Noise Considerations for Tomographic Reconstruction of Single-Projection Digital Holographic Interferometry-Based Radiation Dosimetry
Abstract
:1. Introduction
2. Materials and Methods
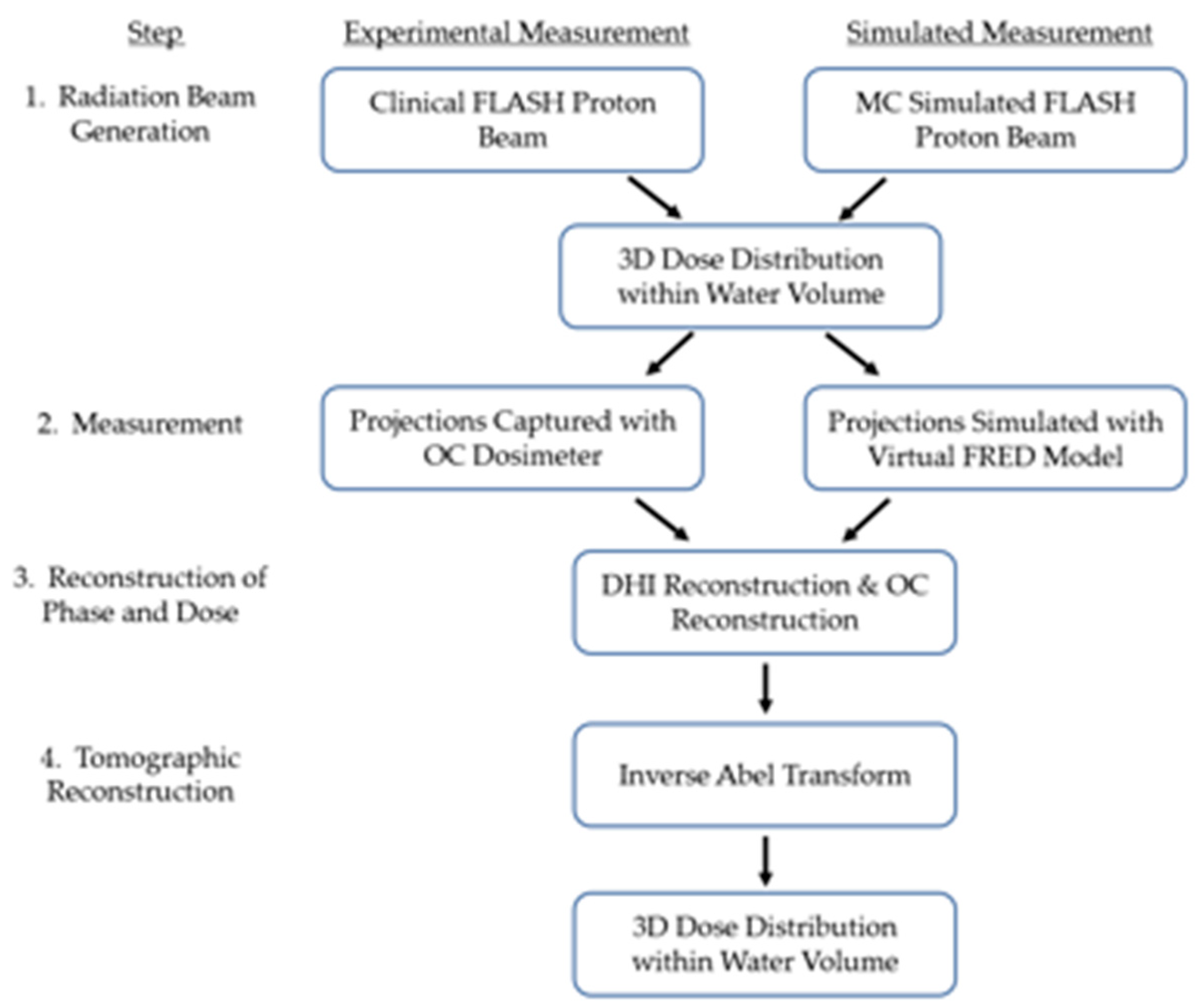
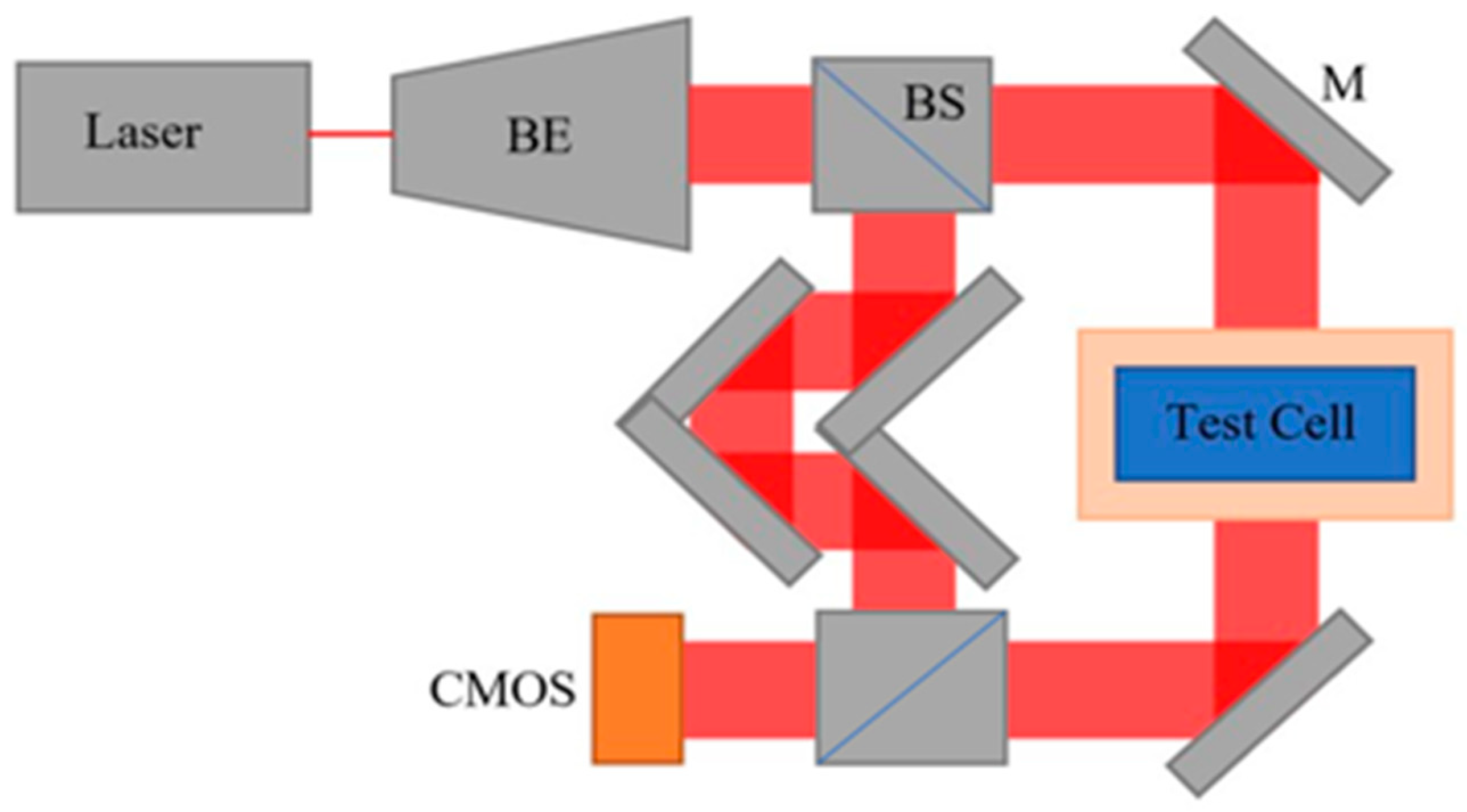
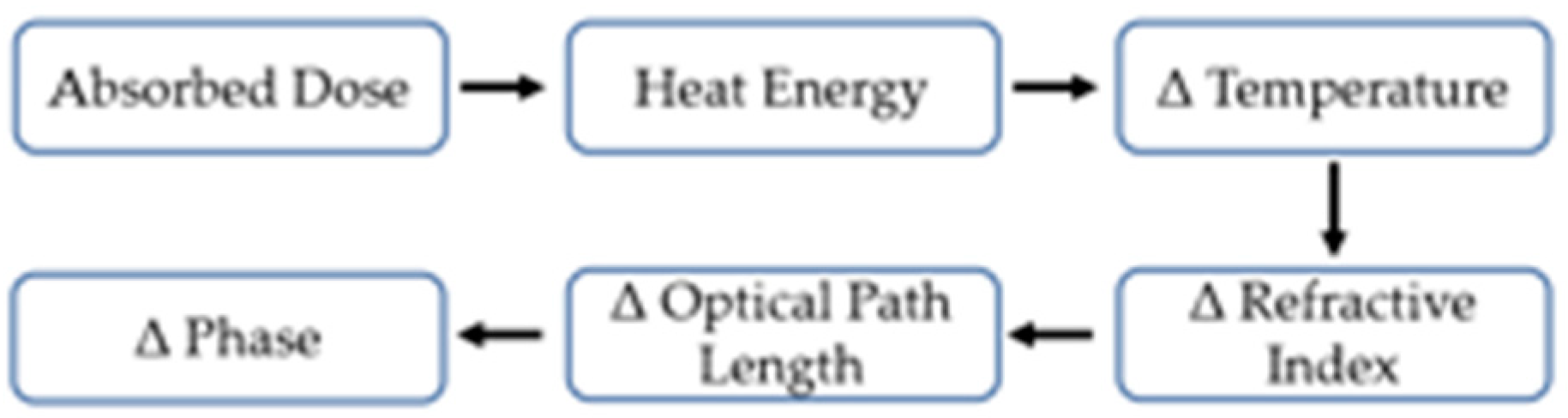
2.1. Data Generation and Workflow
- No-noise simulation—the theoretical best level of performance, where no information is lost due to the modelled sources of noise within the dosimeter. Interferograms were generated by the virtual OC dosimeter with the modelled refractive index distribution within the water cell perturbed based on the MC-simulated dose distribution, and the noise contribution due to atmospheric turbulence and mechanical vibration within the DHI measurement system was removed.
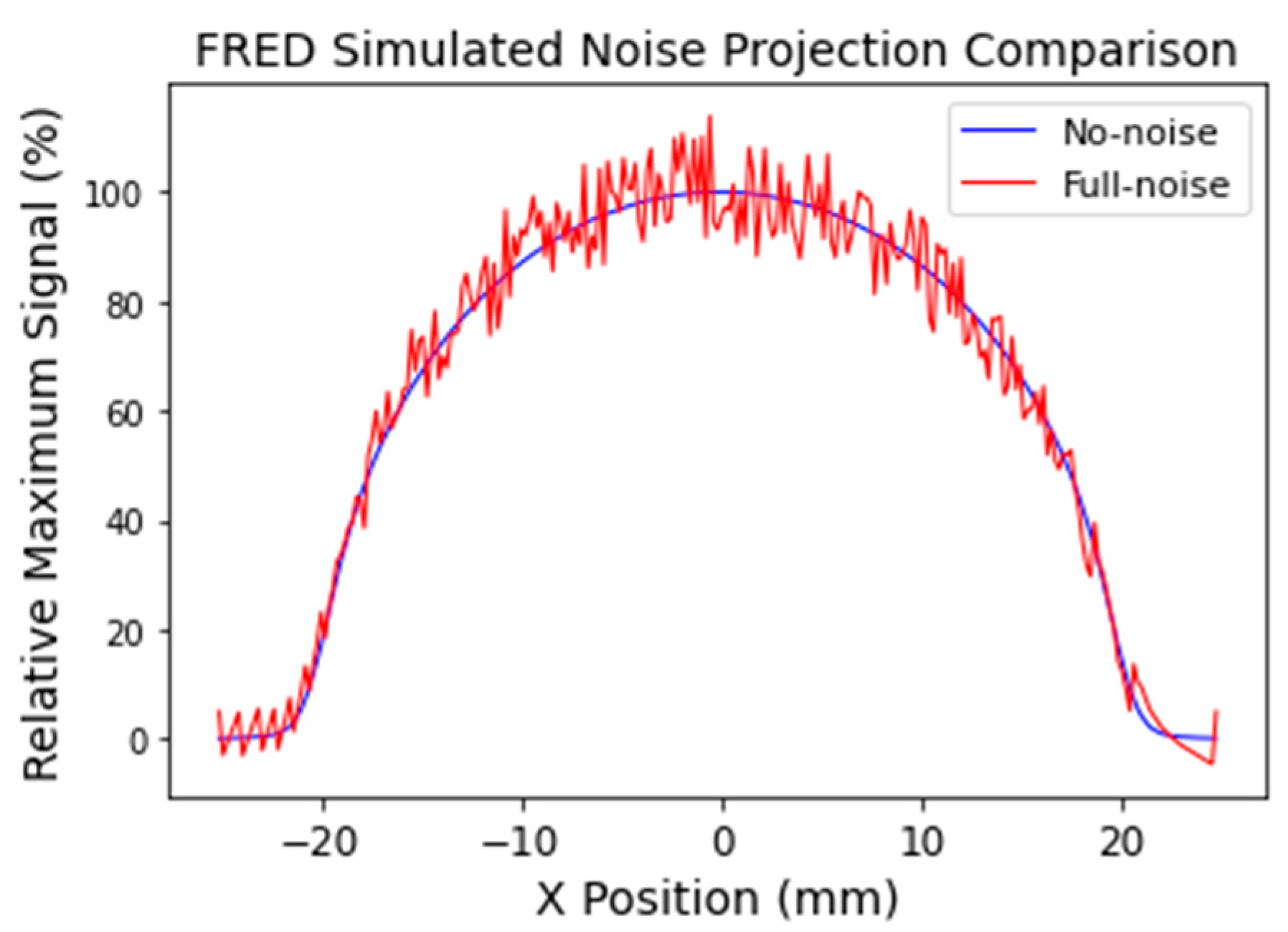
- Full-noise simulation—representing the current level of dosimeter performance. Interferograms were generated by the virtual OC dosimeter as for the no-noise simulation, but the full noise contribution due to atmospheric turbulence and mechanical vibration within the DHI measurement system was modelled.
- Reduced atmospheric turbulence simulation—interferograms were generated as for the full-noise simulation, but the noise contribution from the modelled atmospheric turbulence was reduced to half the maximum level by reducing the range of the temperature, pressure, and humidity fluctuations by half.
- Reduced mechanical vibration simulation—interferograms were generated as for the full-noise simulation, but the noise contribution from the modelled mechanical vibration was reduced to half the maximum level by reducing the maximum amplitude of the vibrations by half.
2.2. Data Analysis
3. Results
3.1. Beam Data Simulation
3.2. No-Noise Simulation Reconstruction
3.3. Full-Noise Simulation Reconstruction
3.4. Reduced Noise Simulation Reconstruction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hariharan, P. Optical interferometry. Rep. Prog. Phys. 1991, 54, 339–390. [Google Scholar]
- Schnars, U.; Jüptner, W.P.O. Digital recording and reconstruction of holograms in hologram interferometry and shearography. Appl. Opt. 1994, 33, 4373. [Google Scholar] [CrossRef] [PubMed]
- Schnars, U.; Jueptner, W. Digital Holography; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Petrov, V.; Pogoda, A.; Sementin, V.; Sevryugin, A.; Shalymov, E.; Venediktov, D.; Venediktov, V. Advances in Digital Holographic Interferometry. J. Imaging 2022, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montes, M.d.S.; Mendoza-Santoyo, F.; Flores Moreno, M.; de la Torre-Ibarra, M.; Silva Acosta, L.; Palacios-Ortega, N. Macro to nano specimen measurements using photons and electrons with digital holographic interferometry: A review. J. Eur. Opt. Soc. Rapid Publ. 2020, 16, 16. [Google Scholar] [CrossRef]
- Cubreli, G.; Psota, P.; Dančová, P.; Lédl, V.; Vít, T. Digital Holographic Interferometry for the Measurement of Symmetrical Temperature Fields in Liquids. Photonics 2021, 8, 200. [Google Scholar] [CrossRef]
- Hubley, L.; Roberts, J.; Meyer, J.; Moggré, A.; Marsh, S. Optical-radiation-calorimeter refinement by virtual-sensitivity analysis. Sensors 2019, 19, 1167. [Google Scholar] [CrossRef] [PubMed]
- Cavan, A.; Meyer, J. Digital holographic interferometry: A novel optical calorimetry technique for radiation dosimetry. Med. Phys. 2014, 41, 022102. [Google Scholar] [CrossRef]
- Roberts, J.; Moggré, A.; Marsh, S.; Meyer, J. Optical Calorimetry for Radiation Dosimetry. In Proceedings of NZPEM, Hamilton, New Zealand, 9 March 2020. [Google Scholar]
- Roberts, J.; Moggré, A.; Marsh, S.; Juergen, M. Optical Calorimetry for Radiation Dosimetry. In Proceedings of AAPM|COMP, Virtual Meeting, 12–16 July 2020. [Google Scholar]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef]
- Esplen, N.; Mendonca, M.S.; Bazalova-Carter, M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: A topical review. Phys. Med. Biol. 2020, 65, 23TR03. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Özşahin, M.; Bochud, F.; Germond, J.F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Romano, F.; Bailat, C.; Jorge, P.G.; Lerch, M.L.F.; Darafsheh, A. Ultra-high dose rate dosimetry: Challenges and opportunities for FLASH radiation therapy. Med. Phys. 2022, 49, 4912–4932. [Google Scholar] [CrossRef]
- McManus, M.; Romano, F.; Lee, N.D.; Farabolini, W.; Gilardi, A.; Royle, G.; Palmans, H.; Subiel, A. The challenge of ionisation chamber dosimetry in ultra-short pulsed high dose-rate Very High Energy Electron beams. Sci. Rep. 2020, 10, 9089. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.; Owen, H.; Schippers, M.; Welsch, C. Technical challenges for FLASH proton therapy. Phys. Med. 2020, 78, 71–82. [Google Scholar] [CrossRef]
- Roberts, J.; Moggré, A.; Marsh, S.; Meyer, J. Optical Calorimetry, a Promising Dosimetry Technique for FLASH Radiotherapy. In Proceedings of FRPT, Vienna, Austria, 1–3 December 2021. [Google Scholar]
- Abel, N.H. Auflösung einer mechanischen Aufgabe. J. Für Die Reine Und Angew. Math. 1826, 1, 153–157. [Google Scholar]
- Ashraf, M.R.; Rahman, M.; Zhang, R.; Cao, X.; Williams, B.B.; Hoopes, P.J.; Gladstone, D.J.; Pogue, B.W.; Bruza, P. Technical Note: Single-pulse beam characterization for FLASH-RT using optical imaging in a water tank. Med. Phys. 2021, 48, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Bashkatov, A.N.; Genina, E.A. Water refractive index in dependence on temperature and wavelength: A simple approximation. In Saratov Fall Meeting 2002: Optical Technologies in Biophysics and Medicine IV; SPIE: Bellingham, WA, USA, 2003; Volume 5068, pp. 393–395. [Google Scholar] [CrossRef]
- Lide, D. CRC Handbook of Chemistry and Physics; CRC press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Rashidian Vaziri, M.R.; Beigzadeh, A.M.; Ziaie, F.; Yarahmadi, M. Digital holographic interferometry for measuring the absorbed three-dimensional dose distribution. Eur. Phys. J. Plus 2020, 135, 436. [Google Scholar] [CrossRef]
- Perl, J.; Shin, J.; Schümann, J.; Faddegon, B.; Paganetti, H. TOPAS: An innovative proton Monte Carlo platform for research and clinical applications. Med. Phys. 2012, 39, 6818–6837. [Google Scholar] [CrossRef] [PubMed]
- Faddegon, B.; Ramos-Méndez, J.; Schuemann, J.; McNamara, A.; Shin, J.; Perl, J.; Paganetti, H. The TOPAS tool for particle simulation, a Monte Carlo simulation tool for physics, biology and clinical research. Phys. Med. 2020, 72, 114–121. [Google Scholar] [CrossRef]
- International Electrotechnical Commission. IEC 60976: Medical Electrical Equipment—Medical Electron Accelerators—Functional Performance Characteristics; International Electrotechnical Commission: Geneva, Switzerland, 2007. [Google Scholar]
- International Atomic Energy Agency. IAEA TRS-398: Absorbed Dose Determination in External Beam Radiotherapy; International Atomic Energy Agency: Vienna, Austria, 2000. [Google Scholar]
- Harvey, J.E.; Irvin, R.G.; Pfisterer, R.N. Modeling physical optics phenomena by complex ray tracing. Opt. Eng. 2015, 54, 035105. [Google Scholar] [CrossRef]
- Hickstein, D.D.; Gibson, S.T.; Yurchak, R.; Das, D.D.; Ryazanov, M. A direct comparison of high-speed methods for the numerical Abel transform. Rev. Sci. Instrum. 2019, 90, 065115. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.; Hickstein, D.D.; Yurchak, R.; Ryazanov, M.; Das, D.; Shih, G. PyAbel/PyAbel: v0.8.5 2022; European Organization for Nuclear Research: Geneva, Switzerland, 2022. [Google Scholar] [CrossRef]
- Owens, S.A.; Spencer, M.F.; Thornton, D.E.; Perram, G.P. Pulsed laser source digital holography efficiency measurements. Appl. Opt. 2022, 61, 4823. [Google Scholar] [CrossRef] [PubMed]
- Owens, S.A.; Spencer, M.F.; Perram, G.P. Digital-holography efficiency measurements using a heterodyne-pulsed configuration. Opt. Eng. 2022, 61, 123101. [Google Scholar] [CrossRef]


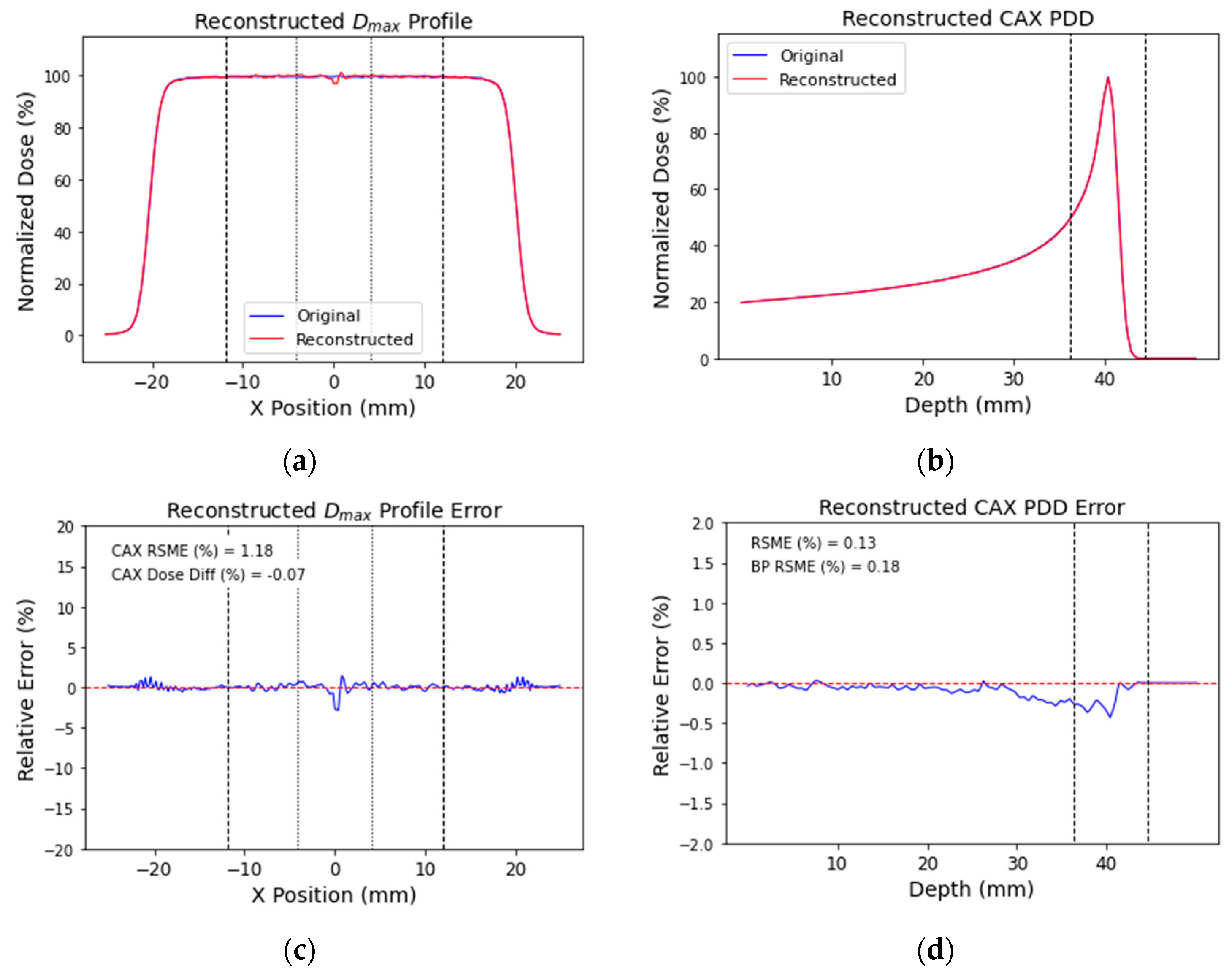


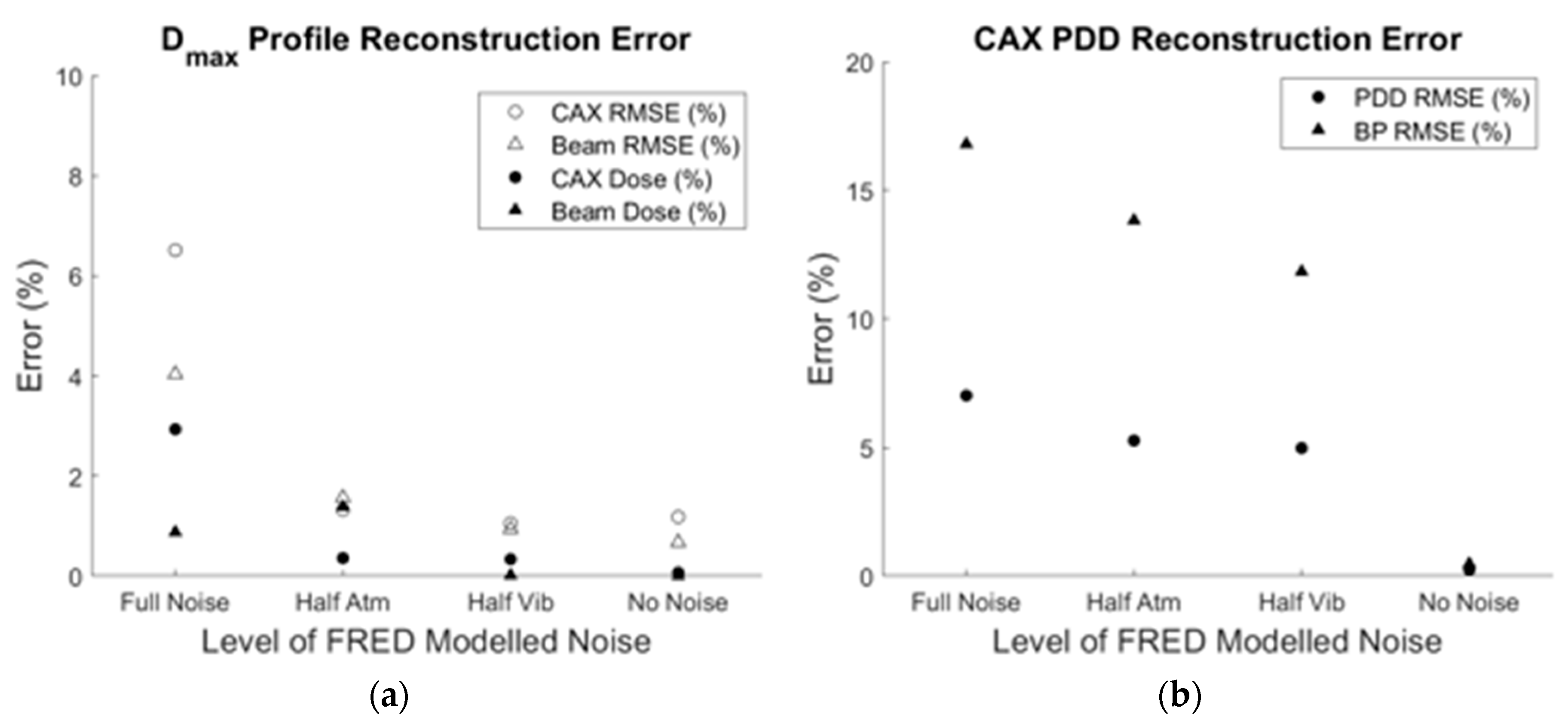
| Metric | RMSE (%) of Dmax Profile | RMSE (%) of PDD | Mean Dose Difference (%) | RMSE (%) of Projection Error | |||
|---|---|---|---|---|---|---|---|
| Region | Beam | CAX | Full | BP | Beam | CAX | Beam |
| No-noise | 0.67 | 1.18 | 0.13 | 0.18 | 0.01 | −0.07 | 0.03 |
| Metric | RMSE (%) of Dmax Profile | RMSE (%) of PDD | Mean Dose Difference (%) | RMSE (%) of Projection Error | |||
|---|---|---|---|---|---|---|---|
| Region | Beam | CAX | Full | BP | Beam | CAX | Beam |
| Mean of Simulations | 6.82 | 10.16 | 7.20 | 16.60 | −2.92 | 0.87 | 1.08 |
| Averaged Projection | 4.04 | 6.51 | 7.02 | 16.78 | −2.93 | 0.88 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telford, T.; Roberts, J.; Moggré, A.; Meyer, J.; Marsh, S. Noise Considerations for Tomographic Reconstruction of Single-Projection Digital Holographic Interferometry-Based Radiation Dosimetry. Photonics 2023, 10, 188. https://doi.org/10.3390/photonics10020188
Telford T, Roberts J, Moggré A, Meyer J, Marsh S. Noise Considerations for Tomographic Reconstruction of Single-Projection Digital Holographic Interferometry-Based Radiation Dosimetry. Photonics. 2023; 10(2):188. https://doi.org/10.3390/photonics10020188
Chicago/Turabian StyleTelford, Tom, Jackson Roberts, Alicia Moggré, Juergen Meyer, and Steven Marsh. 2023. "Noise Considerations for Tomographic Reconstruction of Single-Projection Digital Holographic Interferometry-Based Radiation Dosimetry" Photonics 10, no. 2: 188. https://doi.org/10.3390/photonics10020188
APA StyleTelford, T., Roberts, J., Moggré, A., Meyer, J., & Marsh, S. (2023). Noise Considerations for Tomographic Reconstruction of Single-Projection Digital Holographic Interferometry-Based Radiation Dosimetry. Photonics, 10(2), 188. https://doi.org/10.3390/photonics10020188





