A Facile Aqueous Solution Route for the Growth of Chalcogenide Perovskite BaZrS3 Films
Abstract
1. Introduction
2. Materials and Methods
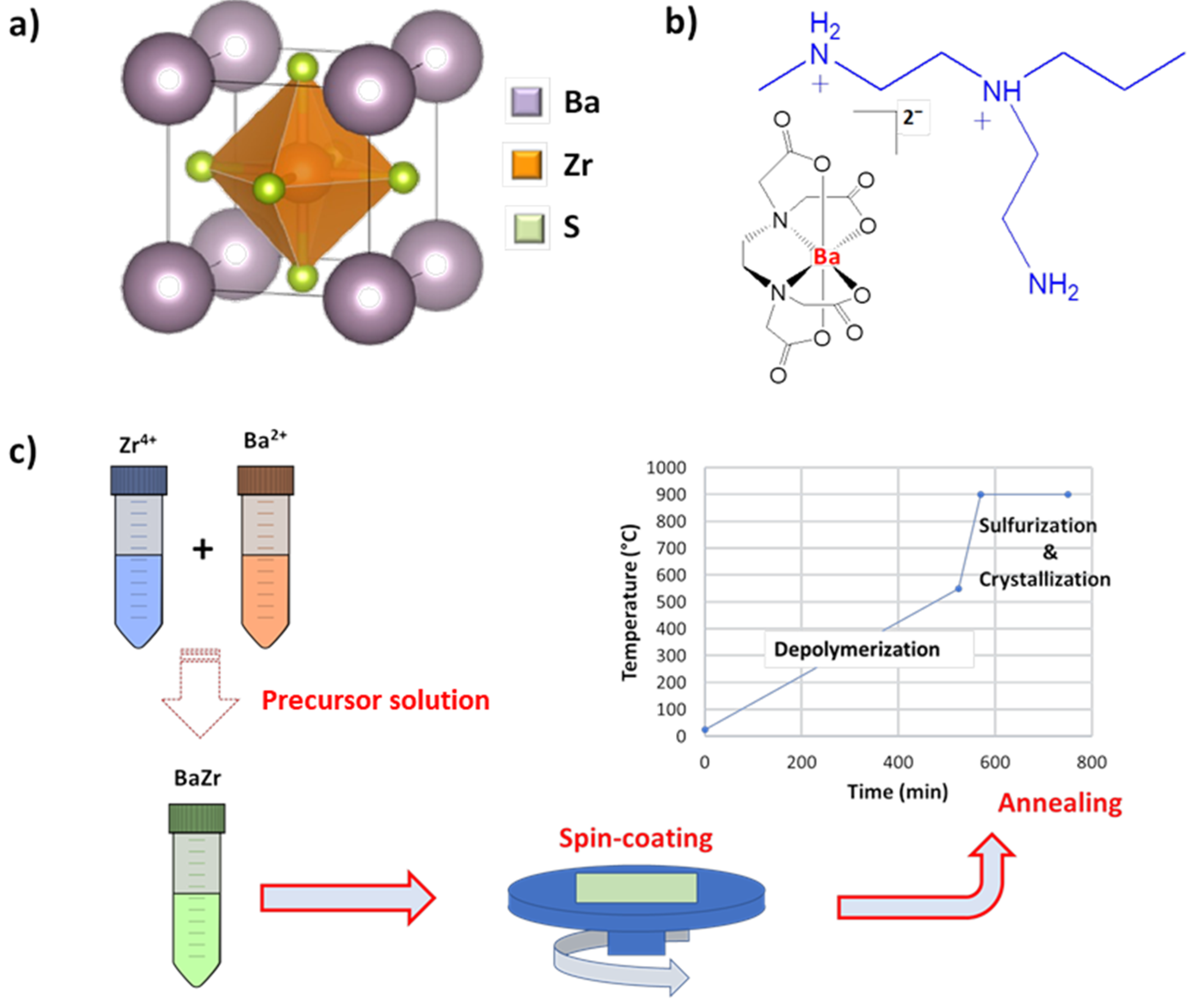
2.1. Precursor Synthesis and BZS Film Growth
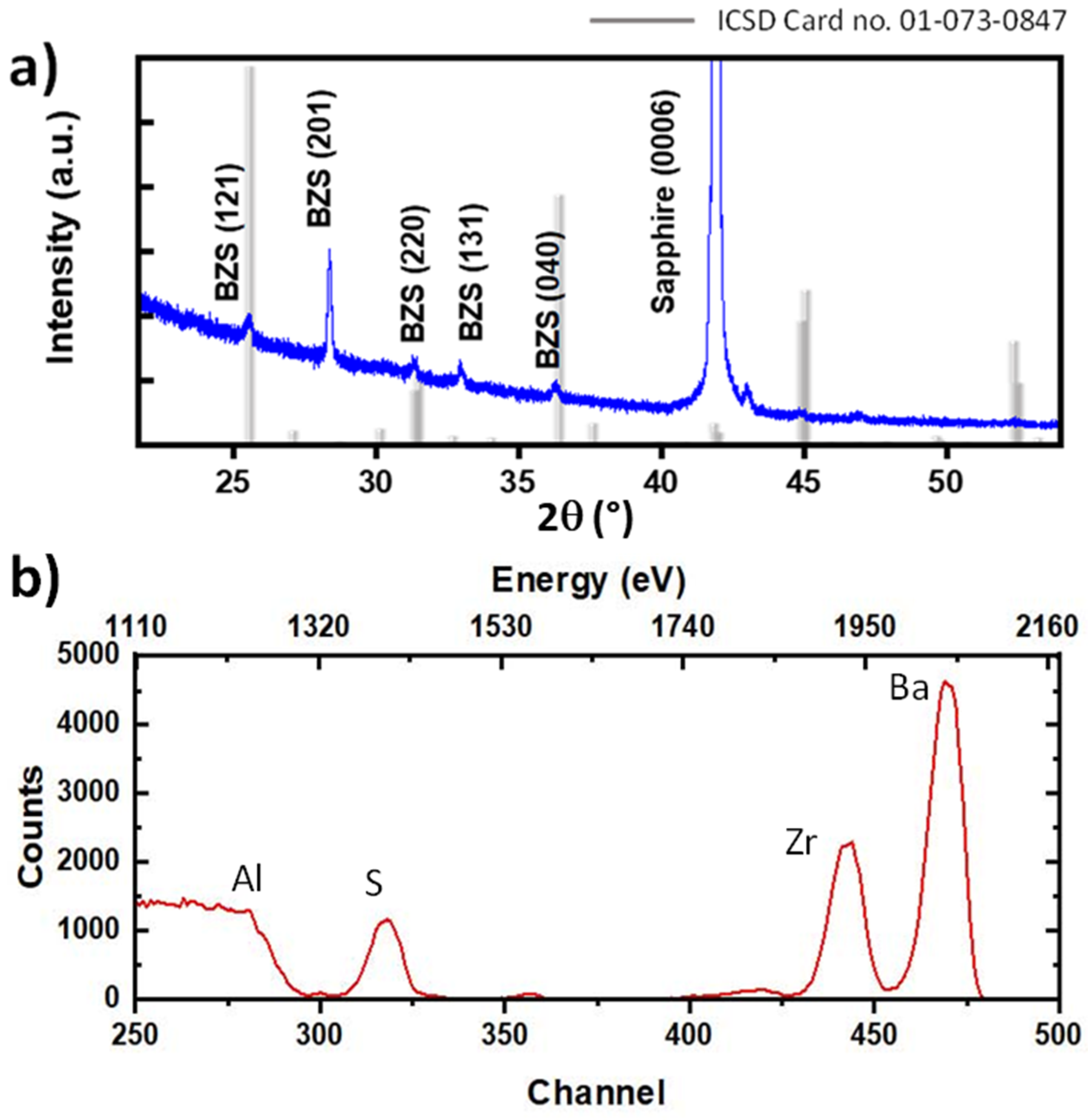
2.2. Characterization of Structure and Chemical Composition of the BZS Films
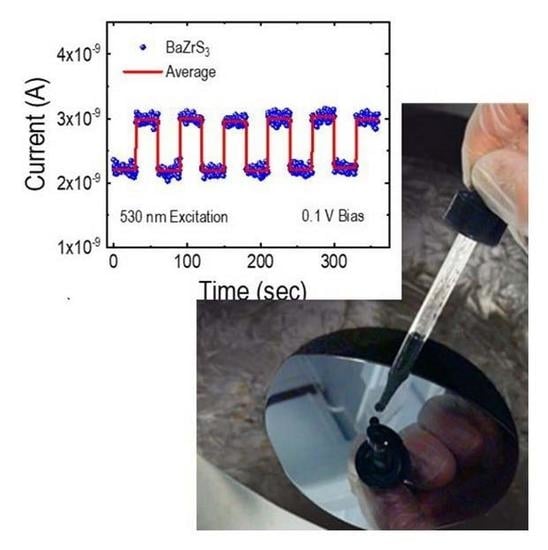
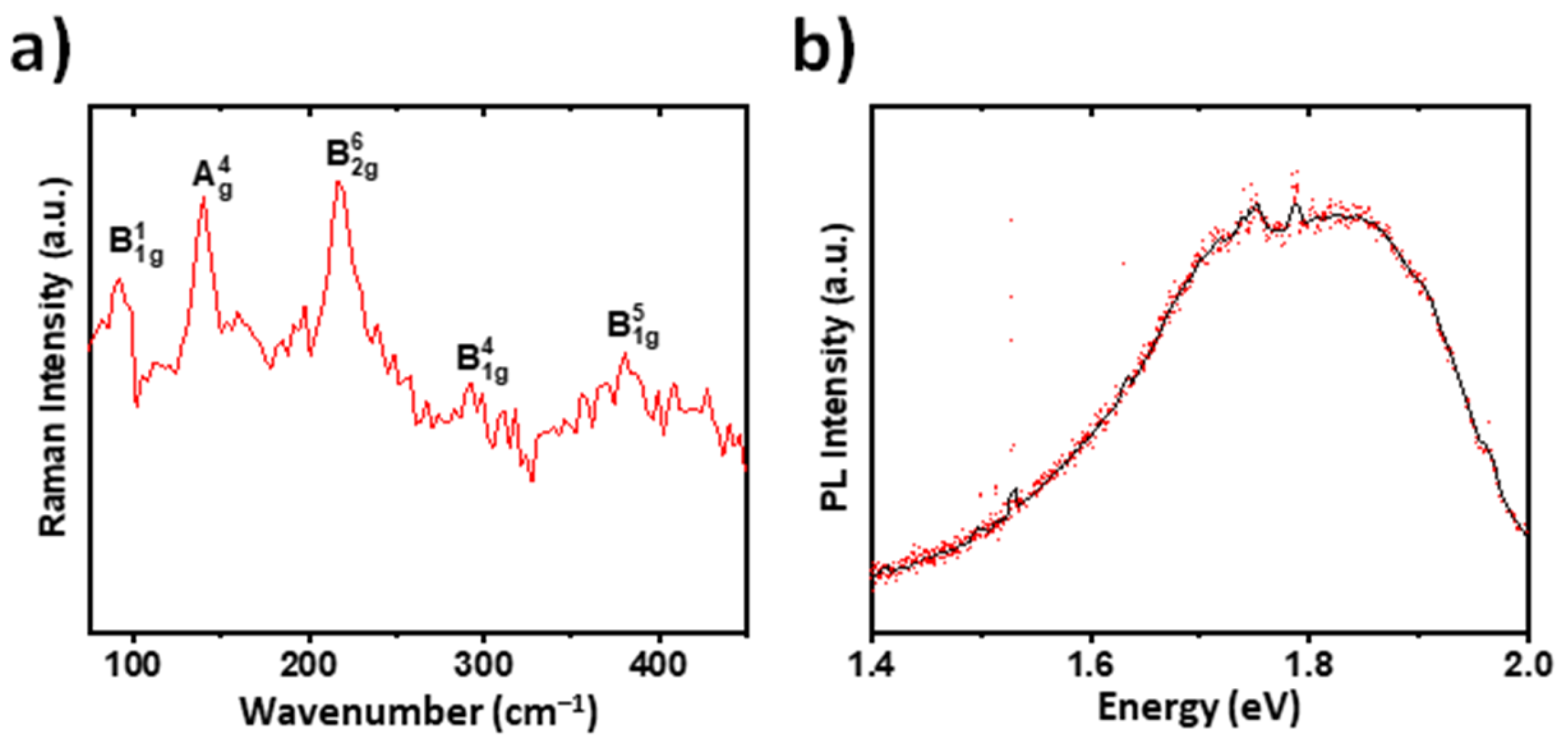
2.3. Raman Spectra, Photoluminescence, and Photocurrent Measurements of the BZS Films
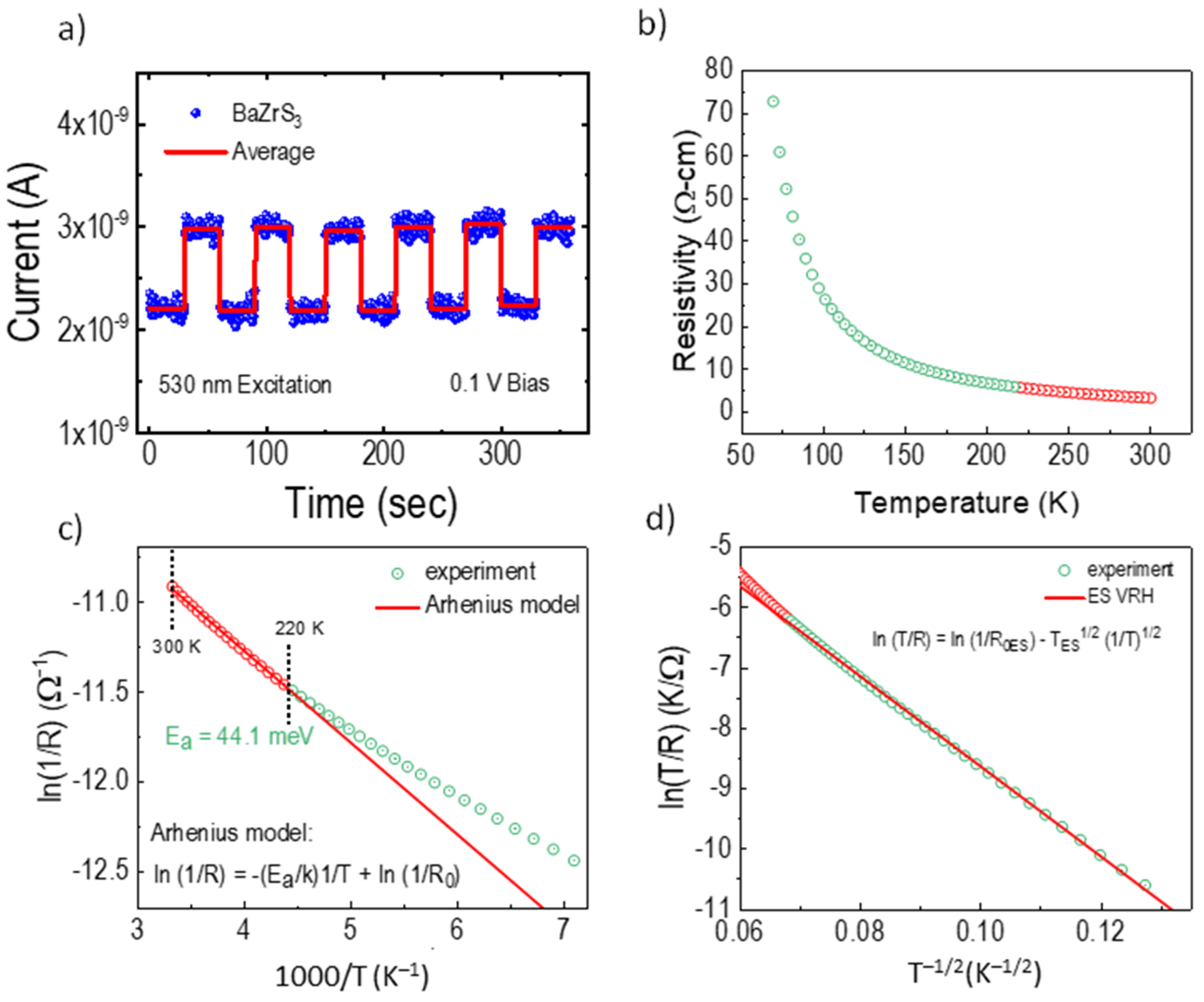
2.4. Temperature-Dependence of Resistance Measurements
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Bresolin, B.-M.; Park, Y.; Bahnemann, D.W. Recent Progresses on Metal Halide Perovskite-Based Material as Potential Photocatalyst. Catalysts 2020, 10, 709. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef]
- Rondinelli, J.M.; May, S.J.; Freeland, J.W. Control of octahedral connectivity in perovskite oxide heterostructures: An emerging route to multifunctional materials discovery. MRS Bull. 2012, 37, 261–270. [Google Scholar] [CrossRef]
- Raveau, B.; Maignan, A.; Martin, C.; Hervieu, M. Colossal Magnetoresistance Manganite Perovskites: Relations between Crystal Chemistry and Properties. Chem. Mater. 1998, 10, 2641–2652. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Müller, K.A. Perovskite-type oxides—The new approach to high-Tcsuperconductivity. Rev. Mod. Phys. 1988, 60, 585–600. [Google Scholar] [CrossRef]
- Tress, W. Metal Halide Perovskites as Mixed Electronic–Ionic Conductors: Challenges and Opportunities—From Hysteresis to Memristivity. J. Phys. Chem. Lett. 2017, 8, 3106–3114. [Google Scholar] [CrossRef]
- Dhole, S.; Chen, A.; Nie, W.; Park, B.; Jia, Q. Strain Engineering: A Pathway for Tunable Functionalities of Perovskite Metal Oxide Films. Nanomaterials 2022, 12, 835. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Ai, Q.; Gao, G.; Yuan, L.; Fang, Q.; Tian, X.; Zhang, X.; Egap, E.; Ajayan, P.M.; et al. In Situ Synthesis of Lead-Free Halide Perovskite–COF Nanocomposites as Photocatalysts for Photoinduced Polymerization in Both Organic and Aqueous Phases. ACS Mater. Lett. 2022, 4, 464–471. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Miller, K.A.; Zhu, H.; Egap, E. Lead Halide Perovskite Nanocrystals as Photocatalysts for PET-RAFT Polymerization under Visible and Near-Infrared Irradiation. ACS Macro Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xia, P.; Zhao, G.; Nie, C.; Gao, K.; He, S.; Wang, L.; Wu, K. Energy-Transfer Photocatalysis Using Lead Halide Perovskite Nanocrystals: Sensitizing Molecular Isomerization and Cycloaddition. Angew. Chem. Int. Edit. 2022, 61, e202208241. [Google Scholar]
- Sun, Y.-Y.; Agiorgousis, M.L.; Zhang, P.; Zhang, S. Chalcogenide Perovskites for Photovoltaics. Nano Lett. 2015, 15, 581–585. [Google Scholar] [CrossRef]
- Zilevu, D.; Creutz, S.E. Shape-Controlled Synthesis of Colloidal Nanorods and Nanoparticles of Barium Titanium Sulfide. Chem. Mater. 2021, 33, 5137–5146. [Google Scholar] [CrossRef]
- Adjogri, S.J.; Meyer, E.L. Chalcogenide Perovskites and Perovskite-Based Chalcohalide as Photoabsorbers: A Study of Their Properties, and Potential Photovoltaic Applications. Materials 2021, 14, 7857. [Google Scholar] [CrossRef]
- Sharma, S.; Ward, Z.; Bhimani, K.; Li, K.; Lakhnot, A.; Jain, R.; Shi, S.-F.; Terrones, H.; Koratkar, N. Bandgap Tuning in BaZrS3 Perovskite Thin Films. ACS Appl. Electron. Mater. 2021, 3, 3306–3312. [Google Scholar] [CrossRef]
- Yu, Z.; Wei, X.; Zheng, Y.; Hui, H.; Bian, M.; Dhole, S.; Seo, J.-H.; Sun, Y.-Y.; Jia, Q.; Zhang, S.; et al. Chalcogenide perovskite BaZrS3 thin-film electronic and optoelectronic devices by low temperature processing. Nano Energy 2021, 85, 105959. [Google Scholar] [CrossRef]
- Wu, X.; Gao, W.; Chai, J.; Ming, C.; Chen, M.; Zeng, H.; Zhang, P.; Zhang, S.; Sun, Y.-Y. Defect tolerance in chalcogenide perovskite photovoltaic material BaZrS3. Sci. China Mater. 2021, 64, 2976–2986. [Google Scholar] [CrossRef]
- Surendran, M.; Chen, H.; Zhao, B.; Thind, A.S.; Singh, S.; Orvis, T.; Zhao, H.; Han, J.-K.; Htoon, H.; Kawasaki, M.; et al. Epitaxial Thin Films of a Chalcogenide Perovskite. Chem. Mater. 2021, 33, 7457–7464. [Google Scholar] [CrossRef]
- Osei-Agyemang, E.; Koratkar, N.; Balasubramanian, G. Examining the electron transport in chalcogenide perovskite BaZrS3. J. Mater. Chem. C 2021, 9, 3892–3900. [Google Scholar] [CrossRef]
- Nishigaki, Y.; Nagai, T.; Nishiwaki, M.; Aizawa, T.; Kozawa, M.; Hanzawa, K.; Kato, Y.; Sai, H.; Hiramatsu, H.; Hosono, H.; et al. Extraordinary Strong Band-Edge Absorption in Distorted Chalcogenide Perovskites. Sol. RRL 2020, 4, 1900555. [Google Scholar] [CrossRef]
- Wei, X.; Hui, H.; Zhao, C.; Deng, C.; Han, M.; Yu, Z.; Sheng, A.; Roy, P.; Chen, A.; Lin, J.; et al. Realization of BaZrS3 chalcogenide perovskite thin films for optoelectronics. Nano Energy 2019, 68, 104317. [Google Scholar] [CrossRef]
- Pandey, J.; Ghoshal, D.; Dey, D.; Gupta, T.; Taraphder, A.; Koratkar, N.; Soni, A. Local ferroelectric polarization in antiferroelectric chalcogenide perovskite BaZrS3 thin films. Phys. Rev. B 2020, 102, 205308. [Google Scholar] [CrossRef]
- Zitouni, H.; Tahiri, N.; El Bounagui, O.; Ez-Zahraouy, H. Electronic, optical and transport properties of perovskite BaZrS3 compound doped with Se for photovoltaic applications. Chem. Phys. 2020, 538, 110923. [Google Scholar] [CrossRef]
- Filippone, S.; Zhao, B.; Niu, S.; Koocher, N.Z.; Silevitch, D.; Fina, I.; Rondinelli, J.M.; Ravichandran, J.; Jaramillo, R. Discovery of highly polarizable semiconductors BaZrS3 and Ba3Zr2S7. Phys. Rev. Mater. 2020, 4, 091601. [Google Scholar] [CrossRef]
- Wei, X.; Hui, H.; Perera, S.; Sheng, A.; Watson, D.F.; Sun, Y.-Y.; Jia, Q.; Zhang, S.; Zeng, H. Ti-Alloying of BaZrS3 Chalcogenide Perovskite for Photovoltaics. ACS Omega 2020, 5, 18579–18583. [Google Scholar] [CrossRef]
- Niu, S.; Zhao, B.; Ye, K.; Bianco, E.; Zhou, J.; McConney, M.E.; Settens, C.; Haiges, R.; Jaramillo, R.; Ravichandran, J. Crystal growth and structural analysis of perovskite chalcogenide BaZrS3 and Ruddlesden–Popper phase Ba3Zr2S7. J. Mater. Res. 2019, 34, 3819–3826. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Q.; Chen, H.; Yin, W.-J. Disparity of the Nature of the Band Gap between Halide and Chalcogenide Single Perovskites for Solar Cell Absorbers. J. Phys. Chem. Lett. 2019, 10, 4566–4570. [Google Scholar] [CrossRef]
- Agiorgousis, M.L.; Sun, Y.; Choe, D.; West, D.; Zhang, S. Machine Learning Augmented Discovery of Chalcogenide Double Perovskites for Photovoltaics. Adv. Theory Simul. 2019, 2, 1800173. [Google Scholar] [CrossRef]
- Niu, S.; Milam-Guerrero, J.; Zhou, Y.; Ye, K.; Zhao, B.; Melot, B.C.; Ravichandran, J. Thermal stability study of transition metal perovskite sulfides. J. Mater. Res. 2018, 33, 4135–4143. [Google Scholar] [CrossRef]
- Meng, W.; Saparov, B.; Hong, F.; Wang, J.; Mitzi, D.B.; Yan, Y. Alloying and Defect Control within Chalcogenide Perovskites for Optimized Photovoltaic Application. Chem. Mater. 2016, 28, 821–829. [Google Scholar] [CrossRef]
- Xu, J.; Fan, Y.; Tian, W.; Ye, L.; Zhang, Y.; Tian, Y.; Han, Y.; Shi, Z. Enhancing the optical absorption of chalcogenide perovskite BaZrS3 by optimizing the synthesis and post-processing conditions. J. Solid State Chem. 2022, 307, 122872. [Google Scholar] [CrossRef]
- Gupta, T.; Ghoshal, D.; Yoshimura, A.; Basu, S.; Chow, P.K.; Lakhnot, A.S.; Pandey, J.; Warrender, J.M.; Efstathiadis, H.; Soni, A.; et al. An Environmentally Stable and Lead-Free Chalcogenide Perovskite. Adv. Funct. Mater. 2020, 30, 2001387. [Google Scholar] [CrossRef]
- Ravi, V.K.; Yu, S.H.; Rajput, P.K.; Nayak, C.; Bhattacharyya, D.; Chung, D.S.; Nag, A. Colloidal BaZrS3 chalcogenide perovskite nanocrystals for thin film device fabrication. Nanoscale 2020, 13, 1616–1623. [Google Scholar] [CrossRef]
- Márquez, J.A.; Rusu, M.; Hempel, H.; Ahmet, I.Y.; Kölbach, M.; Simsek, I.; Choubrac, L.; Gurieva, G.; Gunder, R.; Schorr, S.; et al. BaZrS3 Chalcogenide Perovskite Thin Films by H2S Sulfurization of Oxide Precursors. J. Phys. Chem. Lett. 2021, 12, 2148–2153. [Google Scholar] [CrossRef]
- Sadeghi, I.; Ye, K.; Xu, M.; Li, Y.; LeBeau, J.M.; Jaramillo, R. Making BaZrS3 Chalcogenide Perovskite Thin Films by Molecular Beam Epitaxy. Adv. Funct. Mater. 2021, 31, 202105563. [Google Scholar] [CrossRef]
- Filippone, S.; Song, S.; Jaramillo, R. High densification of BaZrS3 powder inspired by the cold-sintering process. J. Mater. Res. 2021, 36, 4404–4412. [Google Scholar] [CrossRef]
- Comparotto, C.; Davydova, A.; Ericson, T.; Riekehr, L.; Moro, M.V.; Kubart, T.; Scragg, J.J.S. Chalcogenide Perovskite BaZrS3: Thin Film Growth by Sputtering and Rapid Thermal Processing. ACS Appl. Energy Mater. 2020, 3, 2762–2770. [Google Scholar] [CrossRef]
- Yang, R.; Jess, A.D.; Fai, C.; Hages, C.J. Low-Temperature, Solution-Based Synthesis of Luminescent Chalcogenide Perovskite BaZrS3 Nanoparticles. J. Am. Chem. Soc. 2022, 144, 15928–15931. [Google Scholar] [CrossRef]
- Luo, H.; Wang, H.; Zou, G.; Bauer, E.; Mccleskey, T.M.; Burrell, A.K.; Jia, Q. A Review of Epitaxial Metal-Nitride Films by Polymer-Assisted Deposition. Trans. Electr. Electron. Mater. 2010, 11, 54–60. [Google Scholar] [CrossRef]
- Zou, G.; Wang, H.; Mara, N.; Luo, H.; Li, N.; Di, Z.; Bauer, E.; Wang, Y.; McCleskey, T.; Burrell, A.; et al. Chemical Solution Deposition of Epitaxial Carbide Films. J. Am. Chem. Soc. 2010, 132, 2516–2517. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lin, Y.; Wang, H.; Lee, J.H.; Suvorova, N.A.; Mueller, A.H.; Burrell, A.K.; McCleskey, T.M.; Bauer, E.; Usov, I.O.; et al. A Chemical Solution Approach to Epitaxial Metal Nitride Thin Films. Adv. Mater. 2009, 21, 193–197. [Google Scholar] [CrossRef]
- Burrell, A.K.; McCleskey, T.M.; Jia, Q.X. Polymer assisted deposition. Chem. Commun. 2008, 1271–1277. [Google Scholar] [CrossRef]
- Jia, Q.X.; Mccleskey, T.; Burrell, A.K.; Lin, Y.; Collis, G.E.; Wang, H.; Li, A.D.Q.; Foltyn, S.R. Polymer-assisted deposition of metal-oxide films. Nat. Mater. 2004, 3, 529–532. [Google Scholar] [CrossRef] [PubMed]
- McCleskey, T.M.; Shi, P.; Bauer, E.; Highland, M.J.; Eastman, J.A.; Bi, Z.X.; Fuoss, P.H.; Baldo, P.M.; Ren, W.; Scott, B.L.; et al. Nucleation and growth of epitaxial metal-oxide films based on polymer-assisted deposition. Chem. Soc. Rev. 2013, 43, 2141–2146. [Google Scholar] [CrossRef]
- Zou, G.F.; Zhao, J.; Luo, H.M.; McCleskey, T.M.; Burrell, A.K.; Jia, Q.X. Polymer-assisted-deposition: A chemical solution route for a wide range of materials. Chem. Soc. Rev. 2012, 42, 439–449. [Google Scholar] [CrossRef]
- Perera, S.; Hui, H.; Zhao, C.; Xue, H.; Sun, F.; Deng, C.; Gross, N.; Milleville, C.; Xu, X.; Watson, D.F.; et al. Chalcogenide perovskites—An emerging class of ionic semiconductors. Nano Energy 2016, 22, 129–135. [Google Scholar] [CrossRef]
- Gross, N.; Sun, Y.-Y.; Perera, S.; Hui, H.; Wei, X.; Zhang, S.; Zeng, H.; Weinstein, B.A. Stability and Band-Gap Tuning of the Chalcogenide Perovskite BaZrS3 in Raman and Optical Investigations at High Pressures. Phys. Rev. Appl. 2017, 8, 044014. [Google Scholar] [CrossRef]
- Niu, S.; Huyan, H.; Liu, Y.; Yeung, M.; Ye, K.; Blankemeier, L.; Orvis, T.; Sarkar, D.; Singh, D.J.; Kapadia, R.; et al. Bandgap Control via Structural and Chemical Tuning of Transition Metal Perovskite Chalcogenides. Adv. Mater. 2016, 29, 201604733. [Google Scholar] [CrossRef]
- Efros, A.L.; Shklovskii, B.I. Coulomb gap and low temperature conductivity of disordered systems. J. Phys. C Solid State Phys. 1975, 8, L49–L51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhole, S.; Wei, X.; Hui, H.; Roy, P.; Corey, Z.; Wang, Y.; Nie, W.; Chen, A.; Zeng, H.; Jia, Q. A Facile Aqueous Solution Route for the Growth of Chalcogenide Perovskite BaZrS3 Films. Photonics 2023, 10, 366. https://doi.org/10.3390/photonics10040366
Dhole S, Wei X, Hui H, Roy P, Corey Z, Wang Y, Nie W, Chen A, Zeng H, Jia Q. A Facile Aqueous Solution Route for the Growth of Chalcogenide Perovskite BaZrS3 Films. Photonics. 2023; 10(4):366. https://doi.org/10.3390/photonics10040366
Chicago/Turabian StyleDhole, Samyak, Xiucheng Wei, Haolei Hui, Pinku Roy, Zachary Corey, Yongqiang Wang, Wanyi Nie, Aiping Chen, Hao Zeng, and Quanxi Jia. 2023. "A Facile Aqueous Solution Route for the Growth of Chalcogenide Perovskite BaZrS3 Films" Photonics 10, no. 4: 366. https://doi.org/10.3390/photonics10040366
APA StyleDhole, S., Wei, X., Hui, H., Roy, P., Corey, Z., Wang, Y., Nie, W., Chen, A., Zeng, H., & Jia, Q. (2023). A Facile Aqueous Solution Route for the Growth of Chalcogenide Perovskite BaZrS3 Films. Photonics, 10(4), 366. https://doi.org/10.3390/photonics10040366