Recent Advances in Optical Hydrogen Sensor including Use of Metal and Metal Alloys: A Review
Abstract
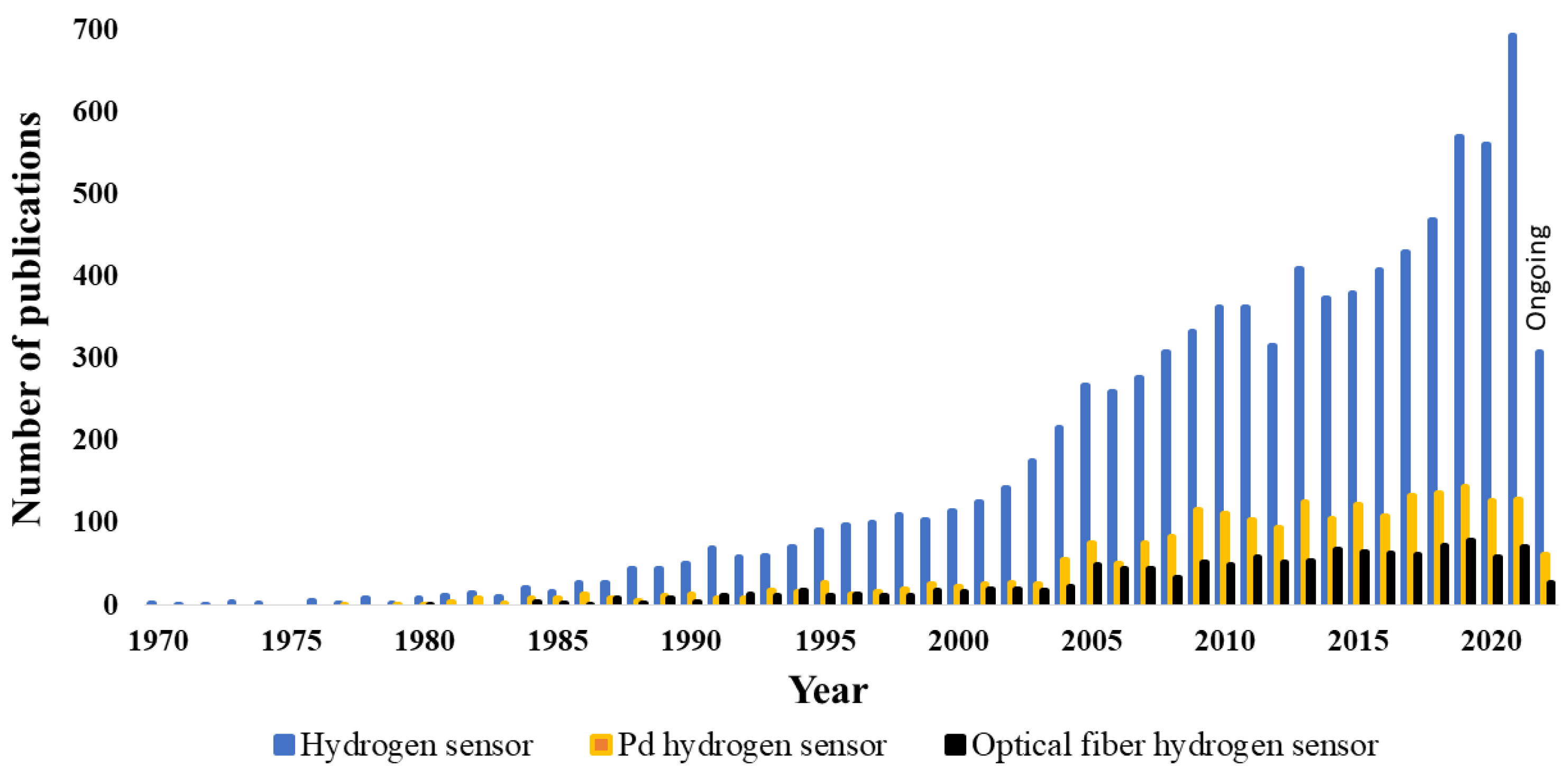
1. Introduction
2. Sensing Materials
2.1. Tungsten Oxide (WO3)
2.2. Palladium (Pd)
2.3. Advantage and Limitations
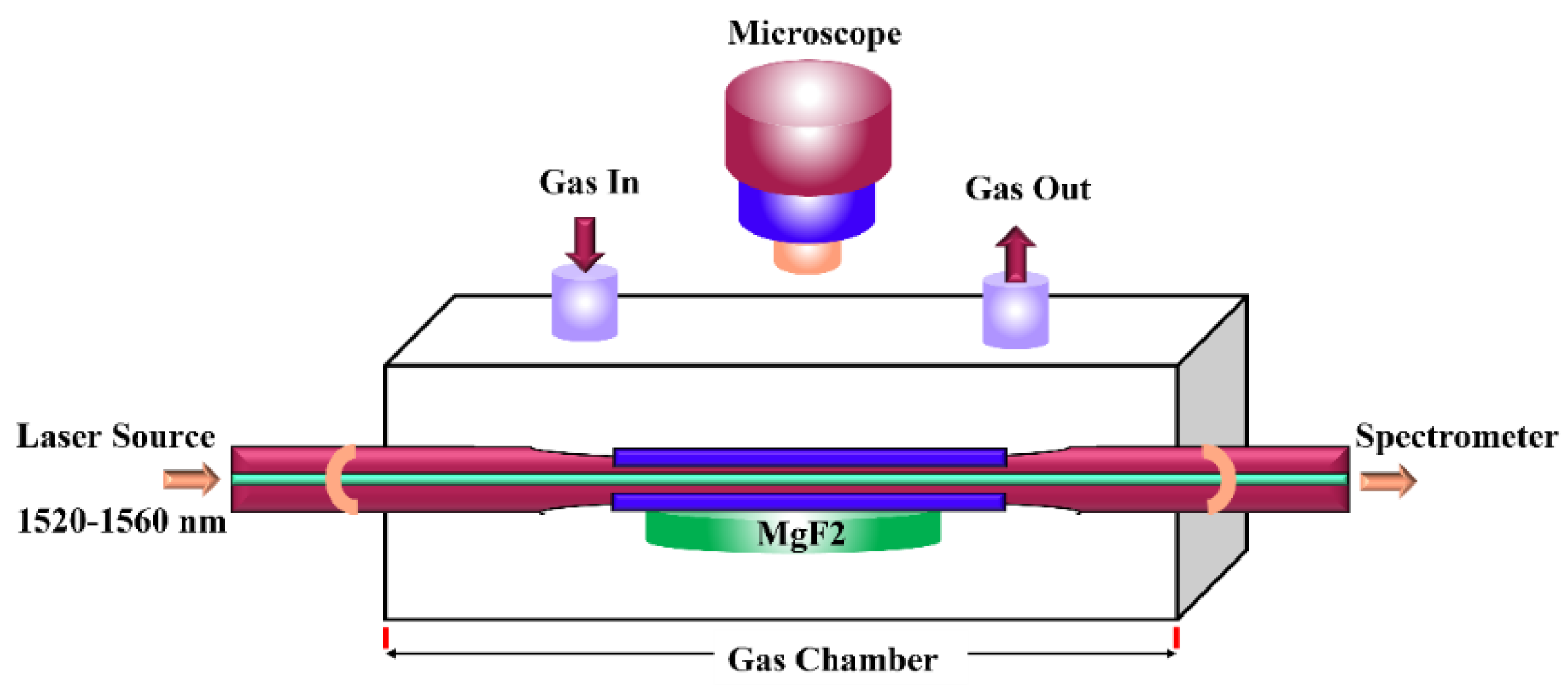
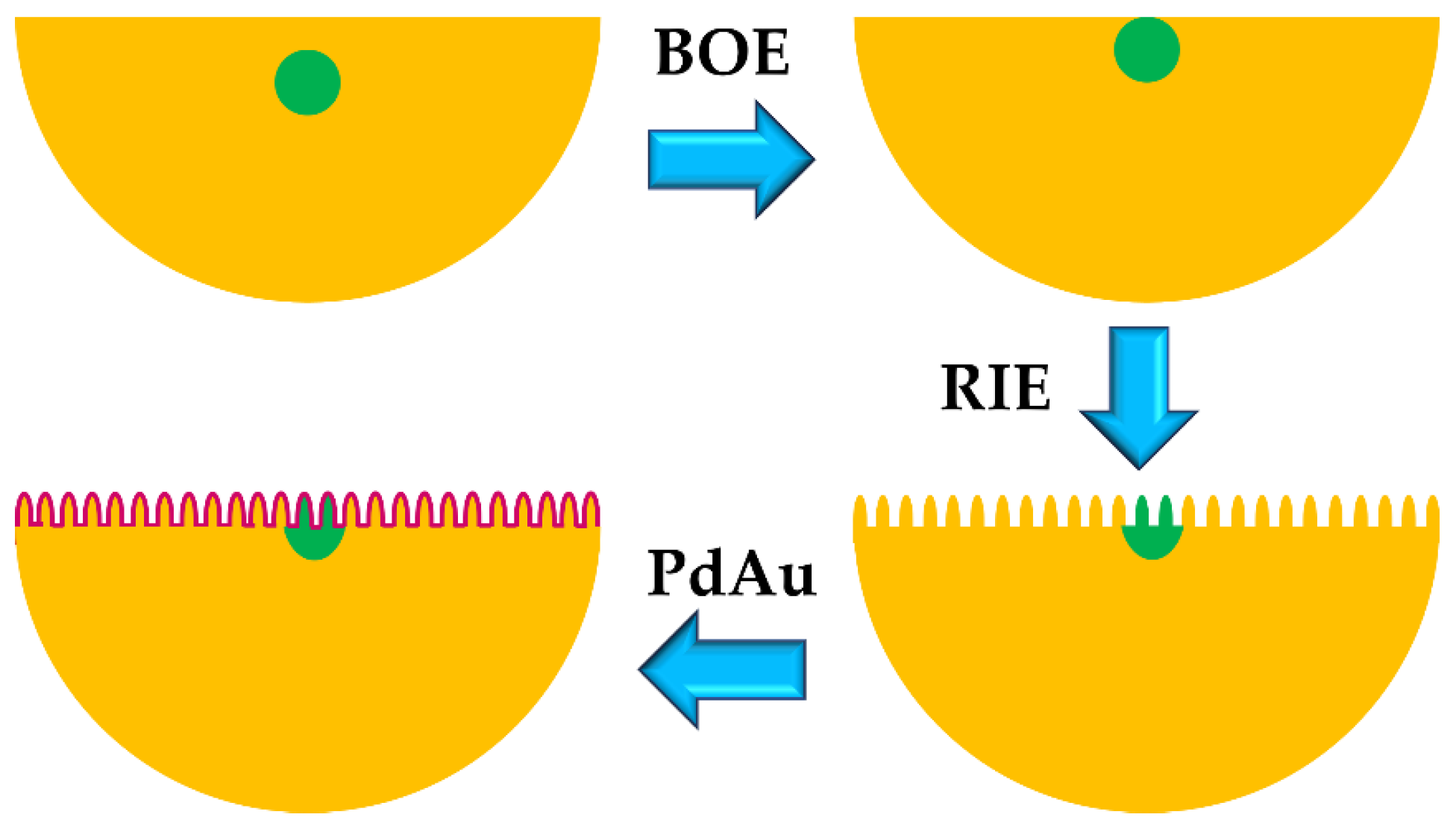
3. Measurement Method
3.1. Evanescent Field (EF) Based Hydrogen Sensor (Side Polished/Tapered/Etched Standard Fiber)
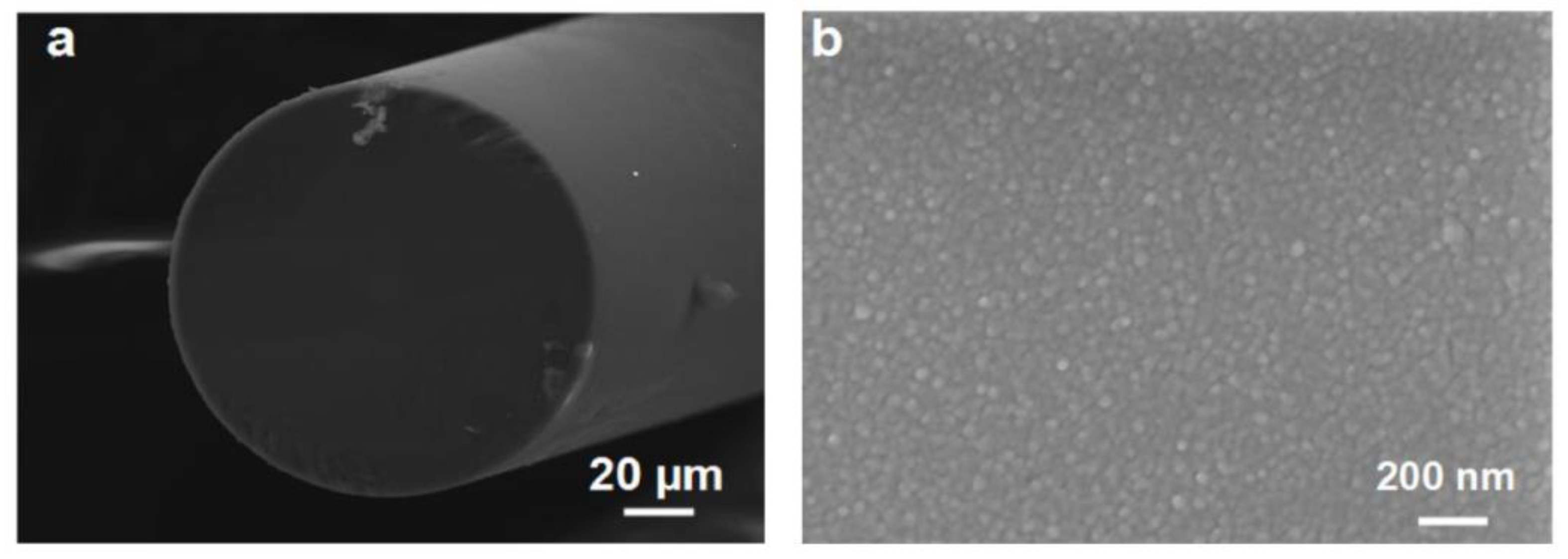
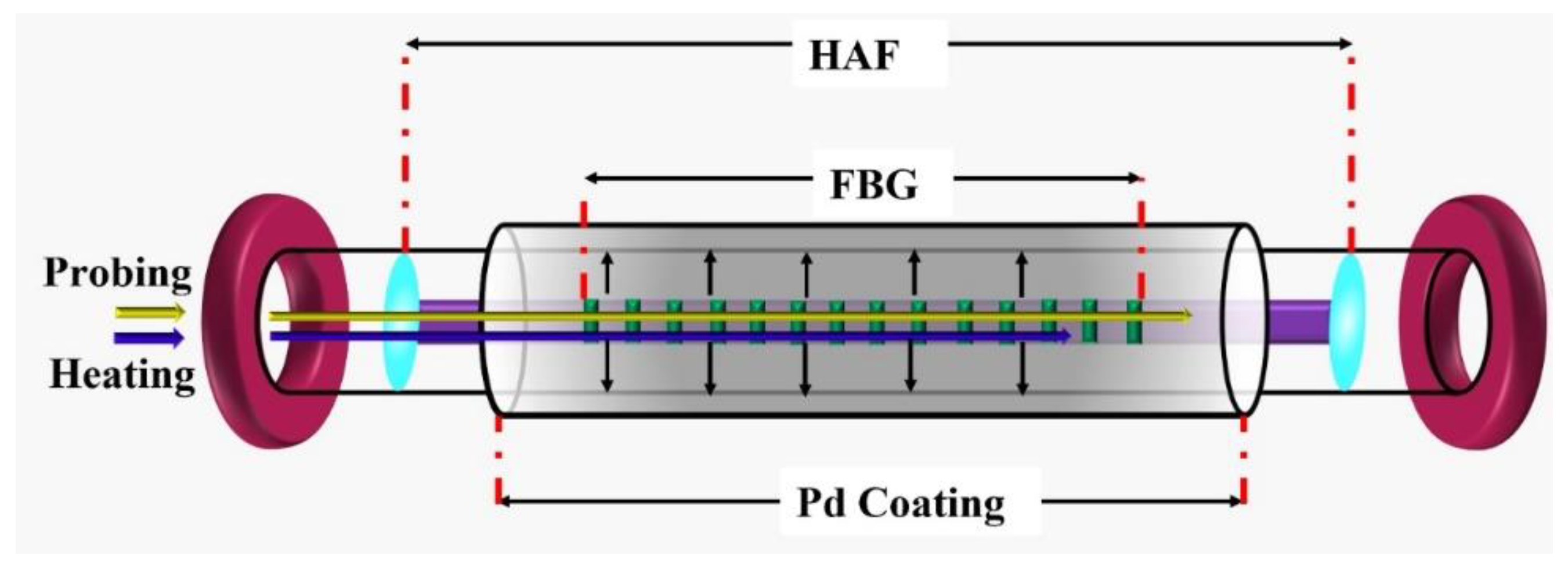
3.2. Grating Based Hydrogen Sensor
3.3. Microstructured Optical Fiber (MOF) Hydrogen Sensor
3.4. Plasmonic Fiber Hydrogen Sensor
4. Other Approaches for Hydrogen Sensing
4.1. SAW Based Hydrogen Sensor
4.2. Hi-Birefringence (Hi-Bi) Based Hydrogen Sensor
4.3. Pd Embedded Hydrogen Sensor
5. Future Prospective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caliendo, C.; Russo, P.; Ciambelli, P. Hydrogen safety, state of the art, perspectives, risk assessment, and engineering solutions. Util. Hydrog. Sustain. Energy Fuels 2021, 433–450. [Google Scholar] [CrossRef]
- Chowdhury, M.; Qubbaj, A. A review of storage mechanisms for hydrogen economy. In AIAA SCITECH 2022 Forum; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2022. [Google Scholar]
- Hübert, T.; Boon-Brett, L.; Palmisano, V.; Bader, M.A. Developments in gas sensor technology for hydrogen safety. Int. J. Hydrogen Energy 2014, 39, 20474–20483. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H. Hydrogen-A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Vega, L.F.; Kentish, S.E. The Hydrogen economy preface. Ind. Eng. Chem. Res. 2022, 61, 6065–6066. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Moon, H.; Lee, W. Hydrogen gas sensors using palladium nanogaps on an elastomeric substrate. Adv. Mater. 2021, 33, 2005929. [Google Scholar] [CrossRef] [PubMed]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors–A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Sansone, F.J. Fuel cell hydrogen sensor for marine applications. Mar. Chem. 1992, 37, 3–14. [Google Scholar] [CrossRef]
- Min, R.; Liu, Z.; Pereira, L.; Yang, C.; Sui, Q.; Marques, C. Optical fiber sensing for marine environment and marine structural health monitoring: A review. Opt. Laser Technol. 2021, 140, 107082. [Google Scholar] [CrossRef]
- Miliutina, E.; Guselnikova, O.; Chufistova, S.; Kolska, Z.; Elashnikov, R.; Burtsev, V.; Postnikov, P.; Svorcik, V.; Lyutakov, O. Fast and all-optical hydrogen sensor based on gold-coated optical fiber functionalized with metal–organic framework layer. ACS Sens. 2019, 4, 3133–3140. [Google Scholar] [CrossRef]
- Norwegian Hydrogen Will Deliver 100% Emission-Free Energy Solutions to the Aquaculture Industry. 2022. Available online: https://fuelcellsworks.com/news/norwegian-hydrogen-will-deliver-100-emission-free-energy-solutions-to-the-aquaculture-industry/ (accessed on 9 January 2023).
- Aristokleous, N.; Charalambides, M.; Menikou, M. Powering aquaculture operations at sea: Can hydrogen be a sustainable solution? SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Pathak, A.K.; Viphavakit, C. A review on all-optical fiber-based VOC sensors: Heading towards the development of promising technology. Sens. Actuators A Phys. 2022, 338, 113455. [Google Scholar] [CrossRef]
- Aslani, S.; Armstrong, D.W. High information spectroscopic detection techniques for gas chromatography. J. Chromatogr. A 2022, 1676, 463255. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of electrochemical hydrogen sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.C.N.; Cavdar, O.; Haliński, Ł.P.; Miodyńska, M.; Parnicka, P.; Bajorowicz, B.; Kobylański, M.; Lewandowski, Ł.; Zaleska-Medynska, A. Hydrogen detection during photocatalytic water splitting: A tutorial. Int. J. Hydrogen Energy 2022, 47, 15783–15788. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrog. Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Synthesis methods of obtaining materials for hydrogen sensors. Sensors 2021, 21, 5758. [Google Scholar] [CrossRef]
- Yang, M.; Dai, J. Fiber optic hydrogen sensors: A review. Photonic Sens. 2014, 4, 300–324. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, H.; Qian, X.; Zhang, Y.; An, G.; Zhao, Y. Recent advancements in optical fiber hydrogen sensors. Sens. Actuators B Chem. 2017, 244, 393–416. [Google Scholar] [CrossRef]
- Thathsara, S.K.T.; Harrison, C.J.; Hocking, R.K.; Shafiei, M. Photoactive semiconducting metal oxides: Hydrogen gas sensing mechanisms. Int. J. Hydrog. Energy 2022, 47, 18208–18227. [Google Scholar] [CrossRef]
- Lei, G.; Lou, C.; Liu, X.; Xie, J.; Li, Z.; Zheng, W.; Zhang, J. Thin films of tungsten oxide materials for advanced gas sensors. Sens. Actuators B Chem. 2021, 341, 129996. [Google Scholar] [CrossRef]
- Graham, T. On the absorption and dialytic separation of gases by colloid septa. Philos. Trans. R. Soc. London 1866, 156, 399–439. [Google Scholar] [CrossRef]
- Avila, J.I.; Matelon, R.J.; Trabol, R.; Favre, M.; Lederman, D.; Volkmann, U.G.; Cabrera, A.L. Optical properties of Pd thin films exposed to hydrogen studied by transmittance and reflectance spectroscopy. J. Appl. Phys. 2010, 107, 23504. [Google Scholar] [CrossRef]
- Hung, C.-W.; Tsai, T.-H.; Tsai, Y.-Y.; Lin, K.-W.; Chen, H.-I.; Chen, T.-P.; Liu, W.-C. A hydrogen sensor based on InAlAs material with Pt catalytic thin film. Phys. Scr. 2007, T129, 345–348. [Google Scholar] [CrossRef]
- Ando, M. Recent advances in optochemical sensors for the detection of H2, O2, O3, CO, CO2 and H2O in air. TrAC Trends Anal. Chem. 2006, 25, 937–948. [Google Scholar] [CrossRef]
- Buttner, W.J.; Post, M.B.; Burgess, R.; Rivkin, C. An overview of hydrogen safety sensors and requirements. Int. J. Hydrog. Energy 2011, 36, 2462–2470. [Google Scholar] [CrossRef]
- Boon-Brett, L.; Bousek, J.; Black, G.; Moretto, P.; Castello, P.; Hübert, T.; Banach, U. Identifying performance gaps in hydrogen safety sensor technology for automotive and stationary applications. Int. J. Hydrog. Energy 2010, 35, 373–384. [Google Scholar] [CrossRef]
- Chen, K.; Yuan, D.; Zhao, Y. Review of optical hydrogen sensors based on metal hydrides: Recent developments and challenges. Opt. Laser Technol. 2021, 137, 106808. [Google Scholar] [CrossRef]
- Gullapalli, S.K.; Vemuri, R.S.; Ramana, C.V. Structural transformation induced changes in the optical properties of nanocrystalline tungsten oxide thin films. Appl. Phys. Lett. 2010, 96, 171903. [Google Scholar] [CrossRef]
- Ranjbar, M.; Iraji zad, A.; Mahdavi, S.M. Gasochromic tungsten oxide thin films for optical hydrogen sensors. J. Phys. D Appl. Phys. 2008, 41, 55405. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Gasochromic WO3 nanostructures for the detection of hydrogen gas: An Overview. Appl. Sci. 2019, 9, 1775. [Google Scholar] [CrossRef]
- Hurlbert, R.C.; Konecny, J.O. Diffusion of hydrogen through palladium. J. Chem. Phys. 1961, 34, 655–658. [Google Scholar] [CrossRef]
- Fedtke, P.; Wienecke, M.; Bunescu, M.-C.; Pietrzak, M.; Deistung, K.; Borchardt, E. Hydrogen sensor based on optical and electrical switching. Sens. Actuators B Chem. 2004, 100, 151–157. [Google Scholar] [CrossRef]
- Oates, W.A.; Flanagan, T.B. The reaction of hydrogen atoms with palladium and its alloys. Can. J. Chem. 1975, 53, 694–701. [Google Scholar] [CrossRef]
- Perrotton, C.; Javahiraly, N.; Slaman, M.; Dam, B.; Meyrueis, P. Fiber optic surface plasmon resonance sensor based on wavelength modulation for hydrogen sensing. Opt. Express 2011, 19, A1175. [Google Scholar] [CrossRef] [PubMed]
- Downes, F.; Taylor, C.M. Theoretical investigation into the optimisation of an optical fibre surface plasmon resonance hydrogen sensor based on a PdY alloy. Meas. Sci. Technol. 2017, 28, 15104. [Google Scholar] [CrossRef]
- Von Rottkay, K.; Rubin, M.; Duine, P.A. Refractive index changes of Pd-coated magnesium lanthanide switchable mirrors upon hydrogen insertion. J. Appl. Phys. 1999, 85, 408–413. [Google Scholar] [CrossRef]
- Yang, M.; Li, Z.; Dai, J.; Yang, Z.; Zhang, Y.; Zhuang, Z. Comparison of optical fiber Bragg grating hydrogen sensors with Pd-based thin films and sol–gel WO3 coatings. Meas. Sci. Technol. 2013, 24, 94009. [Google Scholar] [CrossRef]
- Silva, S.F.; Coelho, L.; Frazao, O.; Santos, J.L.; Malcata, F.X. A review of palladium-based fiber-optic sensors for molecular hydrogen detection. IEEE Sens. J. 2012, 12, 93–102. [Google Scholar] [CrossRef]
- Jimenez, G.; Dillon, E.; Dahlmeyer, J.; Garrison, T.; Garrison, T.; Darkey, S.; Wald, K.; Kubik, J.; Paciulli, D.; Talukder, M.; et al. A comparative assessment of hydrogen embrittlement: Palladium and palladium-silver (25 weight% silver) subjected to hydrogen absorption/desorption cycling. Adv. Chem. Eng. Sci. 2016, 6, 246–261. [Google Scholar] [CrossRef]
- Alwan, A.M.; Wali, L.A.; Zayer, M.Q. A new approach of pH-IEGFET sensor based on the surface modification of macro porous silicon with palladium nanoparticles. Opt. Quantum Electron. 2020, 52, 227. [Google Scholar] [CrossRef]
- Favier, F.; Walter, E.C.; Zach, M.P.; Benter, T.; Penner, R.M. Hydrogen sensors and switches from electrodeposited palladium mesowire arrays. Science. 2001, 293, 2227–2231. [Google Scholar] [CrossRef]
- Hu, Y.; Lei, J.; Wang, Z.; Yang, S.; Luo, X.; Zhang, G.; Chen, W.; Gu, H. Rapid response hydrogen sensor based on nanoporous Pd thin films. Int. J. Hydrog. Energy 2016, 41, 10986–10990. [Google Scholar] [CrossRef]
- Le Huy, B.; Kumar, S.; Kim, G.-H. Manipulation of palladium nanoparticles in a 20 nm gap between electrodes for hydrogen sensor application. J. Phys. D Appl. Phys. 2011, 44, 325402. [Google Scholar] [CrossRef]
- Yi, J.; Kim, S.H.; Lee, W.W.; Kwon, S.S.; Sung, W.N.; Park, W. Il Graphene meshes decorated with palladium nanoparticles for hydrogen detection. J. Phys. D Appl. Phys. 2015, 48, 475103. [Google Scholar] [CrossRef]
- Javahiraly, N. Review on hydrogen leak detection: Comparison between fiber optic sensors based on different designs with palladium. Opt. Eng. 2015, 54, 030901. [Google Scholar] [CrossRef]
- Yang, M.; Dai, J. Review on optical fiber sensors with sensitive thin films. Photonic Sens. 2012, 2, 14–28. [Google Scholar] [CrossRef]
- Mamatkulov, M.; Zhdanov, V.P. Partial or complete suppression of hysteresis in hydride formation in binary alloys of Pd with other metals. J. Alloys Compd. 2021, 885, 160956. [Google Scholar] [CrossRef]
- Ndaya, C.C.; Javahiraly, N.; Brioude, A. Recent advances in palladium nanoparticles-based hydrogen sensors for leak detection. Sensors 2019, 19, 4478. [Google Scholar] [CrossRef]
- Westerwaal, R.J.; Rooijmans, J.S.A.; Leclercq, L.; Gheorghe, D.G.; Radeva, T.; Mooij, L.; Mak, T.; Polak, L.; Slaman, M.; Dam, B.; et al. Nanostructured Pd–Au based fiber optic sensors for probing hydrogen concentrations in gas mixtures. Int. J. Hydrog. Energy 2013, 38, 4201–4212. [Google Scholar] [CrossRef]
- Luna-Moreno, D.; Monzón-Hernández, D. Effect of the Pd–Au thin film thickness uniformity on the performance of an optical fiber hydrogen sensor. Appl. Surf. Sci. 2007, 253, 8615–8619. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Yu, X.; Lu, H. Optical hydrogen sensor based on etched fiber Bragg grating sputtered with Pd/Ag composite film. Opt. Fiber Technol. 2013, 19, 26–30. [Google Scholar] [CrossRef]
- Zou, M.; Dai, Y.; Zhou, X.; Dong, K.; Yang, M. Femtosecond laser ablated FBG with composite microstructure for hydrogen sensor application. Sensors 2016, 16, 2040. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y. Enhanced sensitivity of transmission based optical fiber hydrogen sensor with multi-layer Pd–Y alloy thin film. Sens. Actuators B Chem. 2016, 227, 178–184. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Yang, Z.; Li, Z.; Wang, Y.; Wang, G.; Zhang, Y.; Zhuang, Z. Enhanced sensitivity of fiber Bragg grating hydrogen sensor using flexible substrate. Sens. Actuators B Chem. 2014, 196, 604–609. [Google Scholar] [CrossRef]
- Bannenberg, L.; Schreuders, H.; Dam, B. Tantalum-palladium: Hysteresis-free optical hydrogen sensor over 7 orders of magnitude in pressure with sub-second response. Adv. Funct. Mater. 2021, 31, 2010483. [Google Scholar] [CrossRef]
- Zhu, C.-C.; Yu, Y.-S.; Zhang, X.-Y.; Chen, C.; Liang, J.-F.; Liu, Z.-J.; Meng, A.-H.; Jing, S.-M.; Sun, H.-B. Compact Mach–Zehnder interferometer based on tapered hollow optical fiber. IEEE Photonics Technol. Lett. 2015, 27, 1277–1280. [Google Scholar] [CrossRef]
- Taha, B.A.; Ali, N.; Sapiee, N.M.; Fadhel, M.M.; Mat Yeh, R.M.; Bachok, N.N.; Al Mashhadany, Y.; Arsad, N. Comprehensive review tapered optical fiber configurations for sensing application: Trend and challenges. Biosensors 2021, 11, 253. [Google Scholar] [CrossRef]
- Pathak, A.K.; Chaudhary, D.K.; Singh, V.K. Broad range and highly sensitive optical pH sensor based on Hierarchical ZnO microflowers over tapered silica fiber. Sens. Actuators A Phys. 2018, 280, 399–405. [Google Scholar] [CrossRef]
- Dong, H.; Chen, L.; Zhou, J.; Yu, J.; Guan, H.; Qiu, W.; Dong, J.; Lu, H.; Tang, J.; Zhu, W.; et al. Coreless side-polished fiber: A novel fiber structure for multimode interference and highly sensitive refractive index sensors. Opt. Express 2017, 25, 5352. [Google Scholar] [CrossRef]
- De Acha, N.; Socorro-Leránoz, A.B.; Elosúa, C.; Matías, I.R. Trends in the design of intensity-based optical fiber biosensors (2010–2020). Biosensors 2021, 11, 197. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Wei, Z.; Lou, X. Evanescent wave optical-fiber aptasensor for rapid detection of zearalenone in corn with unprecedented sensitivity. Biosensors 2022, 12, 438. [Google Scholar] [CrossRef]
- Tabib-Azar, M.; Sutapun, B.; Petrick, R.; Kazemi, A. Highly sensitive hydrogen sensors using palladium coated fiber optics with exposed cores and evanescent field interactions. Sens. Actuators B Chem. 1999, 56, 158–163. [Google Scholar] [CrossRef]
- Sekimoto, S.; Nakagawa, H.; Okazaki, S.; Fukuda, K.; Asakura, S.; Shigemori, T.; Takahashi, S. A fiber-optic evanescent-wave hydrogen gas sensor using palladium-supported tungsten oxide. Sens. Actuators B Chem. 2000, 66, 142–145. [Google Scholar] [CrossRef]
- Villatoro, J.; Diez, A.; Cruz, J.L.; Andres, M.V. In-line highly sensitive hydrogen sensor based on palladium-coated single-mode tapered fibers. IEEE Sens. J. 2003, 3, 533–537. [Google Scholar] [CrossRef]
- Kim, K.T.; Song, H.S.; Mah, J.P.; Hong, K.B.; Im, K.; Baik, S.-J.; Yoon, Y.-I. Hydrogen sensor based on palladium coated side-polished single-mode fiber. IEEE Sens. J. 2007, 7, 1767–1771. [Google Scholar] [CrossRef]
- Luna-Moreno, D.; Monzón-Hernández, D.; Villatoro, J.; Badenes, G. Optical fiber hydrogen sensor based on core diameter mismatch and annealed Pd–Au thin films. Sens. Actuators B Chem. 2007, 125, 66–71. [Google Scholar] [CrossRef]
- Monzón-Hernández, D.; Luna-Moreno, D.; Martínez-Escobar, D. Fast response fiber optic hydrogen sensor based on palladium and gold nano-layers. Sens. Actuators B Chem. 2009, 136, 562–566. [Google Scholar] [CrossRef]
- Li, J.; Fan, R.; Hu, H.; Yao, C. Hydrogen sensing performance of silica microfiber elaborated with Pd nanoparticles. Mater. Lett. 2018, 212, 211–213. [Google Scholar] [CrossRef]
- Dai, J.; Li, Y.; Ruan, H.; Ye, Z.; Chai, N.; Wang, X.; Qiu, S.; Bai, W.; Yang, M. Fiber optical hydrogen sensor based on WO3-Pd2Pt-Pt nanocomposite films. Nanomaterials 2021, 11, 128. [Google Scholar] [CrossRef]
- Alkhabet, M.; Girei, S.H.; Paiman, S.; Arsad, N.; Alresheedi, M.T.; Mahdi, M.A.; Yaacob, M.H. Room temperature operated hydrogen sensor using palladium coated on tapered optical fiber. SSRN Electron. J. 2022, 287, 116092. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, H.-J.; Yang, S.-C.; Park, J.-H.; Lee, S.-K. Fiber optic localized surface plasmon resonance hydrogen sensor based on gold nanoparticles capped with palladium. J. Ind. Eng. Chem. 2022, 111, 281–288. [Google Scholar] [CrossRef]
- Alkhabet, M.M.; Girei, S.H.; Paiman, S.; Arsad, N.; Mahdi, M.A.; Yaacob, M.H. Tapered optical fiber for hydrogen sensing application based on molybdenum trioxide (MoO3). Eng. Proc. 2021, 10, 75. [Google Scholar] [CrossRef]
- Villatoro, J.; Díez, A.; Cruz, J.L.; Andrés, M.V. Highly sensitive optical hydrogen sensor using circular Pd-coated singlemode tapered fibre. Electron. Lett. 2001, 37, 1011. [Google Scholar] [CrossRef]
- Sumida, S.; Okazaki, S.; Asakura, S.; Nakagawa, H.; Murayama, H.; Hasegawa, T. Distributed hydrogen determination with fiber-optic sensor. Sens. Actuators B Chem. 2005, 108, 508–514. [Google Scholar] [CrossRef]
- Cao, R.; Wu, J.; Liang, G.; Ohodnicki, P.R.; Chen, K.P. Functionalized PdAu alloy on nanocones fabricated on optical fibers for hydrogen sensing. IEEE Sens. J. 2020, 20, 1922–1927. [Google Scholar] [CrossRef]
- Tang, Y.; Peng, T.; Sirkis, J.S.; Childers, B.A.; Moore, J.P.; Melvin, L.D. Characterization of a fiber Bragg grating (FBG)-based palladium tube hydrogen sensor. In Smart Structures and Materials 1999: Sensory Phenomena and Measurement Instrumentation for Smart Structures and Materials; SPIE: Bellingham, WA, USA, 1999; Volume 3670, pp. 532–540. [Google Scholar]
- Trouillet, A.; Marin, E.; Veillas, C. Fibre gratings for hydrogen sensing. Meas. Sci. Technol. 2006, 17, 1124–1128. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, M.J.; Park, M.S.; Jang, J.H.; Lee, B.H.; Kim, T.K. Hydrogen sensor based on a palladium-coated long-period fiber grating pair. J. Opt. Soc. Korea 2008, 12, 221–225. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, M.J.; Rho, B.S.; Park, M.-S.; Jang, J.-H.; Lee, B.H. Ultra sensitive fiber-optic hydrogen sensor based on high order cladding mode. IEEE Sens. J. 2011, 11, 1423–1426. [Google Scholar] [CrossRef]
- Buric, M.; Chen, K.P.; Bhattarai, M.; Swinehart, P.R.; Maklad, M. Active fiber Bragg grating hydrogen sensors for all-temperature operation. IEEE Photonics Technol. Lett. 2007, 19, 255–257. [Google Scholar] [CrossRef]
- Buric, M.; Chen, T.; Maklad, M.; Swinehart, P.R.; Chen, K.P. Multiplexable low-temperature fiber Bragg grating hydrogen sensors. IEEE Photonics Technol. Lett. 2009, 21, 1594–1596. [Google Scholar] [CrossRef]
- Rahman, B.M.A.; Viphavakit, C.; Chitaree, R.; Ghosh, S.; Pathak, A.K.; Verma, S.; Sakda, N. Optical fiber, nanomaterial, and THz-metasurface-mediated nano-biosensors: A review. Biosensors 2022, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.B.; Raghavan, V.S.; Shorie, M.; Sabherwal, P.; Gorthi, S.S.; Asokan, S.; Sood, A.K. Enhanced optical sensitivity of polyvinyl alcohol–reduced graphene oxide electrospun nanofiber coated etched fiber Bragg grating sensor for detection of myoglobin a cardiac biomarker. Adv. Photonics Res. 2021, 2, 2000138. [Google Scholar] [CrossRef]
- Ayupova, T.; Shaimerdenova, M.; Tosi, D. Shallow-tapered chirped fiber Bragg grating sensors for dual refractive index and temperature sensing. Sensors 2021, 21, 3635. [Google Scholar] [CrossRef]
- Silva, S.; Coelho, L.; Almeida, J.M.; Frazao, O.; Santos, J.L.; Malcata, F.X.; Becker, M.; Rothhardt, M.; Bartelt, H. H2 sensing based on a Pd-coated tapered-FBG fabricated by DUV femtosecond laser technique. IEEE Photonics Technol. Lett. 2013, 25, 401–403. [Google Scholar] [CrossRef]
- Ma, G.; Li, C.; Luo, Y.; Mu, R.; Wang, L. High sensitive and reliable fiber Bragg grating hydrogen sensor for fault detection of power transformer. Sens. Actuators B Chem. 2012, 169, 195–198. [Google Scholar] [CrossRef]
- Ma, G.-M.; Li, C.-R.; Mu, R.-D.; Jiang, J.; Luo, Y.-T. Fiber bragg grating sensor for hydrogen detection in power transformers. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 380–385. [Google Scholar] [CrossRef]
- Zhao, Z.; Carpenter, M.A.; Xia, H.; Welch, D. All-optical hydrogen sensor based on a high alloy content palladium thin film. Sens. Actuators B Chem. 2006, 113, 532–538. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Yu, X.; Cao, K.; Liao, J. Greatly etched fiber Bragg grating hydrogen sensor with Pd/Ni composite film as sensing material. Sens. Actuators B Chem. 2012, 174, 253–257. [Google Scholar] [CrossRef]
- Ma, G.M.; Jiang, J.; Li, C.R.; Song, H.T.; Luo, Y.T.; Wang, H.B. Pd/Ag coated fiber Bragg grating sensor for hydrogen monitoring in power transformers. Rev. Sci. Instrum. 2015, 86, 45003. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, M.R.; Shee, Y.G.; Adikan, F.R.M.; Razak, B.B.A.; Dahari, M. Fiber Bragg gratings hydrogen sensor for monitoring the degradation of transformer oil. IEEE Sens. J. 2016, 16, 2993–2999. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, H.; Wang, C.; Sun, Z.; Yin, G.; Zhou, K.; Wang, Y.; Zhao, W.; Zhang, L. Theoretical and experimental analysis of excessively tilted fiber gratings. Opt. Express 2016, 24, 12107. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Ge, Y.-M.; Wu, S.-T.; Hwang, S.-J. Ultra-sensitive curvature sensor based on liquid crystal-infiltrated fiber interferometer. J. Phys. D Appl. Phys. 2017, 50, 15102. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L. Wavelength tuning of fiber Bragg grating based on fiber side polishing. In Proceedings of the 2008 International Conference on Optical Instruments and Technology: Advanced Sensor Technologies and Applications, Beijing, China, 27 January 2009. [Google Scholar]
- Pathak, A.K.; Singh, V.K. SPR based optical fiber refractive index sensor using silver nanowire assisted CSMFC. IEEE Photonics Technol. Lett. 2020, 32, 465–468. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, L.; Wang, G.; Xiang, F.; Qin, Y.; Wang, M.; Yang, M. Optical fiber grating hydrogen sensors: A review. Sensors 2017, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yang, M.; Chen, Y.; Cao, K.; Liao, H.; Zhang, P. Side-polished fiber Bragg grating hydrogen sensor with WO3-Pd composite film as sensing materials. Opt. Express 2011, 19, 6141. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-T.; Wang, H.-B.; Ma, G.-M.; Song, H.-T.; Li, C.; Jiang, J. Research on high sensitive D-shaped FBG hydrogen sensors in power transformer oil. Sensors 2016, 16, 1641. [Google Scholar] [CrossRef] [PubMed]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Hunze, A. Improving the sensitivity of palladium-based fiber optic hydrogen sensors. J. Light. Technol. 2018, 36, 2166–2174. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Janssens, S.; Hunze, A. Palladium-based hydrogen sensors using fiber Bragg gratings. J. Light. Technol. 2018, 36, 850–856. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Hunze, A. Optimizing the sensitivity of palladium based hydrogen sensors. Sens. Actuators B Chem. 2018, 259, 10–19. [Google Scholar] [CrossRef]
- Yu, J.; Wu, Z.; Yang, X.; Han, X.; Zhao, M. Tilted fiber Bragg grating sensor using chemical plating of a palladium membrane for the detection of hydrogen leakage. Sensors 2018, 18, 4478. [Google Scholar] [CrossRef]
- Shen, C.; Huang, Z.; Chen, X.; Chen, H.; Wang, Z.; Li, Y.; Liu, J.; Zhou, J. Reflective-type high sensitivity optical fiber hydrogen sensor based on enlarged taper cascaded with tilted fiber grating. J. Light. Technol. 2022, 40, 1–6. [Google Scholar] [CrossRef]
- Tang, Y. Characterization of fiber Bragg grating (FBG) based palladium tube hydrogen sensors. In Proceedings of the Smart Structures and Materials 1999: Sensory Phenomena and Measurement Instrumentation for Smart Structures and Materials, Newport Beach, CA, USA, 31 May 1999. [Google Scholar]
- Zhong, X.; Yang, M.; Huang, C.; Wang, G.; Dai, J.; Bai, W. Water photolysis effect on the long-term stability of a fiber optic hydrogen sensor with Pt/WO3. Sci. Rep. 2016, 6, 39160. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, G.-M.; Li, C.-R.; Song, H.-T.; Luo, Y.-T.; Wang, H.-B. Highly sensitive dissolved hydrogen sensor based on side-polished fiber Bragg grating. IEEE Photonics Technol. Lett. 2015, 27, 1453–1456. [Google Scholar] [CrossRef]
- Coelho, L.; de Almeida, J.M.M.M.; Santos, J.L.; Viegas, D. Fiber optic hydrogen sensor based on an etched Bragg grating coated with palladium. Appl. Opt. 2015, 54, 10342. [Google Scholar] [CrossRef] [PubMed]
- Karanja, J.M.; Dai, Y.; Zhou, X.; Liu, B.; Yang, M. Micro-structured femtosecond laser assisted FBG hydrogen sensor. Opt. Express 2015, 23, 31034. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, L.; Chen, L.; Li, J.; Ran, Y.; Guan, B.-O. Microfiber Bragg grating hydrogen sensors. IEEE Photonics Technol. Lett. 2015, 27, 2575–2578. [Google Scholar] [CrossRef]
- Xiang, F.; Wang, G.; Qin, Y.; Yang, S.; Zhong, X.; Dai, J.; Yang, M. Improved performance of fiber Bragg hydrogen sensors assisted by controllable optical heating system. IEEE Photonics Technol. Lett. 2017, 29, 1233–1236. [Google Scholar] [CrossRef]
- Yang, S.; Dai, J.; Qin, Y.; Xiang, F.; Wang, G.; Yang, M. Improved performance of fiber optic hydrogen sensor based on MoO3 by ion intercalation. Sens. Actuators B Chem. 2018, 270, 333–340. [Google Scholar] [CrossRef]
- Zhou, F.; Qiu, S.-J.; Luo, W.; Xu, F.; Lu, Y.-Q. An all-fiber reflective hydrogen sensor based on a photonic crystal fiber in-line interferometer. IEEE Sens. J. 2014, 14, 1133–1136. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, F.; Wang, H.; Yang, W.; Jin, W. Temperature-insensitive hydrogen sensor with polarization-maintaining photonic crystal fiber-based sagnac interferometer. J. Light. Technol. 2015, 33, 2566–2571. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhou, T.-M.; Han, B.; Zhang, L.; Wu, Q.-L. Simultaneous measurement of hydrogen concentration and temperature based on fiber loop mirror combined with PCF. IEEE Sens. J. 2018, 18, 2369–2376. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhang, A.; Han, B.; E, S. A reflective hydrogen sensor based on fiber ring laser with PCF modal interferometer. J. Opt. 2018, 20, 65401. [Google Scholar] [CrossRef]
- Aazi, M.; Kudinova, M.; Kinet, D.; Auguste, J.-L.; Delépine-Lesoille, S.; Mégret, P.; Humbert, G. Impact of H2 gas on disruptive birefringence optical fibers with embedded Palladium particles for developing robust sensors. J. Phys. Photonics 2020, 2, 14005. [Google Scholar] [CrossRef]
- Liu, R.-X.; Zhang, X.; Zhu, X.-S.; Shi, Y.-W. Optical fiber hydrogen sensor based on the EVA/Pd coated hollow fiber. IEEE Photonics J. 2022, 14, 1–6. [Google Scholar] [CrossRef]
- Liu, H.; Wang, M.; Wang, Q.; Li, H.; Ding, Y.; Zhu, C. Simultaneous measurement of hydrogen and methane based on PCF-SPR structure with compound film-coated side-holes. Opt. Fiber Technol. 2018, 45, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Peng, H.; Zhao, Y. Photonic crystal fiber modal interferometer with Pd/WO3 coating for real-time monitoring of dissolved hydrogen concentration in transformer oil. Rev. Sci. Instrum. 2016, 87, 125002. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, Y.; Qi, Y.; Tan, Y.Z.; Ho, H.L.; Jin, W. Towards label-free distributed fiber hydrogen sensor with stimulated Raman spectroscopy. Opt. Express 2019, 27, 12869. [Google Scholar] [CrossRef] [PubMed]
- Minkovich, V.P.; Sotsky, A.B.; Shilov, A.V.; Sotskaya, L.I. Taper with palladium coating in photonic crystal fiber as a sensitive element of hydrogen sensor. J. Appl. Spectrosc. 2019, 86, 112–119. [Google Scholar] [CrossRef]
- Luo, J.; Liu, S.; Chen, P.; Chen, Y.; Zhong, J.; Wang, Y. Highly sensitive hydrogen sensor based on an optical driven nanofilm resonator. ACS Appl. Mater. Interfaces 2022, 14, 29357–29365. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, B.; Zhang, X.; Zhao, B.; Zhang, H.; Tang, S. A highly sensitive sensor of methane and hydrogen in tellurite photonic crystal fiber based on four-wave mixing. Opt. Quantum Electron. 2022, 54, 215. [Google Scholar] [CrossRef]
- Wadell, C.; Syrenova, S.; Langhammer, C. Plasmonic hydrogen sensing with nanostructured metal hydrides. ACS Nano 2014, 8, 11925–11940. [Google Scholar] [CrossRef]
- Ai, B.; Sun, Y.; Zhao, Y. Plasmonic hydrogen sensors. Small 2022, 18, 2107882. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, R.; Gupta, B.D. Fiber optic hydrogen gas sensor utilizing surface plasmon resonance and native defects of zinc oxide by palladium. J. Opt. 2016, 18, 015004. [Google Scholar] [CrossRef]
- Kumari, S.; Gupta, S. Performance estimation of hybrid plasmonic waveguide in presence of stress. Plasmonics 2021, 16, 359–370. [Google Scholar] [CrossRef]
- Bévenot, X.; Trouillet, A.; Veillas, C.; Gagnaire, H.; Clément, M. Surface plasmon resonance hydrogen sensor using an optical fibre. Meas. Sci. Technol. 2002, 13, 118–124. [Google Scholar] [CrossRef]
- Perrotton, C.; Westerwaal, R.J.; Javahiraly, N.; Slaman, M.; Schreuders, H.; Dam, B.; Meyrueis, P. A reliable, sensitive and fast optical fiber hydrogen sensor based on surface plasmon resonance. Opt. Express 2013, 21, 382. [Google Scholar] [CrossRef]
- Hosoki, A.; Nishiyama, M.; Igawa, H.; Seki, A.; Choi, Y.; Watanabe, K. A surface plasmon resonance hydrogen sensor using Au/Ta2O5/Pd multi-layers on hetero-core optical fiber structures. Sens. Actuators B Chem. 2013, 185, 53–58. [Google Scholar] [CrossRef]
- Hosoki, A.; Nishiyama, M.; Igawa, H.; Watanabe, K. Multipoint hydrogen sensing of hetero-core fiber SPR tip sensors with pseudorandom noise code correlation reflectometry. IEEE Sens. J. 2016, 16, 2447–2452. [Google Scholar] [CrossRef]
- Hosoki, A.; Nishiyama, M.; Igawa, H.; Seki, A.; Watanabe, K. A hydrogen curing effect on surface plasmon resonance fiber optic hydrogen sensors using an annealed Au/Ta2O5/Pd multi-layers film. Opt. Express 2014, 22, 18556. [Google Scholar] [CrossRef]
- Wadell, C.; Nugroho, F.A.A.; Lidström, E.; Iandolo, B.; Wagner, J.B.; Langhammer, C. Hysteresis-free nanoplasmonic Pd–Au alloy hydrogen sensors. Nano Lett. 2015, 15, 3563–3570. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Silkin, V.M.; Muiño, R.D.; Chernov, I.P.; Chulkov, E.V.; Echenique, P.M. Tuning the plasmon energy of palladium–hydrogen systems by varying the hydrogen concentration. J. Phys. Condens. Matter 2012, 24, 104021. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, C.; Zorić, I.; Kasemo, B.; Clemens, B.M. Hydrogen storage in Pd nanodisks characterized with a novel nanoplasmonic sensing scheme. Nano Lett. 2007, 7, 3122–3127. [Google Scholar] [CrossRef]
- Zoric´, I.; Larsson, E.M.; Kasemo, B.; Langhammer, C. Localized surface plasmons shed light on nanoscale metal hydrides. Adv. Mater. 2010, 22, 4628–4633. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.M.; Edvardsson, M.E.M.; Langhammer, C.; Zorić, I.; Kasemo, B. A combined nanoplasmonic and electrodeless quartz crystal microbalance setup. Rev. Sci. Instrum. 2009, 80, 125105. [Google Scholar] [CrossRef]
- Wadell, C.; Langhammer, C. Drift-corrected nanoplasmonic hydrogen sensing by polarization. Nanoscale 2015, 7, 10963–10969. [Google Scholar] [CrossRef] [PubMed]
- Poyli, M.A.; Silkin, V.M.; Chernov, I.P.; Echenique, P.M.; Muiño, R.D.; Aizpurua, J. Multiscale theoretical modeling of plasmonic sensing of hydrogen uptake in palladium nanodisks. J. Phys. Chem. Lett. 2012, 3, 2556–2561. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, C.; Larsson, E.M.; Zhdanov, V.P.; Zorić, I. Asymmetric hysteresis in nanoscopic single-metal hydrides: Palladium nanorings. J. Phys. Chem. C 2012, 116, 21201–21207. [Google Scholar] [CrossRef]
- Niu, W.; Zhang, W.; Firdoz, S.; Lu, X. Controlled synthesis of palladium concave nanocubes with sub-10-nanometer edges and corners for tunable plasmonic property. Chem. Mater. 2014, 26, 2180–2186. [Google Scholar] [CrossRef]
- Sil, D.; Gilroy, K.D.; Niaux, A.; Boulesbaa, A.; Neretina, S.; Borguet, E. Seeing is believing: Hot electron based gold nanoplasmonic optical hydrogen sensor. ACS Nano 2014, 8, 7755–7762. [Google Scholar] [CrossRef]
- Mukherjee, S.; Libisch, F.; Large, N.; Neumann, O.; Brown, L.V.; Cheng, J.; Lassiter, J.B.; Carter, E.A.; Nordlander, P.; Halas, N.J. Hot electrons do the impossible: Plasmon-induced dissociation of H2 on Au. Nano Lett. 2013, 13, 240–247. [Google Scholar] [CrossRef]
- Strohfeldt, N.; Tittl, A.; Schäferling, M.; Neubrech, F.; Kreibig, U.; Griessen, R.; Giessen, H. Yttrium hydride nanoantennas for active plasmonics. Nano Lett. 2014, 14, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Steffes, H.; Chabicovsky, R.; Haruta, M.; Stangl, G. Optical and electrical H2 and NO2 sensing properties of Au/InxOyNz films. IEEE Sens. J. 2004, 4, 232–236. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M.L.; Hentschel, M.; Giessen, H.; Alivisatos, A.P. Nanoantenna-enhanced gas sensing in a single tailored nanofocus. In CLEO:2011-Laser Applications to Photonic Applications; OSA: Washington, DC, USA, 2011. [Google Scholar]
- Shegai, T.; Langhammer, C. Hydride formation in single palladium and magnesium nanoparticles studied by nanoplasmonic dark-field scattering spectroscopy. Adv. Mater. 2011, 23, 4409–4414. [Google Scholar] [CrossRef]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Pundt, A.; Sachs, C.; Winter, M.; Reetz, M.; Fritsch, D.; Kirchheim, R. Hydrogen sorption in elastically soft stabilized Pd-clusters. J. Alloys Compd. 1999, 293–295, 480–483. [Google Scholar] [CrossRef]
- Züttel, A.; Nützenadel, C.; Schmid, G.; Chartouni, D.; Schlapbach, L. Pd-cluster size effects of the hydrogen sorption properties. J. Alloys Compd. 1999, 293–295, 472–475. [Google Scholar] [CrossRef]
- Nugroho, F.A.A.; Eklund, R.; Nilsson, S.; Langhammer, C. A fiber-optic nanoplasmonic hydrogen sensor via pattern-transfer of nanofabricated PdAu alloy nanostructures. Nanoscale 2018, 10, 20533–20539. [Google Scholar] [CrossRef] [PubMed]
- Darmadi, I.; Nugroho, F.A.A.; Kadkhodazadeh, S.; Wagner, J.B.; Langhammer, C. Rationally designed PdAuCu ternary alloy nanoparticles for intrinsically deactivation-resistant ultrafast plasmonic hydrogen sensing. ACS Sensors 2019, 4, 1424–1432. [Google Scholar] [CrossRef]
- Matuschek, M.; Singh, D.P.; Jeong, H.-H.; Nesterov, M.; Weiss, T.; Fischer, P.; Neubrech, F.; Liu, N. Chiral plasmonic hydrogen sensors. Small 2018, 14, 1702990. [Google Scholar] [CrossRef]
- Hosoki, A.; Nishiyama, M.; Igawa, H.; Choi, Y.; Watanbea, K. Surface plasmon resonance hydrogen sensor based on hetero-core optical fiber structure. Procedia Eng. 2012, 47, 128–131. [Google Scholar] [CrossRef]
- Bhatia, P.; Gupta, B.D. Surface plasmon resonance based fiber optic hydrogen sensor utilizing wavelength interrogation. In Proceedings of the Third Asia Pacific Optical Sensors Conference, Sydney, Australia, 30 January 2012. [Google Scholar]
- Wang, X.; Tang, Y.; Zhou, C.; Liao, B. Design and optimization of the optical fiber surface plasmon resonance hydrogen sensor based on wavelength modulation. Opt. Commun. 2013, 298–299, 88–94. [Google Scholar] [CrossRef]
- Song, H.; Luo, Z.; Liu, M.; Zhang, G.; Peng, W.; Wang, B.; Zhu, Y. Centrifugal deposited Au-Pd core-shell nanoparticle film for room-temperature optical detection of hydrogen gas. Sensors 2018, 18, 1448. [Google Scholar] [CrossRef]
- Downes, F.; Taylor, C.M. Theoretical investigation of a multi-channel optical fiber surface plasmon resonance hydrogen sensor. Opt. Commun. 2021, 490, 126916. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Cao, W.; Wang, M.; Hao, H.; Xia, W.; Su, F. Fiber optic coupled surface plasmon resonance sensor based Ag-TiO2 films for hydrogen detection. Opt. Fiber Technol. 2021, 65, 102616. [Google Scholar] [CrossRef]
- Luo, J.; Liu, S.; Chen, P.; Lu, S.; Zhang, Q.; Chen, Y.; Du, B.; Tang, J.; He, J.; Liao, C.; et al. Fiber optic hydrogen sensor based on a Fabry–Perot interferometer with a fiber Bragg grating and a nanofilm. Lab Chip 2021, 21, 1752–1758. [Google Scholar] [CrossRef]
- King, W.H. Piezoelectric sorption detector. Anal. Chem. 1964, 36, 1735–1739. [Google Scholar] [CrossRef]
- Anisimkin, V.; Kotelyanskii, I.; Verardi, P.; Verona, E. Elastic properties of thin-film palladium for surface acoustic wave (SAW) sensors. Sens. Actuators B Chem. 1995, 23, 203–208. [Google Scholar] [CrossRef]
- Colin Campbell. Surface Acoustic Wave Devices and Their Signal Processing Applications; Academic Press: London, UK, 1989. [Google Scholar]
- Liu, B.; Chen, X.; Cai, H.; Ali, M.M.; Tian, X.; Tao, L.; Yang, Y.; Ren, T. Surface acoustic wave devices for sensor applications. J. Semicond. 2016, 37, 21001. [Google Scholar] [CrossRef]
- Lucklum, R. Acoustic wave-based sensors. Meas. Sci. Technol. 2003, 14, E01. [Google Scholar] [CrossRef]
- Jakubik, W.P.; Urbańczyk, M.W.; Kochowski, S.; Bodzenta, J. Bilayer structure for hydrogen detection in a surface acoustic wave sensor system. Sens. Actuators B Chem. 2002, 82, 265–271. [Google Scholar] [CrossRef]
- Marcu, A.; Viespe, C. Surface acoustic wave sensors for hydrogen and deuterium detection. Sensors 2017, 17, 1417. [Google Scholar] [CrossRef]
- Huang, F.-C.; Chen, Y.-Y.; Wu, T.-T. A room temperature surface acoustic wave hydrogen sensor with Pt coated ZnO nanorods. Nanotechnology 2009, 20, 065501. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Tourlog, A. Propagation characteristics of leaky surface acoustic waves and surface acoustic waves on LiNbO3 substrates with a ferrroelectric inversion layer. Jpn. J. Appl. Phys. 1995, 34, 5273–5275. [Google Scholar] [CrossRef]
- Fechete, A.C.; Wlodarski, W.; Kalantar-Zadeh, K.; Holland, A.S.; Antoszewski, J.; Kaciulis, S.; Pandolfi, L. SAW-based gas sensors with rf sputtered InOx and PECVD SiNx films: Response to H2 and O3 gases. Sens. Actuators B Chem. 2006, 118, 362–367. [Google Scholar] [CrossRef]
- D’Amico, A.; Palma, A.; Verona, E. Surface acoustic wave hydrogen sensor. Sens. Actuators 1982, 3, 31–39. [Google Scholar] [CrossRef]
- Li, D.; Le, X.; Pang, J.; Peng, L.; Xu, Z.; Gao, C.; Xie, J. A SAW hydrogen sensor based on decoration of graphene oxide by palladium nanoparticles on AlN/Si layered structure. J. Micromechanics Microengineering 2019, 29, 045007. [Google Scholar] [CrossRef]
- Caliendo, C.; Imperatori, P. High-frequency, high-sensitivity acoustic sensor implemented on AlN/Si substrate. Appl. Phys. Lett. 2003, 83, 1641–1643. [Google Scholar] [CrossRef]
- Hu, F.; Cheng, L.; Fan, S.; Xue, X.; Liang, Y.; Lu, M.; Wang, W. Chip-level orthometric surface acoustic wave device with AlN/metal/Si multilayer structure for sensing strain at high temperature. Sens. Actuators A Phys. 2022, 333, 113298. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Mei, S.; Jia, Y.; Liu, M.; Xue, X.; Yang, D. Development of a Pd/Cu nanowires coated SAW hydrogen gas sensor with fast response and recovery. Sens. Actuators B Chem. 2019, 287, 157–164. [Google Scholar] [CrossRef]
- Devkota, J.; Mao, E.; Greve, D.W.; Ohodnicki, P.R.; Baltrus, J. A surface acoustic wave hydrogen sensor with tin doped indium oxide layers for intermediate temperatures. Sens. Actuators B Chem. 2022, 354, 131229. [Google Scholar] [CrossRef]
- Wan, J.K.S.; Ioffe, M.S.; Depew, M.C. A novel acoustic sensing system for on-line hydrogen measurements. Sens. Actuators B Chem. 1996, 32, 233–237. [Google Scholar] [CrossRef]
- Lomperski, S.; Anselmi, M.; Huhtiniemi, I. Ultrasonic and resistive hydrogen sensors for inert gas-water vapour atmospheres. Meas. Sci. Technol. 2000, 11, 518–525. [Google Scholar] [CrossRef]
- Wang, X.; Du, L.; Cheng, L.; Zhai, S.; Zhang, C.; Wang, W.; Liang, Y.; Yang, D.; Chen, Q.; Lei, G. Pd/Ni nanowire film coated SAW hydrogen sensor with fast response. Sens. Actuators B Chem. 2022, 351, 130952. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, C.L.; Yang, F.; Gong, H.; Wang, D.N.; Dai, J.; Yang, M. Sagnac interferometer hydrogen sensor based on panda fiber with Pt-loaded WO3/SiO2 coating. Opt. Lett. 2016, 41, 1594. [Google Scholar] [CrossRef]
- Liu, Y.; Rahman, B.M.A.; Grattan, K.T.V. Thermal-stress-induced birefringence in bow-tie optical fibers. Appl. Opt. 1994, 33, 5611. [Google Scholar] [CrossRef] [PubMed]
- Darmadi, I.; Stolaś, A.; Östergren, I.; Berke, B.; Nugroho, F.A.A.; Minelli, M.; Lerch, S.; Tanyeli, I.; Lund, A.; Andersson, O.; et al. Bulk-processed Pd nanocube–poly(methyl methacrylate) nanocomposites as plasmonic plastics for hydrogen sensing. ACS Appl. Nano Mater. 2020, 3, 8438–8445. [Google Scholar] [CrossRef]
- Wang, G.; Dai, J.; Yang, M. Fiber-optic hydrogen sensors: A review. IEEE Sens. J. 2021, 21, 12706–12718. [Google Scholar] [CrossRef]
- Villatoro, J.; Luna-Moreno, D.; Monzón-Hernández, D. Optical fiber hydrogen sensor for concentrations below the lower explosive limit. Sens. Actuators B Chem. 2005, 110, 23–27. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Kishor, K.; Sharma, A.C. Tapered optical fiber geometries and sensing applications based on Mach-Zehnder Interferometer: A review. Opt. Fiber Technol. 2020, 58, 102302. [Google Scholar] [CrossRef]
- Summary and Findings from the NREL/DOE Hydrogen Sensor Workshop (8 June 2011); Report No.: NREL/TP-5600-55645, 1048994; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2012. Available online: https://www.osti.gov/biblio/1048994 (accessed on 9 January 2023).
- Hydrogen Safety Sensor Performance and Use Gap Analysis: Preprint. 15 November 2017; Report No.: NREL/CP-5400-68773. Available online: https://www.osti.gov/biblio/1409729 (accessed on 9 January 2023).




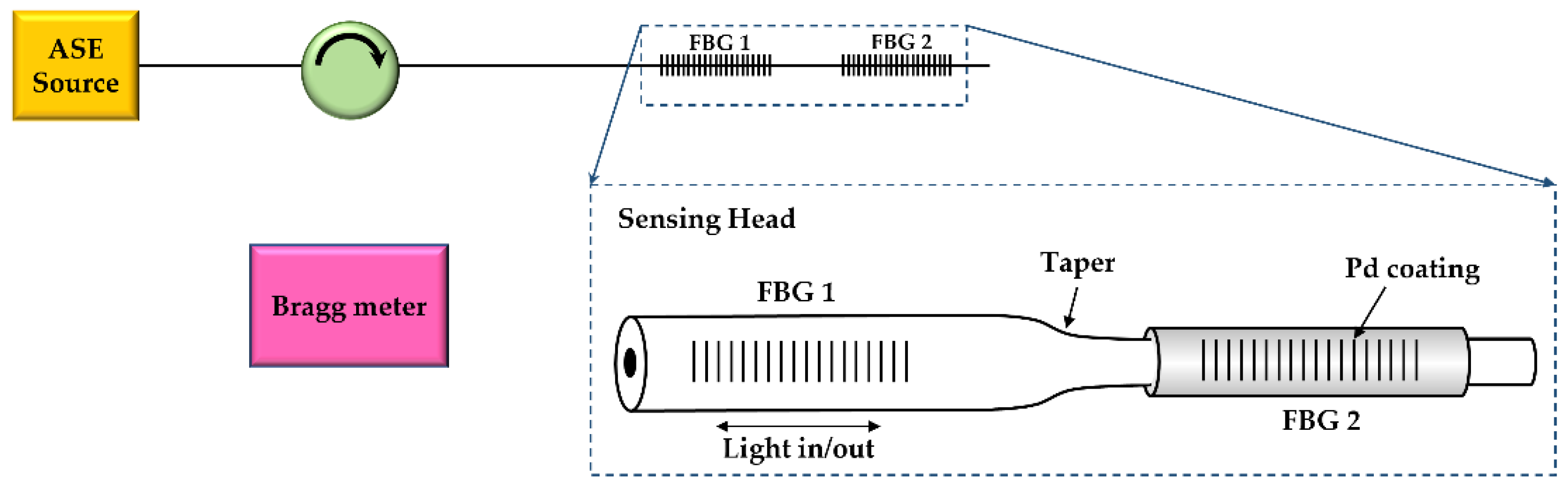
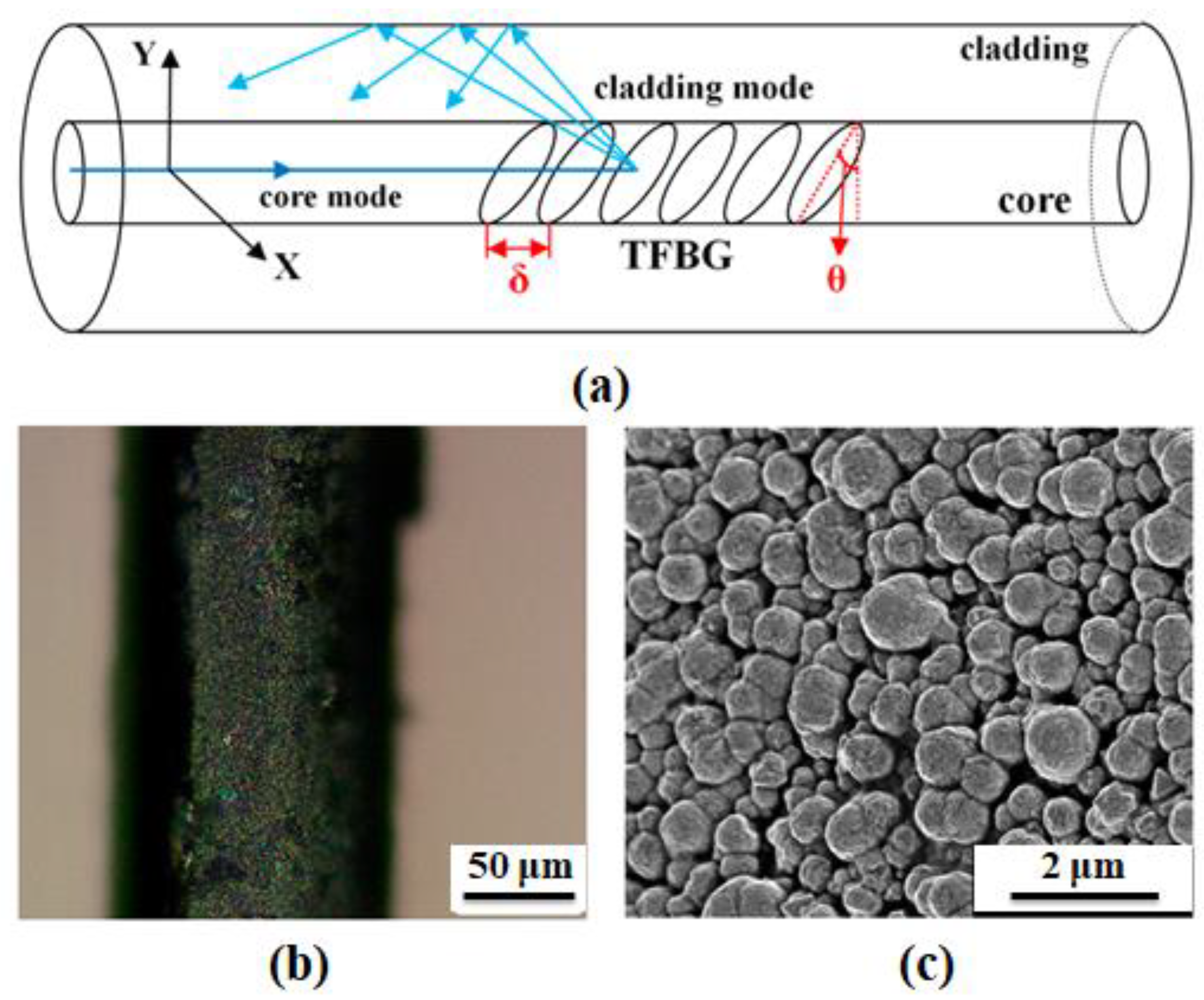

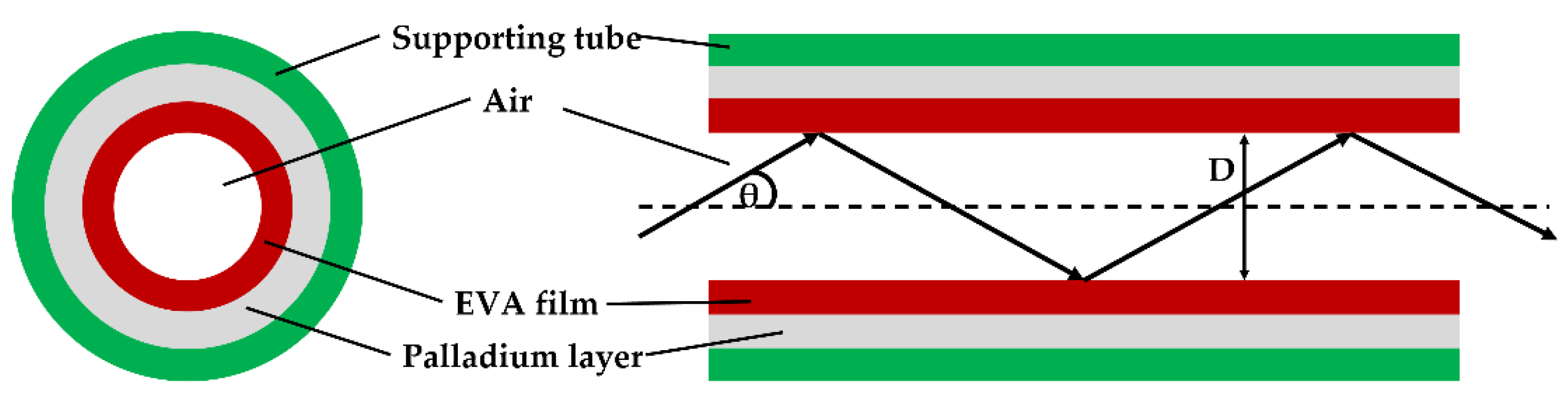
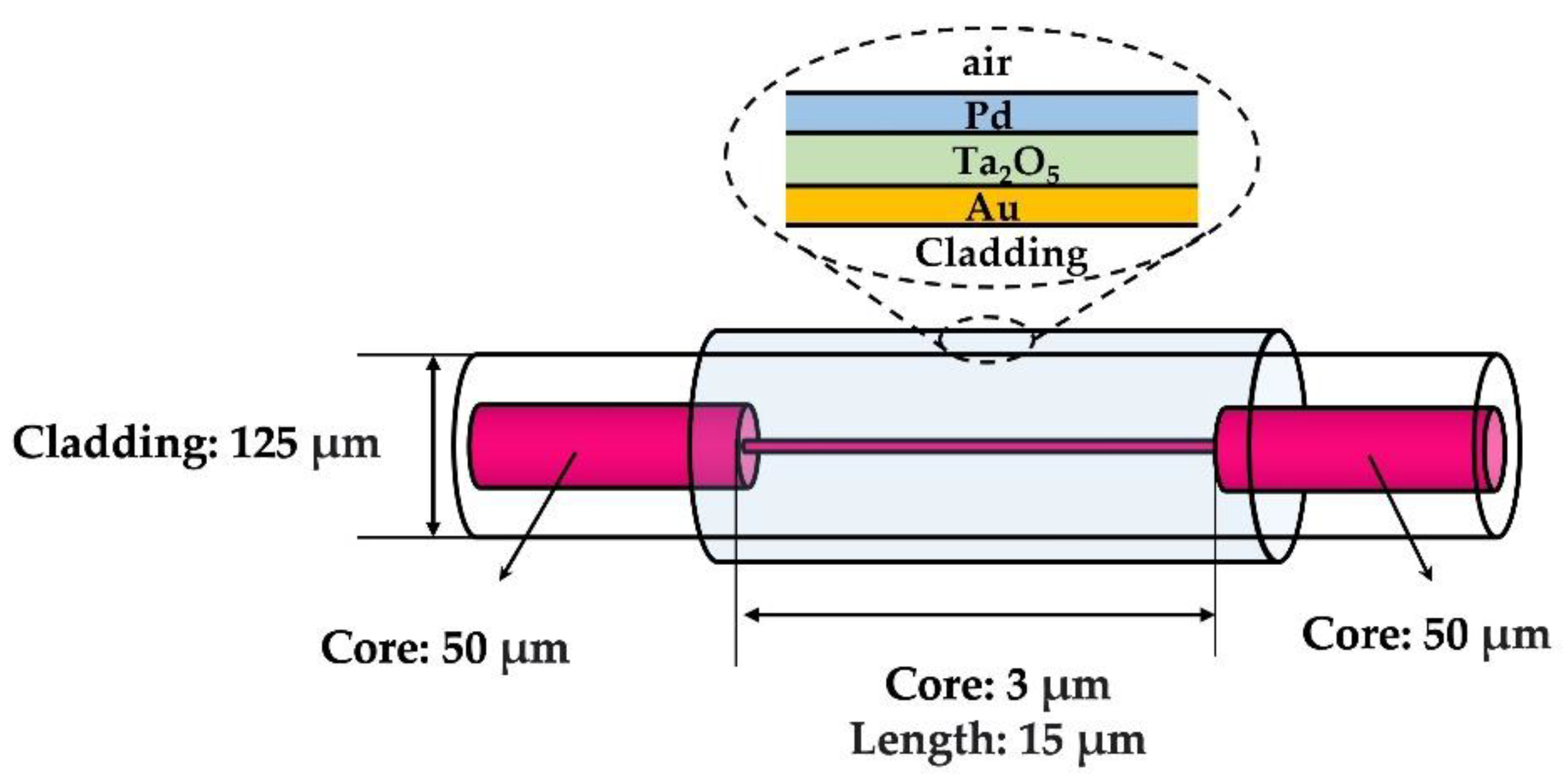


| Sensing Material | Advantage | Limitations | Commercialization |
|---|---|---|---|
| WO3 |
|
|
|
|
|
|
| Sensing Material | Sensor | Detection Range | Sensitivity | Response Time | LOD | Reference |
|---|---|---|---|---|---|---|
| Pd film | Etched MMF | 0.2% 0.6% | NA | 30 s 20 s | N/A | [64] |
| Pd/WO3 | Etched MMF | 100% | NA | 10–20 min | N/A | [65] |
| Pd film | Tapered SMF | 0–10% | NA | <100 s | 4% | [66] |
| Pd film | Polished SMF | 4% | NA | Response time 100 s/recovery time 150 s | 4% | [67] |
| Pd/Au film | SMF sandwiched by 2 MMFs | <4% | NA | 15 s | N/A | [68] |
| Pd/Au multilayer stack | Hetero core | 4% | NA | Response 4.5 s/recovery 13 s | 4% | [69] |
| Pd particle embedded in PMMA | Tapered SMF | 0.2–1% | 5.58 nm/% | 5 s | 35.8 ppm | [70] |
| Molybdenum Trioxide | Tapered MMF | 0.125–2.00% | 11.96 vol/% | 220 s | N/A | [74] |
| Pd particle | Tapered MMF | 2% | NA | Response 50 s/ Recovery 230 s | 2% | [72] |
| Pd | Tapered SMF | 1.8–10% | NA | <100 s | 2% | [75] |
| Pt/WO3 | Etched MMF | 1% | NA | 5 min | N/A | [76] |
| Pd/Au | Etched D-shape fiber | 0.25–20% | NA | ≈30 s | N/A | [77] |
| Au capped with Pd | MMF | 0.8–4% | NA | 116 s | 0.086% | [63] |
| Sensing Material | Sensor | Detection Range | Sensitivity | Response Time | LOD | Reference |
|---|---|---|---|---|---|---|
| Pd:Ag = 76:24 | Etched FBG | 4% in volume | 10 pm/% | 280–300 s | 4% | [53] |
| Pd:Ni = 91:9 | Etched FBG | 0.5–4% | 36.5 pm/% | 5–6 min | N/A | [56] |
| Pd film | LPFG | 4% | NA | NA | NA | [69] |
| Pd film | LPFG | 4% | −0.29 nm/min | NA | NA | [70] |
| Pd film | FBG/w HAF | 1–10% | 27 pm/% | - | N/A | [83] |
| Pd | Tapered FBG | 0.1–1% (v/v) | 81.8 pm/% | 30 s | N/A | [87] |
| Pd/Ti/polyimide | FBG | 0.25–2% | 13.5 ppm/pm | ≈1 h | N/A | [88] |
| Pd/Ti/polyimide | FBG | 1791.46 ppm | 0.042–0.044 pm/ppm | - | N/A | [89] |
| Pd91Ni9 | Etched FBG | 1% | 15 pm/% | 5 min | N/A | [91] |
| Pd/Ag | FBG | 0–2000 µL/L | 0.055 pm/(µL/L) | 24 min | 18 µL/L | [92] |
| Pd:Cr = 58:42 (with TiO2) | Standard FBG | 0–650 ppm | NA | 10 min | 4% | [93] |
| Pd/WO3 | Polished FBG | 0–8% | 6.5 pm/% | 40–90 s | N/A | [99] |
| Pd/WO3 | Polished FBG | 0.2–1.4% | 196 µL/L | - | N/A | [100] |
| Pd | FBG | 1%/5% | NA | Response 20–30 min/recovery 50 min | 1% | [102] |
| Pd foil | Etched FBG | 1–5% | 212.6 pm/% | 4 h | 1% | [103] |
| Pd membrane | Titled FBG | 1–4% | NA | 5 min | 1% | [104] |
| Pd film | FBG | 4% | NA | 2 min | N/A | [106] |
| Pt/WO3 | FBG | 1500–20000 ppm | NA | 55–80 s | N/A | [107] |
| Pd/Ag | Side-polished FBG | 0.08% | 4770 pm/% | <1 h | N/A | [108] |
| Pd | Two Etched FGBs | 1% | 20 pm/% | 2 min | 1% | [109] |
| Pd75Ag25/Ni | FBG/w microgroove | 0–4% | 16.5 pm/% | 10 min | N/A | [110] |
| Pd | Tapered FBG | 5% | 216 pm/% | 1 min | N/A | [111] |
| Pd91Ni9 | FBG | 0–1% | 0.01 pm/ppm | ≈200 s | 4% | [112] |
| Pt/MoO3 | FBG | 1500–15000 ppm | 0.022–0.031 pm/ppm | Response 100 s/recovery 110 s | N/A | [113] |
| Sensing Material | Sensor | Detection Range | Sensitivity | Response Time | LOD | Reference |
|---|---|---|---|---|---|---|
| Pd film | SMF-PCF | 0–5% | NA | - | N/A | [114] |
| Pd/Ag | PM-PCF | 1–4% | 131 pm/% | - | N/A | [115] |
| Pd/WO3 | Fiber loop mirror-PCF | 0–1% | 1.12 nm/% | - | 0.14% | [116] |
| Pd/WO3 | Ring laser-PCF | 0–1% | 1.28 nm/% | - | 0.0133% | [117] |
| Pd particle embedded | PM-Panda | 100% | NA | 20 h | N/A | [118] |
| EVA/Pd | Hollow core fiber (HCF) | 0–4% | 2.66 nm/% | - | N/A | [119] |
| Au/Pd/WO3 | PCF | 0–3% | 0.19 nm/% | - | N/A | [120] |
| Pd/WO3 | PCF | 0–10,000 μL/L | 0.109 pm/(μL/L) | 33 min | N/A | [121] |
| Pd-Pt | Hollow core-PCF | 0–100% | NA | 46 s | N/A | [122] |
| Pd | Taper-PCF | 0–6% | NA | - | N/A | [123] |
| Pd-Au-Graphene | Capillary-HCF | 0–1000 ppm | NA | 120 s | 741 ppb | [124] |
| NA | Tellurite PCF | 0–3% | −0.236 nm/% | - | 2500 ppm | [125] |
| Sensing Material | Platform | Detection Range | Sensitivity | Response Time | LOD | Reference |
|---|---|---|---|---|---|---|
| Au-IRMOF-20 | MMF | 0–50% | NA | 5 s–10 s | N/A | [10] |
| Ag/SiO2/Pd | MMF | 4% | NA | - | N/A | [36] |
| Ag/SiO2/PdY | MMF | 4% | NA | NA | NA | [37] |
| Ag/ZnO(1-x) Pdx | MMF | 4% | NA | 1 min | N/A | [128] |
| Au/silica/Pd | MMF | 0.5–4% | NA | 15 s | 0.5% | [131] |
| Au/Ta2O5/Pd | Hetero core | 4% | NA | 15 s | N/A | [132] |
| Au/Ta2O5/Pd | MMF | 4% | NA | 25 s | N/A | [133] |
| Pd75Au25 | MMF | 0–4% | NA | Response time 90 s/Recovery time 10 s | N/A | [154] |
| Au/Ta2O5/Pd | Hetero-core fiber | 0–4% | NA | 40 s | N/A | [157] |
| Ag/Si/Pd | Plastic clad MMF | 4% | NA | - | N/A | [158] |
| Ag/Si/WO3/Pt | MMF | 2% | NA | - | N/A | [159] |
| Au-Pd nano cube | Fiber bundle | 4% | NA | Response time 30 s/recovery time 4 s | N/A | [160] |
| Ag/SiO2/Pd | MMF | 0–4% | NA | - | N/A | [161] |
| Ag/TiO2 | MMF | 14.7% | 523 nW/% | - | N/A | [162] |
| Graphene-Au-Pd nanofilm | FBG | 0–4.5%, | 290 pm/% | - | N/A | [163] |
| Parameter | System Safety Requirements |
|---|---|
| Detection range | 0.1 to 10 vol% |
| Operating temperature | −30 °C to 80 °C |
| Gas environment | Ambient air, 10% to 98% relative humidity |
| Accuracy | 5% of full scale |
| Response time | ˂1 s |
| Life time | 10 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathak, A.K.; Verma, S.; Sakda, N.; Viphavakit, C.; Chitaree, R.; Rahman, B.M.A. Recent Advances in Optical Hydrogen Sensor including Use of Metal and Metal Alloys: A Review. Photonics 2023, 10, 122. https://doi.org/10.3390/photonics10020122
Pathak AK, Verma S, Sakda N, Viphavakit C, Chitaree R, Rahman BMA. Recent Advances in Optical Hydrogen Sensor including Use of Metal and Metal Alloys: A Review. Photonics. 2023; 10(2):122. https://doi.org/10.3390/photonics10020122
Chicago/Turabian StylePathak, Akhilesh Kumar, Sneha Verma, Natsima Sakda, Charusluk Viphavakit, Ratchapak Chitaree, and B. M. Azizur Rahman. 2023. "Recent Advances in Optical Hydrogen Sensor including Use of Metal and Metal Alloys: A Review" Photonics 10, no. 2: 122. https://doi.org/10.3390/photonics10020122
APA StylePathak, A. K., Verma, S., Sakda, N., Viphavakit, C., Chitaree, R., & Rahman, B. M. A. (2023). Recent Advances in Optical Hydrogen Sensor including Use of Metal and Metal Alloys: A Review. Photonics, 10(2), 122. https://doi.org/10.3390/photonics10020122









