Insights on Potential Photoprotective Activity of Two Butylchalcone Derivatives: Synthesis, Spectroscopic Characterization and Molecular Modeling
Abstract
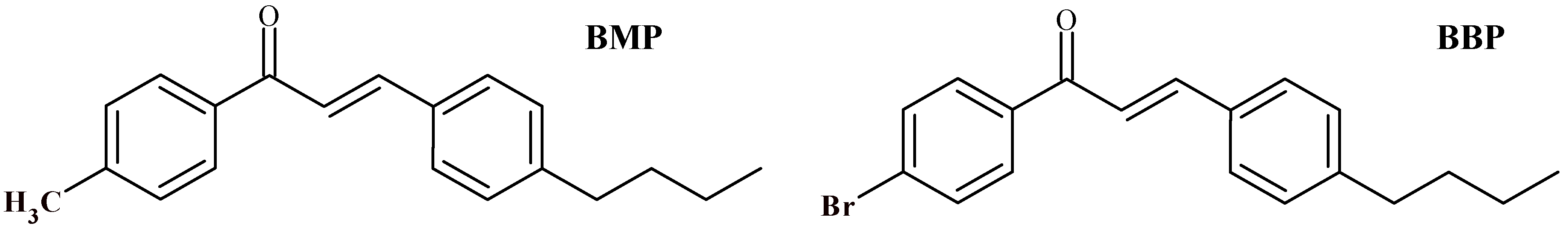
1. Introduction
2. Materials and Methods
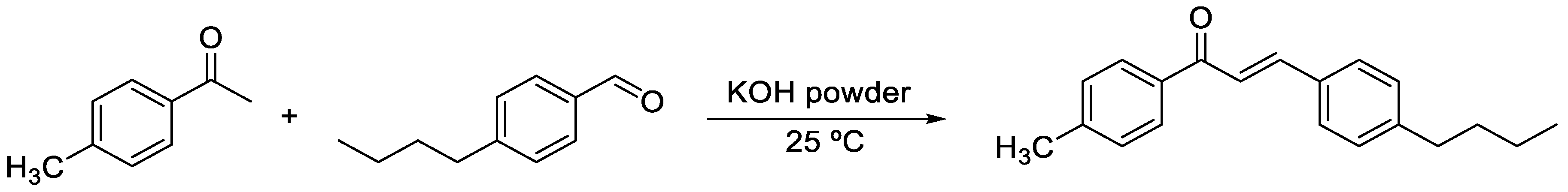
2.1. Synthesis and Crystallization
2.2. Characterization
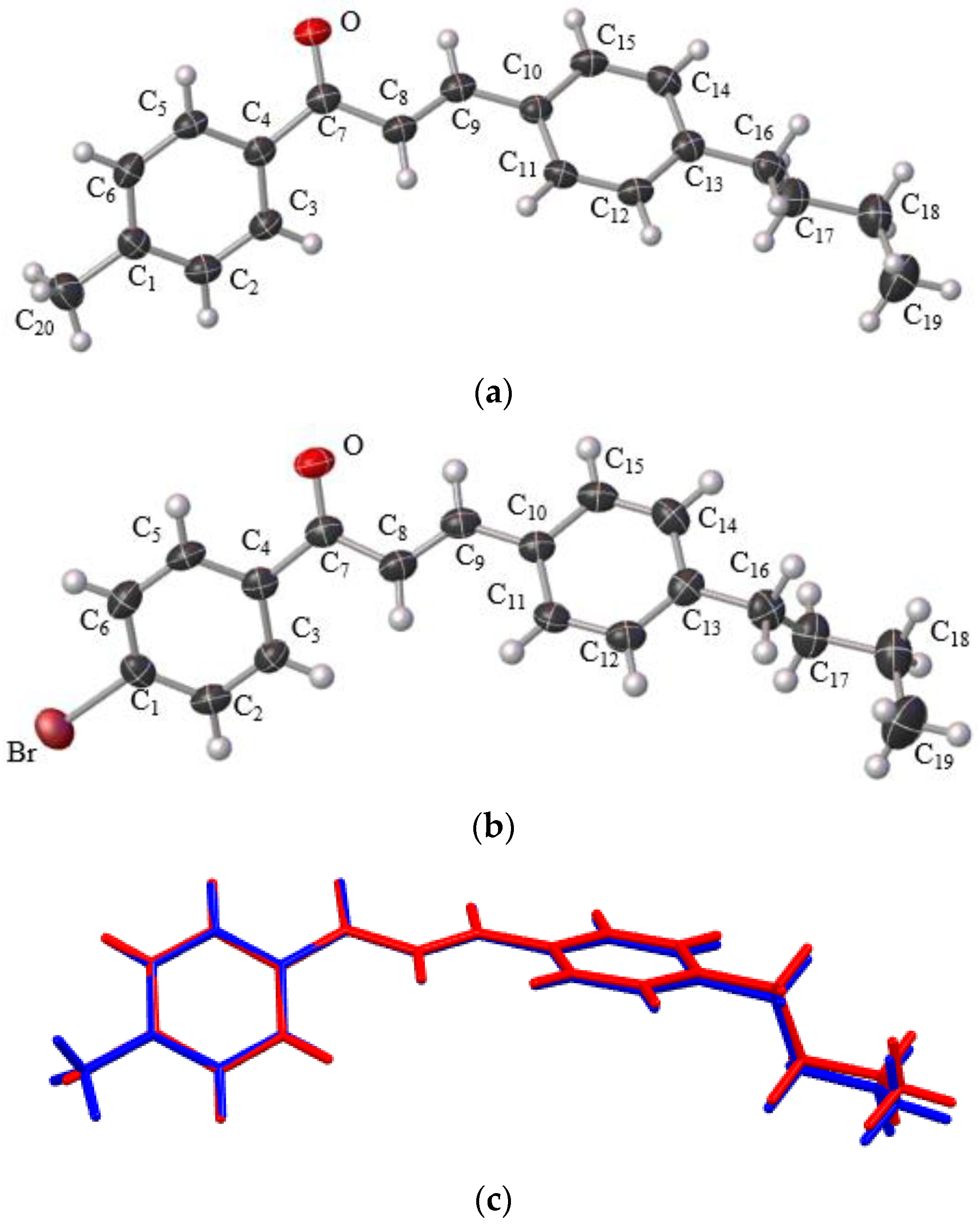
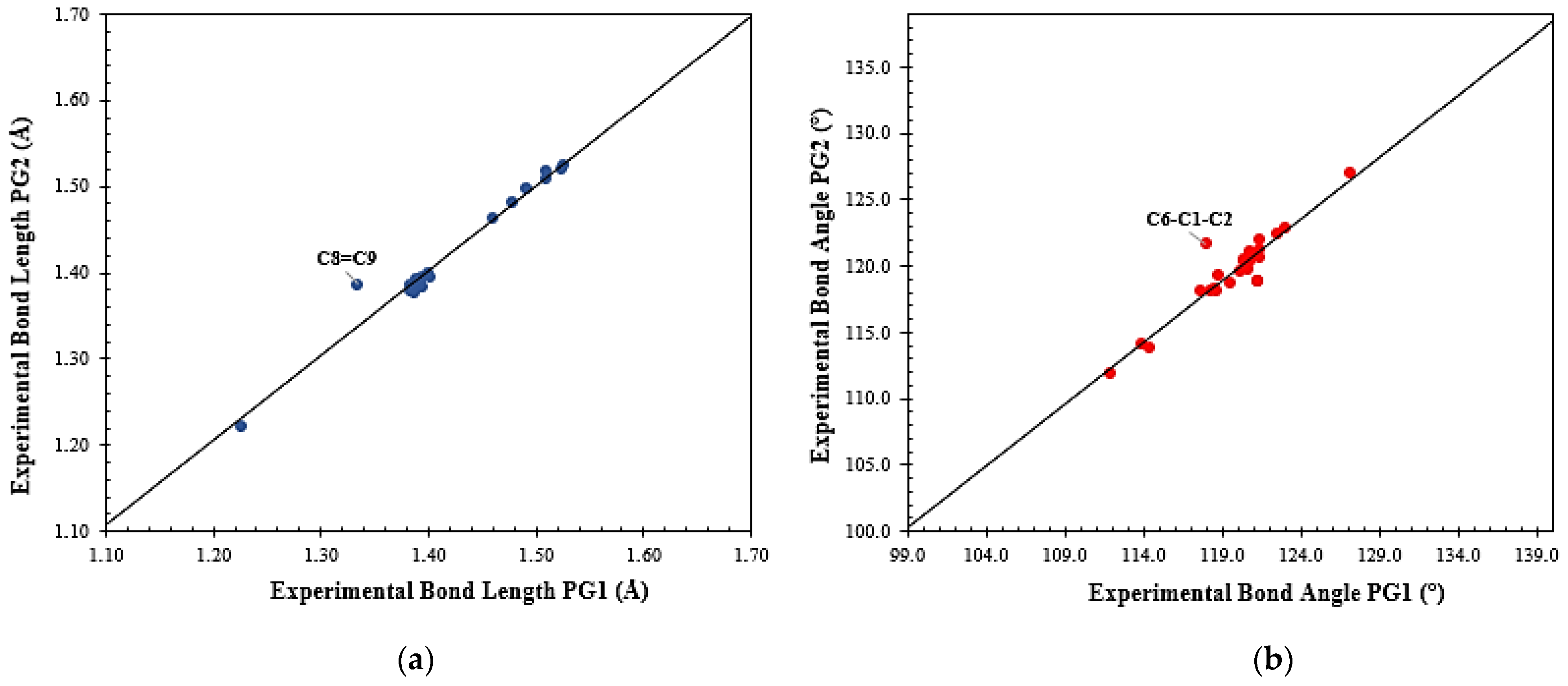
2.3. X-ray Diffraction Analysis
2.4. Molecular Modeling
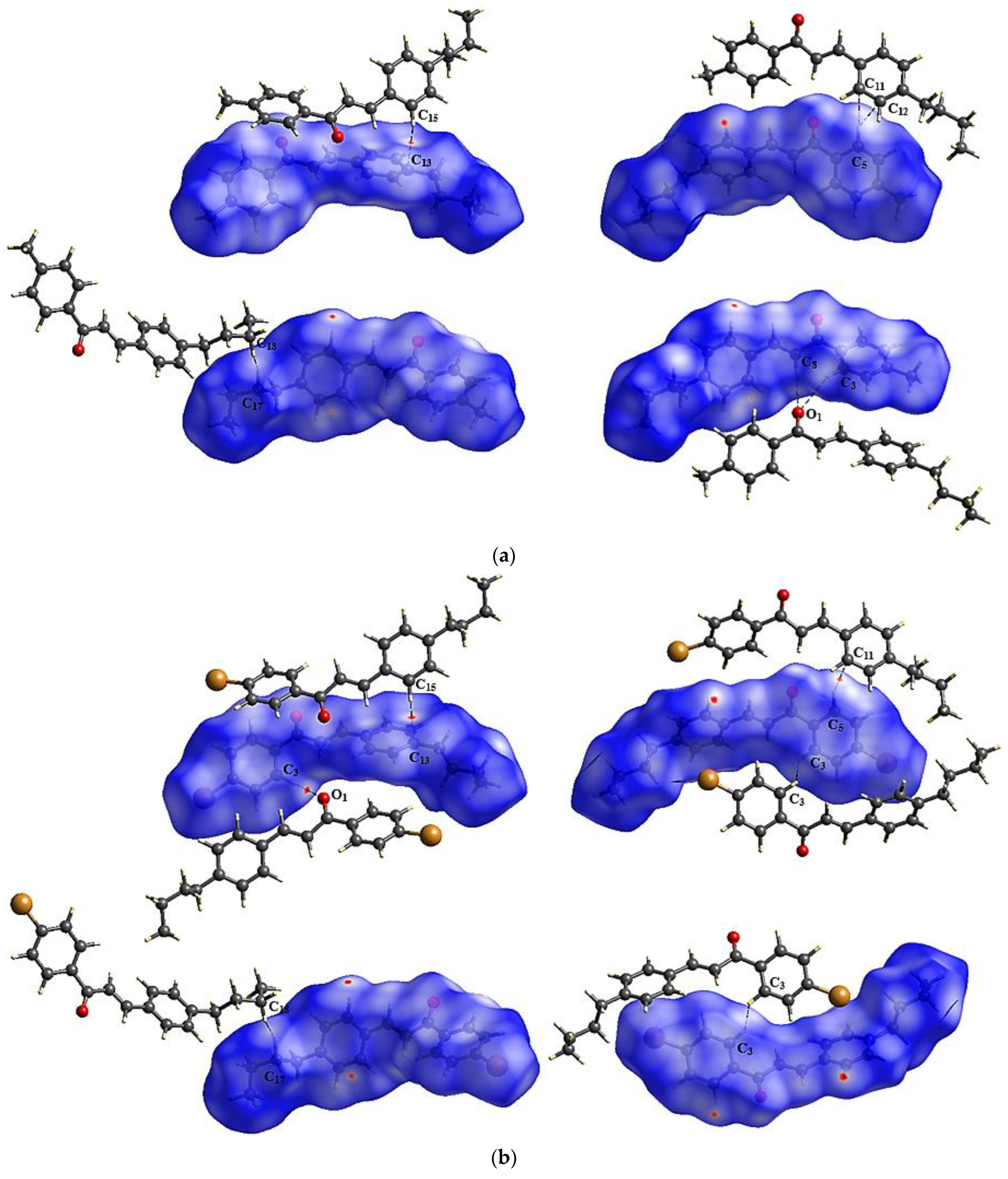
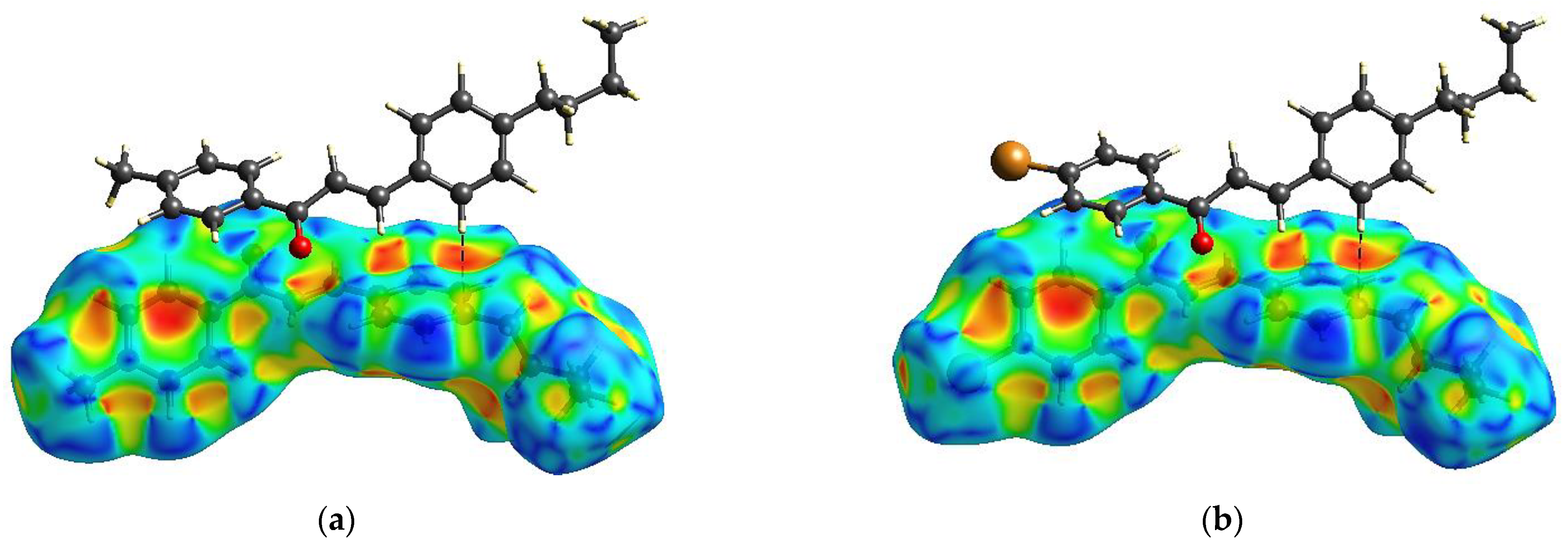
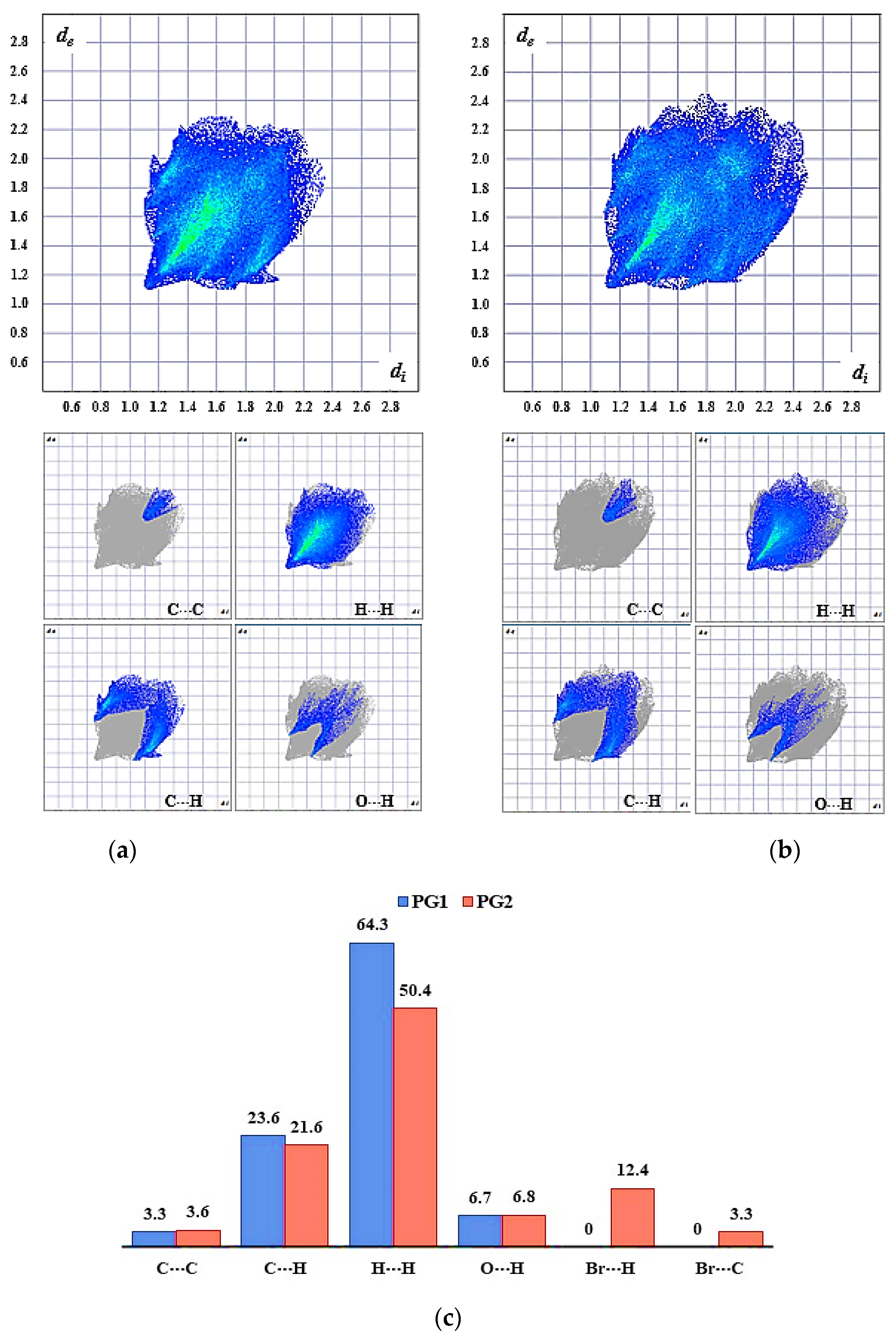
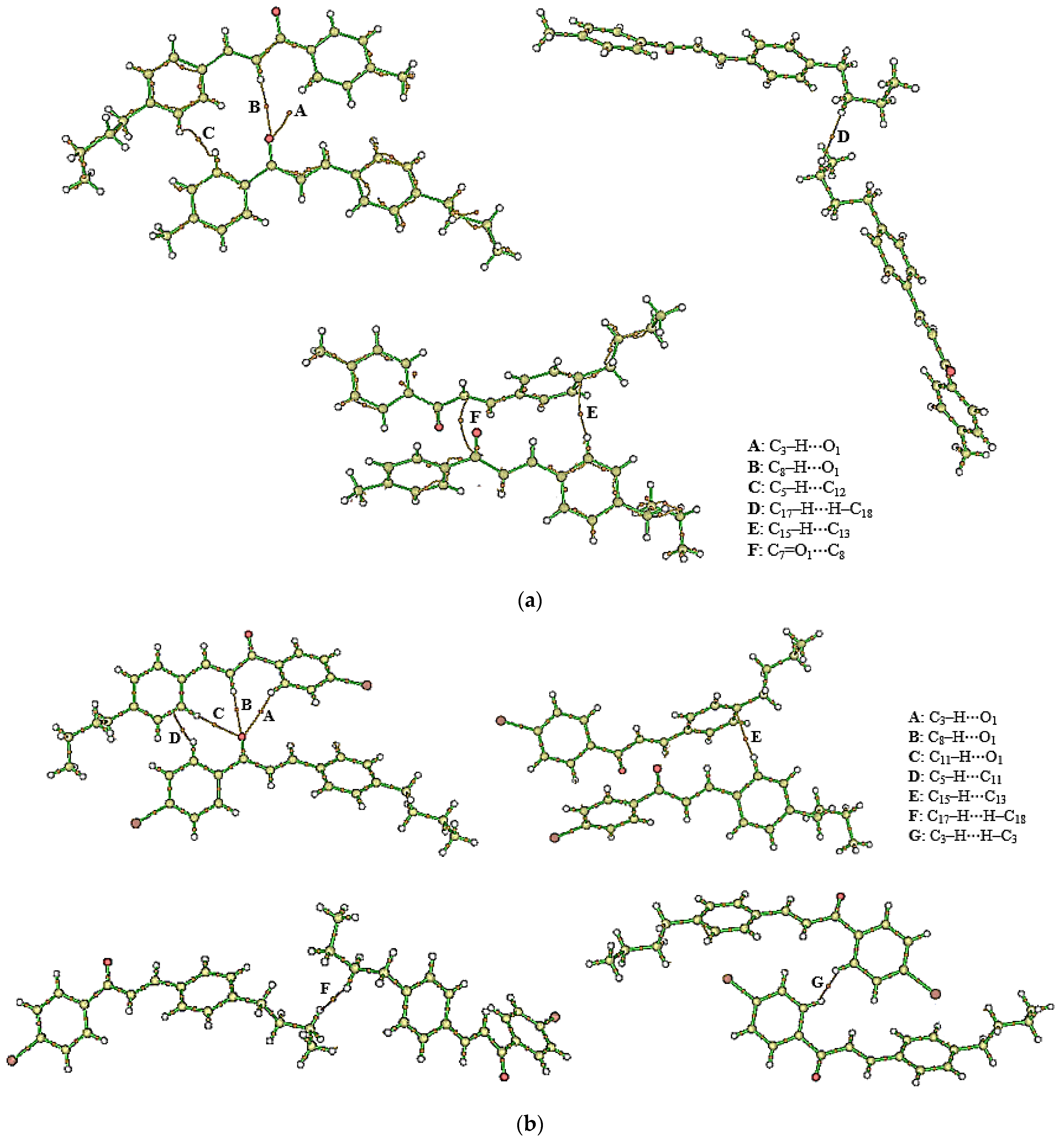
2.5. Supramolecular Arrangement
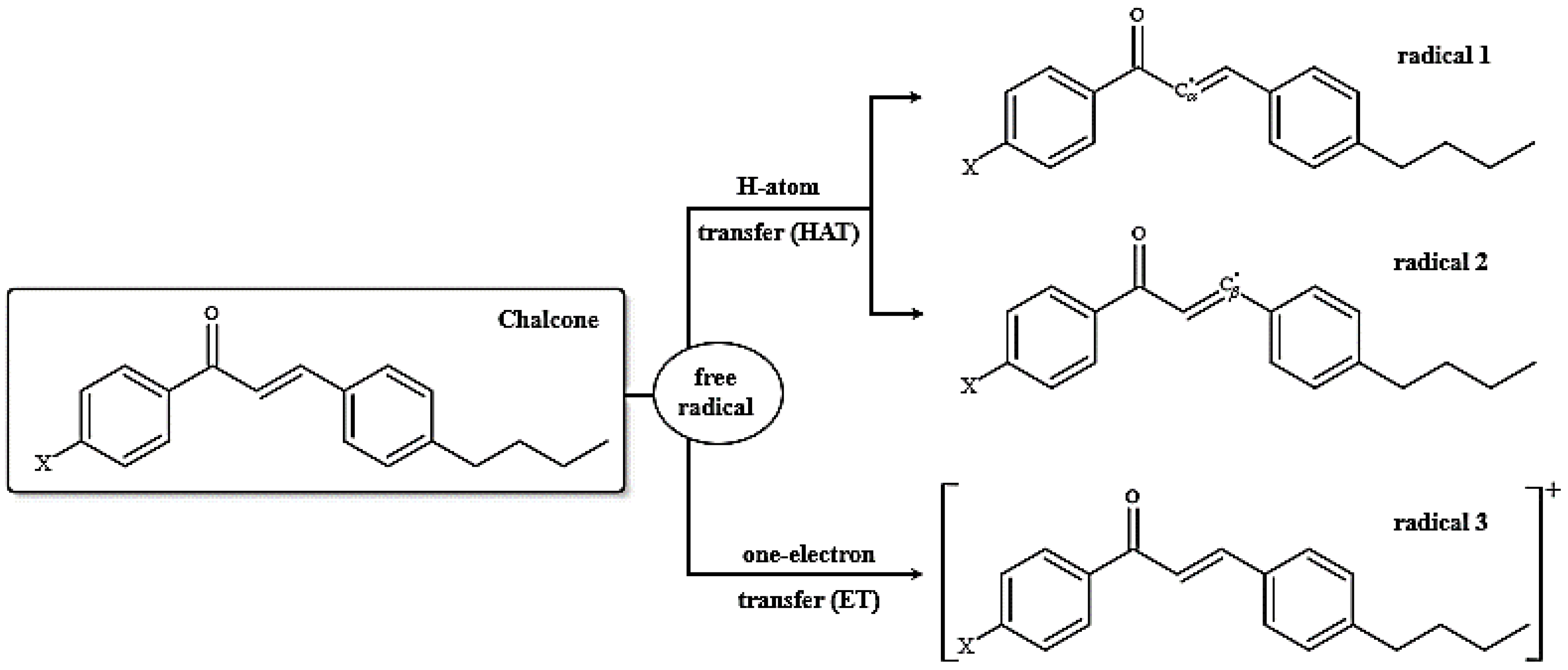
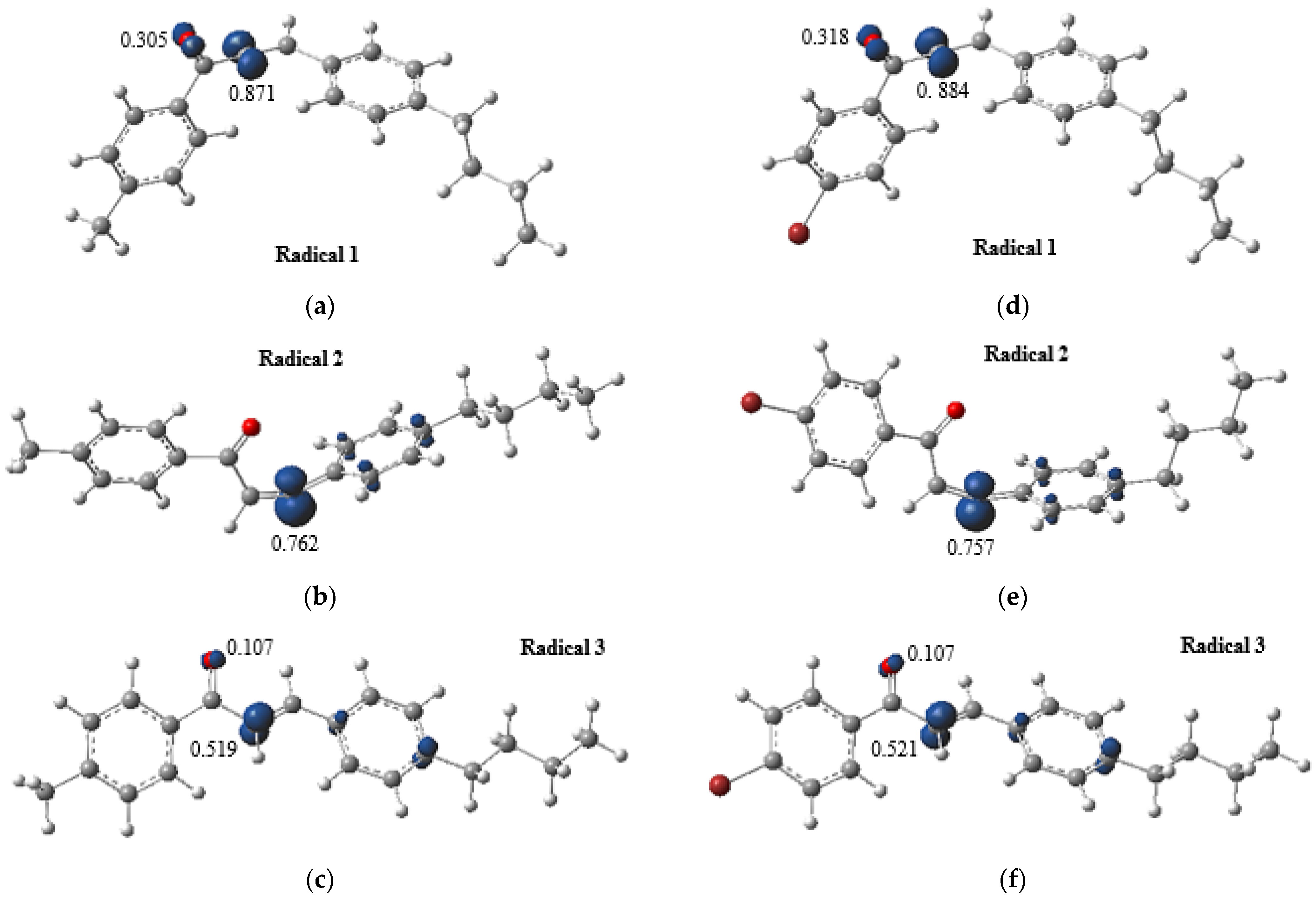
2.6. Analysis of Photoprotective Property
3. Results
3.1. Synthesis and Characterization
3.2. Solid State Description
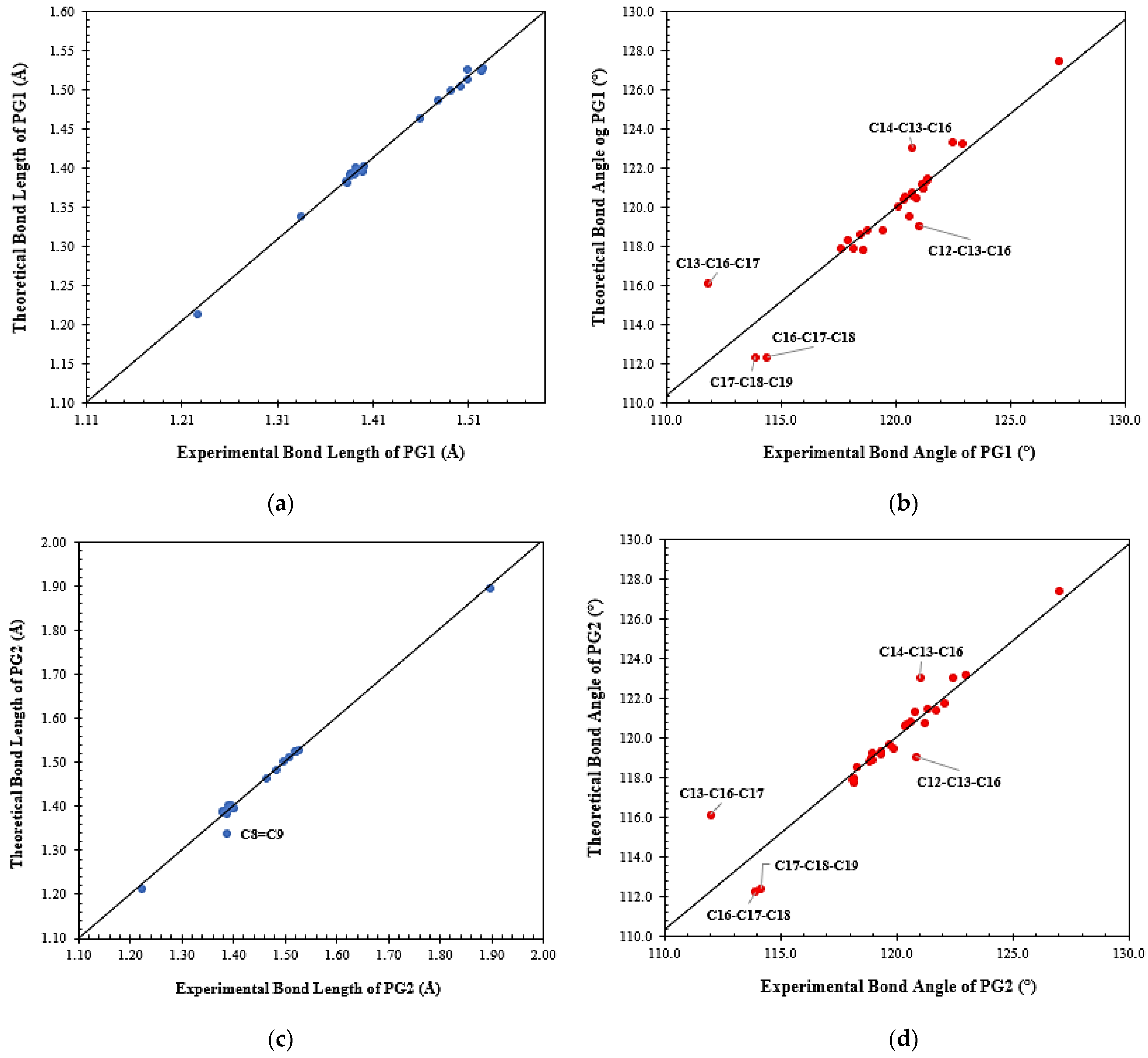
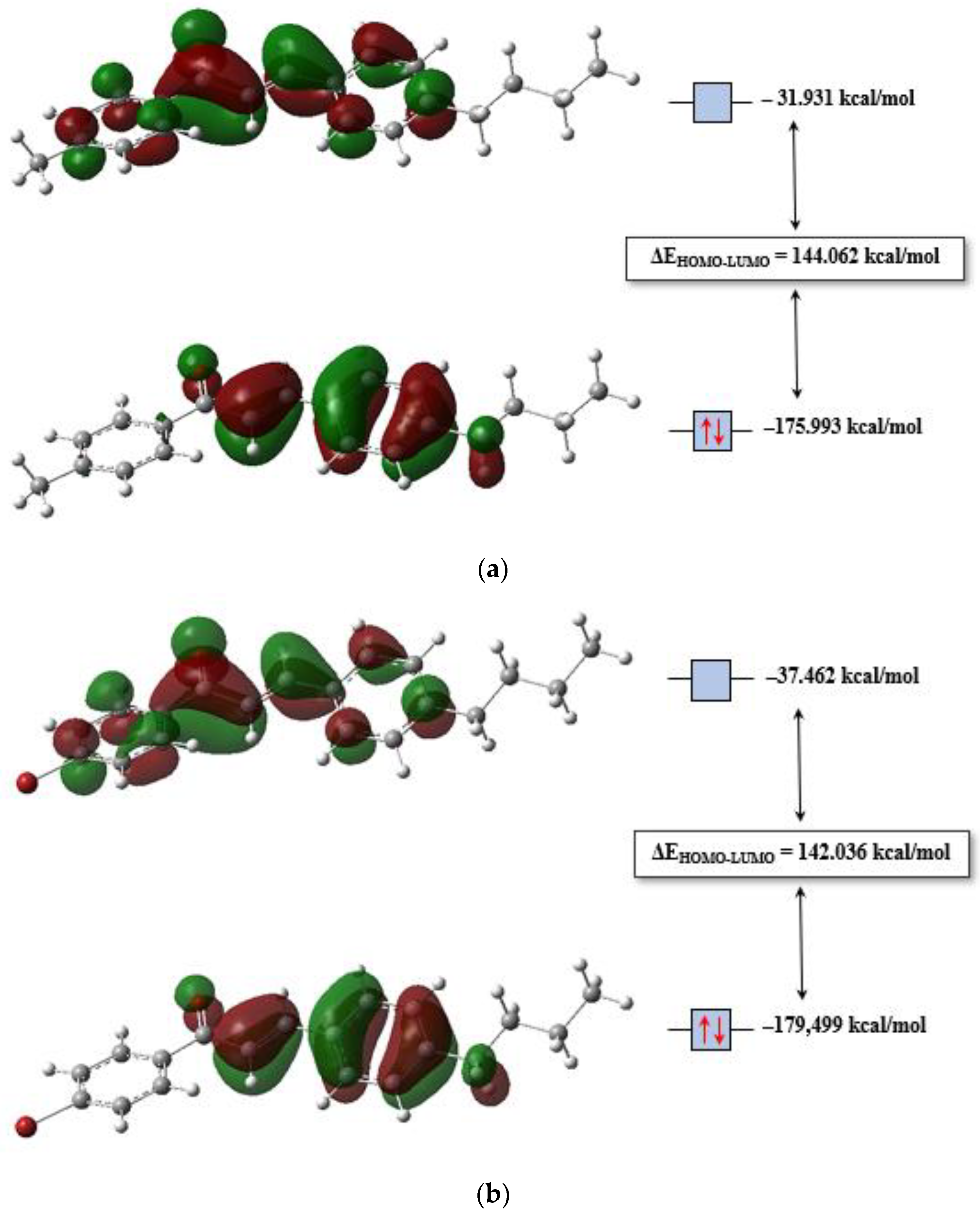
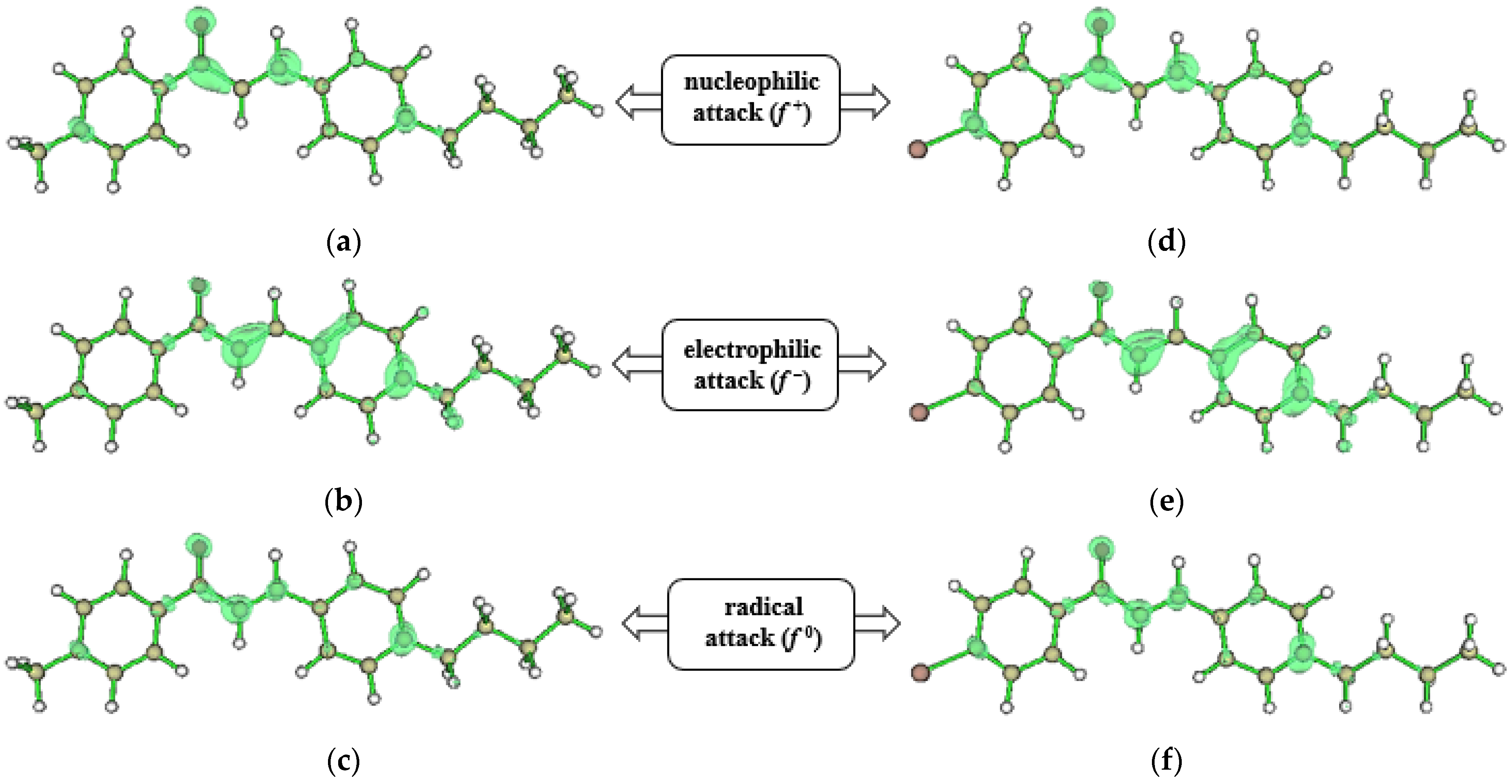
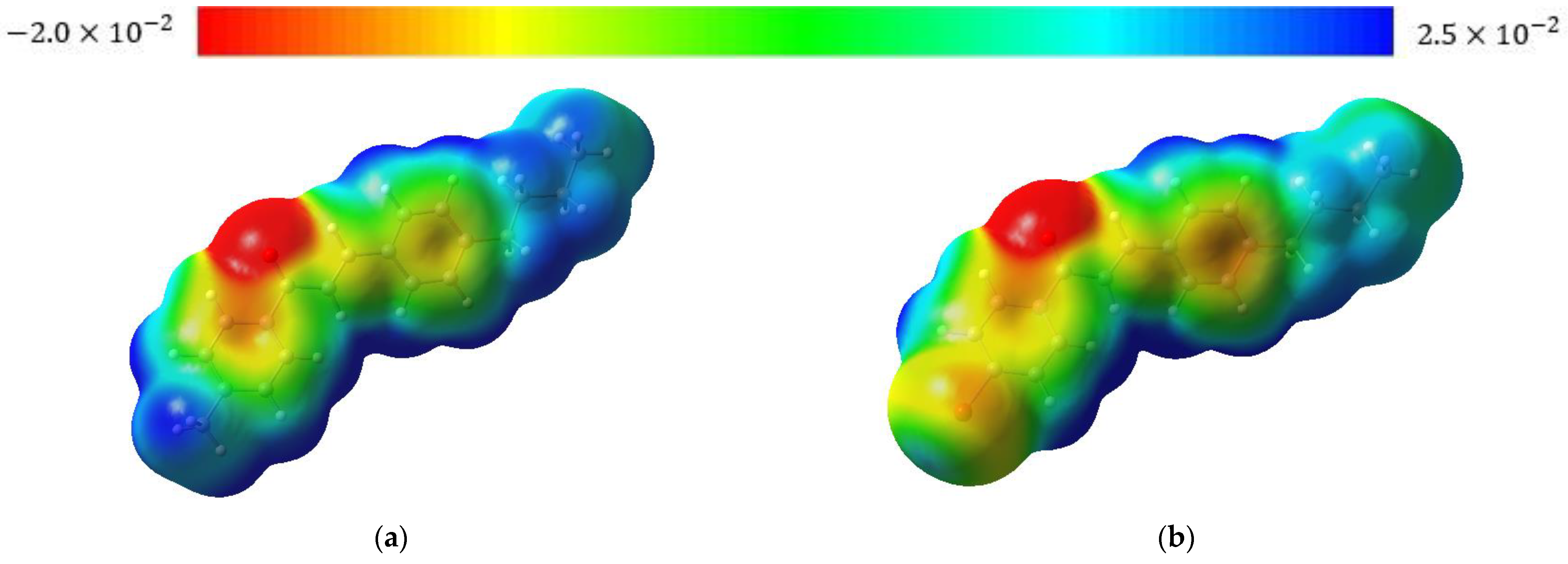
3.3. Molecular Modeling Analysis



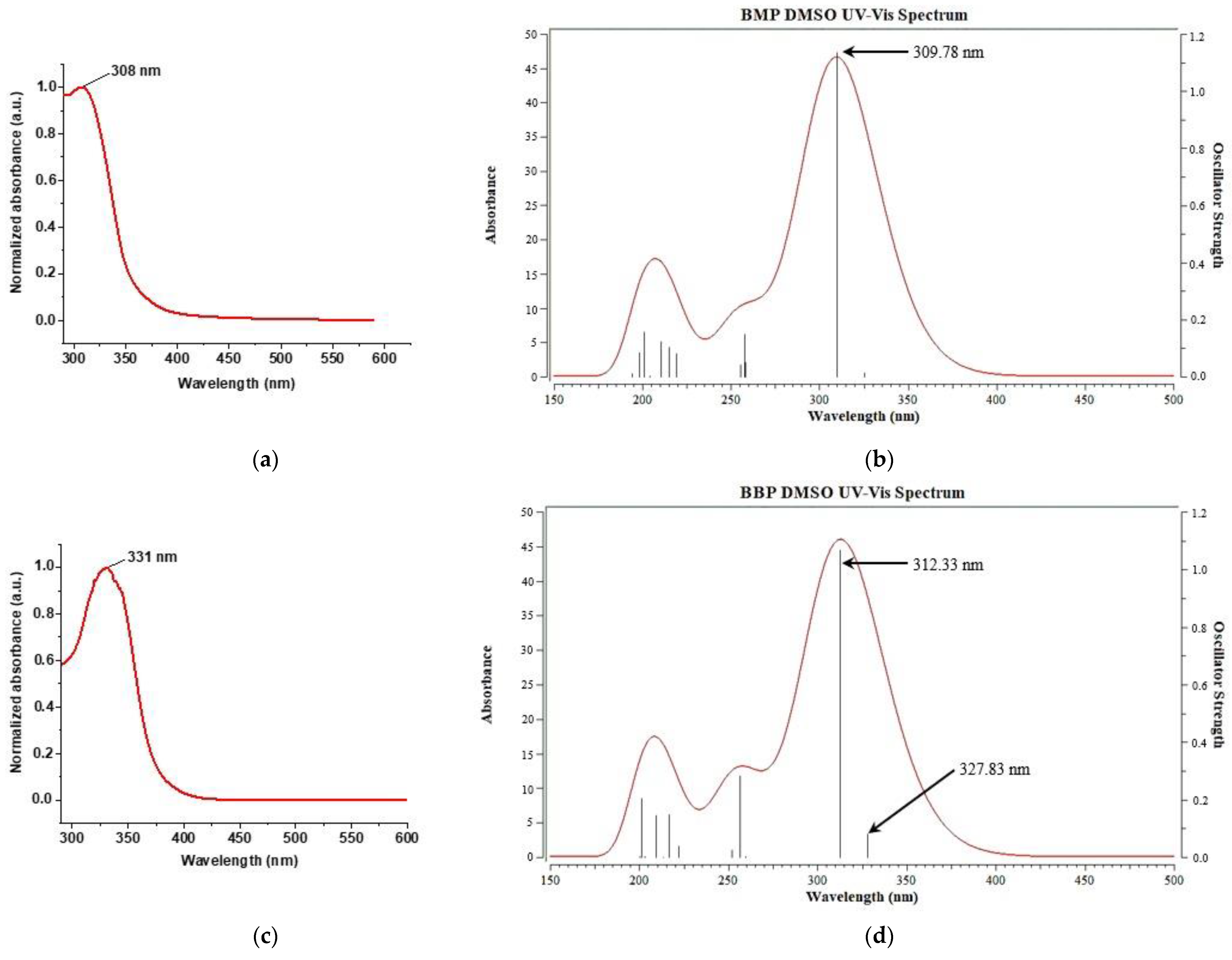
3.4. Analysis of Photoprotective Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.Q.; Balagula, Y.; Osterwalder, U. Photoprotection: A Review of the Current and Future Technologies. Dermatol. Ther. 2010, 23, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F. Natural agents: Cellular and molecular mechanisms of photoprotection. Arch. Biochem. Biophys. 2011, 508, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Gregoris, E.; Fabris, S.; Bertelle, M.; Grassato, L.; Stevanato, R. Propolis as potential cosmeceutical sunscreen agent for its combined photoprotective and antioxidant properties. Int. J. Pharm. 2011, 405, 97–101. [Google Scholar] [CrossRef]
- Wijayanti, L.W.; Swasono, R.T.; Lee, W.; Jumina, J. Synthesis and Evaluation of Chalcone Derivatives as Novel Sunscreen Agent. Molecules 2021, 26, 2698. [Google Scholar] [CrossRef]
- Lahsasni, S.A.; Al Korbi, F.H.; Aljaber, N.A.A. Synthesis, characterization and evaluation of antioxidant activities of some novel chalcones analogues. Chem. Cent. J. 2014, 8, 32. [Google Scholar] [CrossRef]
- Kozlowska, J.; Potaniec, B.; Baczynska, D.; Zarowska, B.; Aniol, M. Synthesis and Biological Evaluation of Novel Aminochalcones as Potential Anticancer and Antimicrobial Agents. Molecules 2019, 24, 4129. [Google Scholar] [CrossRef]
- Rocha, S.; Ribeiro, D.; Fernandes, E.; Freitas, M. A Systematic Review on Anti-diabetic Properties of Chalcones. Curr. Med. Chem. 2020, 27, 2257–2321. [Google Scholar] [CrossRef]
- Targanski, S.K.; Sousa, J.R.; de Padua, G.M.S.; de Sousa, J.M.; Vieira, L.C.C.; Soares, M.A. Larvicidal activity of substituted chalcones against Aedes aegypti (Diptera: Culicidae) and non-target organisms. Pest Manag. Sci. 2021, 77, 325–334. [Google Scholar] [CrossRef]
- Kozlowska, J.; Potaniec, B.; Zarowska, B.; Aniol, M. Microbial transformations of 4-methylchalcones as an efficient method of obtaining novel alcohol and dihydrochalcone derivatives with antimicrobial activity. RSC Adv. 2018, 8, 30379–30386. [Google Scholar] [CrossRef]
- Roussaki, M.; Hall, B.; Lima, S.C.; da Silva, A.C.; Wilkinson, S.; Detsi, A. Synthesis and anti-parasitic activity of a novel quinolinone-chalcone series. Bioorganic Med. Chem. Lett. 2013, 23, 6436–6441. [Google Scholar] [CrossRef]
- Herencia, F.; Ferrandiz, M.L.; Ubeda, A.; Dominguez, J.N.; Charris, J.E.; Lobo, G.M.; Alcaraz, M.J. Synthesis and anti-inflammatory activity of chalcone derivatives. Bioorganic Med. Chem. Lett. 1998, 8, 1169–1174. [Google Scholar] [CrossRef]
- Sooknual, P.; Pingaew, R.; Phopin, K.; Ruankham, W.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and neuroprotective effects of novel chalcone-triazole hybrids. Bioorganic Chem. 2020, 105, 104384. [Google Scholar] [CrossRef]
- Gaonkar, S.L.; Vignesh, U.N. Synthesis and pharmacological properties of chalcones: A review. Res. Chem. Intermed. 2017, 43, 6043–6077. [Google Scholar] [CrossRef]
- Custodio, J.M.F.; Guimaraes-Neto, J.J.A.; Awad, R.; Queiroz, J.E.; Verde, G.M.V.; Mottin, M.; Neves, B.J.; Andrade, C.H.; Aquino, G.L.B.; Valverde, C.; et al. Molecular modelling and optical properties of a novel fluorinated chalcone. Arab. J. Chem. 2020, 13, 3362–3371. [Google Scholar] [CrossRef]
- Custodio, J.M.F.; Moreira, C.A.; Valverde, C.; de Aquino, G.L.B.; Baseia, B.; Napolitano, H.B. Hirshfeld Surfaces and Nonlinear Optics on Two Conformers of a Heterocyclic Chalcone. J. Braz. Chem. Soc. 2018, 29, 258–268. [Google Scholar] [CrossRef]
- Borges, I.D.; Danielli, J.A.V.; Silva, V.E.G.; Sallum, L.O.; de Queiroz, J.E.; Dias, L.D.; Iermak, I.; Aquino, G.L.B.; Camargo, A.J.; Valverde, C.; et al. Synthesis and structural studies on (E)-3-(2,6-difluorophenyl)-1-(4-fluorophenyl)prop-2-en-1-one: A promising nonlinear optical material. RSC Adv. 2020, 10, 22542–22555. [Google Scholar] [CrossRef] [PubMed]
- Fatmasari, E.; Zulkarnain, A.K.; Kuswahyuning, R. 3,4-dimethoxychalcone novel ultraviolet-A-protection factor in conventional sunscreen cream. J. Adv. Pharm. Technol. Res. 2021, 12, 279–284. [Google Scholar] [CrossRef]
- Fayed, T.A.; Gaber, M.; El-Nahass, M.N.; Diab, H.A.; El-Gamil, M.M. Synthesis, Structural characterization, thermal, molecular modeling and biological studies of chalcone and Cr(III), Mn(II), Cu(II) Zn(II) and Cd(II) chelates. J. Mol. Struct. 2020, 1221, 128742. [Google Scholar] [CrossRef]
- Carvalho, P.S.; Sallum, L.O.; Cidade, A.F.; Aquino, G.L.B.; Napolitano, H.B. (E)-1-(4-Methoxyphenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one. Acta Crystallogr. Sect. E-Struct. Rep. Online 2011, 67, O2126–U1337. [Google Scholar] [CrossRef]
- Firmino, P.P.; Queiroz, J.E.; Dias, L.D.; Wenceslau, P.R.S.; de Souza, L.M.; Iermak, I.; Vaz, W.F.; Custodio, J.M.F.; Oliver, A.G.; de Aquino, G.L.B.; et al. Synthesis, Molecular Structure, Thermal and Spectroscopic Analysis of a Novel Bromochalcone Derivative with Larvicidal Activity. Crystals 2022, 12, 440. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Found. Adv. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C-Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D-Struct. Biol. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, 1133. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Zhang, G.; Musgrave, C.B. Comparison of DFT methods for molecular orbital eigenvalue calculations. J. Phys. Chem. A 2007, 111, 1554–1561. [Google Scholar] [CrossRef]
- Narayszabo, G.; Ferenczy, G.G. Molecular electrostatics. Chem. Rev. 1995, 95, 829–847. [Google Scholar] [CrossRef]
- Fukui, K. The role of frontier orbitals in chemical-reactions (nobel lecture). Angew. Chem.-Int. 1982, 21, 801–809. [Google Scholar] [CrossRef]
- Fukui, K. Role of frontier orbitals in chemical-reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Overhauser, A.W. Spin density waves in an electron gas. Phys. Rev. 1962, 128, 1437. [Google Scholar] [CrossRef]
- Jacob, C.R.; Reiher, M. Spin in density-functional theory. Int. J. Quantum Chem. 2012, 112, 3661–3684. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Dehkordi, M.M.; Asgarshamsi, M.H. Density functional theory studies of the antioxidants-a review. J. Mol. Model. 2021, 27, 271. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R.; Glendening, E.D. What is NBO analysis and how is it useful? Int. Rev. Phys. Chem. 2016, 35, 399–440. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Gilmore, K.M.; Peterson, P.W. Hyperconjugation. Wiley Interdiscip. Rev.-Comput. Mol. Sci. 2011, 1, 109–141. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Crystengcomm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Crystengcomm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge-densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Moreira, C.A.; Custodio, J.M.F.; Vaz, W.F.; D’Oliveira, G.D.C.; Perez, C.N.; Napolitano, H.B. A comprehensive study on crystal structure of a novel sulfonamide-dihydroquinolinone through experimental and theoretical approaches. J. Mol. Model. 2019, 25, 205. [Google Scholar] [CrossRef]
- Custodio, J.M.F.; Gotardo, F.; Vaz, W.F.; D’Oliveira, G.D.C.; de Almeida, L.R.; Fonseca, R.D.; Cocca, L.H.Z.; Perez, C.N.; Oliver, A.G.; de Boni, L.; et al. Benzenesulfonyl incorporated chalcones: Synthesis, structural and optical properties. J. Mol. Struct. 2020, 1208, 127845. [Google Scholar] [CrossRef]
- Perez, P.; Domingo, L.R.; Aurell, A.J.; Contreras, R. Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1,3-dipolar cycloaddition reactions. Tetrahedron 2003, 59, 3117–3125. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Perez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W.T. Density functional-approach to the frontier-electron theory of chemical-reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Gacche, R.N.; Dhole, N.A.; Kamble, S.G.; Bandgar, B.P. In-vitro evaluation of selected chalcones for antioxidant activity. J. Enzym. Inhib. Med. Chem. 2008, 23, 28–31. [Google Scholar] [CrossRef]
- Anto, R.J.; Sukumaran, K.; Kuttan, G.; Rao, M.N.A.; Subbaraju, V.; Kuttan, R. Anticancer and antioxidant activity of synthetic chalcones and related-compounds. Cancer Lett. 1995, 97, 33–37. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Xue, Y.S.; Zheng, Y.G.; Zhang, L.; Wu, W.Y.; Yu, D.; Liu, Y. Theoretical study on the antioxidant properties of 2′-hydroxychalcones: H-atom vs. electron transfer mechanism. J. Mol. Model. 2013, 19, 3851–3862. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S.N.; Borges, I.D.; Borges, L.L.; Dias, L.D.; Camargo, A.J.; Perjesi, P.; Napolitano, H.B. New Insights on Glutathione’s Supramolecular Arrangement and Its In Silico Analysis as an Angiotensin-Converting Enzyme Inhibitor. Molecules 2022, 27, 7958. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W.; Macdougall, P.J. Toward a theory of chemical-reactivity based on the charge-density. J. Am. Chem. Soc. 1985, 107, 6788–6795. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Itoh, N. Extended hypervalent 5c-6e interactions: Linear alignment of five C-Se---O---Se-C atoms in anthraquinone and 9-methoxyanthracene bearing arylselanyl groups at the 1,8-positions. J. Org. Chem. 2004, 69, 1676–1684. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Narahara, K. Polar Coordinate Representation of H-b(r(c)) versus ((h)over-bar(2)/8m)del(2)rho(b)(r(c)) at BCP in AIM Analysis: Classification and Evaluation of Weak to Strong Interactions. J. Phys. Chem. A 2009, 113, 10050–10057. [Google Scholar] [CrossRef]
- Wheeler, O.H.; Gore, P.H.; Baez, R.; Santiago, M. Ultraviolet absorption of substituted phenyl + polycyclic aryl chalcones. Can. J. Chem. -Rev. Can. Chim. 1964, 42, 2580. [Google Scholar] [CrossRef]
- Maragatham, G.; Selvarani, S.; Rajakumar, P.; Lakshmi, S. Structure determination and quantum chemical analysis of chalcone derivatives. J. Mol. Struct. 2019, 1179, 568–575. [Google Scholar] [CrossRef]
- Lavakumar, S.; Vivekanand, P.A.; Prince, A.A.M. Simultaneous analysis of octylmethoxycinnamate and butylmethoxydibenzoylmethane in sunscreen products by a validated UV-spectrophotometric method. In Proceedings of National Conference on Chemistry and Materials (NCCM), Chennai, India, 22–23 March 2019; pp. 893–897. [Google Scholar]
- Manaia, E.B.; Kaminski, R.C.K.; Correa, M.A.; Chiavacci, L.A. Inorganic UV filters. Braz. J. Pharm. Sci. 2013, 49, 201–209. [Google Scholar] [CrossRef]
| Crystal Data | BMP | BBP |
|---|---|---|
| Chemical formula | C20H22O | C19H19OBr |
| Molecular weight (g/mol) | 278.37 | 343.25 |
| Crystal system | orthorhombic | orthorhombic |
| Space group | Pbca | Pbca |
| a (Å) | 11.341(3) | 11.3065(12) |
| b (Å) | 8.1445(19) | 8.1831(9) |
| c (Å) | 33.628(8) | 33.892(4) |
| α (°) | 90 | 90 |
| β (°) | 90 | 90 |
| γ (°) | 90 | 90 |
| Volume (Å3) | 3106.2(12) | 3135.8(6) |
| Z | 8 | 8 |
| Radiation type | Mo Kα | Mo Kα |
| R[F2 > 2σ(F2)]; wR(F2) | R1 = 0.0545, wR2 = 0.1346 | R1 = 0.0317, wR2 = 0.0620 |
| S | 1.025 | 1.035 |
| Dimentions (mm) | 0.23 × 0.21 × 0.04 | 0.226 × 0.197 × 0.044 |
| DESCRIPTORS | CNP-OM (kcal/mol) | CNP-CL (kcal/mol) |
|---|---|---|
| EHOMO | −175.993 | −179.499 |
| ELUMO | −31.931 | −37.462 |
| ΔEHOMO-LUMO * | 144.062 | 142.036 |
| Ionization Energy () | 175.993 | 179.499 |
| Electronic Afinity () | 31.931 | 37.462 |
| Electronegativity () | 103.962 | 108.480 |
| Chemical potential () | −103.962 | −108.480 |
| Chemical hardness () | 144.062 | 142.036 |
| Electrophilicity index () | 37.512 | 41.426 |
| Interaction | HA (Å) | D–HA (°) | (a.u.) | (a.u.) | (a.u.) | (a.u.) | (a.u.) | |
|---|---|---|---|---|---|---|---|---|
| BMP | ||||||||
| C3–HO1 | 2.753 | 148.44 | 0.0219 | 0.0504 | 0.0107 | −0.0089 | 0.0019 | 0.8 |
| C8–HO1 | 2.767 | 158.31 | 0.0194 | 0.0217 | 0.0060 | −0.0066 | −0.0006 | 1.1 |
| C15–HC13 | 2.877 | 148.87 | 0.0264 | 0.0388 | 0.0121 | −0.0144 | −0.0024 | 1.2 |
| C5–HC11 | 2.884 | 138.40 | - | - | - | - | - | - |
| C5–HC12 | 2.886 | 121.22 | 0.0215 | 0.0314 | 0.0078 | −0.0078 | 0.0000 | 1.0 |
| C17–HH–C18 | 2.391 | 140.07 | 0.0048 | 0.0179 | 0.0034 | −0.0022 | 0.0011 | 0.7 |
| C7=O1C8 | 3.467 | 85.93 | 0.0286 | 0.0040 | 0.0033 | −0.0056 | −0.0023 | 1.7 |
| BBP | ||||||||
| C3–HO1 | 2.666 | 147.47 | 0.0060 | 0.0202 | 0.0043 | −0.0035 | 0.0008 | 0.8 |
| C8–HO1 | 2.723 | 157.43 | 0.0046 | 0.0166 | 0.0035 | −0.0028 | 0.0007 | 0.8 |
| C15–HC13 | 2.846 | 146.19 | 0.0058 | 0.0160 | 0.0034 | −0.0028 | 0.0006 | 0.8 |
| C5–HC11 | 2.845 | 135.33 | 0.0058 | 0.0184 | 0.0037 | −0.0028 | 0.0009 | 0.8 |
| C17–HH–C18 | 2.373 | 140.37 | 0.0051 | 0.0149 | 0.0032 | −0.0027 | 0.0005 | 0.8 |
| C11–HO1 | 2.929 | 140.32 | 0.0039 | 0.0134 | 0.0028 | −0.0022 | 0.0006 | 0.8 |
| C3–HH–C3 | 2.397 | 120.68 | 0.0054 | 0.0178 | 0.0036 | −0.0028 | 0.0008 | 0.8 |
| Excited State | (kcal/mol) | λtheor (nm) | λexper (nm) | f | Excitation | Transition | % |
|---|---|---|---|---|---|---|---|
| BMP | |||||||
| S1 | 87.878 | 325.36 | 0.0148 | H–4 L | σ →π* | 80.03 | |
| S2 | 92.299 | 309.78 | 308 | 1.1349 | H L | π →π* | 90.45 |
| S3 | 110.757 | 258.15 | 0.0506 | H–2 L | π →π* | 36.6 | |
| H–1 L | π →π* | 32.81 | |||||
| H L + 5 | π →π* | 13.94 | |||||
| S4 | 111.075 | 257.41 | 0.1487 | H–1 L | π →π* | 49.6 | |
| H–2 L | π →π* | 25.01 | |||||
| S5 | 111.947 | 255.41 | 0.0419 | H–3 L | π →π* | 65.58 | |
| H–3 L + 1 | π →π* | 12.06 | |||||
| H–1 L + 4 | π →π* | 11.68 | |||||
| S6 | 130.488 | 219.12 | 0.0812 | H L + 1 | π →π* | 45.77 | |
| H L + 5 | π →π* | 22.32 | |||||
| H–2 L | π →π* | 15.32 | |||||
| S7 | 133.024 | 214.94 | 214 | 0.1035 | H L + 5 | π →π* | 41.96 |
| H L + 1 | π →π* | 22.58 | |||||
| H–2 L | π →π* | 13.85 | |||||
| S8 | 136.039 | 210.17 | 0.1243 | H–1 L + 4 | π →π* | 38.8 | |
| H-3 L | π →π* | 25.35 | |||||
| H–1 L + 1 | π →π* | 11.57 | |||||
| S9 | 140.109 | 204.07 | 0.0024 | H L + 2 | π →σ* | 60.69 | |
| S10 | 142.413 | 200.77 | 0.1578 | H–1 L + 1 | π →π* | 26.2 | |
| H–3 L + 4 | π →π* | 23.39 | |||||
| S11 | 144.223 | 198.25 | 0.0848 | H–5 L | π →π* | 49.33 | |
| S12 | 147.428 | 193.94 | 0.0118 | H–4 L + 1 | σ →π* | 67.71 | |
| BBP | |||||||
| S1 | 87.216 | 327.83 | 331 | 0.0805 | H–4 L | π →π* | 62.58 |
| H–3 L | π →π* | 13.56 | |||||
| H L | π →π* | 11.61 | |||||
| S2 | 91.545 | 312.33 | 1.0669 | H L | π →π* | 80.49 | |
| S3 | 110.162 | 259.55 | 0.0039 | H–2 L | π →π* | 61.74 | |
| S4 | 111.439 | 256.57 | 0.2825 | H–1 L | π →π* | 81.83 | |
| S5 | 113.612 | 251.66 | 0.0264 | H–3 L | π →π* | 50.84 | |
| H–1 L + 2 | π →π* | 16.85 | |||||
| H–4 L | π →π* | 11.39 | |||||
| H–3 L + 1 | π →π* | 11.03 | |||||
| S6 | 128.744 | 222.08 | 0.038 | H L + 1 | π →π* | 61.96 | |
| S7 | 132.137 | 216.38 | 0.1495 | H L + 6 | π →σ* | 51.22 | |
| H–2 L | π →π* | 20.01 | |||||
| H L + 1 | π →π* | 11.13 | |||||
| S8 | 134.069 | 213.26 | 213 | 0.0007 | H–1 L + 6 | π →σ* | 44.9 |
| H–1 L + 2 | π →π* | 11.49 | |||||
| S9 | 136.592 | 209.32 | 0.1464 | H–1 L + 2 | π →π* | 43.55 | |
| H–3 L | π →π* | 26.25 | |||||
| S10 | 140.725 | 203.18 | 0.0034 | H L + 2 | π →π* | 55.62 | |
| H L + 8 | π →σ* | 19.42 | |||||
| S11 | 142.182 | 201.09 | 0.2044 | H–6 L | π →π* | 58.15 | |
| H L + 1 | π →π* | 11.05 | |||||
| S12 | 142.855 | 200.15 | 0.0016 | H–1 L + 1 | π →π* | 45.17 | |
| H–3 L + 2 | π →π* | 14.57 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiar, A.S.N.; Dias, P.G.M.; Queiroz, J.E.; Firmino, P.P.; Custódio, J.M.F.; Dias, L.D.; Aquino, G.L.B.; Camargo, A.J.; Napolitano, H.B. Insights on Potential Photoprotective Activity of Two Butylchalcone Derivatives: Synthesis, Spectroscopic Characterization and Molecular Modeling. Photonics 2023, 10, 228. https://doi.org/10.3390/photonics10030228
Aguiar ASN, Dias PGM, Queiroz JE, Firmino PP, Custódio JMF, Dias LD, Aquino GLB, Camargo AJ, Napolitano HB. Insights on Potential Photoprotective Activity of Two Butylchalcone Derivatives: Synthesis, Spectroscopic Characterization and Molecular Modeling. Photonics. 2023; 10(3):228. https://doi.org/10.3390/photonics10030228
Chicago/Turabian StyleAguiar, Antônio S. N., Pablo G. M. Dias, Jaqueline E. Queiroz, Pollyana P. Firmino, Jean M. F. Custódio, Lucas D. Dias, Gilberto L. B. Aquino, Ademir J. Camargo, and Hamilton B. Napolitano. 2023. "Insights on Potential Photoprotective Activity of Two Butylchalcone Derivatives: Synthesis, Spectroscopic Characterization and Molecular Modeling" Photonics 10, no. 3: 228. https://doi.org/10.3390/photonics10030228
APA StyleAguiar, A. S. N., Dias, P. G. M., Queiroz, J. E., Firmino, P. P., Custódio, J. M. F., Dias, L. D., Aquino, G. L. B., Camargo, A. J., & Napolitano, H. B. (2023). Insights on Potential Photoprotective Activity of Two Butylchalcone Derivatives: Synthesis, Spectroscopic Characterization and Molecular Modeling. Photonics, 10(3), 228. https://doi.org/10.3390/photonics10030228





