the synthesis.
2.3. Methods
The dependence of the luminescence and excitation spectra of samples of YAG:Ce ceramics prepared by radiation-assisted synthesis from oxide materials with different prehistory has been studied. The measurements were performed using an SM 2203 SOLAR spectrophotometer. To conduct the measurements, several sampling operations were performed. The samples were mechanically crushed, and the resulting phosphor powder was poured into the cuvette of a spectrophotometer.
To assess the conversion efficiency of excitation radiation into luminescence, we measured the brightness of a series of synthesized ceramic samples using an experimental setup based on a CS-200 luminance colorimeter. All optical elements of the setup were mounted on a rigid base, ensuring that the mutual position of the elements remained constant. The power supply for the excitation sources and light receivers was provided by stabilized sources, which did not change during each measurement series. Measurements were conducted under excitation by LED chip radiation at 450 nm. In brightness measurements, the angle of view was 0.2°. The scattered light background brightness was determined by the measurements and typically did not exceed 1% of the phosphor brightness.
3. Results
Figure 3 shows the luminescence and excitation spectra (insets) of YAG ceramic samples synthesized using initial materials with distinct prehistories from the four most characteristic groups described in Table 1. The similarity of all luminescence spectra presented in Figure 3 suggests that the spectra’s shape is independent of the yttrium oxide utilized. Y2O3 powders had sizes of 2–4 microns and varied in purity levels: samples 270, 284, and 289 on one side, and the rest on the other. The spectra’s shape was not influenced by the initial particle size of aluminum oxide: samples 276 and 300 were derived from powders with grain sizes of 9.5–6.5 µm and 4.5–3 µm, while samples 289 and 291 were obtained from powders with grain sizes of 1 nm–200 nm. Moreover, the spectra’s shape was unaffected by the activation impurity introduced (samples 300, 301, 302).
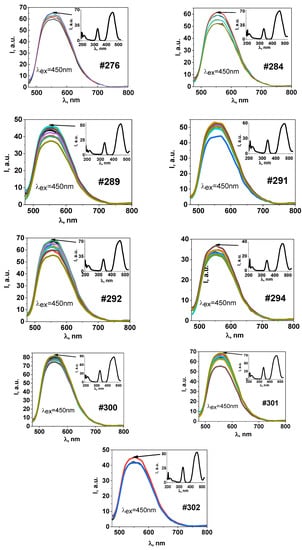
Figure 3.
Luminescence and excitation spectra (insets) of YAG:Ce ceramic samples.
As a result, the shape of the luminescence spectra of the ceramic samples remained consistent regardless of the initial material’s purity, as long as the purity was at least 99%. The spectra’s shape was also independent of the powder grain morphology used for synthesis and the concentration of the introduced activation impurity, ranging from 0.2–1.0%. Notably, there was a difference in the intensities’ ratio in the region of 340 and 450 nm; however, the dependence of this ratio on the properties of the starting materials could not be determined.
Absolute measurements of excitation fluxes are only feasible for narrow, specific tasks. Measuring and comparing fluxes that vary in intensity and spectrum is generally impracticable. Relative measurements of radiation fluxes can be performed by comparing them to known sources, which are taken as standards. Such sources can include commercial phosphors, which are manufactured according to strictly controlled technologies, ensuring their luminescent properties remain constant over time.
Our results demonstrate that the relative brightness values of the tested phosphors are directly proportional to their flux (1):
where Φe1, Φe2, Φν1, Φν2, L1, L2 are the energy and light fluxes, the brightness of the test and reference phosphors; φ(λ) and ν(λ) are the relative spectral density and spectral luminous efficiency of radiation. These are essential parameters for evaluating the conversion efficiency of excitation radiation into luminescence. This condition is met when the luminescence spectra of the test and reference phosphors are similar, the measurement geometry remains constant, and the electrical characteristics of the measuring systems are unchanged.
The relative conversion efficiency of chip radiation into luminescence was measured for all sample series listed in Table 1. To measure the brightness, the ceramic samples were mechanically crushed. The resulting powders were poured into 1 mm deep cuvettes, and the powder surface was leveled. Each sample was measured ten times, and the average values were determined.
The relative brightness values of all sample series are presented in Figure 4. The relative brightness values of commercial luminophores YAG-01 and YAG-02, which were used as references, are also included. The measurements demonstrate consistent conversion efficiency across the samples, providing valuable insights into the performance of synthesized ceramic samples in terms of luminescence and excitation radiation conversion.
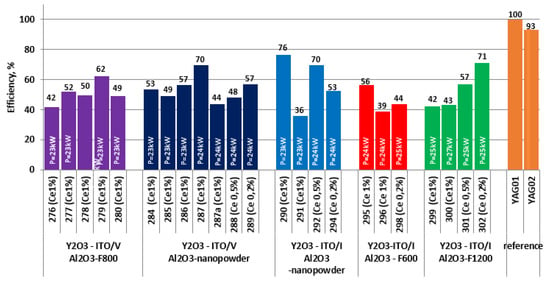
Figure 4.
Relative luminescence brightness of the studied ceramic samples upon LED 450 nm excitation.
4. Discussion
We have determined that the overall luminescence brightness of samples prepared by radiation-assisted synthesis is comparable to reference samples. For instance, the brightness of sample #290 is 76%, #292 is 70%, and #302 is 71% of the maximum value of the reference phosphor. The minimum values of the luminescence brightness of the synthesized samples reach 36%. There is significant variability in luminescence brightness values for samples with the same initial compositions, but there is no substantial difference between brightness sets of samples with different initial precursors.
The large variability in measured brightness values for synthesized samples is primarily due to the uncontrolled presence of residue from the mixture of oxides adhering to the ceramics. The presented results do not reveal any clear dependence of brightness on the activator concentration.
The obtained results suggest that certain factors can impact the efficiency of converting chip radiation into luminescence. In our case, the insufficient cleaning of samples from adhering charged particles may be one such factor. Y2O3 and Al2O3 powders, when excited at 450 nm, do not luminesce, but scatter the exciting radiation. This likely explains the absence of a regular pattern in the dependence of brightness on activator concentration.
The lack of a pronounced dependence on the conversion efficiency of LED radiation into luminescence on the particle size distribution of precursors is well supported by the results displayed in Figure 4. The relative luminescence brightness of ceramics obtained from precursors with distinctly different particle size distributions falls within the same range of values.
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
5. Conclusions
The conducted studies examining the dependence of morphology and luminescent properties of YAG:Ce ceramics on the prehistory of the initial substances have unveiled two crucial effects that could enhance the synthesis process.
The morphology of the synthesized ceramic samples is primarily influenced by the prehistory of the initial Y2O3 and Al2O3 oxide powders, specifically their grain size. Large samples, with dimensions up to 10 cm2 and thicknesses ranging from 0.6 to 0.8 cm, can be effectively produced using powders with diverse grain sizes greater than 1 µm. Conversely, smaller samples are obtained from nanopowders. The most likely cause for this effect is the dispersion of fine charge fractions due to the electron flux charging. It is important to note that the morphology of the synthesized samples is not affected by the concentration of introduced activators, as long as it remains below 1% and the purity of starting materials is above 99% regarding Y2O3 and Al2O3.
The luminescent properties of the synthesized ceramic samples, such as excitation luminescence spectra and conversion efficiency of chip radiation into luminescence, are not influenced by the prehistory of the initial Y2O3 and Al2O3 powders. In every instance, ceramics containing YAG crystallites activated by cerium exhibit a consistent structure in the radiation field. This uniformity can be attributed to the synthesis processes occurring within the high-energy electron flux beam. Synthesis takes place in the region where the electron beam impact leads to the maximum density of absorbed energy and the presence of elements constituting the bulk of the mixture. This conclusion is consistent for both nanopowder-based synthesis and the synthesis from larger grain sizes. It is likely that in the region with the highest absorbed energy density [14,15,16,17,18,19,20,21], the rate of new YAG phase formation is so rapid that charged particles do not have the opportunity to leave the reactor zone before the decomposition of the initial particles occurs.
Author Contributions
Conceptualization, V.M.L.; methodology, V.M.L. and M.G.G.; validation, Z.S.Z. and V.M.L.; formal analysis, A.M.Z.; investigation, A.M.Z.; resources, M.G.G.; data curation, Z.S.Z.; writing—original draft preparation, V.M.L.; writing—review and editing, V.M.L., A.T.T. and Z.T.K.; visualization, Z.S.Z.; supervision, Z.S.Z.; project administration, V.M.L., Z.T.K. and A.M.Z.; funding acquisition, V.M.L., Z.T.K. and A.M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP14871114). This research was funded by the Russian Science Foundation of the Russian Federation. (Grant No. 23-73-00108).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ueda, J.; Tanabe, S. Review of Luminescent Properties of Ce3+-Doped Garnet Phosphors: New Insight into the Effect of Crystal and Electronic Structure. Opt. Mater. X 2019, 1, 100018. [Google Scholar] [CrossRef]
- Smet, P.; Parmentier, A.; Poelman, D. Selecting Conversion Phosphors for White Light-Emtting Diodes. J. Electrochem. Soc. 2011, 158, R37–R54. [Google Scholar] [CrossRef]
- Ju, Y.; Lisitsyn, V.; Lukash, V.S. Losses of Energy in Phosphor of LED at Transformation of Emission Spectrum. DEStech Trans. Eng. Technol. Res. 2017, 794–799. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, M.; Su, Q. Comparative Investigation on Synthesis and Photoluminescence of YAG:Ce Phosphor. Mater. Sci. Eng. B 2004, 106, 251. [Google Scholar] [CrossRef]
- Xia, Z.; Meijerink, A. Ce3+-Doped Garnet Phosphors: Composition Modification, Luminescence Properties and Applications. Chem. Soc. Rev. 2017, 46, 275–299. [Google Scholar] [CrossRef]
- Sharma, S.K.; James, J.; Gupta, S.K.; Hussain, S. UV-A, B, C Emitting Persistent Luminescent Materials. Materials 2022, 16, 236. [Google Scholar] [CrossRef]
- Marius, M.; Popovici, E.-J.; Barbu-Tudoran, L.; Indrea, E.; Mesaros, A. Cerium-Doped Yttrium Aluminate-Based Phosphors Prepared by Wet-Chemical Synthesis Route: Modulation of the Luminescence Color by Changing the Host-Lattice Composition. Ceram. Int. 2014, 40, 6233–6239. [Google Scholar] [CrossRef]
- Murai, S.; Fujita, K.; Iwata, K.; Tanaka, K. Scattering-Based Hole Burning in Y3Al5O12:Ce3+ Monoliths with Hierarchical Porous Structures Prepared via the Sol–Gel Route. J. Phys. Chem. C 2011, 115, 17676–17681. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Z.-J.; Zhao, J.; Zhang, J.; Liu, Z. Low Temperature Synthesis of Monodispersed YAG:Eu Crystallites by Hydrothermal Method. J. Alloy. Compd. 2015, 647, 1075–1080. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Pankratova, V.; Popov, A.I.; Pankratov, V. Study of Phase Composition, Photocatalytic Activity, and Photoluminescence of TiO2 with Eu Additive Produced by the Extraction-Pyrolytic Method. J. Mater. Res. Technol. 2021, 13, 2350–2360. [Google Scholar] [CrossRef]
- Huczko, A.; Kurcz, M.; Baranowski, P.; Bystrzejewski, M.; Bhattarai, A.; Dyjak, S.; Bhatta, R.; Pokhrel, B.; Kafle, B.P. Fast Combustion Synthesis and Characterization of YAG:Ce3+ Garnet Nanopowders. Phys. Status Solidi (B) 2013, 250, 2702–2708. [Google Scholar] [CrossRef]
- Ohyama, J.; Zhu, C.; Saito, G.; Haga, M.; Nomura, T.; Sakaguchi, N.; Akiyama, T. Combustion Synthesis of YAG:Ce Phosphors via the Thermite Reaction of Aluminum. J. Rare Earths 2018, 36, 248–256. [Google Scholar] [CrossRef]
- Serrano-Bayona, R.; Chu, C.; Liu, P.; Roberts, W.L. Flame Synthesis of Carbon and Metal-Oxide Nanoparticles: Flame Types, Effects of Combustion Parameters on Properties and Measurement Methods. Materials 2023, 16, 1192. [Google Scholar] [CrossRef] [PubMed]
- Le Godec, Y.; Le Floch, S. Recent Developments of High-Pressure Spark Plasma Sintering: An Overview of Current Applications, Challenges and Future Directions. Materials 2023, 16, 997. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyn, V.; Tulegenova, A.; Kaneva, E.; Mussakhanov, D.; Gritsenko, B. Express Synthesis of YAG: Ce Ceramics in the High-Energy Electrons Flow Field. Materials 2023, 16, 1057. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyn, V.; Lisitsyna, L.; Golkovskii, M.; Mussakhanov, D.; Ermolaev, A. Formation of Luminescing High-Temperature Ceramics upon Exposure to Powerful High-Energy Electron Flux. Russ. Phys. J. 2021, 63, 1615–1621. [Google Scholar] [CrossRef]
- Karipbayev, Z.T.; Lisitsyn, V.M.; Mussakhanov, D.A.; Alpyssova, G.K.; Popov, A.I.; Polisadova, E.F.; Elsts, E.; Akilbekov, A.T.; Kukenova, A.B.; Kemere, M.; et al. Time-Resolved Luminescence of YAG:Ce and YAGG:Ce Ceramics Prepared by Electron Beam Assisted Synthesis. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 479, 222–228. [Google Scholar] [CrossRef]
- Polisadova, E.; Valiev, D.; Vaganov, V.; Oleshko, V.; Han, T.; Zhang, C.; Burachenko, A.; Popov, A. Time-Resolved Cathodoluminescence Spectroscopy of YAG and YAG:Ce3+ Phosphors. Opt. Mater. 2019, 96, 109289. [Google Scholar] [CrossRef]
- Lisitsyn, V.; Lisitsyna, L.; Dauletbekova, A.; Golkovskii, M.; Karipbayev, Z.; Musakhanov, D.; Akilbekov, A.; Zdorovets, M.; Kozlovskiy, A.; Polisadova, E. Luminescence of the Tungsten-Activated MgF2 Ceramics Synthesized under the Electron Beam. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 435, 263–267. [Google Scholar] [CrossRef]
- Lisitsyn, V.; Golkovskii, M.; Lisiysyna, L.; Dauletbekova, A.; Mussakhanov, D.; Vaganov, V.; Tulegenova, A.; Karipbayev, Z. MgF2-Based Luminescing Ceramics. Russ. Phys. J. 2019, 61, 1908–1913. [Google Scholar] [CrossRef]
- Lisitsyn, V.; Lisitsyna, L.; Tulegenova, A.; Ju, Y.; Polisadova, E.; Lipatov, E.; Vaganov, V. Nanodefects in YAG:Ce-Based Phosphor Microcrystals. Crystals 2019, 9, 476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).